The transcription factor nuclear factor erythroid-2 (NF-E2) is overexpressed in the majority of polycythemia vera (PV) patients. In this study, elevation of NF-E2 expression in healthy CD34+ cells to levels observed in PV caused Epo-independent erythroid maturation and expansion of hematopoietic stem cell (HSC) and common myeloid progenitor (CMP) cell numbers. Silencing NF-E2 in PV patients reverted both aberrancies, demonstrating for the first time that NF-E2 overexpression is both required and sufficient for Epo independence and HSC/CMP expansion in PV.
Keywords: Adult hematopoietic stem cells, Hematopoietic progenitors, Myeloproliferative disorders, Transcription factors
Abstract
The molecular etiology of polycythemia vera (PV) remains incompletely understood. Patients harbor increased numbers of hematopoietic stem cells and display Epo-independent erythroid maturation. However, the molecular mechanism underlying Epo hypersensitivity and stem cell expansion is unclear. We have previously shown that the transcription factor nuclear factor erythroid-2 (NF-E2) is overexpressed in the majority of PV patients. Here we demonstrated that elevation of NF-E2 expression in healthy CD34+ cells to levels observed in PV caused Epo-independent erythroid maturation and expansion of hematopoietic stem cell (HSC) and common myeloid progenitor (CMP) cell numbers. Silencing NF-E2 in PV patients reverted both aberrancies, demonstrating for the first time that NF-E2 overexpression is both required and sufficient for Epo independence and HSC/CMP expansion in PV.
Introduction
Despite advances incurred through the discovery of a point mutation in the JAK2 kinase (JAK2V617F), the molecular etiology of polycythemia vera (PV) remains incompletely understood. Analyzing both purified cell populations and individual colonies grown from sorted populations, several groups have demonstrated that the JAK2V617F mutation occurs at the level of the hematopoietic stem cell (HSC) in PV patients [1, 2]. Furthermore, Jamieson et al. have shown that PV patients harbor increased numbers of cells with an HSC phenotype (defined as CD34+, CD38−, CD90+, Lin−) and that PV patients with leukocytosis in addition show an increase in the number of common myeloid progenitors (CMPs) [2]. Therefore, the stem and progenitor pools are expanded in PV patients, but the authors hypothesize that additional alterations “are likely responsible … for expansion of the CMP compartment, … as has been demonstrated with other [myeloproliferative diseases]” [2]. Anand et al. recently reported that the presence of JAK2V617F alone does not alter HSC compartment size and likewise suggested “regulation of the [hematopoietic stem and progenitor cell] compartment through additional mechanisms” [3].
Epo-independent colony formation (so-called endogenous erythroid colonies [EECs]) is a pathognomonic hallmark of PV, and CD34+ cells from PV patients display Epo-independent erythroid differentiation. In cell line and murine models, expression of mutant JAK2V617F is clearly sufficient to promote Epo-independent growth and colony formation [4, 5]. However, the molecular mechanism by which the JAK2V617F mutation elicits these aberrations remains unclear. Several lines of evidence support the hypothesis that additional aberrations, either preceding or following acquisition of the JAK2V617F mutation, contribute to the pathophysiology of these disorders [6–8].
We have recently shown that nuclear factor erythroid-2 (NF-E2), a hematopoietic transcription factor, is overexpressed in a large majority of patients with PV and other myeloproliferative neoplasms (MPNs) [9, 10]. In a murine model, elevated NF-E2 levels lead to an MPN phenotype with expansion of the stem and progenitor compartments [11]. Furthermore, in the presence of Epo, NF-E2 overexpression in isolated human CD34+ cells alters erythroid differentiation, leading to a significant increase in the number of mature erythroid progeny generated per CD34+ cell [12]. However, it is unknown whether NF-E2 can promote either Epo independence or stem cell expansion in human cells. We therefore investigated whether, in the absence of Epo, elevated NF-E2 levels in CD34+ cells contribute to Epo-independent erythroid differentiation and, furthermore, promote the expansion of the HSC and CMP compartment observed in PV patients.
Materials and Methods
MPN Patients and Healthy Controls
Peripheral blood samples were obtained from therapeutic phlebotomies of PV patients fulfilling the World Health Organization criteria for diagnosis [13], as well as from patients with secondary polycythemia due to histologically documented pulmonary fibrosis or chronic obstructive pulmonary disease. Buffy coats of healthy volunteer blood donors were obtained from the University Hospital Freiburg Center for Blood Transfusion. The study protocol was approved by the local ethics committees, and informed consent was obtained from all patients in accordance with the Declaration of Helsinki. All patients were tested for the presence of the JAK2V617F mutation by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) as previously described [14].
CD34+ Cell and Granulocyte Isolation
CD34+ cells and peripheral blood granulocytes were isolated as previously described [12].
Cell Culture and Lentiviral Infections
CD34+ cells were cultured in StemSpan serum-free expansion medium supplemented with 20 ng/ml thrombopoietin (TPO), 100 ng/ml stem cell factor (SCF), 100 ng/ml FMS-related-tyrosine-kinase-3-ligand (Flt3L), and 20 ng/ml interleukin-6 (IL6) (all from Peprotech, Hamburg, Germany, http://www.peprotech.com). Viral transduction was performed using pLeGO-iG or pLeGO-G [15] expressing the human NF-E2 cDNA or a short hairpin RNA (shRNA) against NF-E2, respectively, as previously described [16]. Green fluorescence protein-expressing cells were sorted by fluorescence-activated cell sorting (FACS) to obtain pure populations.
FACS Analysis
Progenitor populations were determined by FACS staining using the following antibodies: CD34-PacificBlue (catalog no. 343511; BioLegend, San Diego, CA, http://www.biolegend.com), CD38-Alexa Fluor 700 (catalog no. 303523; BioLegend), CD45RA-APC (catalog no. MAB4624; Abnova, Taipei City, Taiwan, http://www.abnova.com), CD123-PE (catalog no. 555644; BD Biosciences, San Diego, CA, http://www.bdbiosciences.com), Flt3R (CD135)-biotin (catalog no. 313312; BioLegend) revealed with streptavidin-PE/Cy7 (catalog no. 405206; BioLegend), and Thy-1 (CD90)-PerCP-Cy5.5 (catalog no. 561557; BD Biosciences). Erythroid differentiation was determined with CD36-APC (catalog no. 550956; BD Pharmingen, San Diego, CA, http://www.bdbiosciences.com) and CD235a-PE (catalog no. 555570; BD Pharmingen).
qRT-PCR Assays
NF-E2-specific qRT-PCR was done as previously described [10].
Data Analysis
The Student t test (paired and unpaired, two-sided) was used to determine whether a significant (p < .05) difference existed between two groups. These analyses were performed using GraphPad Prism 5.0 software (GraphPad Software, Inc., San Diego, CA, http://www.graphpad.com).
Results and Discussion
NF-E2 Overexpression in CD34+ Cells Promotes Epo-Independent Erythroid Maturation
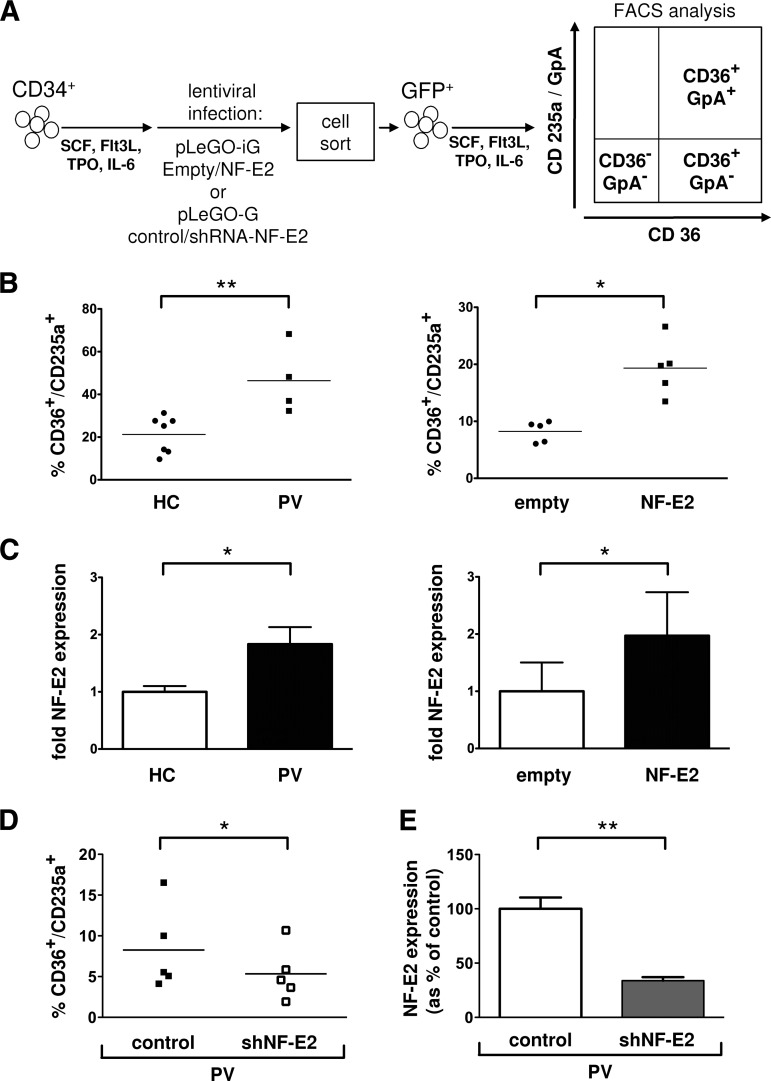
In order to test our hypothesis that elevated NF-E2 levels can promote Epo-independent erythroid maturation in human cells, we transduced purified healthy control CD34+ cells either with an empty control pLeGO-iG lentivirus or with pLeGO-iG-expressing human NF-E2 (pLeGO-iG-hNF-E2). Subsequently, cells were FACS sorted to obtain populations of >98% transduced cells and cultured in a cytokine mix lacking Epo (SCF, Flt3L, IL6, TPO) for 8 days. We assessed erythroid maturation in the liquid culture by FACS, using CD36 and GpA (Glycophorin A; CD235a), as established by Dr. Vainchenker's laboratory [17, 18] (Fig. 1A).
Figure 1.
NF-E2 overexpression in CD34+ cells promotes Epo-independent erythroid differentiation. (A): Experimental design: NF-E2 overexpression or silencing. Peripheral blood CD34+ cells were transduced either with pLeGO-iG-NF-E2 (NF-E2) controlled for by an empty pLeGO-iG (empty) or with pLeGO-G-shRNA-NF-E2 (shNF-E2) controlled for by a pLeGO-G control (control), sorted for GFP expression, and cultured in SCF, Flt3 ligand, IL6, and TPO for 8 days. (B): Erythroid differentiation. Cells were analyzed for CD36 and GpA expression by FACS on day 8 following lentiviral transduction. The percentage of CD36+/GpA+ double-positive erythroid cells is shown. Mean and SE of n = 7 healthy controls and n = 4 PV patients (left panel) and of n = 5 independent experiments of NF-E2 overexpression (right panel) are shown. *, p < .05; **, p < .01. (C): NF-E2 mRNA expression was determined by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) on day 8. Mean and SE of n = 6 healthy controls and n = 3 PV patients (left panel) and five independent experiments (right panel) are shown. *, p < .05. (D): NF-E2 silencing. CD34+ cells of n = 5 PV patients were transduced either with control pLeGO-G or the NF-E2 silencing pLeGO-G-shNF-E2 and analyzed for CD36 and GpA expression by FACS on day 8 following lentiviral transduction. The percentage of CD36+/GpA+ double-positive erythroid cells is shown. Mean and SE of n = 5 PV patients, analyzed in five independent experiments, are shown. *, p < .05. (E): NF-E2 mRNA expression was determined by qRT-PCR on day 8. Mean and SE of five independent experiments are shown. **, p < .01. Abbreviations: FACS, fluorescence-activated cell sorting; Flt3L, FMS-related-tyrosine-kinase-3 ligand; GFP, green fluorescence protein; HC, healthy controls; IL, interleukin; NF-E2, nuclear factor erythroid-2; PV, polycythemia vera; SCF, stem cell factor; shNF-E2, short hairpin RNA against NF-E2; shRNA, short hairpin RNA; TPO, thrombopoietin.
Ugo et al. [17] have previously demonstrated enhanced Epo-independent erythroid maturation of PV patient CD34+ cells in liquid culture, albeit using a different cytokine mixture. We recapitulated this observation in our cytokine mix, observing significantly elevated Epo-independent erythroid maturation of PV patient CD34+ cells compared with healthy control CD34+ cells (Fig. 1B, left panel). Elevation of NF-E2 levels in healthy control CD34+ cells by lentiviral transduction led to a similar increase in Epo-independent erythroid maturation (Fig. 1B, right panel). Since lentiviral transduction can lead to an unphysiologically high expression of the transduced gene, we compared NF-E2 mRNA expression in healthy control and PV CD34+ cells with that in healthy control CD34+ cells transduced with NF-E2 or control virus. Figure 1C shows that NF-E2 levels were elevated a mean of twofold in PV CD34+ cells compared with healthy controls. In addition, we have recently demonstrated a 1.5–3.5-fold increase in NF-E2 levels in FACS-sorted stem and progenitor populations from PV patients (long-term HSCs, short-term HSCs, and granulocyte monocyte progenitors [GMPs]) [11]. The same degree of overexpression was recapitulated in healthy control CD34+ by lentiviral transduction with the hNF-E2 cDNA (Fig. 1C; supplemental online Fig. 1A). Increased NF-E2 expression was not observed in patients with secondary polycythemia due to hypoxia (supplemental online Fig. 1B). However, these data do not demonstrate the specificity of elevated NF-E2 levels in PV compared with other, related hematological disorders. In particular, because of the rarity of these patients, we did not examine polycythemic patients with mutations of the Epo-receptor [19–22] or patients with hypoxia-inducible factor-driven primary polycythemias, such as Chuvash polycythemia [23]. Nonetheless, elevation of NF-E2 expression in healthy control CD34+ cells to the same levels as the NF-E2 overexpression observed in PV CD34+ cells promotes Epo-independent erythroid maturation.
NF-E2 Silencing in PV CD34+ Cells Abrogates Epo-Independent Maturation
If elevated NF-E2 levels contribute critically to Epo-independent maturation, reducing NF-E2 expression by RNA interference technology should abrogate the unphysiological erythroid differentiation of PV. We therefore transduced isolated CD34+ cells from PV patients with a lentivirus carrying an shRNA against NF-E2, which we have previously demonstrated to efficiently silence gene expression [12, 16] (pLeGO-G-shNF-E2) or with a control virus carrying an ineffective, scrambled shRNA (pLeGO-G-control). The NF-E2 shRNA reduced NF-E2 gene expression in PV cells to those levels observed in healthy controls (Fig. 1E, and compare Fig. 1E with Fig. 1C). At the same time, the number of erythroid, CD36+/GpA+ double-positive cells produced in the absence of Epo was significantly reduced (Fig. 1D). Hence, elevated NF-E2 levels are required for the Epo-independent erythroid maturation that is a pathognomonic hallmark of PV. Taken together, these data demonstrate that NF-E2 overexpression is both necessary and sufficient for Epo independence.
NF-E2 Overexpression in CD34+ Cells Promotes Expansion of the Number of HSCs, CMPs, and GMPs
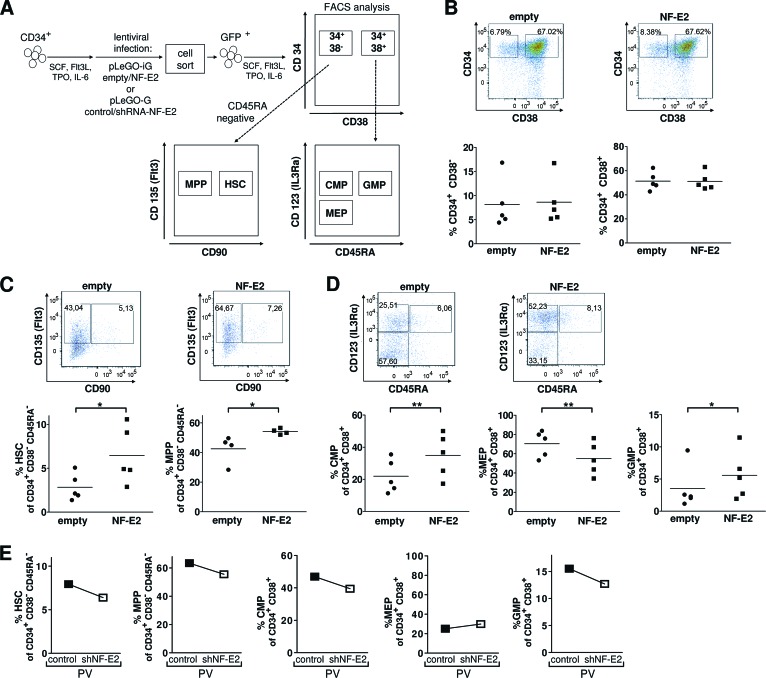
We subsequently used the same liquid culture system to examine the number of HSCs, multipotent progenitors (MPPs), CMPs, megakaryocyte-erythroid progenitors (MEPs), and GMPs elaborated from healthy control CD34+ cells transduced either with empty pLeGO-iG or with pLeGO-iG-hNF-E2. A schematic representation of the cell surface markers and the gating strategy used for FACS analysis is shown in Figure 2A. HSCs, MPPs, CMPs, MEPs, and GMPs were defined as previously described [2, 24, 25].
Figure 2.
NF-E2 overexpression in CD34+ cells promotes expansion of HSCs, MPPs, CMPs, and GMPs. (A): Experimental design and FACS analysis of stem and progenitor populations. CD34+ cells were transduced either with pLeGO-iG-NF-E2 (NF-E2) controlled for by an empty pLeGO-iG (empty) or with pLeGO-G-shRNA-NF-E2 (shNF-E2) controlled for by a pLeGO-G control (control), sorted for GFP expression, and cultured in SCF, Flt3L, IL6, and TPO for 5 days. Stem and progenitor cells were analyzed according to cell surface marker profiles as depicted. (B): CD34 and CD38 staining. Top: representative results of empty and NF-E2 transduced cells; bottom: statistical analysis of n = 5 independent experiments. No significant difference was observed. (C): CD90 and CD135 (FLT3) staining of CD34+/CD38−/CD45RA− cells. Top: representative results of empty and NF-E2-transduced cells; bottom: statistical analysis of n = 4 or 5 independent experiments, respectively. *, p < .05. (D): CD45RA and CD123 (IL3Rα) staining of CD34+/CD38+ cells. Top: representative results of empty and NF-E2 transduced cells; bottom: statistical analysis of n = 5 independent experiments. *, p < .05; **, p < .01. (E): NF-E2 silencing. Primary PV CD34+ cells were transduced either with control pLeGO-G or the NF-E2 silencing pLeGO-G-shNF-E2 and analyzed for the presence of HSCs, MPPs, CMPs, MEPs, and GMPs as detailed in (B–D). Abbreviations: CMP, common myeloid progenitor; FACS, fluorescence-activated cell sorting; Flt3L, FMS-related-tyrosine-kinase-3-ligand; GFP, green fluorescence protein; GMP, granulocyte monocyte progenitor; HSC, hematopoietic stem cell; IL, interleukin IL3Rα, IL-3 receptor α; MEP, megakaryocyte-erythroid progenitor; MPP, multipotent progenitor; NF-E2, nuclear factor erythroid-2; PV, polycythemia vera; SCF, stem cell factor; shNF-E2, short hairpin RNA against NF-E2; shRNA, short hairpin RNA; TPO, thrombopoietin.
Five days following lentiviral transduction, both control and NF-E2 transduced cells contained a similar proportion of CD34+/CD38− cells, as well as CD34+/CD38+ double-positive cells (Fig. 2B). However, CD34+ cells overexpressing NF-E2 gave rise to a significantly higher percentage and absolute number of HSCs compared with control CD34+ cells (Fig. 2C; supplemental online Fig. 2A). In addition, the percentage and absolute number of MPPs were also significantly increased (Fig. 2C; supplemental online Fig. 2A). Among the progenitor cells, both the percentage and the absolute number of CMPs and GMPs were significantly increased (Fig. 2D; supplemental online Fig. 2B). The percentage and number of MEPs decreased correspondingly. Elevation of NF-E2 expression in healthy control CD34+ cells to the levels observed in PV CD34+ cells is therefore sufficient to cause an expansion of the HSC, CMP, and GMP cell numbers, precisely those compartments observed to be enlarged in PV patient samples [2]. Although we have previously demonstrated that NF-E2 overexpression causes HSC and progenitor cell expansion in a murine model [11], this constitutes the first report of an NF-E2 effect on human stem cells.
If elevated NF-E2 levels are required for stem and progenitor cell expansion, reducing NF-E2 expression should abrogate this phenotype. Therefore, we silenced NF-E2 expression in primary PV CD34+ cells, using the NF-E2 shRNA lentivirus described above. Decreased NF-E2 levels led to a reduction in the percentages of HSCs, MPPs, CMPs, and GMPs formed, whereas the MEPs remained nearly unchanged (Fig. 2E). Elevated NF-E2 expression therefore appears to be a prerequisite for aberrant expansion of the stem and progenitor cell compartments. This observation strongly argues that NF-E2 overexpression is both required and sufficient for the stem cell alterations seen in PV patients.
Conclusion
Our data support the hypothesis that the elevated NF-E2 levels observed in PV CD34+ cells contribute to disease pathology by promoting both growth factor independence and stem and progenitor cell expansion. Our data did not address the question of the specificity of elevated NF-E2 levels in PV. However, either the aberrant transcription factor expression itself or its downstream consequences constitute a rational target for novel drug design in PV. We are currently investigating novel NF-E2 target genes in CD34+ cells in order to define pharmacologically amenable targets.
Acknowledgments
We gratefully acknowledge technical assistance by Klaus Geiger in the Core Facility for FACS and by Sven Schwemmers and Laura Siegwart for the NF-E2 qRT-PCR. This work was supported by grants from the National Cancer Institute (P01-CA108671, to H.L.P.) and the Deutsche Forschungsgemeinschaft (Pa 611/6-1, to H.L.P.). H.L.P. is a member of the Myeloproliferative Disease Research Consortium. R.B.'s work was funded by the Excellence Initiative of the German Federal and State Governments (GSC-4, Spemann Graduate School).
Author Contributions
R.B.: conception and design, collection and assembly of data; H.L.P.: conception and design, collection and assembly of data, financial support, manuscript writing.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Lasho TL, Mesa R, Gilliland DG, et al. Mutation studies in CD3+, CD19+ and CD34+ cell fractions in myeloproliferative disorders with homozygous JAK2(V617F) in granulocytes. Br J Haematol. 2005;130:797–799. doi: 10.1111/j.1365-2141.2005.05682.x. [DOI] [PubMed] [Google Scholar]
- 2.Jamieson CH, Gotlib J, Durocher JA, et al. The JAK2 V617F mutation occurs in hematopoietic stem cells in polycythemia vera and predisposes toward erythroid differentiation. Proc Natl Acad Sci USA. 2006;103:6224–6229. doi: 10.1073/pnas.0601462103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anand S, Stedham F, Beer P, et al. Effects of the JAK2 mutation on the hematopoietic stem and progenitor compartment in human myeloproliferative neoplasms. Blood. 2011;118:177–181. doi: 10.1182/blood-2010-12-327593. [DOI] [PubMed] [Google Scholar]
- 4.James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 5.Lacout C, Pisani DF, Tulliez M, et al. JAK2V617F expression in murine hematopoietic cells leads to MPD mimicking human PV with secondary myelofibrosis. Blood. 2006;108:1652–1660. doi: 10.1182/blood-2006-02-002030. [DOI] [PubMed] [Google Scholar]
- 6.Kralovics R, Teo SS, Li S, et al. Acquisition of the V617F mutation of JAK2 is a late genetic event in a subset of patients with myeloproliferative disorders. Blood. 2006;108:1377–1380. doi: 10.1182/blood-2005-11-009605. [DOI] [PubMed] [Google Scholar]
- 7.Cario H, Goerttler PS, Steimle C, et al. The JAK2V617F mutation is acquired secondary to the predisposing alteration in familial polycythaemia vera. Br J Haematol. 2005;130:800–801. doi: 10.1111/j.1365-2141.2005.05683.x. [DOI] [PubMed] [Google Scholar]
- 8.Jones AV, Chase A, Silver RT, et al. JAK2 haplotype is a major risk factor for the development of myeloproliferative neoplasms. Nat Genet. 2009;41:446–449. doi: 10.1038/ng.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goerttler PS, Kreutz C, Donauer J, et al. Gene expression profiling in polycythaemia vera: Overexpression of transcription factor NF-E2. Br J Haematol. 2005;129:138–150. doi: 10.1111/j.1365-2141.2005.05416.x. [DOI] [PubMed] [Google Scholar]
- 10.Wang W, Schwemmers S, Hexner EO, et al. AML1 is overexpressed in patients with myeloproliferative neoplasms and mediates JAK2V617F-independent overexpression of NF-E2. Blood. 2010;116:254–266. doi: 10.1182/blood-2009-11-254664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaufmann K, Gründer A, Hadlich T, et al. A novel murine model of myeloproliferative disorders generated by overexpression of the transcription factor NF-E2. J Exp Med. 2012;209:35–50. doi: 10.1084/jem.20110540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mutschler M, Magin AS, Buerge M, et al. NF-E2 overexpression delays erythroid maturation and increases erythrocyte production. Br J Haematol. 2009;146:203–217. doi: 10.1111/j.1365-2141.2009.07742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swerdlow S, Campo E, Harris N, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IRAC Press; 2008. [Google Scholar]
- 14.Steimle C, Lehmann U, Temerinac S, et al. Biomarker analysis in polycythemia vera under interferon-alpha treatment: Clonality, EEC, PRV-1, and JAK2 V617F. Ann Hematol. 2007;86:239–244. doi: 10.1007/s00277-006-0214-1. [DOI] [PubMed] [Google Scholar]
- 15.Weber K, Bartsch U, Stocking C, et al. A multicolor panel of novel lentiviral “gene ontology” (LeGO) vectors for functional gene analysis. Mol Ther. 2008;16:698–706. doi: 10.1038/mt.2008.6. [DOI] [PubMed] [Google Scholar]
- 16.Roelz R, Pilz IH, Mutschler M, et al. Of mice and men: Human RNA polymerase III promoter U6 is more efficient than its murine homologue for shRNA expression from a lentiviral vector in both human and murine progenitor cells. Exp Hematol. 2010;38:792–797. doi: 10.1016/j.exphem.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Ugo V, Marzac C, Teyssandier I, et al. Multiple signaling pathways are involved in erythropoietin-independent differentiation of erythroid progenitors in polycythemia vera. Exp Hematol. 2004;32:179–187. doi: 10.1016/j.exphem.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Garçon L, Lacout C, Svinartchouk F, et al. Gfi-1B plays a critical role in terminal differentiation of normal and transformed erythroid progenitor cells. Blood. 2005;105:1448–1455. doi: 10.1182/blood-2003-11-4068. [DOI] [PubMed] [Google Scholar]
- 19.Sokol L, Luhovy M, Guan Y, et al. Primary familial polycythemia: A frameshift mutation in the erythropoietin receptor gene and increased sensitivity of erythroid progenitors to erythropoietin. Blood. 1995;86:15–22. [PubMed] [Google Scholar]
- 20.Kralovics R, Indrak K, Stopka T, et al. Two new EPO receptor mutations: Truncated EPO receptors are most frequently associated with primary familial and congenital polycythemias. Blood. 1997;90:2057–2061. [PubMed] [Google Scholar]
- 21.Kralovics R, Sokol L, Prchal JT. Absence of polycythemia in a child with a unique erythropoietin receptor mutation in a family with autosomal dominant primary polycythemia. J Clin Invest. 1998;102:124–129. doi: 10.1172/JCI2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perrotta S, Cucciolla V, Ferraro M, et al. EPO receptor gain-of-function causes hereditary polycythemia, alters CD34 cell differentiation and increases circulating endothelial precursors. PLoS One. 2010;5:e12015. doi: 10.1371/journal.pone.0012015. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Ang SO, Chen H, Hirota K, et al. Disruption of oxygen homeostasis underlies congenital Chuvash polycythemia. Nat Genet. 2002;32:614–621. doi: 10.1038/ng1019. [DOI] [PubMed] [Google Scholar]
- 24.Randrianarison-Huetz V, Laurent B, Bardet V, et al. Gfi-1B controls human erythroid and megakaryocytic differentiation by regulating TGF-beta signaling at the bipotent erythro-megakaryocytic progenitor stage. Blood. 2010;115:2784–2795. doi: 10.1182/blood-2009-09-241752. [DOI] [PubMed] [Google Scholar]
- 25.Doulatov S, Notta F, Eppert K, et al. Revised map of the human progenitor hierarchy shows the origin of macrophages and dendritic cells in early lymphoid development. Nat Immunol. 2010;11:585–593. doi: 10.1038/ni.1889. [DOI] [PubMed] [Google Scholar]