Abstract
Notch signaling is an evolutionarily-conserved, intercellular signaling mechanism that plays myriad roles during vascular development and physiology in vertebrates. These roles include the regulation of arteriovenous specification and differentiation in both endothelial cells and vascular smooth muscle cells, regulation of blood vessel sprouting and branching during normal and pathological angiogenesis, and the physiological responses of vascular smooth muscle cells. Defects in Notch signaling also cause inherited vascular diseases, such as the degenerative vascular disorder Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL). This review summarizes recent studies that highlight the multiple roles the Notch signaling pathway plays during vascular development and physiology.
1. Introduction
The Notch signaling pathway plays myriad roles during vascular development and physiology in vertebrates. This review will summarize recent work highlighting the multiple roles the Notch pathway plays during vascular development, physiology and disease, emphasizing its role in mammals. Additional views on the role of Notch signaling during these processes can be found in a number of previously published reviews (Gridley, 2007; Hofmann and Iruela-Arispe, 2007; Kume, 2009; Phng and Gerhardt, 2009; Roca and Adams, 2007). Notch signaling also plays an important role in development of the vertebrate heart, which is not addressed here. Details on the roles of Notch signaling in heart development can be found in several recent reviews (High and Epstein, 2008; Nemir and Pedrazzini, 2008; Niessen and Karsan, 2008).
Notch family receptors are large single-pass Type I transmembrane proteins (Figure 1). In mammals, four Notch family receptors have been described: NOTCH1 through NOTCH4. The extracellular domain of Notch family proteins contains up to 36 tandemly-repeated copies of an epidermal growth factor (EGF)-like motif. A Notch family receptor exists at the cell surface as a proteolytically-cleaved heterodimer consisting of a large ectodomain and a membrane-tethered intracellular domain. Notch receptors interact with ligands that are also single-pass Type I transmembrane proteins. This restricts the Notch pathway to regulating signaling interactions between physically adjacent cells (which has been termed juxtacrine signaling). In mammals, the Notch ligands are encoded by the Jagged (JAG1 and JAG2) and Delta-like (DLL1, DLL3, and DLL4) gene families.
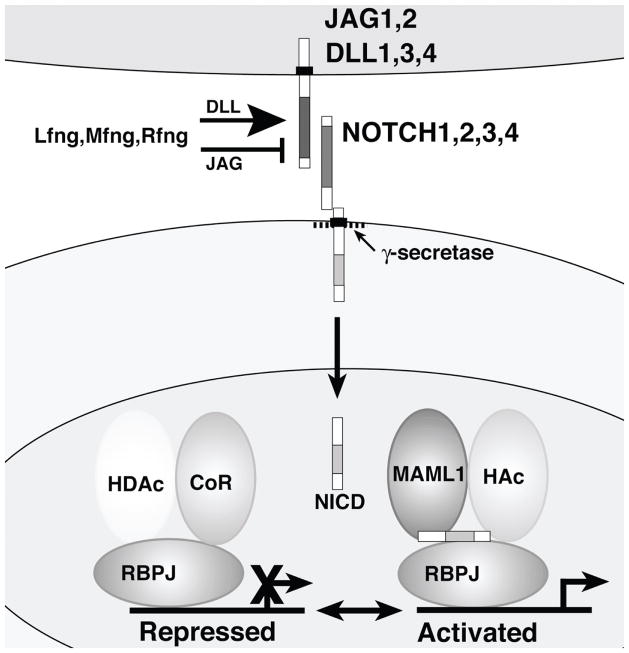
Figure 1. Core components of the canonical Notch signaling pathway.
Ligands of the Jagged (JAG1, JAG2) and Delta-like (DLL1, DLL3, DLL4) families (upper cell) interact with Notch family receptors (NOTCH1 through NOTCH4) on an adjacent cell. The Notch receptor exists at the cell surface as a proteolytically-cleaved heterodimer consisting of a large ectodomain and a membrane-tethered intracellular domain. The Fringe proteins (Lfng, Mfng and Rfng) glycosylate Notch family receptors, potentiating signaling from DLL family ligands and suppressing signaling from JAG family ligands. The receptor/ligand interaction induces additional proteolytic cleavages in the membrane-tethered intracellular domain. The final cleavage, catalyzed by the γ–secretase complex, frees the Notch intracellular domain (NICD) from the cell membrane. NICD translocates to the nucleus, where it forms a complex with the RBPJ protein, displacing a histone deacetylase (HDAc)/corepressor (CoR) complex from the RBPJ protein. Components of an activation complex, such as MAML1 and histone acetyltransferases (HAc) are recruited to the NICD/RBPJ complex, leading to the transcriptional activation of Notch target genes.
The signal induced by ligand binding is transmitted intracellularly by a process involving proteolytic cleavage of the receptor and nuclear translocation of the intracellular domain of the Notch family protein. The receptor/ligand interaction induces two additional proteolytic cleavages in the membrane-tethered fragment of the Notch heterodimer. The final cleavage, catalyzed by the γ-secretase complex, frees the intracellular domain of the Notch receptor from the cell membrane. The cleaved fragment translocates to the nucleus due to the presence of nuclear localization signals located in the Notch intracellular domain (NICD). Once in the nucleus, NICD forms a complex with the RBPJ protein, a sequence-specific DNA binding protein (also known in mammals as CSL and CBF1). In the absence of NICD, the RBPJ protein binds to specific DNA sequences in the regulatory elements of various target genes and represses transcription by recruiting histone deacetylases and other components of a corepressor complex. Nuclear translocation of the Notch intracellular domain displaces the histone deacetylase/corepressor complex from the RBPJ protein. The NICD/RBPJ complex recruits other proteins, such as MAML1 and histone acetyltransferases, leading to the transcriptional activation of Notch target genes. Among the most commonly induced Notch target genes are basic helix-loop-helix (bHLH) transcriptional repressors of the Hes/Hey family (Borggrefe and Oswald, 2009). For additional details on the biochemistry of the Notch signaling pathway and references to the primary literature, please refer to a number of excellent review articles (Bray, 2006; Ehebauer et al., 2006a; Ehebauer et al., 2006b; Fortini, 2009; Ilagan and Kopan, 2007; Kageyama et al., 2007; Kopan and Ilagan, 2009; Le Borgne, 2006).
2. Arteriovenous differentiation
2.1. Notch signaling is downstream of the Vegf pathway during vascular development
A role for the Notch pathway in regulating vascular development was demonstrated initially from analysis of targeted mouse mutants in Notch pathway components. Mouse mutants for which targeted mutagenesis and transgenic studies have demonstrated a role in embryonic vascular development include the receptors Notch1 (Huppert et al., 2000; Krebs et al., 2000; Limbourg et al., 2005) and Notch4 (Carlson et al., 2005; Krebs et al., 2000; Uyttendaele et al., 2001), the ligands Jag1 (Xue et al., 1999) and Dll4 (Duarte et al., 2004; Gale et al., 2004; Krebs et al., 2004), the Notch transcriptional regulator Rpbj (Krebs et al., 2004), the E3 ubiquitin ligase Mib1 (Barsi et al., 2005; Koo et al., 2005), components of the γ-secretase complex such as nicastrin (Li et al., 2003) and presenilin 1 and 2 (Herreman et al., 1999), and the Notch pathway downstream effector bHLH proteins Hey1 and Hey2 (Fischer et al., 2004; Kokubo et al., 2005). Most of these mutants exhibit a similar phenotype characterized by the absence of angiogenic vascular remodeling in the extraembryonic yolk sac, placenta, and embryo proper.
However, analysis of zebrafish embryos with reduced Notch signaling gave the first clues that a primary function of the Notch pathway during vascular development was to regulate the specification of arterial fate in endothelial cells. It had long been believed that the primary factor regulating differentiation of arteries and veins was blood flow. Endothelial cells lining arteries experience higher blood pressures, higher rates of hemodynamic flow, and higher oxygen tensions than endothelial cells lining veins. However, it has recently become clear that genetic prepatterning, mediated in large part by the Notch pathway, plays a primary role in regulating arteriovenous differentiation. The role of the Notch pathway in regulating early embryonic vascular development is intertwined with that of another major regulator of vascular development and physiology, the vascular endothelial growth factor-A (VEGF-A) pathway (Figure 2). VEGF-A is a secreted glycoprotein that is a potent inducer of angiogenesis, that also regulates multiple other aspects of blood vessel homeostasis (Byrne et al., 2005; Coultas et al., 2005; Shibuya and Claesson-Welsh, 2006). The roles and interdependence of the Notch and VEGF-A pathways in regulating formation of the large axial blood vessels of the trunk, the dorsal aorta and the posterior cardinal vein, was studied first in zebrafish (Lawson et al., 2001; Lawson et al., 2002). Notch signaling-deficient embryos exhibited a poorly formed dorsal aorta and posterior cardinal vein with accompanying arteriovenous malformations (the fusion of arteries and veins without an intervening capillary bed). These embryos exhibited loss of expression of arterial markers such as ephrinB2 from arterial vessels with an accompanying expansion of venous markers into normally arterial domains. Embryos in which Notch signaling had been ectopically activated exhibited the reverse phenotype: suppression of vein-specific markers with ectopic expression of arterial markers in venous vessels (Lawson et al., 2001). A similar phenotype was observed in embryos mutant for some Notch target genes, such as the bHLH transcriptional repressor Hey2 (referred to in zebrafish as the gridlock gene) (Zhong et al., 2001; Zhong et al., 2000).
Figure 2. Model for genetic regulation of artery-vein differentiation by the Notch and PLCγ/MAPK pathways.
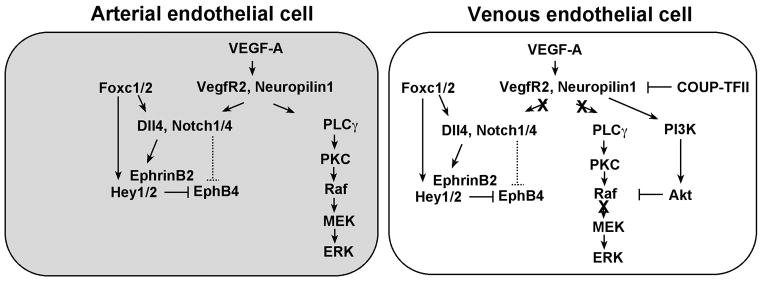
An arterial endothelial cell is shown on the left, while a venous endothelial cell is depicted on the right. Two main signaling pathways operate downstream of VEGF-A to induce arterial differentiation: the Notch pathway and the Phospholipase Cγ (PLCγ) / Mitogen-activated protein kinase (MAPK) pathway. The transcription factors Foxc1 and Foxc2 directly induce Dll4 and Hey2 transcription. VEGF-A signaling, by an unknown mechanism, augments Foxc1/Foxc2 induction of Dll4 and Hey2 gene expression. During venous differentiation, two different mechanisms inhibit artery differentiation. The orphan nuclear receptor COUP-TFII suppresses neuropilin1 expression, thereby suppressing reception of the VEGF-A signal and activation of Notch signaling. In addition, activation of PI3K/Akt signaling antagonizes promotion of arterial cell differentiation by blocking ERK activation.
This analysis of formation of the major trunk vessels of the zebrafish embryo revealed a signaling cascade responsible for determining arterial and venous cell fates in these vessels (Lawson et al., 2002). Reduction of Vegf activity resulted in loss of expression of arterial markers from the dorsal aorta and ectopic arterial expression of vein markers. Injection of Vegf mRNA induced ectopic expression of the arterial marker ephrinB2 in the posterior cardinal vein. Vegf expression was regulated by expression of the secreted morphogen Sonic hedgehog (Shh) along the axial midline. Similar to what was observed in Vegf-deficient embryos, Shh mutant zebrafish embryos also exhibited loss of arterial differentiation, while injection of Shh mRNA caused ectopic expression of arterial markers. Shh acted upstream of Vegf, since injection of Vegf mRNA into the Shh mutant embryos rescued arterial differentiation. This work also demonstrated that the Notch pathway acted downstream of the Vegf pathway. While injection of Vegf mRNA into Notch signaling-deficient zebrafish embryos could not rescue arterial marker gene expression, expression of an activated Notch1 transgene in Vegf-deficient embryos could rescue expression of arterial markers (Lawson et al., 2002). A new study has demonstrated by high resolution in vivo imaging analysis that formation of the major trunk vessels of the zebrafish embryo takes place by a novel mechanism (Herbert et al., 2009). Angioblasts coalesced along the embryonic midline to form a single vascular cord in the position of the future dorsal aorta, which then lumenized. A subset of angioblasts then sprouted ventrally from this progenitor vessel to form the cardinal vein. Sprouting behaviors were regulated in the embryos by coordinated Vegf, Notch and ephrinB2-EphB4 signaling.
Studies in mammalian cells in culture have also placed the Notch pathway downstream of the Vegf pathway. VEGF-A administration induced expression of mRNA for the Notch1 receptor and the Dll4 ligand in human arterial endothelial cells, but not in venous endothelial cells (Liu et al., 2003). Targeted mutagenesis studies have demonstrated that VEGF-A is essential for vascular development in mice. Embryos heterozygous for a Vegfa targeted mutation exhibited lethal haploinsufficiency (Carmeliet et al., 1996; Ferrara et al., 1996). Blood vessels formed in these embryos, but were severely constricted or atretic. It is not known whether artery-vein differentiation is affected in Vegfa+/− embryos. However, gain of function transgenic experiments have demonstrated a role for Vegfa in regulating arterial endothelial cell differentiation in mice. Alternative splicing of the Vegfa gene results in production of several different protein isoforms (VEGF-A 120, VEGF-A 164 and VEGF-A 188). Genetically-engineered mice expressing only the VEGF-A 164 isoform exhibited normal retinal vascular development. However, mice expressing only VEGF-A 120 exhibited severe defects in vascular outgrowth, while mice expressing only VEGF-A 188 exhibited impaired retinal arterial development, but normal venous development (Stalmans et al., 2002). Overexpression of the VEGF-A 164 isoform in cardiac muscle increased the number of ephrinB2-positive capillaries in the heart while reducing the number of EphB4-positive venules (Visconti et al., 2002). VEGF-A could induce ephrinB2 expression in mouse primary embryonic endothelial cells, and VEGF-A derived from sensory neurons, motor neurons and Schwann cells was required for arterial differentiation of small diameter nerve-associated vessels in mice (Mukouyama et al., 2005; Mukouyama et al., 2002).
2.2. DLL4 is a key regulator of early embryonic vascular development
In mice, DLL4 is the key Notch ligand required for vascular development. Similarly to Vegfa+/− heterozygous embryos, Dll4+/− heterozygous embryos exhibited embryonic lethal haploinsufficiency due to vascular defects on inbred genetic backgrounds (Duarte et al., 2004; Gale et al., 2004; Krebs et al., 2004). However, some Dll4+/− mice were viable on an outbred background, permitting the examination of Dll4−/ − embryos. The phenotype of the Dll4−/ − homozygotes was similar, although more severe, than that of the Dll4+/− heterozygous embryos (Duarte et al., 2004; Gale et al., 2004). Similar to what was observed in Notch signaling-deficient zebrafish embryos, both Dll4-deficient embryos and other types of Notch signaling-deficient mouse embryos such as Rbpj mutant and Hey1/Hey2 double mutant embryos did not express arterial markers (Duarte et al., 2004; Fischer et al., 2004; Gale et al., 2004; Kokubo et al., 2005; Krebs et al., 2004). Supporting a direct role for Notch signaling in regulating expression of important arterially-expressed genes, Dll4-mediated Notch signaling induced ephrinB2 expression in cultured endothelial cells (Iso et al., 2006), and the ephrinB2 gene was demonstrated to be a direct Notch target (Grego-Bessa et al., 2007).
Little is known of the transcriptional regulation of genes that exhibit arterially-restricted expression in early embryos. The winged helix/forkhead (Fox) proteins are a large family of evolutionarily conserved transcription factors (Kaestner et al., 2000). Mouse embryos with compound mutations of the Foxc1 and Foxc2 genes, two related Fox family transcription factors, exhibited defects in vascular remodeling in the yolk sac and embryo (Kume et al., 2001), accompanied by reduced or absent expression of arterial markers and arteriovenous malformations (Hayashi and Kume, 2008; Seo et al., 2006). The mechanism for this failure of arterial specification was likely through disrupted regulation of Dll4 transcription (Figure 2). The Foxc1 and Foxc2 proteins directly activate Dll4 transcription through a Foxc-binding element in the upstream region of the Dll4 gene (Hayashi and Kume, 2008; Seo et al., 2006). The Foxc1 and Foxc2 proteins also bind directly to two Foxc-binding elements to activate transcription of the Notch target gene Hey2, and the Foxc2 protein can form a complex with the RBPJ and NICD proteins (Hayashi and Kume, 2008). In bovine aortic endothelial cells, VEGF-A treatment augmented Foxc-induced activity Dll4 and Hey2 luciferase reporter constructs. These results demonstrate that the Foxc proteins are key transcriptional regulators that act upstream of the Notch pathway during arteriovenous differentiation (Hayashi and Kume, 2008; Seo et al., 2006).
2.3. DLL1 is required for both fetal and postnatal arterial development
During embryogenesis in mice, Dll1 expression in the vasculature is detected at approximately 13 days of gestation in arterial, but not venous, endothelial cells (Sorensen et al., 2009). In Dll1 loss of function mutant embryos (Dll1 hypomorphs, or embryos with endothelial cell-specific Dll1 gene deletion), generation of the Notch1 intracellular domain and expression of arterial markers such as neuropilin1, VEGF receptor 2, and ephrinB2 were lost, despite the fact that DLL4 and JAG1 continued to be expressed in the arterial endothelium. These results established DLL1 as a critical Notch ligand required for maintaining arterial identity of endothelial cells during mouse fetal development, and suggested context-dependent cross-regulation of the VEGF-A and Notch signaling pathways (Sorensen et al., 2009).
Dll1 function also is required for arteriogenesis postnatally. Arterial growth is required for restoration of blood flow following ischemia. During neovascularization induced by a mouse hindlimb ischemia model, DLL1 expression was strongly induced in arterial endothelial cells, and neovascularization was impaired in Dll1+/− heterozygous mutant mice (Limbourg et al., 2007). Blood flow recovery and postnatal neovascularization in response to hindlimb ischemia also were impaired in Notch1+/− heterozygous mice, and in mice heterozygous for an endothelial cell-specific deletion of the Notch1 gene (Takeshita et al., 2007). However, postnatal arteriogenesis and recovery from hindlimb ischemia were normal in Notch4−/ − mice, indicating that expression of the NOTCH1 protein in endothelial cells was critical for these processes (Takeshita et al., 2007).
2.4. Formation of arteriovenous malformations in Notch pathway mutants
Arteries normally connect to veins only through an intervening capillary bed. An aberrant direct communication between an artery and vein is termed an arteriovenous malformation. One model for the formation of arteriovenous malformations is an inability to establish or maintain distinct arterial and venous vascular beds. Both zebrafish (Lawson et al., 2001) and mouse (Duarte et al., 2004; Gale et al., 2004; Krebs et al., 2004) embryos deficient in Notch signaling formed arteriovenous malformations. Notch pathway gain of function mutations can also cause formation of arteriovenous malformations. Ectopic Notch4 (Uyttendaele et al., 2001; Carlson et al., 2005; Kim et al., 2008) or Notch1 (Krebs et al., 2010) activation in endothelial cells, as well as conditional Dll4 overexpression (Trindade et al., 2008) all resulted in formation of arteriovenous malformations and embryonic vascular remodeling defects. Arteriovenous malformations that formed in Notch pathway gain of function mutants were distinct from those exhibited by Notch pathway loss of function mutants (Krebs et al., 2010). Formation of arteriovenous malformations in both Notch pathway gain and loss of function mutants is likely due to an inability of the vascular beds to maintain distinct arterial and venous identities in these mutants.
EphrinB2 and EphB4 loss of function mutants also formed arteriovenous malformations (Kim et al., 2008; Krebs et al., 2010). EphrinB2 is a direct Notch target gene whose expression is induced by Notch signal reception (Grego-Bessa et al., 2007). Surprisingly, arteriovenous malformations in the ephrinB2 and EphB4 loss of function mutants phenocopied the arteriovenous malformations present in embryos with conditional Notch1 activation in endothelial cells, rather than the arteriovenous malformations exhibited by Notch pathway loss of function mutants. This result is contrary to the phenotype expected if the ephrinB2/EphB4 pathway was simply acting downstream of the Notch pathway, and suggests independent mechanisms for formation of arteriovenous malformations in Notch pathway gain of function mutant embryos and in ephrinB2 and EphB4 loss of function mutant embryos. This idea is supported by the finding that ephrinB2 and EphB4 loss of function mutant embryos contain venous endothelial cells mislocalized to the aorta, whereas the aortas of embryos with Notch4 gain of function mutations do not contain these venous endothelial cells (Kim et al., 2008). These data suggest that ephrinB2/EphB4 signaling functions distinctly from Notch signaling, by sorting arterial and venous endothelial cells into their respective vessels.
Inducible expression of an activated Notch4 transgene in adult mice resulted in vessel arterialization, such as induction of venous expression of ephrinB2, and caused arteriovenous malformations in several organs, including liver, uterus and skin (Carlson et al., 2005). Surprisingly, these malformations were reversible if activated Notch4 transgene expression was repressed. These studies demonstrate that the ability of Notch signaling to arterialize blood vessels is not confined to the embryonic period. This inducible Notch4 transgenic line has been utilized to model arteriovenous shunts and malformations in the lung (Miniati et al., 2010) and brain (Murphy et al., 2008). These mouse models have direct clinical relevance, as recent studies have established that NOTCH1 signaling is activated in brain arteriovenous malformations in humans (Murphy et al., 2009; ZhuGe et al., 2009).
2.5. Arterial specification of vascular smooth muscle cells
In addition to regulating arterial specification of endothelial cells, Notch signaling also regulates arterial specification of vascular smooth muscle cells. The Notch3 gene is expressed in vascular smooth muscle cells of arteries, but not veins. Notch3−/ − mice exhibited marked arterial defects, including enlarged arteries with a thinner vascular smooth muscle cell coat than wildtype arteries (Domenga et al., 2004). These defects arose postnatally as a consequence of defects in arterial vessel maturation. Morphologically, vascular smooth muscle cells in arteries of Notch3−/ − mice resembled the vascular smooth muscle cells surrounding veins in wildtype mice. Only a few markers are expressed predominantly in arterial vascular smooth muscle cells, rather than venous cells. These include smoothelin (van der Loop et al., 1997) and a transgenic line expressing the β–galactosidase protein from arterial-specific regulatory elements of the SM22α promoter (Moessler et al., 1996). Expression of both these markers was markedly downregulated in arteries of Notch3−/ − mice. Combined with the morphological data, this indicates that vascular smooth muscle cells surrounding arteries in Notch3−/ − mice have acquired a venous fate. Notably, arteries in Notch3−/ − mice, which did not express arterial markers for vascular smooth muscle cells, exhibited normal expression of several endothelial cell arterial markers (Domenga et al., 2004). These results demonstrate that arterial identity of endothelial cells and the vascular smooth muscle cells surrounding them is specified independently.
3. Endothelial tip cell differentiation
3.1. Notch signaling is a key regulator of endothelial tip cell formation and function
During angiogenesis, new capillaries sprout from previously existing blood vessels. Tip cells are specialized endothelial cells situated at the tips of vascular sprouts (Figure 3). These cells extend filopodia that sense the local extracellular environment and guide growth of these sprouts along VEGF-A gradients (Gerhardt et al., 2003; Gerhardt et al., 2004). The Notch pathway has a primary role in regulating formation and function of endothelial tip cells. Such a role was initially described by Hughes and colleagues (Sainson et al., 2005). In an in vitro angiogenesis culture system utilizing human umbilical vein endothelial cells (HUVECs), Notch signaling suppressed branching at the tip of developing angiogenic sprouts. Suppression of Notch signaling led to tip cell division, with both daughter cells being specified as tip cells. This led to increased branching through vessel bifurcation.
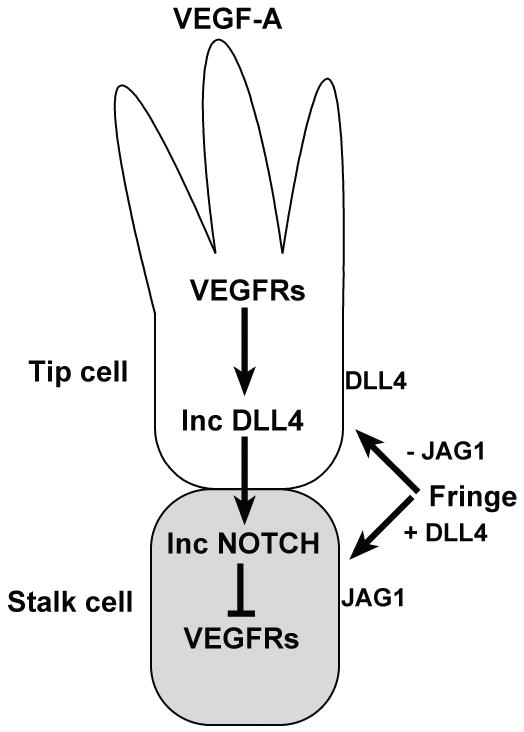
Figure 3. Antagonistic roles of the DLL4 and JAG1 proteins in tip cell selection during sprouting angiogenesis.
An endothelial tip cell (white) at the leading edge of a vascular sprout extends filopodia and migrates towards a VEGF-A gradient. Signal reception by VEGF receptors (VEGFRs) in the tip cell leads to upregulation of DLL4 expression (Inc DLL4). The tip cell signals via DLL4 to the adjacent Notch receptor-expressing cell, downregulating VEGF receptor expression. This suppresses the tip cell fate and promotes differentiation of the adjacent endothelial cell as a stalk cell (gray). Glycosylation of Notch receptors (likely NOTCH1) by Fringe proteins enhances DLL4-Notch signaling and suppresses JAG1-Notch signaling. The DLL4 and JAG1 ligands act in an antagonistic fashion during endothelial tip cell selection and angiogenic sprouting. DLL4, expressed more highly on tip cells, suppresses adoption of the tip cell fate and angiogenic sprouting. JAG1, which is expressed more highly on stalk cells, promotes tip cell differentiation and sprouting behavior by antagonizing DLL4-Notch signaling between stalk cells.
More recent work has confirmed and extended our understanding of the role that Notch signaling plays in tip cell formation. Dll4/Notch signaling regulates tip cell numbers, filopodia extension and branching of angiogenic sprouts in several model systems in addition to HUVECs: the mouse retina and hindbrain (Hellstrom et al., 2007; Lobov et al., 2007; Ridgway et al., 2006; Suchting et al., 2007), the zebrafish embryo (Leslie et al., 2007; Siekmann and Lawson, 2007), and xenograft tumor models (Noguera-Troise et al., 2006; Ridgway et al., 2006; Scehnet et al., 2007). In all of these studies, Notch signal inhibition led to increased sprouting and branching of blood vessels. The Notch pathway regulates sprouting and branching behaviors by influencing the differentiation, migration and proliferation of vascular tip cells. The Notch ligand DLL4 is most highly expressed in endothelial tip cells, where it signals to adjacent Notch receptor-expressing endothelial cells, causing them to adopt the stalk cell fate. Reduced Notch signaling leads to increases in tip cell numbers, filopodia extension and vessel branching. Suppression of tip cell formation and angiogenic sprouting by Notch signaling was downstream of the VEGF-A signal, since pharmacological or genetic manipulations that blocked VEGF-A function reduced both Dll4 expression and blood vessel sprouting.
Several studies have assessed the effects of modulating Notch signaling on differentiation of the postnatal retinal vasculature in mice (Hellstrom et al., 2007; Lobov et al., 2007; Ridgway et al., 2006; Suchting et al., 2007). The mouse retina possesses distinct advantages for analysis of developmental angiogenesis (Dorrell and Friedlander, 2006; Gariano and Gardner, 2005; Uemura et al., 2006). Development of the vascular system of the mouse retina occurs postnatally in a highly reproducible spatial and temporal pattern. The retinal vascular system emerges first in the region of the optic nerve head, then grows radially towards the periphery. The primitive vascular plexus that forms initially is remodeled into large and small arterial and venous vessels. During these stages, the retinal vasculature is accessible both for observation and for experimental administration of exogenous agents.
The Dll4 gene is highly expressed in the developing retinal vasculature. Reduced DLL4/Notch signaling leads to striking defects in the early postnatal retinal vasculature. The observed defects are concordant whether DLL4/Notch signaling is reduced genetically, by assessing Dll4+/− heterozygous mice (Hellstrom et al., 2007; Lobov et al., 2007; Suchting et al., 2007) or mice with temporally-regulated Notch1 deletion in the retinal vasculature (Hellstrom et al., 2007), or by administering anti-DLL4 blocking reagents (Lobov et al., 2007; Ridgway et al., 2006) or γ-secretase inhibitors (Hellstrom et al., 2007; Suchting et al., 2007). The retinal vasculature in these mice exhibited severe patterning defects. The vascular plexus had increased capillary density and diameter, with increased filopodial extensions both at the growing vascular front, and in the interior of the plexus. Portions of the vascular plexus fused to form syncytial sinuses. Markers specific for tip cells, such as pdgfb and unc5b, were also upregulated in mice with reduced DLL4/Notch signaling. These data indicate that DLL4/Notch signaling restricts acquisition of endothelial tip cell fate in angiogenic sprouts, causing adjacent Notch receptor-expressing endothelial cells to acquire the stalk cell fate.
Mathematical and computational modeling of angiogenesis has a long history, and many types of computational approaches have been utilized to model different aspects of angiogenesis (Peirce, 2008). Recently, agent-based modeling of angiogenesis has been utilized to more accurately model endothelial tip cell selection and capillary sprouting, and the roles of VEGF-A and DLL4/Notch signaling in these processes (Bentley et al., 2008; Bentley et al., 2009; Qutub and Popel, 2009). Agent-based modeling, utilized initially in fields such as ecology and the social sciences, has been employed to study a variety of multicellular morphogenic processes occurring during embryonic development (Thorne et al., 2007). The new models (Bentley et al., 2008; Bentley et al., 2009; Qutub and Popel, 2009) specifically incorporate features such as VEGF-A concentration gradients and DLL4/Notch signaling during endothelial tip cell selection, and are leading to novel insights amenable to experimental verification.
3.2. Antagonistic roles of the Notch ligands DLL4 and JAG1 during tip cell formation and function
Recent studies of the postnatal retinal vasculature have demonstrated that the Notch ligands DLL4 and JAG1 play antagonistic roles during tip cell selection and sprouting angiogenesis (Benedito et al., 2009). Contrary to the phenotypes observed by modulation of DLL4-mediated Notch signaling, JAG1 loss of function mutants in the retina reduced sprouting angiogenesis, while JAG1 overexpression enhanced angiogenesis and resulted in increased number of tip cells. JAG1 expression therefore acted as a proangiogenic signal during postnatal retinal angiogenesis. JAG1 expression antagonized DLL4-mediated Notch signaling, and required expression of Fringe family glycosyltransferases to do so. Glycosylation of Notch family receptors by the three mammalian Fringe proteins (Lfng, Lunatic fringe; Mfng, Manic fringe; Rfng, Radical fringe) potentiates Notch signal transmission by DLL family ligands, but suppresses signaling by JAG family ligands (Figure 3). During retinal angiogenesis, Fringe-mediated glycosylation of Notch receptors enhanced DLL4-Notch signaling, while simultaneously diminishing JAG1 signaling ability (Benedito et al., 2009). This work demonstrated that the equilibrium between two Notch ligands with distinct spatial expression patterns and opposing functional roles regulates postnatal angiogenesis in the retina.
3.3. Tip cell function in zebrafish
Additional insights into endothelial tip cell formation, migration and behavior have been obtained from analysis of zebrafish embryos (Leslie et al., 2007; Siekmann and Lawson, 2007). The optical clarity of these embryos and the availability of transgenic lines expressing fluorescent proteins in endothelial cells makes this model system ideal for high resolution fluorescent microscopy, including time-lapse confocal microscopy on living embryos. Mosaic analysis revealed that transplanted cells lacking Rbpj did not contribute to the dorsal aorta, but were preferentially located in the posterior cardinal vein or the most dorsal position of the segmental arteries. Conversely, transplanted cells expressing activated Notch1 preferentially located in the dorsal aorta or the base of developing sprouts (Siekmann and Lawson, 2007). These results indicate that Notch signaling is required cell-autonomously for determination of endothelial cell fate in segmental artery sprouts. Utilization of time-lapse confocal microscopy on living embryos revealed that in both Dll4 morphant and Rbpj morphant embryos, segmental artery sprouts contained more cells than controls. These additional cells were incorporated via both increased migration of endothelial cells into the initial sprout, and by proliferation of normally quiescent stalk cells. Interestingly, vascular defects in Rbpj morphant embryos were more severe than those in Dll4 morphant embryos, suggesting that additional Notch ligands play important roles during early vascular development (Leslie et al., 2007; Siekmann and Lawson, 2007). Blocking Vegf signaling with a small molecule inhibitor blocked both normal endothelial sprouting, as well as the ectopic sprouting observed in Dll4 morphant embryos (Leslie et al., 2007). In addition, reducing levels of Vegf receptor 3 in Rbpj morphant embryos partially rescued the Rbpj knockdown phenotype, suggesting that Notch activation might normally repress Vegf receptor 3 to limit angiogenic cell behavior in developing segmental artery sprouts (Siekmann and Lawson, 2007). Taken together, the studies in both the mouse retina and the zebrafish embryo indicate that Notch signaling acts as a negative regulator of VEGF-A induced angiogenesis, and is essential for proper vascular morphogenesis.
4. Tumor angiogenesis
4.1. Use of DLL4 blocking reagents to disrupt tumor angiogenesis
The maintenance, growth and metastasis of solid tumors require the recruitment of host blood vessels into the tumor. Many solid tumors express VEGF-A, and therapies utilizing anti-VEGF-A antibodies or other blocking reagents are effective in inhibiting solid tumor growth in preclinical rodent models (Ferrara and Kerbel, 2005; Jain et al., 2006). Given the prominent role of the Notch pathway in regulating vascular development, components of the Notch pathway may provide novel drug targets during tumor angiogenesis (Dufraine et al., 2008; Li and Harris, 2009). The Notch ligand DLL4 is expressed at high levels in tumor vasculature (Gale et al., 2004; Hainaud et al., 2006; Mailhos et al., 2001; Patel et al., 2005), and recent studies have identified the DLL4 protein as a potential drug target (Noguera-Troise et al., 2006; Ridgway et al., 2006; Scehnet et al., 2007). Systemic administration of neutralizing anti-DLL4 antibodies (Noguera-Troise et al., 2006; Ridgway et al., 2006), and systemic (Noguera-Troise et al., 2006) or localized (Scehnet et al., 2007) administration of recombinant forms of the DLL4 protein that had been modified to block DLL4/Notch signaling, inhibited growth of several different solid tumors in mice. Similar to the findings in zebrafish embryos and mouse retinas, anti-DLL4 treatment (also termed DLL4 blockade) increased blood vessel sprouting and branching, and led to a marked increase in tumor blood vessel density in the treated tumors. Paradoxically, tumor growth was inhibited despite the increased blood vessel density. Testing of the vascular network in the anti-DLL4-treated tumors by perfusion assays with fluorescent lectins or assessment of hypoxic regions in the tumors revealed that the newly-induced vessels functioned inefficiently. Many of these vessels were not connected to the vascular network in the tumors, leading to poor perfusion, increased hypoxia and an overall inhibition of tumor growth. This mechanism of inhibition of tumor growth has been termed nonproductive angiogenesis (Dufraine et al., 2008; Sainson and Harris, 2007; Thurston et al., 2007; Yan and Plowman, 2007). One complication of anti-VEGF-A therapies is acquired resistance of tumors that initially respond to the anti-VEGF-A treatments (Azam et al., 2010). Importantly, anti-DLL4 therapies were effective against tumors that were resistant to anti-VEGF-A treatments, and could provide synergistic effects against certain tumors when combined with anti-VEGF-A therapies (Noguera-Troise et al., 2006; Ridgway et al., 2006).
An important new study has dissected mechanisms involved in inhibition of tumor growth by DLL4 blockade in xenograft mouse models (Hoey et al., 2009). Specific and selective anti-human DLL4 and anti-mouse DLL4 antibodies were generated, enabling the selective targeting of DLL4 expression in the tumor, in the host vasculature and stromal cells, or both. These studies demonstrated that blocking DLL4 signaling inhibited tumor growth through multiple mechanisms. Administration of anti-mouse DLL4 antibodies inhibited tumor growth in a similar fashion to the studies described above (through nonproductive angiogenesis resulting in increased density of poorly-perfusing vessels). However, administration of anti-human DLL4 antibodies also inhibited tumor growth, but by different mechanisms. Anti-human DLL4 administration inhibited tumor growth and the expression of Notch pathway target genes, and reduced proliferation of tumor cells. Significantly, anti-human DLL4 administration reduced the frequency of tumor-initiating cancer stem cells. Combined anti-human and anti-mouse DLL4 antibody treatment had an additive effect on inhibiting tumor growth, as did combined treatment with the chemotherapeutic agent irinotecan (Hoey et al., 2009).
4.2. Safety concerns regarding anti-DLL4 therapies
While the studies described above are quite promising, a number of issues remain before anti-DLL4 therapies reach the clinic. For example, despite the efficacy of anti-VEGF-A therapies in treatment of xenograft tumor models in rodents, in clinical trials anti-VEGF-A antibody treatment of several types of cancer only provided an overall survival benefit for patients when it was combined with conventional chemotherapy treatment (Ferrara and Kerbel, 2005; Jain et al., 2006). Similar issues may arise as anti-DLL4 treatments progress from preclinical models into clinical trials. Phase one clinical trials of the use of two different anti-DLL4 human monoclonal antibodies in treatment of solid tumors are currently in progress (A multiple-ascending-dose study of the safety and tolerability of REGN421 in patients with advanced solid malignancies, http://clinicaltrials.gov/ct2/show/NCT00871559; A Phase 1 dose escalation study of OMP-21M18 in subjects with solid tumors, http://clinicaltrials.gov/ct2/show/NCT00744562).
A potentially much more serious problem was revealed in a recent study that demonstrated chronic DLL4 blockade caused pathological activation of endothelial cells, resulted in histopathological changes in several organs, and induced the formation of vascular neoplasms (Yan et al., 2010). Affected organs included liver and thymus, and deleterious effects of chronic DLL4 blockade were observed in mouse, rat and cynomolgus monkey models. Chronic DLL4 blockade in male rats resulted in a dose-dependent increase in ulcerating subcutaneous tumors that exhibited histopathological features characteristic of vascular neoplasms, although the tumors did not appear to be malignant. These studies raise concerns about the safety of chronic DLL4 blockade, and suggest that refined strategies and treatment regimens may be required to safely utilize anti-DLL4 reagents. Despite these issues, anti-DLL4 treatments remain a novel therapeutic approach for cancer treatment and retain much promise, particularly for treatment of tumors unresponsive to anti-VEGF-A therapies.
5. Notch signaling and vascular smooth muscle cells
5.1 Notch signaling is a key regulator of vascular smooth muscle cell differentiation and response to vascular injury
Notch signaling plays an important role in the differentiation, physiology and function of vascular smooth muscle cells (Morrow et al., 2008). However, contradictory results suggest that its role may be context, temporally, or cell line dependent. Several groups have published studies indicating that Notch signaling represses smooth muscle cell differentiation during in vitro culture (Doi et al., 2005; Morrow et al., 2005a; Proweller et al., 2005), and that this repressive effect is likely mediated through induction of the Hey2 protein (a demonstrated Notch target gene). More recent studies, however, have indicated that Notch signaling induces smooth muscle cell differentiation (Doi et al., 2006; High et al., 2007). Jag1-mediated Notch signaling promoted smooth muscle cell differentiation in both human aortic smooth muscle cells and a murine embryonic fibroblast cell line (Doi et al., 2006). Both smooth muscle myosin heavy chain (Doi et al., 2006) and smooth muscle α-actin (Noseda et al., 2006; Tang et al., 2008) have been demonstrated to be direct Notch target genes. In vivo studies in which Notch signaling was inactivated specifically in mouse neural crest cells demonstrated that Notch signaling plays an essential role in differentiation of cardiac neural crest cells into smooth muscle cells (High et al., 2007).
Analysis of an in vitro angiogenesis model involving coculture of human vascular endothelial cells and mural cells (progenitors for vascular smooth muscle cells) revealed that expression of the NOTCH3 gene was strongly induced in mural cells by coculture (Lilly and Kennard, 2009; Liu et al., 2009). Knockdown by small interfering RNA revealed that NOTCH3 expression was required for endothelial-dependent mural cell differentiation, whereas NOTCH3 overexpression promoted smooth muscle gene expression. NOTCH3 promoted its own expression, as well as that of the ligand JAG1 in mural cells. These findings suggested that NOTCH3 has the capacity to establish and maintain a differentiated phenotype in vascular mural cells through a positive-feedback loop that includes both NOTCH3 autoregulation and induction of JAG1 expression (Liu et al., 2009). Such a model is consistent with the finding that endothelial cell-specific Jag1 gene deletion in mice leads to a deficiency of vascular smooth muscle cell recruitment and differentiation, causing hemorrhages and early embryonic lethality (High et al., 2008).
Several studies have characterized expression of Notch pathway genes during the response to vascular injury (Campos et al., 2002; Doi et al., 2005; Lindner et al., 2001; Wang et al., 2002). After vascular injury such as carotid artery ligation, smooth muscle cells in the vascular wall of the injured area proliferate and form a thickened layer of smooth muscle cells termed the neointima. Expression of several Notch pathway components, including Notch1, Notch3, Jag1, Jag2, Hey1 and Hey2, is modulated after experimentally-induced vascular injury. Expression of these genes is downregulated within the first two days following vascular injury, but is upregulated compared to uninjured contralateral control vessels by 7 to 14 days after injury. Supporting a functional role for the modulation of Notch pathway components during the response to vascular injury, neointima formation after vascular injury was significantly decreased in Hey2−/ − mice (Sakata et al., 2004). Culture of primary aortic vascular smooth muscle cells from Hey2−/ − mice revealed that the mutant cells exhibited a reduced proliferation rate compared to wildtype cells. Overexpression of Hey1 (Wang et al., 2003) or Hey2 (Havrda et al., 2006) in vascular smooth muscle cells led to increased vascular smooth muscle cell proliferation associated with reduced levels of the cyclin-dependent kinase inhibitors p21waf1/cip1 (Wang et al., 2003) or p27kip1 (Havrda et al., 2006). The Hey2 protein interacted directly with the p27kip1 promoter to repress transcription (Havrda et al., 2006). The Hey1 and Hey2 proteins suppress NICD/RBPJ binding to the smooth muscle α-actin promoter, providing further evidence that temporally regulated induction of Hey1/Hey2 expression by Notch signaling may constitute a negative feedback mechanism involved in the regulation of vascular smooth muscle cell differentiation (Tang et al., 2008).
Neointima formation after carotid artery ligation also was decreased in heterozygous Notch1+/− mice, as well as in mice heterozygous for a conditional deletion of the Notch1 gene in smooth muscle cells (Li et al., 2009). Smooth muscle cells explanted from the aortas of these mice exhibited decreased migration and proliferation, and increased apoptosis compared to control littermate mice. Surprisingly, neointima formation after carotid artery ligation in Notch3−/ − mice was unaffected. These data indicate that Notch1 function in smooth muscle cells of the vascular wall, rather than Notch3 function, mediates smooth muscle cell proliferation and neointima formation subsequent to vascular injury.
5.2. Notch signaling and mechanical stress
Mechanical forces are one of a number of factors implicated in regulating vascular smooth muscle cell differentiation and physiology. Adult vascular smooth muscle cells are not terminally differentiated, and can exhibit substantial plasticity in their phenotype in response to changes in the local environment (Owens et al., 2004). Exposure of primary human or rat vascular smooth muscle cells to cyclic strain during in vitro culture led to significant reductions in Notch1 and Notch3 receptor expression, concomitant with an increase in expression of vascular smooth muscle cell differentiation markers (Morrow et al., 2005a; Morrow et al., 2005b). Strain-exposed vascular smooth muscle cells also exhibited reduced proliferation and increased apoptosis. These changes could be reversed by overexpression of the Notch1 or Notch3 intracellular domains. These results indicate that cyclic strain inhibits vascular smooth muscle cell growth while increasing apoptosis, and that these effects are mediated at least in part by modulation of Notch signaling. Interestingly, cyclic strain led to an upregulation of Notch receptor expression in endothelial cells (Morrow et al., 2007). Cyclic strain also caused increased endothelial cell vascular network formation in Matrigel, indicating that effects of mechanical forces on Notch signaling in the vasculature are not restricted to vascular smooth muscle cells.
6. Medical consequences of aberrant NOCH3 signaling in vascular smooth muscle cells: CADASIL
6.1 Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL)
Mutations in the NOTCH3 gene cause an inherited degenerative vascular disease that affects vascular smooth muscle cells. CADASIL (Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy) is the most common genetic form of stroke and vascular dementia (Chabriat et al., 2009; Kalaria et al., 2004). Affected individuals exhibit a variety of symptoms, including migraines, mood disorders, recurrent subcortical ischemic strokes, progressive cognitive decline, dementia and premature death. CADASIL is characterized by progressive degeneration of vascular smooth muscle cells with accumulation of granular osmiophilic material (GOM) within the smooth muscle cell basement membrane (Chabriat et al., 2009; Kalaria et al., 2004). GOM accumulation in vascular smooth muscle cells is one of the hallmark features of this disease.
CADASIL is caused by mutations in the NOTCH3 gene (Joutel et al., 1996). All mutations associated with CADASIL result in a gain or loss of a cysteine residue in one of the 34 EGF-like repeats in the extracellular domain of the NOTCH3 protein. The characteristic nature of these mutations, in addition to the absence of any examples of obviously inactivating mutations or deletions of the NOTCH3 gene of CADASIL patients, strongly suggests that mutations causing CADASIL do not create NOTCH3 null alleles. In CADASIL patients, the ectodomain of the NOTCH3 protein accumulates in the cerebral microvasculature (Joutel et al., 2000). The NOTCH3 ectodomain accumulates at the cytoplasmic membrane of vascular smooth muscle cells, although it is controversial whether the NOTCH3 ectodomain is a constituent of the GOM deposits (Ishiko et al., 2006; Joutel et al., 2000).
6.2. Mouse models of CADASIL
Mice homozygous for a targeted null mutation of the Notch3 gene are viable and fertile (Krebs et al., 2003). Notch3−/ − mice exhibited marked arterial defects, including enlarged arteries with a thinner vascular smooth muscle cell coat than wildtype arteries (Domenga et al., 2004) (Table 1). These defects arose postnatally as a consequence of defects in arterial vessel maturation. However, Notch3−/ − mice did not exhibit age-dependent accumulation of the NOTCH3 ectodomain and GOM deposits in vascular smooth muscle cells, or any CADASIL-related brain pathology. The Notch3−/ − mice did, however, exhibit a reduction in pressure-induced myogenic tone that was associated with a higher flow-mediated dilation of tail and cerebral resistance arteries (Belin de Chantemele et al., 2008). Another group analyzed an independently generated Notch3 gene trap null allele (Mitchell et al., 2001). Mice homozygous for this Notch3 null allele exhibited enhanced susceptibility in a proximal middle cerebral artery ischemia model (Arboleda-Velasquez et al., 2008). Notch3−/ − mice developed ischemic lesions approximately twice as large as those observed in heterozygous or wildtype littermates, and developed a 60% larger area of severe cerebral blood flow deficit than wildtype mice. The results of these studies indicate that while Notch3 null mice exhibit altered vascular maturation and function in at least some arteries, they do not develop CADASIL-related pathologies.
Table 1.
Phenotypes of Notch3 mutant and CADASIL mouse models
| Notch3 mutation | Allele type | Species of Notch3 coding sequences | Phenotypes | References |
|---|---|---|---|---|
| Null | knockout | NA | Vascular smooth muscle cells surrounding arteries acquire venous fate; decreased myogenic tone in tail and cerebral resistance arteries; no CADASIL- related brain pathology | Domenga et al., 2004; Belin de Chantemele et al., 2008 |
| Null | gene trap | NA | Enhanced susceptibility in middle cerebral artery ischemia model | Arboleda-Velasquez et al., 2008 |
| Arg142Cys | knockin | mouse | No phenotype or CADASIL-related brain pathology in mice heterozygous or homozygous for the knockin allele | Lundkvist et al., 2005 |
| Arg90Cys | transgenic | human | Age-dependent accumulation of NOTCH3 ectodomain and of GOM deposits in vascular smooth muscle cells; impaired cerebral vasoreactivity; impaired vascular mechanotransduction | Ruchoux et al., 2003; Lacombe et al., 2005; Dubroca et al., 2005 |
| Cys428Ser | transgenic | human | Age-dependent accumulation of NOTCH3 ectodomain and of GOM deposits in vascular smooth muscle cells; mild dominant-negative activity | Monet-Lepetre et al., 2009 |
| Arg169Cys | PAC transgenic | rat | NOTCH3 extracellular domain aggregates and GOM deposits in brain vessels; progressive degeneration of the white matter; reduced cerebral blood flow; most accurate CADASIL model reported to date | Joutel et al., 2010 |
Abbreviations: NA, not applicable; PAC, P1-derived artificial chromosome
Several mouse models expressing NOTCH3 proteins with CADASIL mutations have been developed (Table 1). An Arg142Cys knock-in mutation was introduced into the endogenous mouse Notch3 gene (Lundkvist et al., 2005). These mice did not exhibit any CADASIL-like morphological or behavioral phenotypes, even when homozygous for the Notch3 Arg142Cys mutant allele. Another model more successfully recapitulated the early, preclinical phase of CADASIL. In this model, transgenic mice were generated that expressed the human NOTCH3 cDNA containing a different CADASIL mutation, the Arg90Cys mutation, in vascular smooth muscle cells (Ruchoux et al., 2003). These mice demonstrated age-dependent accumulation of the NOTCH3 ectodomain and of GOM deposits in vascular smooth muscle cells of both cerebral and peripheral arterioles. However, despite GOM accumulation, these mice did not exhibit any evidence of damage to the brain parenchyma. Physiological studies of these NOTCH3 Arg90Cys transgenic mice revealed impaired cerebral vasoreactivity that suggested either decreased relaxation or increased resistance of cerebral blood vessels (Lacombe et al., 2005), and increased pressure-induced contraction and decreased flow-induced dilation of tail arteries (Dubroca et al., 2005). Transgenic mice expressing either the wildtype NOTCH3 protein or the NOTCH3 Arg90Cys mutation were equally effective in rescuing arterial defects of Notch3−/ − mice, and the mutant NOTCH3 Arg90Cys protein exhibited normal activity in regulating in vivo expression of a Notch signaling reporter (Monet et al., 2007). These data suggested a novel pathogenic role for the NOTCH3 Arg90Cys protein, rather than compromised NOTCH3 signaling activity, as the primary defect leading to the CADASIL phenotype.
However, it was not clear whether all CADASIL mutations retained normal NOTCH3 signaling activity, or whether genotype-phenotype correlations were observable in CADASIL patients with different NOTCH3 mutations. In particular, CADASIL mutations in the ligand binding domain, located in EGF-like repeats 10 and 11 of the NOTCH3 protein, abrogated Notch signal transduction in cell-based assays (Joutel et al., 2004; Peters et al., 2004). To assess the characteristics of NOTCH3 mutations in the ligand binding domain in vivo, transgenic mice expressing a human NOTCH3 protein with the Cys428Ser CADASIL mutation were constructed and analyzed (Monet-Lepretre et al., 2009). The NOTCH3 Cys428Ser mice, like those with the more common NOTCH3 Arg90Cys mutation, developed characteristic arterial accumulation of the NOTCH3 extracellular domain and GOM deposits upon aging. However, introducing the mutant Cys428Ser NOTCH3 transgene into a Notch3−/ − null background revealed that, in contrast to the Arg90Cys mutant protein, the Cys428Ser mutant protein had lost wildtype NOTCH3 activity, and exhibited mild dominant-negative activity. This study also revealed genotype-phenotype correlations in patients with EGF repeat 10–11 mutations. From a prospectively recruited cohort of 176 CADASIL patients, ten patients from five distinct pedigrees were identified that carried a NOTCH3 mutation in EGF repeat 10 or 11 (including six patients with the Cys428Ser mutation). These patients had higher cognitive function than patients with mutations in EGF repeats 2–5. The patients with EGF repeat 10–11 mutations also had a distinctive presentation on magnetic resonance imaging analyses. These results revealed distinctive functional and phenotypic features of EGF repeat 10–11 mutations, relative to the common CADASIL mutations (Monet-Lepretre et al., 2009).
In the most recently described mouse CADASIL model (Joutel et al., 2010), bacterial recombineering was used to introduce the Arg169Cys CADASIL mutation into Notch3 coding sequences of a rat P1-derived artificial chromosome (PAC) clone. Transgenic mice containing the wildtype rat Notch3 PAC construct and two independent lines containing the Arg169Cys CADASIL mutation were analyzed. Expression of high levels of the mutant Arg169Cys NOTCH3 transgene reproduced the endogenous NOTCH3 expression pattern and the main pathological features of CADASIL, including NOTCH3 extracellular domain aggregates and GOM deposits in brain vessels, progressive degeneration of the white matter, and reduced cerebral blood flow. The mutant mice exhibited a number of neuropathological changes that occurred in the absence of either histologically detectable alterations in cerebral artery structure or blood-brain barrier breakdown. These finding provide evidence for cerebrovascular dysfunction and microcirculatory failure as the earliest detectable consequences of the expression of the Arg169Cys NOTCH3 mutant protein. The continued development and improvement of these mouse CADASIL models are leading to valuable new insights into the onset and progression of CADASIL.
6.3. Mechanistic insights into the pathogenesis of CADASIL mutations
Recent work has yielded insights into the cellular and molecular mechanisms involved in processing and clearance of the wildtype NOTCH3 protein and NOTCH3 proteins containing CADASIL mutations. Human embryonic kidney (HEK) 293 cell lines with inducible expression of either the wildtype NOTCH3 protein or NOTCH3 proteins containing Arg133Cys and Cys185Arg mutations were analyzed (Takahashi et al., 2010). Both NOTCH3 mutant proteins were prone to aggregation and were retained within the endoplasmic reticulum. The turnover rates of the NOTCH3 proteins containing CADASIL mutations were strikingly slow, with half lives greater than 6 days, whereas the wildtype NOTCH3 protein was rapidly degraded, with a half-life of 0.7 days. Expression of the mutant NOTCH3 proteins also impaired cell proliferation compared with expression of wildtype NOTCH3. Cell lines expressing mutant NOTCH3 proteins also were more sensitive than cell lines expressing wildtype NOTCH3 to proteasome inhibition, resulting in cell death. These findings suggest that prolonged retention of mutant NOTCH3 aggregates in the endoplasmic reticulum can decrease cell growth and increase sensitivity to other cellular stresses. The finding that cell lines expressing mutant NOTCH3 proteins exhibited increased sensitivity to proteosome inhibitors (Takahashi et al., 2010) stands in contrast to another recent study, which concluded that the NOTCH3 protein in transfected HEK293 cells was degraded primarily in lysosomes (Jia et al., 2009).
Another study demonstrated that the extracellular domains of both wildtype NOTCH3 protein and NOTCH3 proteins containing CADASIL mutations spontaneously formed oligomers and higher order multimers in vitro, and that multimerization was mediated by disulfide bonds (Opherk et al., 2009). Three CADASIL mutant proteins (Arg133Cys, Cys183Arg and Cys455Arg) were tested in these studies, with concordant results. Using single-molecule analysis techniques, it was shown that CADASIL-associated mutations significantly enhanced multimerization compared to the wildtype NOTCH3 extracellular domain. These results provide experimental evidence for spontaneous NOTCH3 self-association, and are consistent with a neomorphic effect of CADASIL mutations in disease pathogenesis.
One caveat to the studies described in this section is that they all utilized HEK293 cells, rather than vascular smooth muscle cells. One of the studies reported that expression of either wildtype or mutant NOTCH3 proteins in cultured human aortic smooth muscle cells resulted in cell death as early as two days after transfection (Takahashi et al., 2010). Further studies will be required to assess whether these mechanisms also operate in more physiologically relevant situations, such as the in vivo mouse models.
7. Perspectives and conclusions
I last reviewed the field of Notch signaling in vascular biology three years ago (Gridley, 2007), and in the intervening period great advances have been made. We know substantially more about the roles and requirements for Notch ligands during both embryonic and postnatal arterial development, and during endothelial tip cell selection. Great progress has been made in developing mouse models that have important clinical implications (e.g., models for lung and brain arteriovenous malformations, tumor angiogenesis, and CADASIL models that more accurately reproduce the pathology of the human disease), and in developing computational models that accurately predict novel cellular behaviors that can then be tested experimentally.
However, much remains to be learned. We need to learn much more of the role of Notch signaling during vascular physiology in adults. Areas in which there will continue to be advances in upcoming years include the development and evaluation of anti-DLL4 therapies (as well as other types of “anti-Notch” therapies) for tumor angiogenesis and other vascular diseases, such as macular degeneration. We will also see the continued development and characterization of improved animal models for CADASIL, and the utilization of these models to gain insights into the poorly understood mechanisms underlying the pathogenesis of these NOTCH3 mutations. Another area that will see significant advances will be determining the mechanisms for cross-talk between the Notch pathway and other signaling pathways such as the TGFβ, Wnt, ephrin/Eph receptor and PI3K/Akt pathways, and the developmental and physiological decisions in which such cross-talk is operative.
Acknowledgments
Work in my laboratory on these topics has been supported by the National Institutes of Health.
References
- Arboleda-Velasquez JF, Zhou Z, Shin HK, Louvi A, Kim HH, Savitz SI, Liao JK, Salomone S, Ayata C, Moskowitz MA, et al. Linking Notch signaling to ischemic stroke. Proc Natl Acad Sci U S A. 2008;105:4856–61. doi: 10.1073/pnas.0709867105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam F, Mehta S, Harris AL. Mechanisms of resistance to antiangiogenesis therapy. Eur J Cancer Epublished March. 2010;16:2010. doi: 10.1016/j.ejca.2010.02.020. [DOI] [PubMed] [Google Scholar]
- Barsi JC, Rajendra R, Wu JI, Artzt K. Mind bomb1 is a ubiquitin ligase essential for mouse embryonic development and Notch signaling. Mech Dev. 2005;122:1106–17. doi: 10.1016/j.mod.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Belin de Chantemele EJ, Retailleau K, Pinaud F, Vessieres E, Bocquet A, Guihot AL, Lemaire B, Domenga V, Baufreton C, Loufrani L, et al. Notch3 is a major regulator of vascular tone in cerebral and tail resistance arteries. Arterioscler Thromb Vasc Biol. 2008;28:2216–24. doi: 10.1161/ATVBAHA.108.171751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedito R, Roca C, Sorensen I, Adams S, Gossler A, Fruttiger M, Adams RH. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–35. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- Bentley K, Gerhardt H, Bates PA. Agent-based simulation of notch-mediated tip cell selection in angiogenic sprout initialisation. J Theor Biol. 2008;250:25–36. doi: 10.1016/j.jtbi.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Bentley K, Mariggi G, Gerhardt H, Bates PA. Tipping the balance: robustness of tip cell selection, migration and fusion in angiogenesis. PLoS Comput Biol. 2009;5:e1000549. doi: 10.1371/journal.pcbi.1000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borggrefe T, Oswald F. The Notch signaling pathway: transcriptional regulation at Notch target genes. Cell Mol Life Sci. 2009;66:1631–46. doi: 10.1007/s00018-009-8668-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–89. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- Byrne AM, Bouchier-Hayes DJ, Harmey JH. Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF) J Cell Mol Med. 2005;9:777–94. doi: 10.1111/j.1582-4934.2005.tb00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos AH, Wang W, Pollman MJ, Gibbons GH. Determinants of Notch-3 receptor expression and signaling in vascular smooth muscle cells: implications in cell-cycle regulation. Circ Res. 2002;91:999–1006. doi: 10.1161/01.res.0000044944.99984.25. [DOI] [PubMed] [Google Scholar]
- Carlson TR, Yan Y, Wu X, Lam MT, Tang GL, Beverly LJ, Messina LM, Capobianco AJ, Werb Z, Wang R. Endothelial expression of constitutively active Notch4 elicits reversible arteriovenous malformations in adult mice. Proc Natl Acad Sci U S A. 2005;102:9884–9. doi: 10.1073/pnas.0504391102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–9. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG. Cadasil. Lancet Neurol. 2009;8:643–53. doi: 10.1016/S1474-4422(09)70127-9. [DOI] [PubMed] [Google Scholar]
- Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438:937–45. doi: 10.1038/nature04479. [DOI] [PubMed] [Google Scholar]
- Doi H, Iso T, Sato H, Yamazaki M, Matsui H, Tanaka T, Manabe I, Arai M, Nagai R, Kurabayashi M. Jagged1-selective notch signaling induces smooth muscle differentiation via a RBP-Jkappa-dependent pathway. J Biol Chem. 2006;281:28555–64. doi: 10.1074/jbc.M602749200. [DOI] [PubMed] [Google Scholar]
- Doi H, Iso T, Yamazaki M, Akiyama H, Kanai H, Sato H, Kawai-Kowase K, Tanaka T, Maeno T, Okamoto E, et al. HERP1 inhibits myocardin-induced vascular smooth muscle cell differentiation by interfering with SRF binding to CArG box. Arterioscler Thromb Vasc Biol. 2005;25:2328–34. doi: 10.1161/01.ATV.0000185829.47163.32. [DOI] [PubMed] [Google Scholar]
- Domenga V, Fardoux P, Lacombe P, Monet M, Maciazek J, Krebs LT, Klonjkowski B, Berrou E, Mericskay M, Li Z, et al. Notch3 is required for arterial identity and maturation of vascular smooth muscle cells. Genes Dev. 2004;18:2730–5. doi: 10.1101/gad.308904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrell MI, Friedlander M. Mechanisms of endothelial cell guidance and vascular patterning in the developing mouse retina. Prog Retin Eye Res. 2006;25:277–95. doi: 10.1016/j.preteyeres.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Duarte A, Hirashima M, Benedito R, Trindade A, Diniz P, Bekman E, Costa L, Henrique D, Rossant J. Dosage-sensitive requirement for mouse Dll4 in artery development. Genes Dev. 2004;18:2474–8. doi: 10.1101/gad.1239004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubroca C, Lacombe P, Domenga V, Maciazek J, Levy B, Tournier-Lasserve E, Joutel A, Henrion D. Impaired vascular mechanotransduction in a transgenic mouse model of CADASIL arteriopathy. Stroke. 2005;36:113–7. doi: 10.1161/01.STR.0000149949.92854.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufraine J, Funahashi Y, Kitajewski J. Notch signaling regulates tumor angiogenesis by diverse mechanisms. Oncogene. 2008;27:5132–7. doi: 10.1038/onc.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehebauer M, Hayward P, Martinez-Arias A. Notch signaling pathway. Sci STKE. 2006a;2006:cm7. doi: 10.1126/stke.3642006cm7. [DOI] [PubMed] [Google Scholar]
- Ehebauer M, Hayward P, Martinez-Arias A. Notch, a universal arbiter of cell fate decisions. Science. 2006b;314:1414–5. doi: 10.1126/science.1134042. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–42. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–74. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- Fischer A, Schumacher N, Maier M, Sendtner M, Gessler M. The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev. 2004;18:901–11. doi: 10.1101/gad.291004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortini ME. Notch signaling: the core pathway and its posttranslational regulation. Dev Cell. 2009;16:633–47. doi: 10.1016/j.devcel.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Gale NW, Dominguez MG, Noguera I, Pan L, Hughes V, Valenzuela DM, Murphy AJ, Adams NC, Lin HC, Holash J, et al. Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc Natl Acad Sci U S A. 2004;101:15949–54. doi: 10.1073/pnas.0407290101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariano RF, Gardner TW. Retinal angiogenesis in development and disease. Nature. 2005;438:960–6. doi: 10.1038/nature04482. [DOI] [PubMed] [Google Scholar]
- Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–77. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt H, Ruhrberg C, Abramsson A, Fujisawa H, Shima D, Betsholtz C. Neuropilin-1 is required for endothelial tip cell guidance in the developing central nervous system. Dev Dyn. 2004;231:503–9. doi: 10.1002/dvdy.20148. [DOI] [PubMed] [Google Scholar]
- Grego-Bessa J, Luna-Zurita L, Del Monte G, Bolos V, Melgar P, Arandilla A, Garratt AN, Zang H, Mukouyama YS, Chen H, et al. Notch signaling is essential for ventricular chamber development. Dev Cell. 2007;12:415–29. doi: 10.1016/j.devcel.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gridley T. Notch signaling in vascular development and physiology. Development. 2007;134:2709–18. doi: 10.1242/dev.004184. [DOI] [PubMed] [Google Scholar]
- Hainaud P, Contreres JO, Villemain A, Liu LX, Plouet J, Tobelem G, Dupuy E. The Role of the Vascular Endothelial Growth Factor-Delta-like 4 Ligand/Notch4-Ephrin B2 Cascade in Tumor Vessel Remodeling and Endothelial Cell Functions. Cancer Res. 2006;66:8501–10. doi: 10.1158/0008-5472.CAN-05-4226. [DOI] [PubMed] [Google Scholar]
- Havrda MC, Johnson MJ, O’Neill CF, Liaw L. A novel mechanism of transcriptional repression of p27kip1 through Notch/HRT2 signaling in vascular smooth muscle cells. Thromb Haemost. 2006;96:361–70. doi: 10.1160/TH06-04-0224. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Kume T. Foxc transcription factors directly regulate Dll4 and Hey2 expression by interacting with the VEGF-Notch signaling pathways in endothelial cells. PLoS One. 2008;3:e2401. doi: 10.1371/journal.pone.0002401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–80. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- Herbert SP, Huisken J, Kim TN, Feldman ME, Houseman BT, Wang RA, Shokat KM, Stainier DY. Arterial-venous segregation by selective cell sprouting: an alternative mode of blood vessel formation. Science. 2009;326:294–8. doi: 10.1126/science.1178577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herreman A, Hartmann D, Annaert W, Saftig P, Craessaerts K, Serneels L, Umans L, Schrijvers V, Checler F, Vanderstichele H, et al. Presenilin 2 deficiency causes a mild pulmonary phenotype and no changes in amyloid precursor protein processing but enhances the embryonic lethal phenotype of presenilin 1 deficiency. Proc Natl Acad Sci U S A. 1999;96:11872–7. doi: 10.1073/pnas.96.21.11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High FA, Epstein JA. The multifaceted role of Notch in cardiac development and disease. Nat Rev Genet. 2008;9:49–61. doi: 10.1038/nrg2279. [DOI] [PubMed] [Google Scholar]
- High FA, Lu MM, Pear WS, Loomes KM, Kaestner KH, Epstein JA. Endothelial expression of the Notch ligand Jagged1 is required for vascular smooth muscle development. Proc Natl Acad Sci U S A. 2008;105:1955–9. doi: 10.1073/pnas.0709663105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High FA, Zhang M, Proweller A, Tu L, Parmacek MS, Pear WS, Epstein JA. An essential role for Notch in neural crest during cardiovascular development and smooth muscle differentiation. J Clin Invest. 2007;117:353–63. doi: 10.1172/JCI30070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoey T, Yen WC, Axelrod F, Basi J, Donigian L, Dylla S, Fitch-Bruhns M, Lazetic S, Park IK, Sato A, et al. DLL4 blockade inhibits tumor growth and reduces tumor-initiating cell frequency. Cell Stem Cell. 2009;5:168–77. doi: 10.1016/j.stem.2009.05.019. [DOI] [PubMed] [Google Scholar]
- Hofmann JJ, Iruela-Arispe ML. Notch signaling in blood vessels: who is talking to whom about what? Circ Res. 2007;100:1556–68. doi: 10.1161/01.RES.0000266408.42939.e4. [DOI] [PubMed] [Google Scholar]
- Huppert SS, Le A, Schroeter EH, Mumm JS, Saxena MT, Milner LA, Kopan R. Embryonic lethality in mice homozygous for a processing-deficient allele of Notch1. Nature. 2000;405:966–70. doi: 10.1038/35016111. [DOI] [PubMed] [Google Scholar]
- Ilagan MX, Kopan R. SnapShot: notch signaling pathway. Cell. 2007;128:1246. doi: 10.1016/j.cell.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Ishiko A, Shimizu A, Nagata E, Takahashi K, Tabira T, Suzuki N. Notch3 ectodomain is a major component of granular osmiophilic material (GOM) in CADASIL. Acta Neuropathol (Berl) 2006;112:333–9. doi: 10.1007/s00401-006-0116-2. [DOI] [PubMed] [Google Scholar]
- Iso T, Maeno T, Oike Y, Yamazaki M, Doi H, Arai M, Kurabayashi M. Dll4-selective Notch signaling induces ephrinB2 gene expression in endothelial cells. Biochem Biophys Res Commun. 2006;341:708–14. doi: 10.1016/j.bbrc.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3:24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- Jia L, Yu G, Zhang Y, Wang MM. Lysosome-dependent degradation of Notch3. Int J Biochem Cell Biol. 2009;41:2594–8. doi: 10.1016/j.biocel.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joutel A, Andreux F, Gaulis S, Domenga V, Cecillon M, Battail N, Piga N, Chapon F, Godfrain C, Tournier-Lasserve E. The ectodomain of the Notch3 receptor accumulates within the cerebrovasculature of CADASIL patients. J Clin Invest. 2000;105:597–605. doi: 10.1172/JCI8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, Alamowitch S, Domenga V, Cecillion M, Marechal E, et al. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383:707–10. doi: 10.1038/383707a0. [DOI] [PubMed] [Google Scholar]
- Joutel A, Monet M, Domenga V, Riant F, Tournier-Lasserve E. Pathogenic mutations associated with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy differently affect Jagged1 binding and Notch3 activity via the RBP/JK signaling Pathway. Am J Hum Genet. 2004;74:338–47. doi: 10.1086/381506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joutel A, Monet-Lepretre M, Gosele C, Baron-Menguy C, Hammes A, Schmidt S, Lemaire-Carrette B, Domenga V, Schedl A, Lacombe P, et al. Cerebrovascular dysfunction and microcirculation rarefaction precede white matter lesions in a mouse genetic model of cerebral ischemic small vessel disease. J Clin Invest. 2010;120:433–45. doi: 10.1172/JCI39733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaestner KH, Knochel W, Martinez DE. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000;14:142–6. [PubMed] [Google Scholar]
- Kageyama R, Ohtsuka T, Kobayashi T. The Hes gene family: repressors and oscillators that orchestrate embryogenesis. Development. 2007;134:1243–51. doi: 10.1242/dev.000786. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Viitanen M, Kalimo H, Dichgans M, Tabira T. The pathogenesis of CADASIL: an update. J Neurol Sci. 2004;226:35–9. doi: 10.1016/j.jns.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Kim YH, Hu H, Guevara-Gallardo S, Lam MT, Fong SY, Wang RA. Artery and vein size is balanced by Notch and ephrin B2/EphB4 during angiogenesis. Development. 2008;135:3755–64. doi: 10.1242/dev.022475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokubo H, Miyagawa-Tomita S, Nakazawa M, Saga Y, Johnson RL. Mouse hesr1 and hesr2 genes are redundantly required to mediate Notch signaling in the developing cardiovascular system. Dev Biol. 2005;278:301–9. doi: 10.1016/j.ydbio.2004.10.025. [DOI] [PubMed] [Google Scholar]
- Koo BK, Lim HS, Song R, Yoon MJ, Yoon KJ, Moon JS, Kim YW, Kwon MC, Yoo KW, Kong MP, et al. Mind bomb 1 is essential for generating functional Notch ligands to activate Notch. Development. 2005;132:3459–70. doi: 10.1242/dev.01922. [DOI] [PubMed] [Google Scholar]
- Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–33. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs LT, Shutter JR, Tanigaki K, Honjo T, Stark KL, Gridley T. Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev. 2004;18:2469–73. doi: 10.1101/gad.1239204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs LT, Starling C, Chervonsky AV, Gridley T. Notch1 activation in mice causes arteriovenous malformations phenocopied by ephrinB2 and EphB4 mutants. Genesis. 2010;48:146–50. doi: 10.1002/dvg.20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, Gallahan D, Closson V, Kitajewski J, Callahan R, et al. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000;14:1343–52. [PMC free article] [PubMed] [Google Scholar]
- Krebs LT, Xue Y, Norton CR, Sundberg JP, Beatus P, Lendahl U, Joutel A, Gridley T. Characterization of Notch3-deficient mice: normal embryonic development and absence of genetic interactions with a Notch1 mutation. Genesis. 2003;37:139–43. doi: 10.1002/gene.10241. [DOI] [PubMed] [Google Scholar]
- Kume T. Novel insights into the differential functions of Notch ligands in vascular formation. J Angiogenes Res. 2009;1:8. doi: 10.1186/2040-2384-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume T, Jiang H, Topczewska JM, Hogan BL. The murine winged helix transcription factors, Foxc1 and Foxc2, are both required for cardiovascular development and somitogenesis. Genes Dev. 2001;15:2470–82. doi: 10.1101/gad.907301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacombe P, Oligo C, Domenga V, Tournier-Lasserve E, Joutel A. Impaired cerebral vasoreactivity in a transgenic mouse model of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy arteriopathy. Stroke. 2005;36:1053–8. doi: 10.1161/01.STR.0000163080.82766.eb. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128:3675–83. doi: 10.1242/dev.128.19.3675. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Vogel AM, Weinstein BM. sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3:127–36. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- Le Borgne R. Regulation of Notch signalling by endocytosis and endosomal sorting. Curr Opin Cell Biol. 2006;18:213–22. doi: 10.1016/j.ceb.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Leslie JD, Ariza-McNaughton L, Bermange AL, McAdow R, Johnson SL, Lewis J. Endothelial signalling by the Notch ligand Delta-like 4 restricts angiogenesis. Development. 2007;134:839–44. doi: 10.1242/dev.003244. [DOI] [PubMed] [Google Scholar]
- Li JL, Harris AL. Crosstalk of VEGF and Notch pathways in tumour angiogenesis: therapeutic implications. Front Biosci. 2009;14:3094–110. doi: 10.2741/3438. [DOI] [PubMed] [Google Scholar]
- Li T, Ma G, Cai H, Price DL, Wong PC. Nicastrin is required for assembly of presenilin/gamma-secretase complexes to mediate Notch signaling and for processing and trafficking of beta-amyloid precursor protein in mammals. J Neurosci. 2003;23:3272–7. doi: 10.1523/JNEUROSCI.23-08-03272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Takeshita K, Liu PY, Satoh M, Oyama N, Mukai Y, Chin MT, Krebs L, Kotlikoff MI, Radtke F, et al. Smooth muscle Notch1 mediates neointimal formation after vascular injury. Circulation. 2009;119:2686–92. doi: 10.1161/CIRCULATIONAHA.108.790485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly B, Kennard S. Differential gene expression in a coculture model of angiogenesis reveals modulation of select pathways and a role for Notch signaling. Physiol Genomics. 2009;36:69–78. doi: 10.1152/physiolgenomics.90318.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbourg A, Ploom M, Elligsen D, Sorensen I, Ziegelhoeffer T, Gossler A, Drexler H, Limbourg FP. Notch ligand Delta-like 1 is essential for postnatal arteriogenesis. Circ Res. 2007;100:363–71. doi: 10.1161/01.RES.0000258174.77370.2c. [DOI] [PubMed] [Google Scholar]
- Limbourg FP, Takeshita K, Radtke F, Bronson RT, Chin MT, Liao JK. Essential role of endothelial Notch1 in angiogenesis. Circulation. 2005;111:1826–32. doi: 10.1161/01.CIR.0000160870.93058.DD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner V, Booth C, Prudovsky I, Small D, Maciag T, Liaw L. Members of the Jagged/Notch gene families are expressed in injured arteries and regulate cell phenotype via alterations in cell matrix and cell-cell interaction. Am J Pathol. 2001;159:875–83. doi: 10.1016/S0002-9440(10)61763-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Kennard S, Lilly B. NOTCH3 expression is induced in mural cells through an autoregulatory loop that requires endothelial-expressed JAGGED1. Circ Res. 2009;104:466–75. doi: 10.1161/CIRCRESAHA.108.184846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZJ, Shirakawa T, Li Y, Soma A, Oka M, Dotto GP, Fairman RM, Velazquez OC, Herlyn M. Regulation of Notch1 and Dll4 by vascular endothelial growth factor in arterial endothelial cells: implications for modulating arteriogenesis and angiogenesis. Mol Cell Biol. 2003;23:14–25. doi: 10.1128/MCB.23.1.14-25.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, Wiegand SJ. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci U S A. 2007 doi: 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundkvist J, Zhu S, Hansson EM, Schweinhardt P, Miao Q, Beatus P, Dannaeus K, Karlstrom H, Johansson CB, Viitanen M, et al. Mice carrying a R142C Notch 3 knock-in mutation do not develop a CADASIL-like phenotype. Genesis. 2005;41:13–22. doi: 10.1002/gene.20091. [DOI] [PubMed] [Google Scholar]
- Mailhos C, Modlich U, Lewis J, Harris A, Bicknell R, Ish-Horowicz D. Delta4, an endothelial specific notch ligand expressed at sites of physiological and tumor angiogenesis. Differentiation. 2001;69:135–44. doi: 10.1046/j.1432-0436.2001.690207.x. [DOI] [PubMed] [Google Scholar]
- Miniati D, Jelin EB, Ng J, Wu J, Carlson TR, Wu X, Looney MR, Wang RA. Constitutively active endothelial Notch4 causes lung arteriovenous shunts in mice. Am J Physiol Lung Cell Mol Physiol. 2010;298:L169–77. doi: 10.1152/ajplung.00188.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KJ, Pinson KI, Kelly OG, Brennan J, Zupicich J, Scherz P, Leighton PA, Goodrich LV, Lu X, Avery BJ, et al. Functional analysis of secreted and transmembrane proteins critical to mouse development. Nat Genet. 2001;28:241–9. doi: 10.1038/90074. [DOI] [PubMed] [Google Scholar]
- Moessler H, Mericskay M, Li Z, Nagl S, Paulin D, Small JV. The SM 22 promoter directs tissue-specific expression in arterial but not in venous or visceral smooth muscle cells in transgenic mice. Development. 1996;122:2415–25. doi: 10.1242/dev.122.8.2415. [DOI] [PubMed] [Google Scholar]
- Monet M, Domenga V, Lemaire B, Souilhol C, Langa F, Babinet C, Gridley T, Tournier-Lasserve E, Cohen-Tannoudji M, Joutel A. The archetypal R90C CADASIL-NOTCH3 mutation retains NOTCH3 function in vivo. Hum Mol Genet. 2007 doi: 10.1093/hmg/ddm042. [DOI] [PubMed] [Google Scholar]
- Monet-Lepretre M, Bardot B, Lemaire B, Domenga V, Godin O, Dichgans M, Tournier-Lasserve E, Cohen-Tannoudji M, Chabriat H, Joutel A. Distinct phenotypic and functional features of CADASIL mutations in the Notch3 ligand binding domain. Brain. 2009;132:1601–12. doi: 10.1093/brain/awp049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow D, Cullen JP, Cahill PA, Redmond EM. Cyclic strain regulates the Notch/CBF-1 signaling pathway in endothelial cells: role in angiogenic activity. Arterioscler Thromb Vasc Biol. 2007;27:1289–96. doi: 10.1161/ATVBAHA.107.142778. [DOI] [PubMed] [Google Scholar]
- Morrow D, Guha S, Sweeney C, Birney Y, Walshe T, O’Brien C, Walls D, Redmond EM, Cahill PA. Notch and vascular smooth muscle cell phenotype. Circ Res. 2008;103:1370–82. doi: 10.1161/CIRCRESAHA.108.187534. [DOI] [PubMed] [Google Scholar]
- Morrow D, Scheller A, Birney YA, Sweeney C, Guha S, Cummins PM, Murphy R, Walls D, Redmond EM, Cahill PA. Notch-mediated CBF-1/RBP-J{kappa}-dependent regulation of human vascular smooth muscle cell phenotype in vitro. Am J Physiol Cell Physiol. 2005a;289:C1188–96. doi: 10.1152/ajpcell.00198.2005. [DOI] [PubMed] [Google Scholar]
- Morrow D, Sweeney C, Birney YA, Cummins PM, Walls D, Redmond EM, Cahill PA. Cyclic strain inhibits Notch receptor signaling in vascular smooth muscle cells in vitro. Circ Res. 2005b;96:567–75. doi: 10.1161/01.RES.0000159182.98874.43. [DOI] [PubMed] [Google Scholar]
- Mukouyama YS, Gerber HP, Ferrara N, Gu C, Anderson DJ. Peripheral nerve-derived VEGF promotes arterial differentiation via neuropilin 1-mediated positive feedback. Development. 2005;132:941–52. doi: 10.1242/dev.01675. [DOI] [PubMed] [Google Scholar]
- Mukouyama YS, Shin D, Britsch S, Taniguchi M, Anderson DJ. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 2002;109:693–705. doi: 10.1016/s0092-8674(02)00757-2. [DOI] [PubMed] [Google Scholar]
- Murphy PA, Lam MT, Wu X, Kim TN, Vartanian SM, Bollen AW, Carlson TR, Wang RA. Endothelial Notch4 signaling induces hallmarks of brain arteriovenous malformations in mice. Proc Natl Acad Sci U S A. 2008;105:10901–6. doi: 10.1073/pnas.0802743105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PA, Lu G, Shiah S, Bollen AW, Wang RA. Endothelial Notch signaling is upregulated in human brain arteriovenous malformations and a mouse model of the disease. Lab Invest. 2009;89:971–82. doi: 10.1038/labinvest.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemir M, Pedrazzini T. Functional role of Notch signaling in the developing and postnatal heart. J Mol Cell Cardiol. 2008;45:495–504. doi: 10.1016/j.yjmcc.2008.02.273. [DOI] [PubMed] [Google Scholar]
- Niessen K, Karsan A. Notch signaling in cardiac development. Circ Res. 2008;102:1169–81. doi: 10.1161/CIRCRESAHA.108.174318. [DOI] [PubMed] [Google Scholar]
- Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, Lin HC, Yancopoulos GD, Thurston G. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444:1032–7. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- Noseda M, Fu Y, Niessen K, Wong F, Chang L, McLean G, Karsan A. Smooth Muscle alpha-actin is a direct target of Notch/CSL. Circ Res. 2006;98:1468–70. doi: 10.1161/01.RES.0000229683.81357.26. [DOI] [PubMed] [Google Scholar]
- Opherk C, Duering M, Peters N, Karpinska A, Rosner S, Schneider E, Bader B, Giese A, Dichgans M. CADASIL mutations enhance spontaneous multimerization of NOTCH3. Hum Mol Genet. 2009;18:2761–7. doi: 10.1093/hmg/ddp211. [DOI] [PubMed] [Google Scholar]
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- Patel NS, Li JL, Generali D, Poulsom R, Cranston DW, Harris AL. Up-regulation of delta-like 4 ligand in human tumor vasculature and the role of basal expression in endothelial cell function. Cancer Res. 2005;65:8690–7. doi: 10.1158/0008-5472.CAN-05-1208. [DOI] [PubMed] [Google Scholar]
- Peirce SM. Computational and mathematical modeling of angiogenesis. Microcirculation. 2008;15:739–51. doi: 10.1080/10739680802220331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters N, Opherk C, Zacherle S, Capell A, Gempel P, Dichgans M. CADASIL-associated Notch3 mutations have differential effects both on ligand binding and ligand-induced Notch3 receptor signaling through RBP-Jk. Exp Cell Res. 2004;299:454–64. doi: 10.1016/j.yexcr.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Phng LK, Gerhardt H. Angiogenesis: a team effort coordinated by notch. Dev Cell. 2009;16:196–208. doi: 10.1016/j.devcel.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Proweller A, Pear WS, Parmacek MS. Notch signaling represses myocardin-induced smooth muscle cell differentiation. J Biol Chem. 2005;280:8994–9004. doi: 10.1074/jbc.M413316200. [DOI] [PubMed] [Google Scholar]
- Qutub AA, Popel AS. Elongation, proliferation & migration differentiate endothelial cell phenotypes and determine capillary sprouting. BMC Syst Biol. 2009;3:13. doi: 10.1186/1752-0509-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway J, Zhang G, Wu Y, Stawicki S, Liang WC, Chanthery Y, Kowalski J, Watts RJ, Callahan C, Kasman I, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–7. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- Roca C, Adams RH. Regulation of vascular morphogenesis by Notch signaling. Genes Dev. 2007;21:2511–24. doi: 10.1101/gad.1589207. [DOI] [PubMed] [Google Scholar]
- Ruchoux MM, Domenga V, Brulin P, Maciazek J, Limol S, Tournier-Lasserve E, Joutel A. Transgenic mice expressing mutant Notch3 develop vascular alterations characteristic of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Am J Pathol. 2003;162:329–42. doi: 10.1016/S0002-9440(10)63824-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainson RC, Aoto J, Nakatsu MN, Holderfield M, Conn E, Koller E, Hughes CC. Cell-autonomous notch signaling regulates endothelial cell branching and proliferation during vascular tubulogenesis. Faseb J. 2005;19:1027–9. doi: 10.1096/fj.04-3172fje. [DOI] [PubMed] [Google Scholar]
- Sainson RC, Harris AL. Anti-Dll4 therapy: can we block tumour growth by increasing angiogenesis? Trends Mol Med. 2007;13:389–95. doi: 10.1016/j.molmed.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Sakata Y, Xiang F, Chen Z, Kiriyama Y, Kamei CN, Simon DI, Chin MT. Transcription factor CHF1/Hey2 regulates neointimal formation in vivo and vascular smooth muscle proliferation and migration in vitro. Arterioscler Thromb Vasc Biol. 2004;24:2069–74. doi: 10.1161/01.ATV.0000143936.77094.a4. [DOI] [PubMed] [Google Scholar]
- Scehnet JS, Jiang W, Kumar SR, Krasnoperov V, Trindade A, Benedito R, Djokovic D, Borges C, Ley EJ, Duarte A, et al. Inhibition of Dll4 mediated signaling induces proliferation of immature vessels and results in poor tissue perfusion. Blood. 2007 doi: 10.1182/blood-2006-12-063933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S, Fujita H, Nakano A, Kang M, Duarte A, Kume T. The forkhead transcription factors, Foxc1 and Foxc2, are required for arterial specification and lymphatic sprouting during vascular development. Dev Biol. 2006;294:458–70. doi: 10.1016/j.ydbio.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312:549–60. doi: 10.1016/j.yexcr.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Siekmann AF, Lawson ND. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature. 2007;445:781–4. doi: 10.1038/nature05577. [DOI] [PubMed] [Google Scholar]
- Sorensen I, Adams RH, Gossler A. DLL1-mediated Notch activation regulates endothelial identity in mouse fetal arteries. Blood. 2009;113:5680–8. doi: 10.1182/blood-2008-08-174508. [DOI] [PubMed] [Google Scholar]
- Stalmans I, Ng YS, Rohan R, Fruttiger M, Bouche A, Yuce A, Fujisawa H, Hermans B, Shani M, Jansen S, et al. Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms. J Clin Invest. 2002;109:327–36. doi: 10.1172/JCI14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchting S, Freitas C, le Noble F, Benedito R, Breant C, Duarte A, Eichmann A. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci U S A. 2007 doi: 10.1073/pnas.0611177104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Adachi K, Yoshizaki K, Kunimoto S, Kalaria RN, Watanabe A. Mutations in NOTCH3 cause the formation and retention of aggregates in the endoplasmic reticulum, leading to impaired cell proliferation. Hum Mol Genet. 2010;19:79–89. doi: 10.1093/hmg/ddp468. [DOI] [PubMed] [Google Scholar]
- Takeshita K, Satoh M, Ii M, Silver M, Limbourg FP, Mukai Y, Rikitake Y, Radtke F, Gridley T, Losordo DW, et al. Critical role of endothelial Notch1 signaling in postnatal angiogenesis. Circ Res. 2007;100:70–8. doi: 10.1161/01.RES.0000254788.47304.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Urs S, Liaw L. Hairy-related transcription factors inhibit Notch-induced smooth muscle alpha-actin expression by interfering with Notch intracellular domain/CBF-1 complex interaction with the CBF-1-binding site. Circ Res. 2008;102:661–8. doi: 10.1161/CIRCRESAHA.107.165134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne BC, Bailey AM, DeSimone DW, Peirce SM. Agent-based modeling of multicell morphogenic processes during development. Birth Defects Res C Embryo Today. 2007;81:344–53. doi: 10.1002/bdrc.20106. [DOI] [PubMed] [Google Scholar]
- Thurston G, Noguera-Troise I, Yancopoulos GD. The Delta paradox: DLL4 blockade leads to more tumour vessels but less tumour growth. Nat Rev Cancer. 2007;7:327–31. doi: 10.1038/nrc2130. [DOI] [PubMed] [Google Scholar]
- Trindade A, Kumar SR, Scehnet JS, Lopes-da-Costa L, Becker J, Jiang W, Liu R, Gill PS, Duarte A. Overexpression of delta-like 4 induces arterialization and attenuates vessel formation in developing mouse embryos. Blood. 2008;112:1720–9. doi: 10.1182/blood-2007-09-112748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura A, Kusuhara S, Katsuta H, Nishikawa S. Angiogenesis in the mouse retina: a model system for experimental manipulation. Exp Cell Res. 2006;312:676–83. doi: 10.1016/j.yexcr.2005.10.030. [DOI] [PubMed] [Google Scholar]
- Uyttendaele H, Ho J, Rossant J, Kitajewski J. Vascular patterning defects associated with expression of activated Notch4 in embryonic endothelium. Proc Natl Acad Sci USA. 2001;98:5643–5648. doi: 10.1073/pnas.091584598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Loop FT, Gabbiani G, Kohnen G, Ramaekers FC, van Eys GJ. Differentiation of smooth muscle cells in human blood vessels as defined by smoothelin, a novel marker for the contractile phenotype. Arterioscler Thromb Vasc Biol. 1997;17:665–71. doi: 10.1161/01.atv.17.4.665. [DOI] [PubMed] [Google Scholar]
- Visconti RP, Richardson CD, Sato TN. Orchestration of angiogenesis and arteriovenous contribution by angiopoietins and vascular endothelial growth factor (VEGF) Proc Natl Acad Sci U S A. 2002;99:8219–24. doi: 10.1073/pnas.122109599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Campos AH, Prince CZ, Mou Y, Pollman MJ. Coordinate Notch3-hairy-related transcription factor pathway regulation in response to arterial injury. Mediator role of platelet-derived growth factor and ERK. J Biol Chem. 2002;277:23165–71. doi: 10.1074/jbc.M201409200. [DOI] [PubMed] [Google Scholar]
- Wang W, Prince CZ, Hu X, Pollman MJ. HRT1 modulates vascular smooth muscle cell proliferation and apoptosis. Biochem Biophys Res Commun. 2003;308:596–601. doi: 10.1016/s0006-291x(03)01453-0. [DOI] [PubMed] [Google Scholar]
- Xue Y, Gao X, Lindsell CE, Norton CR, Chang B, Hicks C, Gendron-Maguire M, Rand EB, Weinmaster G, Gridley T. Embryonic lethality and vascular defects in mice lacking the Notch ligand Jagged1. Hum Mol Genet. 1999;8:723–30. doi: 10.1093/hmg/8.5.723. [DOI] [PubMed] [Google Scholar]
- Yan M, Callahan CA, Beyer JC, Allamneni KP, Zhang G, Ridgway JB, Niessen K, Plowman GD. Chronic DLL4 blockade induces vascular neoplasms. Nature. 2010;463:E6–7. doi: 10.1038/nature08751. [DOI] [PubMed] [Google Scholar]
- Yan M, Plowman GD. Delta-like 4/Notch signaling and its therapeutic implications. Clin Cancer Res. 2007;13:7243–6. doi: 10.1158/1078-0432.CCR-07-1393. [DOI] [PubMed] [Google Scholar]
- Zhong TP, Childs S, Leu JP, Fishman MC. Gridlock signalling pathway fashions the first embryonic artery. Nature. 2001;414:216–20. doi: 10.1038/35102599. [DOI] [PubMed] [Google Scholar]
- Zhong TP, Rosenberg M, Mohideen MA, Weinstein B, Fishman MC. gridlock, an HLH gene required for assembly of the aorta in zebrafish. Science. 2000;287:1820–4. doi: 10.1126/science.287.5459.1820. [DOI] [PubMed] [Google Scholar]
- ZhuGe Q, Zhong M, Zheng W, Yang GY, Mao X, Xie L, Chen G, Chen Y, Lawton MT, Young WL, et al. Notch-1 signalling is activated in brain arteriovenous malformations in humans. Brain. 2009;132:3231–41. doi: 10.1093/brain/awp246. [DOI] [PMC free article] [PubMed] [Google Scholar]