The objective of this work was to evaluate the in vitro capacity of CD133+ stem cells from 13 amyotrophic lateral sclerosis (ALS) patients to differentiate into neuron lineages. Stem cells were cultured in a control medium or a neuroinduction medium for 2–48 hours, and the expression of neuronal genes was analyzed. It was found that CD133+ stem cells from ALS patients, like the stem cells of healthy subjects, are capable of differentiating into preneuron cells.
Keywords: Autologous stem cell transplantation, CD133+ stem cells, Stem cell differentiation, Neural induction, Amyotrophic lateral sclerosis, Neurodegenerative diseases
Abstract
Improvements in quality of life and life expectancy have been observed in amyotrophic lateral sclerosis (ALS) patients transplanted with CD133+ stem cells into their frontal motor cortices. However, questions have emerged about the capacity of cells from these patients to engraft and differentiate into neurons. The objective of this work was to evaluate the in vitro capacity of CD133+ stem cells from 13 ALS patients to differentiate into neuron lineage. Stem cells were obtained through leukapheresis and cultured in a control medium or a neuroinduction medium for 2–48 hours. Expression of neuronal genes was analyzed by reverse transcription polymerase chain reaction (RT-PCR) and immunohistochemical techniques. Fluorescence microscopy demonstrated that CD133+ stem cells from ALS patients incubated for 48 hours in a neuroinduction medium increased the detection of neuronal proteins such as nestin, β-tubulin III, neuronal-specific enolase, and glial fibrillary acidic protein. RT-PCR assays demonstrated an increase in the expression of β-tubulin III, nestin, Olig2, Islet-1, Hb9, and Nkx6.1. No correlation was found between age, sex, or ALS functional scale and the CD133+ stem cell response to the neuroinduction medium. We conclude that CD133+ stem cells from ALS patients, like the stem cells of healthy subjects, are capable of differentiating into preneuron cells.
Introduction
Amyotrophic lateral sclerosis (ALS) is a middle age-onset, rapidly deteriorating neurological disorder characterized by the selective death of upper motor neurons in the brain and lower motor neurons in the spinal cord. The mean survival for patients with ALS is 3 years [1–5]. Despite exciting advances in identifying the molecular basis of ALS, the etiology of sporadic cases remains unexplained. Unfortunately, there is no available or effective treatment for this condition. Different drugs have been proposed for treatment, such as lithium, a neuroprotective compound that promotes autophagy and helps to eliminate the α-synuclein and ubiquitin that accumulate in affected neurons. The immunomodulatory agents lenalidomide and cannabinoids have also been proposed as therapeutic agents for ALS because of their anti-inflammatory effects, inflammation being the probable culprit of motor neuron death. Because oxidative stress could also play an important role in neuron damage, coenzyme Q10 has been administered to ALS patients. Riluzole is the drug of choice and the only one approved by the U.S. Food and Drug Administration for the treatment of ALS. This compound is a glutamatergic system modulator that apparently inhibits the presynaptic release of glutamic acid. Unfortunately, only modest survival increases have been reported with the use of any of these drugs [6–11].
Stem cell therapy has been proposed and used as an alternative form of treatment for ALS [12–19] and other neurodegenerative disorders, including Parkinson's disease [20], stroke [21], and spinal cord injury [22–24]. The capacity of adult stem cells for in vitro and in vivo differentiation has been widely demonstrated. Stem cells that bear the surface antigen CD133+ are presently isolated from a wide range of sources, including bone marrow, peripheral blood, and umbilical cord [15, 25, 26]. These cells have been shown to be capable of differentiating into neuron-like cells in vitro [27] and to provide neuroprotection [28], and they have been used in several clinical trials [29–36].
Recently, we reported the effects of autologous transplantation to the motor neuron cortex of CD133+ stem cells in patients with confirmed ALS [15]. The rationale for this study was that these cells were capable of differentiating into neuron-like cells in vitro [27], and therefore their implantation could improve upper motor neuron functions by the renewal of lost neuronal population. Results from this research trial demonstrated a significant delay in ALS progression as well as improvement in life quality. Furthermore, the procedure was defined as safe and well tolerated by study subjects [37].
At the present time there is no technology available to probe whether the cells implanted into brains of our study patients [15] were capable of engraftment and differentiation into neurons. Additionally, some doubts have been raised about the plastic capacity of stem cells from ALS patients to differentiate into other cell lineages. Bossolasco et al. reported a significant diminution response of bone marrow stem cells from ALS patients to convert into adipogenic and osteoblastic tissue [38]. Therefore, it is important to confirm whether CD133+ stem cells from ALS patients have the same capacity as those from healthy subjects to differentiate into neurons. In addition, there is a report that cells from elderly donors (over 45 years of age) have lost this capacity [39]. The aim of this research was, therefore, to test whether CD133+ stem cells from 13 ALS patients were able to respond in vitro, as has been reported for healthy donators.
Materials and Methods
Study Subjects
Thirteen ALS patients, 4 women and 9 men, were included and signed an informed consent form as established in a protocol approved by the Ethics and Research Committees of the School of Biotechnology and Health at Tec de Monterrey and Hospital San José. Patients participating in this study ranged in age from 41 to 77 years, and all had spinal onset ALS. At the time of cell sampling, patients were clinically evaluated with the ALS Functional Rating Scale-Revised (ALSFRS-R) [40], with scores ranging from 24 to 46 points. Two of the 13 patients had been prescribed riluzole (Table 1).
Table 1.
Characteristics of amyotrophic lateral sclerosis patients
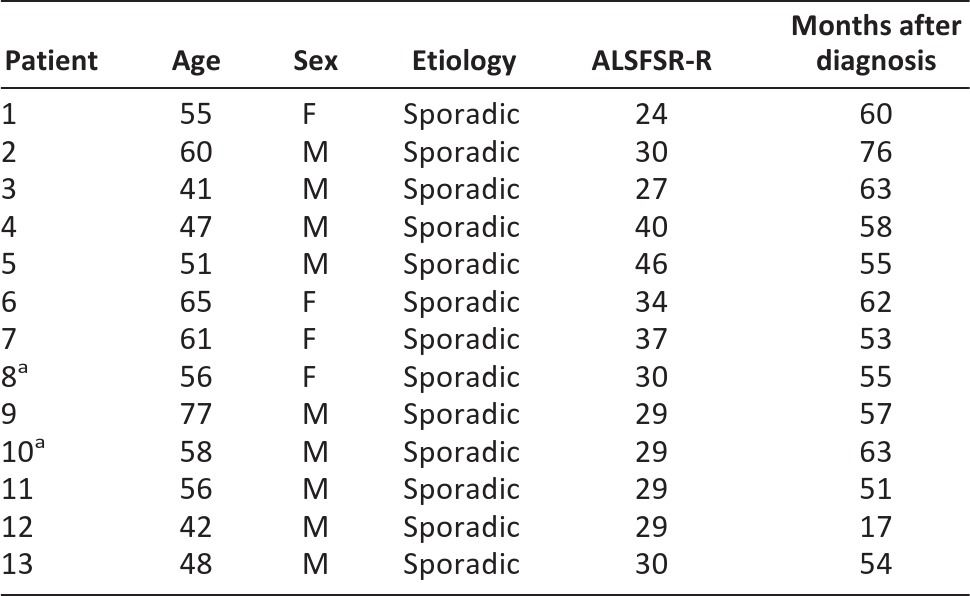
aRiluzole prescribed.
Abbreviations: ALSFSR-R, Amyotrophic Lateral Sclerosis Functional Scale-Revised; F, female; M, male.
CD133 Stem Cell Isolation
Peripheral blood mononuclear cells were obtained by leukapheresis (Baxter CS3000+; Baxter Health Care Systems, Deerfield, IL, http://www.baxter.com) and were initially enriched by Percoll density gradient centrifugation. After being washed three times with phosphate-buffered saline (PBS), cell suspensions were conjugated with anti-human CD133+ microbeads and isolated in a magnetic field over a MiniMACS separation column (Miltenyi Biotec, Bergisch Gladbach, Germany, http://www.miltenyibiotec.com). Cells were counted in a Beckman Z2 Coulter Counter (Beckman Coulter, Fullerton, CA, http://www.beckmancoulter.com), and samples of 1.0 × 106 cells were cultured in 60-mm dishes for 24 hours in Dulbecco's modified Eagle's medium (DMEM-F12; Gibco, Grand Island, NY, http://www.invitrogen.com) supplemented with 20% fetal bovine serum (FBS) and penicillin (100 μg/ml)/streptomycin (250 ng/ml) at 37°C in humid air with 5% CO2. After that, the medium was changed and cells were maintained in DMEM-F12 supplemented with 10% FBS and antibiotics (Gibco) for 1 week until cultures became confluent. Cells were then harvested and seeded into plates containing neuroinduction medium for 2–48 hours. As control medium 1 (C1), cells were incubated in DMEM-F12 supplemented with antibiotics without FBS. Control medium 2 (C2) contained DMEM-F12 supplemented with antibiotics and 5% FBS.
Neuroinduction Medium
Cells were induced to differentiate into neuron-like cells by seeding on poly-l-lysine precoated plates and incubation with neuroinduction medium (NIM): DMEM-F12 supplemented with 5% FBS, 1 μM retinoic acid (trans), 1 mM β-2-mercaptoethanol, 2 mM l-glutamine, 0.2% dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com), 10 ng/ml basic fibroblast growth factor, 40 ng/ml epidermal growth factor (Invitrogen, Grand Island, NY, http://www.invitrogen.com), 10 ng/ml sonic hedgehog (SHH; R&D Systems Inc., Minneapolis, MN, http://www.rndsystems.com), and 0.5 mM 3-isobutyl-1-methylxanthine (Sigma-Aldrich). Detection of mRNA and neuroproteins was conducted after cells were incubated with NIM at 37°C in 5% CO2 for 2, 6, 12, 24, or 48 hours.
Protein Detection by Immunocytochemistry
Cells were fixed using 4% paraformaldehyde 0.1 mM PBS, pH 7.4, for 10 minutes and then washed three times with PBS. Cells were permeabilized using 0.3% Triton X-100 in PBS for 5 minutes. Nonspecific antibody reactions were blocked with 5% bovine serum albumin (BSA) in PBS for 1 hour. Next, cells were incubated overnight at 4°C with primary mouse monoclonal antibodies diluted in PBS containing 1% BSA. The antibodies used were directed to detection of glial fibrillary acidic protein (GFAP; Serotec Kidlington, Oxford, U.K., http://www.abdserotec.com), neuronal-specific enolase (NSE; Millipore, Billerica, MA, http://www.millipore.com), β-tubulin III (TuJ1) (Promega, Madison, WI, http://www.promega.com), and nestin (Serotec). The secondary antibody used was goat anti-mouse fluorescein isothiocyanate (Pierce, Rockford, IL, http://www.piercenet.com). Nuclear contrast was performed with 4′,6-diamidino-2-phenylindole (Santa Cruz Biotechnology Inc., Santa Cruz, CA, http://www.scbt.com).
Gene Expression by Reverse Transcription-Polymerase Chain Reaction
RNA was isolated according to the manufacturer's instructions for the GenElude mammalian total RNA miniPrep kit (Sigma-Aldrich), and reverse transcription-polymerase chain reaction (RT-PCR) was performed according to the instruction manual of the One-Step RT-PCR kit (Qiagen, Crawley, U.K., http://www.qiagen.com). Primers were designed as follows: internal control gene GAPDH (glyceraldehyde-3-phosphate dehydrogenase): sense, 5′-CATGGAGAAGGCTGGGG-3′, antisense, 5′-CAAAGTTGTCATGGATGACC-3′; nestin: sense, 5′-AGGATGTGGAGGTAGTGAGA-3′, antisense, 5′-TGGAGATCTCAGTGGCTCTT-3′; Olig2: sense, 5′-AAGGAGGCAGTGGCTTCAAGTC-3′, antisense, 5′-CGCTCACCAGTCGCTTCATC-3′; β-tubulin III: sense, 5′-CGAGACCTACTGCATCGACA-3′, antisense, 5′-GGGATCCACTCCACGAAGTA-3′; Islet-1: sense, 5′-TGATGAAGCAACTCCAGCAG-3′, antisense, 5′-TTTCCAAGGTGGCTGGTAAC-3′; Nkx6.1: sense, 5′-TCAGGTCAAGGTCTGCTTCC-3′, antisense, 5′-TCAACAGCTGCGTGATTTTC-3′; Hb9: sense, 5′-AAGATGCCCGACTTCAACTC-3′, antisense, 5′-GACAGGTACTTGTTGAGCTTG-3′. RT-PCR products for each target gene were resolved by 2% agarose gel electrophoresis, visualized with SYBR Green (Qiagen) (diluted 1:10,000 in Tris-borate-ethylenediaminetetraacetic acid. The bands were observed under UV light and photographed in a UVP high-performance UV transilluminator, DigiDoc-It (Cambridge, U.K., http://www.uvp.com), and analyzed with the GelAnalyzer program (http://www.gelanalyzer.com).
Statistical Analysis
Statistical analyses were performed with SPSS software (v. 17.0; SPSS, Chicago, IL, http://www.spss.com). For each variable under study, medians, SDs, and ranges were calculated. The Pearson correlation between patient characteristics and gene expression after 48 hours of NIM cell incubation was considered to be significant for values of p < .05. Otherwise, the nonparametric Kendall's τ-b test was performed.
Results
Protein Detection by Immunocytochemistry
The results demonstrated a basal presence of β-tubulin III in cells cultured in control media; with or without 5% FBS, the positive signal was restricted to the area surrounding the nucleus. However, after cells were exposed for 2 hours to NIM, a positive signal for β-tubulin III was more evident, its distribution was observed throughout the cytoplasm, and changes in cell morphology were observed (Fig. 1A).
Figure 1.
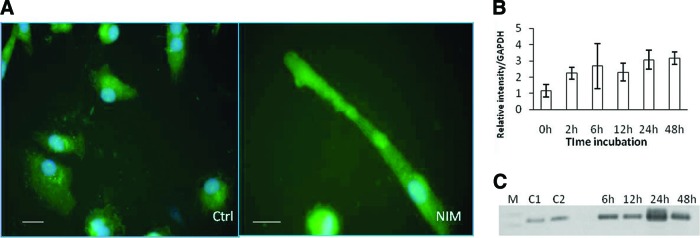
Representative images of immunofluorescence detection of β-tubulin III in CD133+ stem cells from an amyotrophic lateral sclerosis patient. (A): Positive staining for β-tubulin III was detected around the nucleus (green) after 2 hours of incubation in control medium. After 2 h of incubation in NIM, distribution was detected across the cytoplasm. The nucleus was labeled with 4′,6-diamidino-2-phenylindole (blue). Scale bar = 20 μm. (B): Graphic representation of the average expression of β-tubulin III in patient cells detected by reverse transcription-polymerase chain reaction (RT-PCR) after 2–48 hours. Bars represent the SD of patient values. (C): Representative images of agarose electrophoresis of RT-PCR products for β-tubulin III. Lanes show molecular weight markers (M), cells grown in Dulbecco's modified Eagle's medium (DMEM-F12) (C1), cells grown in DMEM-F12 supplemented with 5% fetal bovine serum (FBS) (C2), and cells incubated for 2–48 hours in DMEM-F12 supplemented with 5% FBS and inducers. Abbreviations: C1, control medium 1; C2, control medium 2; Ctrl, control medium; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; M, molecular weight markers; NIM, neuroinduction medium.
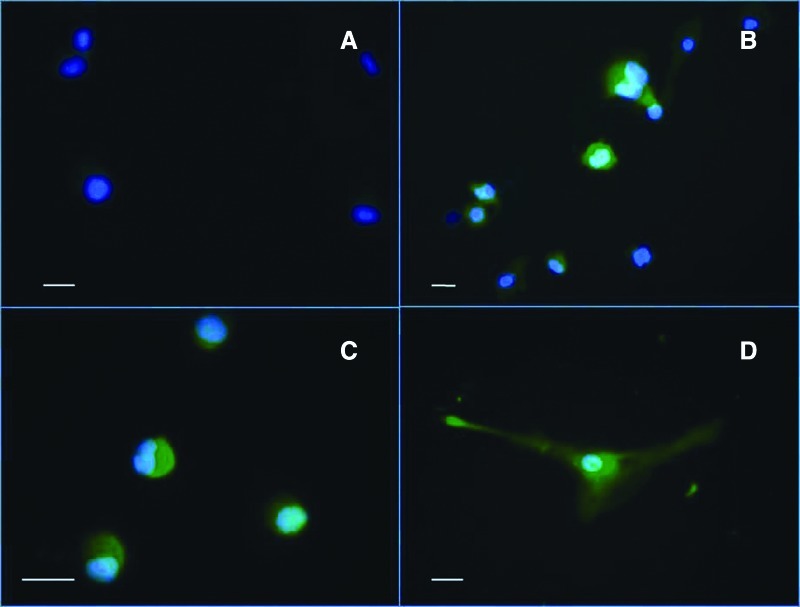
Immunocytochemistry for the neuron marker nestin demonstrated a similar distribution; cells cultivated in control media showed basal staining near the nucleus, consistent with previous reports [30], but a strong staining throughout the cytoplasm from 2 hours after incubation in NIM was observed in at least 50% of the cells (Fig. 2A). In the case of NSE, immunofluorescence microscopy of cells cultivated in control medium showed slight staining surrounding the nucleus (Fig. 3A). After 2 hours of incubation in NIM, a significant increase in staining was observed, but only in areas surrounding the nucleus (Fig. 3B). A similar pattern of expression was observed for GFAP with a distribution in control cultures restricted to the nucleus (Fig. 3C); however, upon NIM addition, a significant increase of the staining throughout the cytoplasm was observed, and cell morphological changes as well (Fig. 3D).
Figure 2.
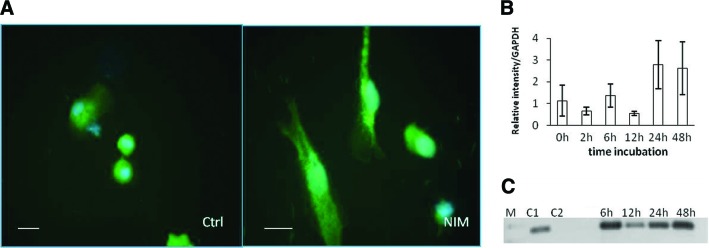
Representative images of immunofluorescence detection of nestin in CD133+ stem cells from an amyotrophic lateral sclerosis patient. (A): Positive staining for nestin was detected around the nucleus (green) after 2 hours of incubation in control medium. After 2 hours of incubation in NIM, distribution was detected across the cytoplasm. The nucleus was labeled with 4′,6-diamidino-2-phenylindole (blue). Scale bars = 20 μm. (B): Graphic representation of the average expression of nestin in patient cells detected by reverse transcription-polymerase chain reaction (RT-PCR) after 2–48 hours. Bars represent the SD of patient values. Under the graph are representative images of agarose electrophoresis of RT-PCR products for nestin. Lanes show molecular weight markers (M), cells grown in Dulbecco's modified Eagle's medium (DMEM-F12) (C1), cells grown in DMEM-F12 supplemented with 5% fetal bovine serum (FBS) (C2), and cells incubated for 2–48 hours in DMEM-F12 supplemented with 5% FBS and inducers. Abbreviations: C1, control medium 1; C2, control medium 2; Ctrl, control medium; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; M, molecular weight markers; NIM, neuroinduction medium.
Figure 3.
Representative images of immunofluorescence detection of neuronal-specific enolase (NSE) and glial fibrillary acidic protein (GFAP) (green) in CD133+ stem cells from an amyotrophic lateral sclerosis patient after 2 hours of cultivation in control medium and neuroinduction medium (NIM). (A): NSE was not detected in cells incubated in control medium. (B): Moderate staining for NSE was observed around the nucleus in cells incubated in NIM; nucleus was labeled with 4′6-diamidino-2-phenylindole (DAPI) (blue). (C): Cells incubated with control medium showed positive staining against GFAP around the nucleus. (D): Cells incubated in NIM showed a cytoplasmic distribution of GFAP; nucleus was labeled with DAPI (blue). Scale bars = 20 μm.
Gene Expression by Reverse Transcription-Polymerase Chain Reaction
Cells cultivated with NIM showed a time-dependent increase in β-tubulin III transcription that was greatest at 48 hours (Fig. 1B), coinciding with the morphological changes observed by microscopy (Fig. 1A).
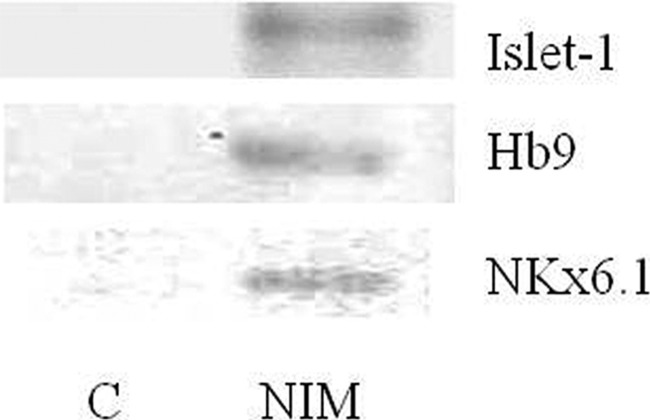
RT-PCR for nestin transcription showed expression correlation with incubation time being greatest at 48 hours (Fig. 2B). No significant differences with respect to control media were observed until 24 hours of incubation in NIM. Olig2 expression in induced CD133+ stem cells from ALS patients was negative in cells incubated with control medium. Some patients' cells incubated in NIM expressed Olig2 after 12 hours of incubation, and the expression level was increased at 48 hours (Fig. 4A, 4B). After 48 hours of incubation in NIM, cells from all patients expressed Olig2 (Fig. 4C, 4D). RT-PCR for Islet-1, Nkx6.1, and Hb9 was performed with a sample from cells of a patient that showed a high expression of Olig2 after 48 hours of incubation in NIM. In this sample, expression of the three genes was observed after 48 hours of incubation in NIM. Cells incubated in control medium were negative (Fig. 5).
Figure 4.
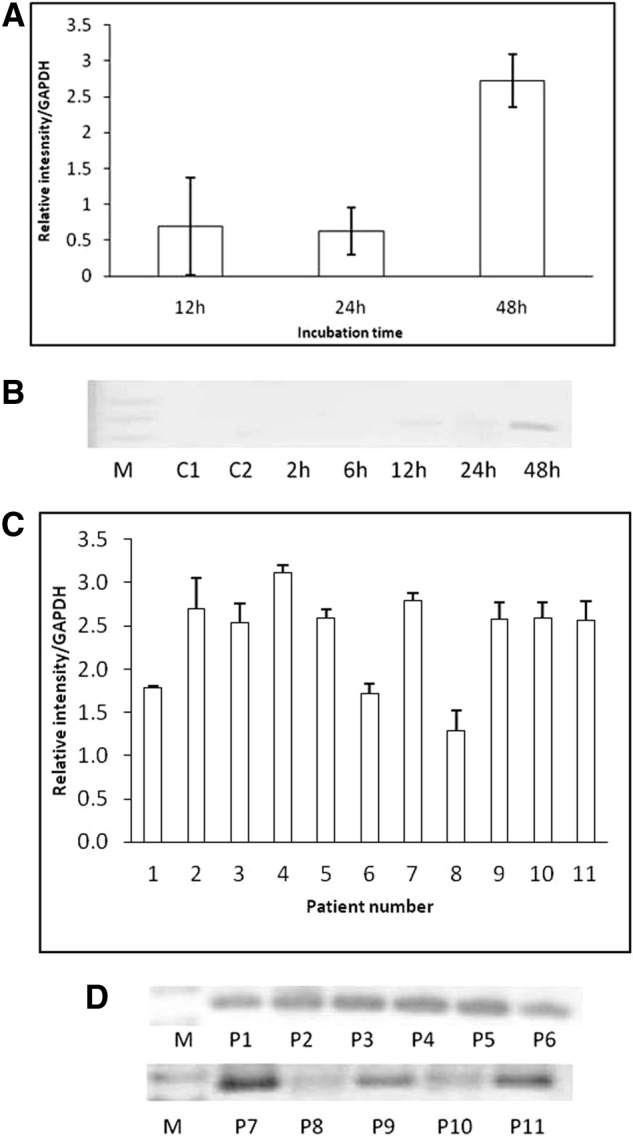
Reverse transcription-polymerase chain reaction (RT-PCR) for Olig2 expression in induced CD133+ stem cells from amyotrophic lateral sclerosis patients. (A): Graphic representation of the band intensity from electrophoresis of RT-PCR products for Olig2 after 12–48 hours of incubation with neuroinduction medium (NIM). (B): Agarose gel imaging of gene expression after 2–48 hours of cell incubation in control medium and NIM. Positive expression was detected after 12 hours and was increased after 48 hours. (C): Graphic representation of the band intensity after electrophoresis of RT-PCR products for Olig2 expression in CD133+ stem cells from 13 patients after 48 hours of incubation in NIM. (D): Agarose gel imaging of gene expression after 48 hours of cell incubation in NIM in cells from 11 patients. Abbreviations: C1, control medium 1; C2, control medium 2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; M, molecular weight markers; P, patient.
Figure 5.
Reverse transcription-polymerase chain reaction for Islet-1, Nkx6.1, and Hb9 expression in induced CD133+ stem cells from an amyotrophic lateral sclerosis patient. Shown is agarose gel imaging of gene expression after 48 hours of cell incubation in control medium and NIM. Abbreviations: C, control medium; NIM, neuroinduction medium.
Statistical Analysis
Statistical analysis did not show any correlation between patient characteristics and β-tubulin III or nestin gene expression after 2–48 hours of incubation in NIM. However, Olig2 expression showed a proportional decrease in intensity with respect to patient age (p < .05). ALSFRS-R, disease duration, and sex showed no correlation with Olig2 expression.
Discussion
Because ALS is a motor neuron degenerative disease, the study was designed to investigate the possibility that CD133+ cells from ALS patients could become neuron-like cells, or at least immature motor neurons. To confirm a possible lineage, RT-PCR was performed to detect the expression of Olig2, a transcription factor characteristic of immature motor neurons [41]. The expression of Olig2 in motor neuron progenitors during embryogenesis is strictly dependent on a precise amount (100 ng/ml) of SHH ligand that is secreted from the notochord during embryological development of the nervous system [42, 43]. For the above, NIM was supplemented with this ligand. Expression of Olig2 was not detected in control samples. However, cells from most patients showed weak expression of this gene after 12 hours of incubation in NIM and a strong expression after 48 hours (Fig. 3). This finding is important because it demonstrates the capacity of stem cells from ALS patients to express a marker for immature motor neurons and therefore suggests that cells from these patients are capable of differentiating into immature motor neurons. In addition, to strengthen the possible motor neuron lineage predifferentiation, one sample that gave a significant signal for Olig2 was analyzed for Islet-1, Nkx6.1, and Hb9 gene expression. Results showed a positive expression for all these genes (Fig. 5). The homeobox gene Hb9 is selectively expressed by motor neurons in the developing vertebrate central nervous system. In embryonic chick spinal cord, the ectopic expression of Hb9 is sufficient to trigger motor-neuron differentiation [44 46]. Furthermore, Hb9+ cells differentiate into electrophysiologically functional motor neuron cells and formed synapses with myotubes a few weeks after all-trans retinoic acid and Shh agonist treatment in human and monkey embryonic cells [45]. It has been demonstrated that Islet-1 expression represents the earliest marker of developing motor neurons and most likely is involved in the establishment of motor neuron fate [46]. Lastly, Nkx6.1-expressing cells give rise to three neuronal subtypes: V3 interneurons, motor neurons, and V2 interneurons. In its absence, loss of two of the three neuroprogenitor cell populations (V2 interneurons and motor neurons) is observed [47]. Therefore, the expression of all these genes in our patient CD133+ stem cells raises the possibility that autologous transplant of stem cells in ALS patients is capable of restoring the upper motor neurons lost in this disease [1–2].
Although the role of neurotrophic factors in ALS is still unclear and clinical trials using different neurotrophic factors did not show encouraging results, there are a number of lines of evidence that loss of specific neurotrophic factors during development results in loss of motor neurons. Studies in animal models of programmed cell death during development, deficient for specific neurotrophic factors, in fact show that motor neurons respond to neurotrophic factors, such as vascular endothelial growth factor, brain-derived neurotrophic factor, glial-derived neurotrophic factor, and NT-3 [48–52]. Unfortunately, at this moment we do not have direct evidence that these cells are capable of secreting neurotrophic factors to improve survival of the existing motor neuron cells in ALS patients or in those cells newly transplanted. However, there is some undirected evidence that the patients enrolled in the transplantation clinical protocol show increases in their pyramidal tracts 6 months after CD133+ stem cell transplantation by means of quantification of the anysotrophic fraction and tractographies obtained by magnetic resonance imaging [37]. This could represent a sign of maintenance and survival of the transplanted cells. Nevertheless, this issue should be addressed with more detail in the future to unequivocally ascertain the permanence and viability of the transplanted cells into ALS patients.
Bossolasco et al. reported a significant delayed response of bone marrow mesenchymal stem cells from ALS patients to differentiate into adipogenic and osteoblastic cell lineages in vitro [38]. We report here that even though some time differences in predifferentiation times can occur, they are not significant. These results are in agreement with the study of Ferrero et al., who showed that mesenchymal stem cells from normal donors and ALS patients do not show significant differences in their potential for osteogenic, adipogenic, chondrogenic, or neurogenic differentiation [52].
Although our results show a delayed Olig2 expression (48 hours after incubation in NIM) that correlates with patient age, all CD133+ stem cell samples were capable of expressing motor neuron associated genes at some level, which suggests that they may have the capacity to become mature motor neurons. Healthy adult stem cells, when grown under specific culture conditions, have shown the capacity to differentiate into various cell lineages, including neurons. However, there is a report that cells from elderly donors (over 45 years of age) have lost this capacity [39]. This fact could not be corroborated in our study. Most of our patients included in our study were included in that elderly category and responded to culture conditions within 2–48 hours.
Despite the small number of patients included in this study, our results show that CD133+ stem cells from ALS patients are capable of responding to external factors to start differentiation at exposures as early as 2–48 hours. This capacity only suggests the possibility of engraftment and differentiation in vitro. At this time, we do not have enough evidence to explain the differences in response among ALS patients. Although stem cells from ALS patients show different kinetics of differentiation into the neuronal lineage, we did not observe a significant association between disease status or patient characteristics and the cell differentiation response.
Conclusion
In this study, we have presented evidence that CD133+ stem cells from the peripheral blood of ALS patients are capable of predifferentiating into neuron-like cells after exposure to an appropriate medium, as has been reported for other stem cell sources.
Acknowledgments
This work was partially funded by endowments from Instituto Tecnológico de Estudios Superiores de Monterrey (cat-134) and the Zambrano-Hellion Foundation. The authors express appreciation to Rosa Maria de la Rosa and Martha Esquivel for technical assistance.
Author Contributions
M.T.G.-G: conception and design, collection and assembly of data, manuscript writing, final approval of manuscript; H.R.M.: data analysis and interpretation, final approval of manuscript; E.C.-O. and M.H.-T.: study design, final approval of manuscript; D.E.C.-V.: collection and asssembly of data, final approval of manuscript; J.E.M.-C.: conception and design, data analysis and interpretation, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Ringel SP, Murphy JR, Alderson MK, et al. The natural history of amyotrophic lateral sclerosis. Neurology. 1993;43:1316–1322. doi: 10.1212/wnl.43.7.1316. [DOI] [PubMed] [Google Scholar]
- 2.Mills KR. The natural history of central motor abnormalities in amyotrophic lateral sclerosis. Brain. 2003;126:2558–2566. doi: 10.1093/brain/awg260. [DOI] [PubMed] [Google Scholar]
- 3.Stambler N, Charatan M, Cedarbaum JM. Prognostic indicators of survival in ALS. ALS CNTF Treatment Study Group. Neurology. 1998;50:66–72. doi: 10.1212/wnl.50.1.66. [DOI] [PubMed] [Google Scholar]
- 4.Zoccolella S, Begh E, Palagano G, et al. Analysis of survival and prognostic factors in amyotrophic lateral sclerosis: A population based study. J Neurol Neurosurg Psychiatry. 2008;79:33–37. doi: 10.1136/jnnp.2007.118018. [DOI] [PubMed] [Google Scholar]
- 5.Martínez HR, Molina-Lopez JF, Cantu-Martinez L, et al. Survival and clinical features in Hispanic amyotrophic lateral sclerosis patients. Amyotroph Lateral Scler. 2011;12:199–205. doi: 10.3109/17482968.2010.550302. [DOI] [PubMed] [Google Scholar]
- 6.Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330:585–591. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- 7.Fornai F, Longone P, Cafaro L, et al. Lithium delays progression of amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2008;105:2052–2057. doi: 10.1073/pnas.0708022105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasquali L, Longone P, Isidoro C, et al. Autophagy, lithium, and amyotrophic lateral sclerosis. Muscle Nerve. 2009;40:173–194. doi: 10.1002/mus.21423. [DOI] [PubMed] [Google Scholar]
- 9.Neymotin A, Petri S, Calingasan NY, et al. Lenalidomide (Revlimid) administration at symptom onset is neuroprotective in a mouse model of amyotrophic lateral sclerosis. Exp Neurol. 2009;220:191–197. doi: 10.1016/j.expneurol.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aggarwal SP, Zinman L, Simpson E, et al. Safety and efficacy of lithium in combination with riluzole for treatment of amyotrophic lateral sclerosis: A randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010;9:481–488. doi: 10.1016/S1474-4422(10)70068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaufmann P, Thompson JL, Levy G, et al. QALS Study Group. Phase II trial of CoQ10 for ALS finds insufficient evidence to justify phase III. Ann Neurol. 2009;66:235–244. doi: 10.1002/ana.21743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cova L, Silani V. Amyotrophic lateral sclerosis: Applications of stem cells—an update. Stem Cells Cloning. 2010;3:145–156. doi: 10.2147/SCCAA.S8662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Zhang SC. Human stem cells as a model of motoneuron development and diseases. Ann NY Acad Sci. 2010;1198:192–200. doi: 10.1111/j.1749-6632.2010.05537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janson CG, Ramesh TM, During MJ, et al. Human intrathecal transplantation of peripheral blood stem cells in amyotrophic lateral sclerosis. J Hematother Stem Cell Res. 2001;10:913–915. doi: 10.1089/152581601317211015. [DOI] [PubMed] [Google Scholar]
- 15.Martinez HR, Gonzalez Garza MT, Moreno Cuevas JE, et al. Stem-cell transplantation into the frontal motor cortex in amyotrophic lateral sclerosis patients. Cytotherapy. 2009;11:26–34. doi: 10.1080/14653240802644651. [DOI] [PubMed] [Google Scholar]
- 16.Mazzini L, Fagioli F, Boccaletti R, et al. Stem cell therapy in amyotrophic lateral sclerosis: A methodological approach in humans. Amyotroph Lateral Scler Other Motor Neuron Disord. 2003;4:158–161. doi: 10.1080/14660820310014653. [DOI] [PubMed] [Google Scholar]
- 17.Silani V, Cova L, Corbo M, et al. Stem cell therapy for amyotrophic lateral sclerosis. Lancet. 2004;364:200–202. doi: 10.1016/S0140-6736(04)16634-8. [DOI] [PubMed] [Google Scholar]
- 18.Swash M. Stem cell therapy in human ALS. Amyotroph Lateral Scler Other Motor Neuron Disord. 2003;4:133–134. doi: 10.1080/aml.4.3.133.134. [DOI] [PubMed] [Google Scholar]
- 19.Soler B, Fadic R, von Bernhardi R. Stem cells therapy in amyotrophic lateral sclerosis treatment. A critical view. Rev Neurol. 2011;52:426–434. [PubMed] [Google Scholar]
- 20.Svendsen C, Langston JW. Stem cells for Parkinson's disease and ALS: Replacement or protection? Nat Med. 2004;10:224–225. doi: 10.1038/nm0304-224. [DOI] [PubMed] [Google Scholar]
- 21.Kondziolka D, Steinberg GK, Wechsler L, et al. Neurotransplantation for patients with subcortical motor stroke: A phase 2 randomized trial. J Neurosurg. 2005;103:38–45. doi: 10.3171/jns.2005.103.1.0038. [DOI] [PubMed] [Google Scholar]
- 22.Lima C, Pratas-Vital J, Escada P, et al. Olfactory mucosa autografts in human spinal cord injury: A pilot clinical study. J Spinal Cord Med. 2006;29:191–203. doi: 10.1080/10790268.2006.11753874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moviglia GA, Fernandez Viña R, Brizuela JA, et al. Combined protocol of cell therapy for chronic spinal cord injury. Report on the electrical and functional recovery of two patients. Cytotherapy. 2006;8:202–209. doi: 10.1080/14653240600736048. [DOI] [PubMed] [Google Scholar]
- 24.Zhou Q, Zhang SZ, Xu RX, et al. Neural stem cell transplantation and postoperative management: Report of 70 cases [in Chinese] Di Yi Jun YI Da Xue XUE Bao. 2004;24:1207–1209. [PubMed] [Google Scholar]
- 25.De Wynter EA, Buck D, Hart C, et al. CD34+AC133+ cells isolated from cord blood are highly enriched in long-term culture-initiating cells, NOD/SCID-repopulating cells and dendritic cell progenitors. Stem Cells. 1998;16:387–396. doi: 10.1002/stem.160387. [DOI] [PubMed] [Google Scholar]
- 26.Quirici N, Soligo D, Caneva L, et al. Differentiation and expansion of endothelial cells from human bone marrow CD133(+) cells. Br J Haematol. 2001;115:186–194. doi: 10.1046/j.1365-2141.2001.03077.x. [DOI] [PubMed] [Google Scholar]
- 27.Kuçi S, Kuçi Z, Schmid S, et al. Efficient in vitro generation of adult multipotent cells from mobilized peripheral blood CD133+ cells. Cell Prolif. 2008;41:12–27. doi: 10.1111/j.1365-2184.2007.00502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bakondi B, Shimada IS, Perry A, et al. CD133 identifies a human bone marrow stem/progenitor cell sub-population with a repertoire of secreted factors that protect against stroke. Mol Ther. 2009;17:1938–1947. doi: 10.1038/mt.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Babin-Ebell J, Sievers HH, Charitos EI, et al. Transmyocardial laser revascularization combined with intramyocardial endothelial progenitor cell transplantation in patients with intractable ischemic heart disease ineligible for conventional revascularization: Preliminary results in a highly selected small patient cohort. Thorac Cardiovasc Surg. 2010;58:11–16. doi: 10.1055/s-0029-1186199. [DOI] [PubMed] [Google Scholar]
- 30.am Esch JS, 2nd, Knoefel WT, Klein M, et al. Portal application of autologous CD133+ bone marrow cells to the liver: A novel concept to support hepatic regeneration. Stem Cells. 2005;23:463–470. doi: 10.1634/stemcells.2004-0283. [DOI] [PubMed] [Google Scholar]
- 31.Bartunek J, Vanderheyden M, Vandekerckhove B, et al. Intracoronary injection of CD133-positive enriched bone marrow progenitor cells promotes cardiac recovery after recent myocardial infarction: Feasibility and safety. Circulation. 2005;112:I178–I183. doi: 10.1161/CIRCULATIONAHA.104.522292. [DOI] [PubMed] [Google Scholar]
- 32.Burt RK, Testori A, Oyama Y, et al. Autologous peripheral blood CD133+ cell implantation for limb salvage in patients with critical limb ischemia. Bone Marrow Transplant. 2010;45:111–116. doi: 10.1038/bmt.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fürst G, Schulte am Esch J, Poll LW, et al. Portal vein embolization and autologous CD133+ bone marrow stem cells for liver regeneration: Initial experience. Radiology. 2007;243:171–179. doi: 10.1148/radiol.2431060625. [DOI] [PubMed] [Google Scholar]
- 34.Sanchez-Guijo FM, Oterino E, Barbado MV, et al. Both CD133(+) cells and monocytes provide significant improvement for hindlimb ischemia, although they do not transdifferentiate into endothelial cells. Cell Transplant. 2010;19:103–112. doi: 10.3727/096368909X476869. [DOI] [PubMed] [Google Scholar]
- 35.Stamm C, Kleine HD, Choi YH, et al. Intramyocardial delivery of CD133+ bone marrow cells and coronary artery bypass grafting for chronic ischemic heart disease: Safety and efficacy studies. J Thorac Cardiovasc Surg. 2007;133:717–725. doi: 10.1016/j.jtcvs.2006.08.077. [DOI] [PubMed] [Google Scholar]
- 36.Torrente Y, Belicchi M, Marchesi C, et al. Autologous transplantation of muscle-derived CD133+ stem cells in Duchenne muscle patients. Cell Transplant. 2007;16:563–577. doi: 10.3727/000000007783465064. [DOI] [PubMed] [Google Scholar]
- 37.Martínez HR, Molina-Lopez JF, Gonzalez Garza MT, et al. Stem cell transplantation in amyotrophic lateral sclerosis patients: Methodological approach, safety and feasibility. Cell Transplant. 2012 doi: 10.3727/096368911X582769. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 38.Bossolasco P, Cova L, Calzarossa C, et al. Metalloproteinase alterations in the bone marrow of ALS patients. J Mol Med. 2010;88:553–564. doi: 10.1007/s00109-009-0584-7. [DOI] [PubMed] [Google Scholar]
- 39.Hermann A, List C, Habisch HJ, et al. Age-dependent neuroectodermal differentiation capacity of human mesenchymal stromal cells: Limitations for autologous cell replacement strategies. Cytotherapy. 2010;12:17–30. doi: 10.3109/14653240903313941. [DOI] [PubMed] [Google Scholar]
- 40.Kaufmann P, Levy G, Thompson JLP, et al. The ALSFRS-R predicts survival time in an ALS clinic population. Neurology. 2005;64:38–43. doi: 10.1212/01.WNL.0000148648.38313.64. [DOI] [PubMed] [Google Scholar]
- 41.Hwang DH, Kim BG, Kim EJ, et al. Transplantation of human neural stem cells transduced with Olig2 transcription factor improves locomotor recovery and enhances myelination in the white matter of rat spinal cord following contusive injury. BMC Neurosci. 2009;10:117. doi: 10.1186/1471-2202-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu QR, Yuk D, Alberta JA, et al. Sonic hedgehog-regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron. 2000;25:317–329. doi: 10.1016/s0896-6273(00)80897-1. [DOI] [PubMed] [Google Scholar]
- 43.Zhou Q, Wang S, Anderson DJ. Identification of a novel family of oligodendrocyte lineage-specific basic helix-loop-helix transcription factors. Neuron. 2000;25:331–343. doi: 10.1016/s0896-6273(00)80898-3. [DOI] [PubMed] [Google Scholar]
- 44.Arber S, Han B, Mendelsohn M, et al. Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron. 1999;23:659–674. doi: 10.1016/s0896-6273(01)80026-x. [DOI] [PubMed] [Google Scholar]
- 45.Wada T, Honda M, Minami I, et al. Highly efficient differentiation and enrichment of spinal motor neurons derived from human and monkey embryonic stem cells. PLoS One. 2009;4:e6722. doi: 10.1371/journal.pone.0006722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ericson J, Thor S, Edlund T, et al. Early stages of motor neuron differentiation revealed by expression of homeobox gene Islet-1. Science. 1992;256:1555–1560. doi: 10.1126/science.1350865. [DOI] [PubMed] [Google Scholar]
- 47.McMahon A. Neural patterning: The role of Nkx genes in the ventral spinal cord. Genes Dev. 2000;14:2261–2264. doi: 10.1101/gad.840800. [DOI] [PubMed] [Google Scholar]
- 48.Kalcheim C, Gendreau M. Brain-derived neurotrophic factor stimulates survival and neuronal differentiation in cultured avian neural crest. Brain Res. 1988;469:79–86. doi: 10.1016/0165-3806(88)90171-x. [DOI] [PubMed] [Google Scholar]
- 49.Kirschenbaum B, Goldman S. Brain-derived neurotrophic factor promotes the survival of neurons arising from the adult rat forebrain subependymal zone. Proc Natl Acad Sci USA. 1995;92:210–214. doi: 10.1073/pnas.92.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ip NY, Li YP, van de Stadt I, et al. Ciliary neurotrophic factor enhances neuronal survival in embryonic rat hippocampal cultures. J Neurosci. 1991;11:3124–3134. doi: 10.1523/JNEUROSCI.11-10-03124.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Logan A, Ahmed Z, Baird A, et al. Neurotrophic factor synergy is required for neuronal survival and disinhibited axon regeneration after CNS injury. Brain. 2006;129:490–502. doi: 10.1093/brain/awh706. [DOI] [PubMed] [Google Scholar]
- 52.Ferrero I, Mazzini L, Rustichelli D, et al. Bone marrow mesenchymal stem cells from healthy donors and sporadic amyotrophic lateral sclerosis patients. Cell Transplant. 2008;17:255–266. doi: 10.3727/096368908784153940. [DOI] [PubMed] [Google Scholar]