Abstract
Purpose
Patients with locally-advanced rectal cancer typically undergo neoadjuvant chemoradiotherapy to decrease the postsurgical recurrence rate. However, neoadjuvant therapy is associated with significant morbidity and not all patients benefit equally. The purpose of this pilot study was to evaluate whether tumor uptake of 18F-labeled 3′-deoxy-3′fluorothymidine (FLT), a proliferative radiotracer, at baseline and early during therapy, is predictive of outcome in locally-advanced rectal cancer.
Methods and Materials
Fourteen patients with rectal cancer underwent positron emission tomography (PET) with FLT before and approximately 2 weeks after initiation of neoadjuvant chemoradiotherapy. All patients underwent PET/CT with 18F-fluorodeoxyglucose (FDG) as part of clinical staging prior to institution of therapy. FLT and FDG uptake were evaluated qualitatively and semiquantitatively by determining the maximum standardized uptake value (SUVmax). Tumor FLT and FDG uptake were correlated with disease-free survival (DFS). Patients were followed for a median of 20 months (range 8 to 37 months).
Results
Thirteen patients underwent surgery following neoadjuvant therapy and one patient died prior to surgery with progressive disease. Overall, pretherapy FDG uptake in the primary tumor was significantly greater than that of pretherapy FLT uptake (p=0.003). FDG-PET/CT was positive for regional lymph node metastases in 5 and FLT-PET detected metastatic disease in only one of these patients. After initiation of therapy, tumor FLT uptake decreased significantly from baseline (p<0.0001). High pretherapy FDG uptake (SUVmax≥14.3), low during-therapy FLT uptake (SUVmax<2.2) and high percentage change in FLT uptake (≥60%) were predictive of improved DFS (p<0.05 for all three values). Pretherapy FLT uptake was not a significant predictor of outcome and did not correlate with DFS (p=NS).
Conclusion
In this pilot study, pretherapy FDG uptake, during-therapy FLT uptake and percentage change in FLT uptake were equally predictive of DFS. In addition, FDG-PET/CT was superior to FLT-PET in detection of metastasis, and thus, in staging rectal cancer.
Keywords: Positron emission tomography, rectal cancer, proliferation, FLT, PET
Introduction
There will be 40,290 new cases of rectal cancer diagnosed in the United States in 2012 [1]. Approximately 80% of patients with newly diagnosed rectal cancer have disease localized to the rectum with or without involvement of regional lymph nodes, making them potential candidates for curative treatment [2]. Patients with locally advanced rectal cancer who are treated by surgery alone are at a high risk of developing local failure. The development of combined-modality therapy (neoadjuvant chemoradiotherapy and surgical resection) over the past few decades has led to improved outcomes in this subset of potentially curable patients. Currently, neoadjuvant chemoradiotherapy is accepted as the standard of care in the United States [3]. However, tumor response to neoadjuvant chemoradiotherapy varies considerably and not all patients benefit equally from such therapy [4]. In high-risk patients, combined-modality therapy has resulted in a decrease in the rate of locoregional recurrence without significant improvement in overall survival [5]. Prospective identification of patients most likely to benefit from preoperative chemoradiotherapy is important in decreasing treatment morbidity and improving survival and local control in patients with locally advanced rectal cancer. Moreover, patients who are unlikely to respond could be identified early and offered alternative treatments. However, prediction of response to combined-modality therapy and outcome has been a challenging problem. TNM staging is limited in predicting which patients will respond to chemoradiotherapy. Several biomarkers have been studied as predictors of response to chemoradiotherapy in rectal cancer, but none is ready for routine clinical use [6].
Pretherapy positron emission tomography (PET) with [18F]fluorodeoxyglucose (FDG) has been shown to be useful in predicting outcome in several solid cancers. In non-small-cell cancer; FDG uptake of the primary tumor has been shown to be the strongest prognostic factor among patients treated by curative surgery or radiotherapy [7, 8]. Similarly in esophageal cancer, the pretherapy FDG uptake was predictive of survival [9]. In rectal cancer, changes in FDG uptake after completion of neoadjuvant therapy have been shown to be accurate in predicting response to neoadjuvant therapy [10, 11]. Limited data suggest that FDG-PET during therapy is useful for predicting response to therapy in various cancers such as breast cancer, esophageal cancer and lung cancer [12–14]. However, FDG is not a tumor-specific tracer and can accumulate at sites of inflammation, including that due to the effects of radiation therapy [15].
The thymidine analogue [18F]-3′-deoxy-3′-fluorothymidine (FLT) has been developed as a specific marker of cellular proliferation in vivo [16]. Like thymidine, FLT is phosphorylated by thymidine kinase 1, a cytosolic enzyme that is upregulated when proliferating cells enter the S-phase of the cell cycle [16]. Several pilot studies have used FDG-PET and FLT-PET as methods for assessing response and predicting survival in various cancers [17, 18]. To date, no studies have compared these two tracers as biomarkers for predicting response and survival after neoadjuvant chemoradiation in rectal cancer. In the current pilot study, we correlated the tumor uptake of FLT and FDG with outcome in patients with locally advanced cancer who were treated with combined modality therapy.
Material and Methods
Patients
We studied 14 patients (12 men, 2 women, mean age 54.1 years; range 39–75 years) with pathologically proven rectal cancer. All patients had tumors that were 4 cm or more in size, and located within 12 cm of the anal verge. All patients were scheduled to undergo surgical resection following neoadjuvant chemoradiotherapy. This investigation was approved by the institutional review board and the Radioactive Drug Research Committee of Washington University School of Medicine. Each patient gave informed consent prior to participating in the study.
All patients were initially evaluated with a history and physical examination, routine laboratory studies, chest radiographs, CT of abdomen and pelvis (6 also had MRI of the abdomen and pelvis), digital and proctoscopic examination, endorectal ultrasonography (9 patients) for local staging of the primary tumor (if ultrasonography was not possible due to obstruction or narrowing of the rectal lumen, tumor measurements were determined by MRI of the pelvis), and whole-body FDG-PET/CT. The FDG-PET/CT images were performed, as previously described [19]. All patients underwent PET imaging with FLT (as described below) prior to and approximately 2 weeks after initiation of therapy as part of the research protocol.
Tumor staging
The primary rectal cancer was measured in 2–3 dimensions (length, width, thickness). The tumor dimensions were obtained from proctoscopic examination, digital rectal examination, endorectal ultrasonography, MRI, radiographs, or any combination of the above. However, the preferred method used for assessing tumor dimensions was proctoscopy (all patients) for tumor length and ultrasonography (9 patients) for tumor thickness. Staging was done with CT and FDG-PET scans in all patients. The clinical tumor (T) stage of the primary tumor was determined based on AJCC guidelines using proctoscopy, endorectal ultrasonography or MRI.
Treatment
The patients were treated in accordance with the standard clinical regimen in use at Washington University during the study interval (2006–2009). Briefly, all patients underwent neoadjuvant chemoradiotherapy, which consisted of 45–50 Gy external beam radiation therapy given in 1.8 Gy fractions to the pelvis with continuous intravenous infusion of 5-fluorouracil (225 mg/m2/day). Radiation was delivered with an equally weighted four-field technique in the prone position. Standard extirpative surgery was performed by a board-certified colorectal surgeon six to eight weeks after completion of neoadjuvant chemoradiotherapy. The surgical specimen was submitted for determination of pathologic tumor stage according to AJCC cancer staging manual by a pathologist who was blinded with respect to the FLT-PET results [20].
Response Evaluation and Follow-up
In addition to routine pathological analysis of the resected tumors, tumor regression was scored by two pathologists (IN, CM) also without the knowledge of patient survival or PET results using an established 5-point tumor regression grade (TRG) [21, 22] as follows: TRG-1, complete regression; TRG-2, presence of rare residual cancer cells scattered through fibrotic tissue; TRG-3, increased number of residual cancer cells, but fibrosis still predominant; TRG-4, residual cancer outgrowing fibrosis; and TRG-5, no regressive changes detectable. TRG-1, 2, and 3 were considered as indicative of responding tumors (TRG 1, complete response; TRG 2, partial response; TRG 3, mild response) and TRG 4 and 5 were considered indicative of nonresponding tumors [23, 24]
Patients were then followed for recurrence according to NCCN guidelines (median of 40 months, range 8 to 37) (http://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf). This typically entails clinical evaluation and serum carcinoembryonic antigen (CEA) assay every 6 months for 5 years, along with chest radiographs, colonoscopy at one year and then every 1–3 years, and annual CT for 3–5 years in patients with high risk for developing recurrence. PET was only obtained if recurrence was highly suspected and conventional imaging was negative. Recurrence was defined as documented tumor recurrence either locally in the pelvis or at a distant site (liver, lung, etc.). Both time to recurrence and survival were measured from the date of surgery for a maximum of 5 years.
Radiopharmaceutical Synthesis
FLT was produced in house at the Washington University cyclotron facility according to a previously described procedure [25, 26]. Each batch of FLT met release standards for radiochemical purity, apyrogenicity, and sterility.
PET Imaging
FLT-PET imaging was performed using either a CTI/Siemens ECAT HR+ scanner or a CTI/Siemens ECAT Exact Scanner. The same scanner was used for the pre-treatment and mid-treatment FLT scans of each patient. The FLT-PET procedure required placement of an angiocather (typically, a 20- or 22-gauge angiocath) or a butterfly needle in a vein of the patient’s arm. The injected dose of FLT was approximately 10 mCi (mean ±standard deviation 9.1 ± 1.7 mCi pretherapy vs. 8.1 ± 3.1 mCi during therapy). Because FLT is excreted via the kidney into the bladder and the area of interest was the pelvis, each patient had a Foley catheter placed in the urinary bladder before the injection of FLT, was given furosemide (20 mg intravenously 20 minutes after FLT injection) and was hydrated intravenously with 500–1000 mL 0.9% saline solution. Beginning 45–60 min after intravenous injection of FLT, a series of overlapping transmission and emission scans (2–5 min transmission and 5–10 min emission scans) was performed to image the torso (typically 5–7 bed positions). Efforts were made to keep the uptake time between each FLT-PET pair studies as close as possible (mean 6.2 min range of 1 to 14 min). Imaging was performed with the patient in the supine position. The emission images were corrected for measured attenuation using a local threshold for segmented attenuation. PET images were reconstructed using an ordered-subset estimation-maximization (OSEM) iterative algorithm. Images were smoothed with an 8-mm post reconstruction filter. FLT-PET was performed 1–18 days (mean 6 days) prior to the start of concurrent chemoradiotherapy as well as 14–19 days (mean 16 days) after the beginning of treatment.
Image Analysis
A nuclear medicine physician, without knowledge of the findings of prior imaging studies, interpreted the FLT-PET images. The pretherapy images were evaluated qualitatively for primary tumor uptake and for the presence or absence of abnormal FLT uptake in pelvic and para-aortic lymph nodes and other possible sites of disease. A re-reading of the FLT-PET images was then done in combination with the conventional imaging studies including the clinical FDG-PET/CT. Pretherapy FLT-PET images were directly compared with FDG-PET to determine whether the lesion(s) seen on FDG-PET also were seen on FLT-PET. In addition, both pretherapy FDG-PET and FLT-PET images were evaluated semiquantitatively by determining the maximum standardized uptake value (SUVmax) of the primary tumor [27]. The second FLT-PET study was evaluated qualitatively as well as semiquantitatively and compared with pretherapy FLT-PET. The change in SUVmax for FLT within the primary tumor was calculated and recorded. The treating physicians were blinded to the results of FLT-PET studies. To assess whether the pretherapy FDG or FLT uptake, or percentage change in FLT uptake was predictive of outcome, the PET results were correlated with the results of clinical follow-up evaluation and response to therapy.
Statistical Analysis
The time to disease recurrence was measured from the completion of treatment. StatView®, SAS Institute Inc. Version 5.0.1 software was used for the analysis. A P < 0.05 was used as the threshold for significance for all study outcomes. Pretreatment FLT and FDG uptake values, as well as pre- and during-treatment FLT uptake values, were compared using paired Student’s t-tests. The percentage change from baseline in tumor FLT uptake during treatment in pathologic responders versus nonresponders was compared using an unpaired Student’s t-test. To assess whether tumor uptake of FLT or FDG was predictive of disease-free survival (DFS), the Kaplan-Meier (product-limit) method was used to derive estimates of recurrence-free and disease- specific survival. Using logistic likelihood–ratio test to maximize the difference in disease-free survival between groups, the best division point for SUVmax for pretherapy FDG and posttherapy FLT uptake and percent change in FLT uptake was determined.
Results
Demographics of patients, TNM stage, imaging data, TRG scores, and outcome data are summarized in the Table 1. Of the 14 patients, 4 had T2 and 10 had T3 primary rectal cancers (Table 1). One patient had progressive disease and died before surgery; the remaining 13 patients completed neoadjuvant therapy and underwent surgery. There was a complete pathologic response by report in one patient (no tumor tissue specimen was available for TRG scoring); this patient died due to intercurrent disease. In the remaining 12 patients, 4 were classified as TRG-5, 1 TRG-4, 5 TRG-3 and 2 patients TRG-2 (Table 1). One of the 5 patients with TRG 4 and 5 died from disease, 2 are alive with disease and 2 patients are alive without disease. All 5 patients with TRG-3 are alive without disease; 1 patient with TRG-2 is alive without disease and 1 is alive with disease.
Table 1.
Patient characteristics and imaging data.
| Patient NO/Age/Sex | Clinical Disease Stage | Pretherapy FDG (SUV) | Pretherapy FLT (SUV) | During-therapy FLT (SUV) | Time of During-therapy FLT-PET* (Days) | Percentage Decrease in SUV for FLT | Pathologic Response to Therapy (TRG Score) | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1/68/M | T2N0M0 | 6.8 | 4.9 | 2.2 | 16 | 55% | 5 | DOD |
| 2/49/M | T3N1M1 | 12.7 | 5.4 | 3.5 | 15 | 35% | ND | DOD |
| 3/60/F | T3N1M0 | 7.1 | 4.0 | 2.1 | 16 | 48% | NA | DICD |
| 4/52/M | T2N1M0 | 9.6 | 5.4 | 2.4 | 17 | 56% | 5 | AWD |
| 5/64/M | T3N0M0 | 24.7 | 6.3 | 2.5 | 16 | 60% | 3 | NED |
| 6/47/M | T2N0M0 | 10.9 | 4.1 | 2.3 | 14 | 39% | 2 | AWD |
| 7/52/F | T2N1M0 | 16.9 | 6.5 | 2.3 | 16 | 65% | 4 | NED |
| 8/51/M | T3N0M0 | 3.9 | 4.2 | 3.6 | 15 | 14% | 2 | NED |
| 9/54/M | T3N1M0 | 13.3 | 8.6 | 3.9 | 15 | 55% | 3 | AWD |
| 10/39/M | T3N0M0 | 26.3 | 10.5 | 1.2 | 15 | 89% | 5 | NED |
| 11/75M | T3N1M0 | 54.5 | 7.6 | 5.7 | 17 | 25% | 5 | NED |
| 12/40/M | T3N2M0 | 22.9 | 6.6 | 1.5 | 19 | 77% | 3 | NED |
| 13/48M | T3N0M0 | 18.5 | 4.5 | 1.5 | 18 | 67% | 3 | NED |
| 14/25/M | T3N1M0 | 14.3 | 6.8 | 1.3 | 17 | 81% | 3 | NED |
Days after beginning of therapy, DOD = died of disease, ND = not done (died before sugary with disease progression), NA= No tumor was available for assessment, DICD = died from intercurrent disease, NED = no evidence of disease, AWD = alive with disease.
All of the primary tumors were readily detectable on both the pretherapy FDG and FLT images, but the average tumor FLT uptake (mean ± standard deviation 6.1 ± 1.9) was significantly less than that of FDG (17.3 ± 12.7) (p = 0.003) (Figure 1). In all but one patient, the primary rectal cancer SUVmax for FDG was greater than that for FLT. There was a weak correlation between tumor FLT and FDG uptake prior to therapy (r2 = 0.3, p = 0.003). Based on pretherapy FDG-PET/CT, 5 patients had pelvic lymph node metastases; FLT-PET in one of these patients also showed increased activity in a perirectal lymph node, but FLT-PET was negative for nodal disease in the other 4 patients. As expected, there was a significant decrease in tumor FLT uptake on the study obtained during the course of neoadjuvant therapy in all patients (2.6 ± 1.2) (p < 0.0001).
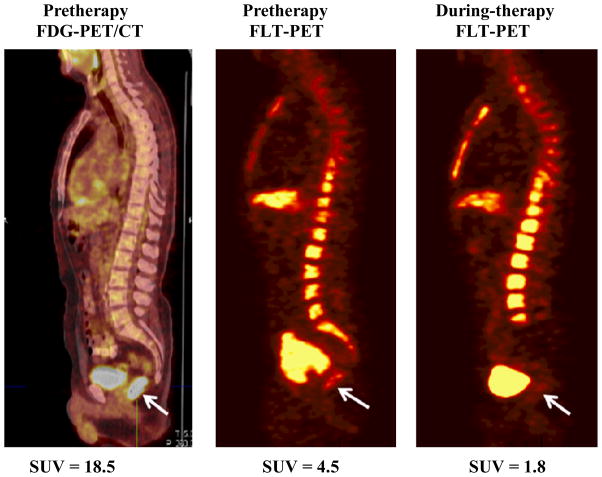
Figure 1.
Representative pretherapy sagittal FDG-PET image registered to CT image (left), pretherapy FLT-PET (middle) and during-therapy FLT-PET images in a patient with rectal cancer (#13) showing increased accumulation in of FDG and FLT prior to initiation of therapy (arrows). Decreased FLT uptake is seen in the rectal cancer and lower lumbar spine and sacrum in mid therapy.
Patients with TRG scores of 1, 2, or 3 (as well as the additional with a pathologic complete response) were considered responders and those with TRG scores of 4 or 5 were considered nonresponders. There was no significant difference in the percentage change in FLT uptake during treatment in the 8 responders versus the 5 nonresponders (58.0 ± 22.9% vs. 56.1 ± −23.3%, p = 0.40) (Figure 2).
Figure 2.
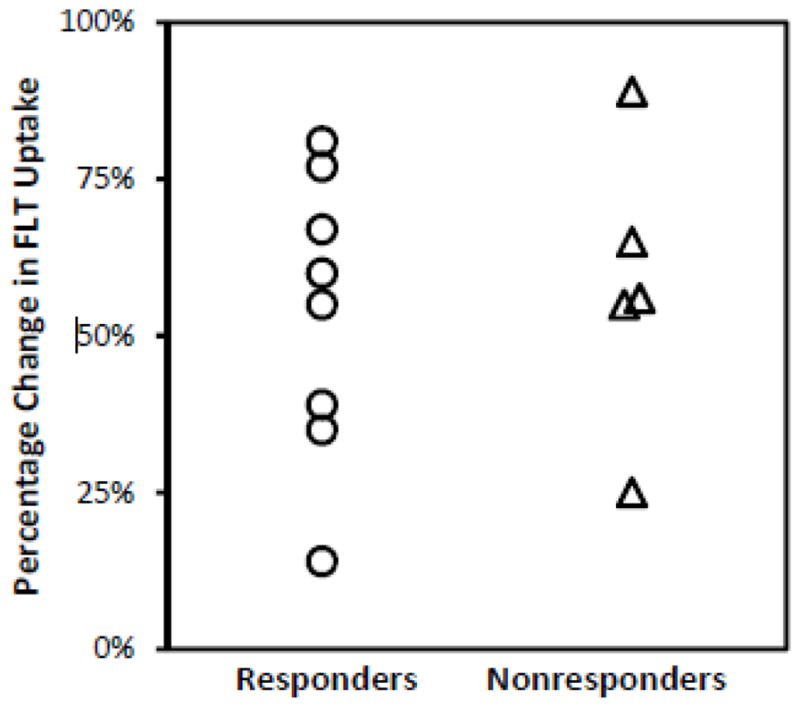
Percentage change in FLT uptake was not significantly different in responders and nonresponders based on TRG score (12 patients) and the patient who had complete response by routine pathologic examination.
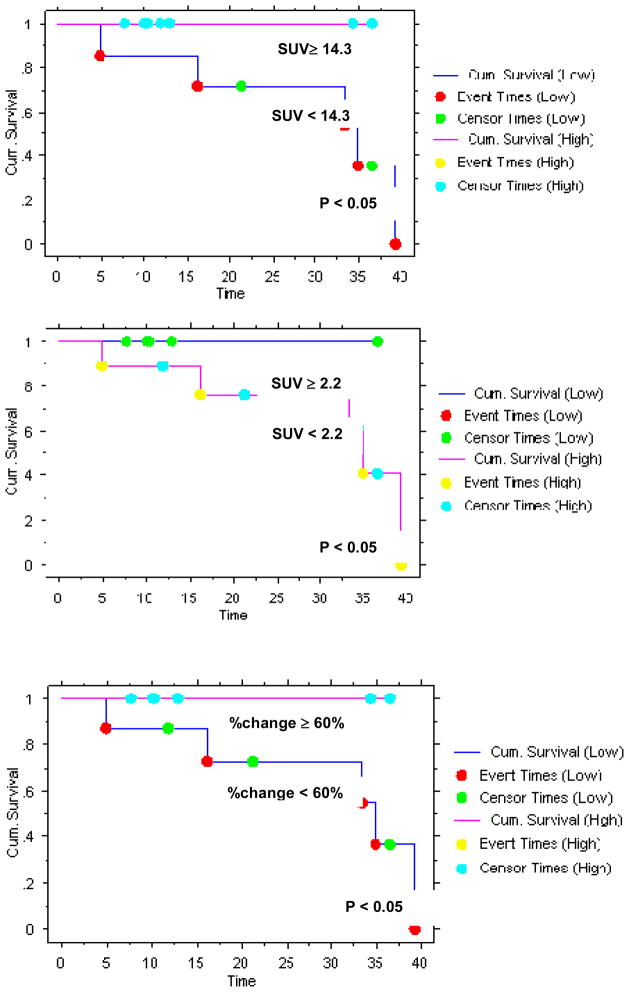
High pretherapy FDG uptake (≥ 14.3), low during-therapy FLT uptake (< 2.2) and high percentage change in FLT uptake (≥ 60%) were predictive of improved DFS (all with p < 0.05) (Figure 3). All 7 patients with FDG uptake ≥ 14.3 SUVmax are alive without disease and 4 of the 7 patients with SUVmax <14.3 are alive without disease. However, 4 of 5 patients with during-therapy FLT uptake < 2.2 SUVmax (1 died of unrelated cause) and all 6 patients with percentage change in tumor FLT uptake ≥ 60% are alive without disease (vs. 2 of 8 with percentage change > 60%). Pretherapy FLT uptake did not correlate with DFS (p = NS). Thus, pretherapy FDG-PET/CT was superior to pretherapy FLT-PET in the initial staging of the disease and was equally predictive of outcome in this pilot study.
Figure 3.
Progression-free survival based on pretherapy FDG uptake (upper left), during-therapy FLT uptake (upper right) and per cent change in FLT uptake (lower left) using Kaplan-Meier method.
Discussion
Rectal cancer is the third most common cancer in both incidence and mortality in the United States [28]. In localized rectal cancer, standard treatment is surgery followed by adjuvant chemoradiotherapy. However in locally advanced disease, multimodality treatment with neoadjuvant chemoradiotherapy before surgical resection has been accepted as the standard of care (http://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf). By comparison with adjuvant radiotherapy, multimodality neoadjuvant therapy of locally advanced disease has been shown to improve local control [3]. Up to 60% of patients treated with neoadjuvant chemoradiotherapy achieve some degree of pathologic downstaging [6]. However, approximately 40% of patients still die from recurrent disease following multimodality therapy [29]. TNM staging, tumor markers, and response to neoadjuvant therapy, although not perfect, provide prognostic information in rectal cancer. Kuremsky et al., in a review of the literature, concluded that some biomarkers such as epidermal growth factor receptor, thymidylate synthase, and p21 appear to be promising and should be evaluated in larger prospective clinical trials to assess their ability in guiding preoperative therapy choices in patients with locally advanced cancer [6].
FDG-PET has been shown to be useful in the management of patients with colorectal cancer [30]. Virens et al. evaluated 19 studies that used FDG-PET in predicting therapy outcome in rectal cancer and found that, while the studies were heterogeneous with regard to the methods applied for PET quantification, evaluation interval, metabolic response criteria and clinical endpoints (histology or survival), most of the studies showed that the pre- to posttherapy change in FDG uptake is a significant predictor of neoadjuvant therapy outcome in patients with rectal cancer [31, 32]. Thus, FDG-PET can be used after neoadjuvant therapy in a preoperative setting to tailor surgical approach for individual patients [33]. In addition, posttherapy FDG-PET can be used to guide adjuvant chemotherapy for rectal cancer after optimal neoadjuvant and local treatments [33]. However, one should be aware of the limitations of FDG-PET performed after therapy, which have been attributed to radiation-associated inflammation shortly after completion of therapy (metabolic response underestimated) or therapy-induced stunning (metabolic response overestimated) [30, 34].
There are limited data regarding the utility of posttherapy FDG-PET in predicting survival [35–37]. Guillem et al. have shown that FDG-PET 4–5 weeks after completion of chemoradiation was the best predictor of recurrence-free survival, while pathologic response was not a significant predictor of either overall or recurrence-free survival in their patient population [38]. Capirci et al. demonstrated that the combination of pathologic stage and the findings of restaging FDG-PET was able to identify a subgroup of patients who had good response to chemoradiotherapy and a more favorable prognosis. Thus, FDG-PET is very useful for staging, assessing and predicting response to neoadjuvant therapy, but it has a limited role in predicting patient outcome [37].
A test that could predict outcome prior to initiation of therapy or early during therapy that is not affected by therapy-related inflammation would be desirable. Only a few studies have evaluated the utility of FDG-PET during chemoradiotherapy as a predictor of response in patients with advanced rectal cancer [35–37]. Several studies have shown that early changes in the FDG uptake during neoadjuvant therapy predict pathologic response. However, none of these studies have evaluated whether FDG-PET early after initiation of therapy is predictive of patient outcome.
FLT, a thymidine analog that enters the salvage pathway of DNA synthesis, has been used to assess tumor proliferation by PET [16]. By comparison with FDG, one of the assumed advantages of FLT is its lack of uptake in inflammatory cells, as has been demonstrated in animal models [39]. There are limited clinical data suggesting that a decrease in FLT uptake shortly after initiation of therapy can predict tumor response and/or patient survival in several solid cancers, including breast, lung and glial neoplasms [40–42]. Wieder et al. studied 10 patients with rectal cancer who underwent FLT-PET before therapy, 2 weeks after initiation and 3–4 weeks after completion of neoadjuvant chemoradiation therapy [43]. They found no correlation between histopathological tumor regression and the change from baseline in FLT uptake, either at 2 weeks after initiation of treatment or after its completion [43]. The authors concluded that the decrease in FLT uptake in nonresponders may reflect treatment-induced growth arrest rather than cell death and suggested that this could represent a limitation of FLT for monitoring treatment response by comparison to FDG.
Similar to Wieder et al, we found no significant correlation between change in FLT uptake during therapy and histopathologic tumor regression. While there may be some pathophysiologic reasons for this lack of the correlation, such as poor delivery of FLT as a result of inadequate blood supply secondary to radiation, the small number of patients included in our study and in the Weider et al. study limit interpretation of the results. Thus, studies with a larger number of patients are needed to address this issue. In addition, we have correlated FDG and FLT uptake prior to therapy as well as percentage change in FLT uptake shortly after initiation of neoadjuvant therapy with survival in our patients. We found that pretherapy FLT was not a significant predictor of outcome and did not correlate with DFS. However, low FLT uptake (< 2.2) and high percentage change in FLT uptake (≥ 60%) during therapy were predictive of improved DFS. These findings are similar to those in other studies with FLT, demonstrating that a decrease in FLT uptake during treatment is predictive of therapy outcome [17, 42, 44].
We also found that high pretherapy FDG uptake (≥ 14.3) was predictive of better DFS. This is in contrast to the findings with FDG-PET in most tumor types, where higher uptake is associated with poorer prognosis. This may be related to greater inflammatory reaction with rectal cancers compared with other cancers or simply that the tumors with higher FDG uptake were more responsive to neoadjuvant therapy, and thus, had better outcome.
The major limitation of this study is our small sample size. Accordingly, study of a larger number of patients is needed to confirm whether above PET-based measures are predictive of outcome in patients with rectal cancer.
Conclusion
In this pilot study we found that the pretherapy FDG uptake, posttherapy FLT uptake and percentage change in FLT uptake during therapy were equally predictive of DFS in patients with advanced rectal cancer who underwent neoadjuvant chemoradiation prior to surgery. However, we found no significant correlation between percentage change in FLT uptake and histopathologic tumor regression. In addition, we found that FDG-PET/CT was superior to FLT-PET in detection of metastatic disease and, thus, in staging of rectal cancer in our patients.
Footnotes
Conflict of Interest:
None of the authors has a conflict of interest associated with this manuscript
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Frederiksen BL, Osler M, Harling H, Jorgensen T. Social inequalities in stage at diagnosis of rectal but not in colonic cancer: a nationwide study. Br J Cancer. 2008;98(3):668–73. doi: 10.1038/sj.bjc.6604215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–40. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 4.Gosens MJ, Dresen RC, Rutten HJ, Nieuwenhuijzen GA, van der Laak JA, Martijn H, et al. Preoperative radiochemotherapy is successful also in patients with locally advanced rectal cancer who have intrinsically high apoptotic tumours. Ann Oncol. 2008;19(12):2026–32. doi: 10.1093/annonc/mdn428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merkel S, Klossek D, Gohl J, Papadopoulos T, Hohenberger W, Hermanek P. Quality management in rectal carcinoma: what is feasible? Int J Colorectal Dis. 2009;24(8):931–42. doi: 10.1007/s00384-009-0736-9. [DOI] [PubMed] [Google Scholar]
- 6.Kuremsky JG, Tepper JE, McLeod HL. Biomarkers for response to neoadjuvant chemoradiation for rectal cancer. Int J Radiat Oncol Biol Phys. 2009;74(3):673–88. doi: 10.1016/j.ijrobp.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Sasaki R, Komaki R, Macapinlac H, Erasmus J, Allen P, Forster, et al. [18F]fluorodeoxyglucose uptake by positron emission tomography predicts outcome of non-small-cell lung cancer. J Clin Oncol. 2005;23(6):1136–43. doi: 10.1200/JCO.2005.06.129. [DOI] [PubMed] [Google Scholar]
- 8.Cerfolio RJ, Bryant AS, Ohja B, Bartolucci AA. The maximum standardized uptake values on positron emission tomography of a non-small cell lung cancer predict stage, recurrence, and survival. J Thorac Cardiovasc Surg. 2005;130(1):151–9. doi: 10.1016/j.jtcvs.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Pan L, Gu P, Huang G, Xue H, Wu S. Prognostic significance of SUV on PET/CT in patients with esophageal cancer: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2009;21(9):1008–15. doi: 10.1097/MEG.0b013e328323d6fa. [DOI] [PubMed] [Google Scholar]
- 10.Calvo FA, Cabezon L, Gonzalez C, Soria A, de la Mata D, Gomez-Espi M, et al. 18F-FDG PET bio-metabolic monitoring of neoadjuvant therapy effects in rectal cancer: focus on nodal disease characteristics. Radiother Oncol. 2010;97(2):212–6. doi: 10.1016/j.radonc.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 11.Martoni AA, Di Fabio F, Pinto C, Castellucci P, Pini S, Ceccarelli C, et al. Prospective study on the FDG-PET/CT predictive and prognostic values in patients treated with neoadjuvant chemoradiation therapy and radical surgery for locally advanced rectal cancer. Ann Oncol. 2011;22(3):650–6. doi: 10.1093/annonc/mdq433. [DOI] [PubMed] [Google Scholar]
- 12.Duch J, Fuster D, Munoz M, Fernandez PL, Paredes P, Fontanillas M, et al. 18F-FDG PET/CT for early prediction of response to neoadjuvant chemotherapy in breast cancer. Eur J Nucl Med Mol Imaging. 2009;36(10):1551–7. doi: 10.1007/s00259-009-1116-y. [DOI] [PubMed] [Google Scholar]
- 13.Weber WA, Ott K, Becker K, Dittler HJ, Helmberger H, Avril NE, et al. Prediction of response to preoperative chemotherapy in adenocarcinomas of the esophagogastric junction by metabolic imaging. J Clin Oncol. 2001;19(12):3058–65. doi: 10.1200/JCO.2001.19.12.3058. [DOI] [PubMed] [Google Scholar]
- 14.Aukema TS, Kappers I, Olmos RA, Codrington HE, van Tinteren H, van Pel R, et al. Is 18F-FDG PET/CT useful for the early prediction of histopathologic response to neoadjuvant erlotinib in patients with non-small cell lung cancer? J Nucl Med. 2010;51(9):1344–8. doi: 10.2967/jnumed.110.076224. [DOI] [PubMed] [Google Scholar]
- 15.Yang W, Fu Z, Yu J, Yuan S, Zhang B, Li D, et al. Value of PET/CT versus enhanced CT for locoregional lymph nodes in non-small cell lung cancer. Lung Cancer. 2008;61(1):35–43. doi: 10.1016/j.lungcan.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Shields AF, Grierson JR, Dohmen BM, Machulla HJ, Stayanoff JC, Lawhorn-Crews JM, et al. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med. 1998;4(11):1334–6. doi: 10.1038/3337. [DOI] [PubMed] [Google Scholar]
- 17.Pio BS, Park CK, Pietras R, Hsueh WA, Satyamurthy N, Pegram MD, et al. Usefulness of 3′-[F-18]fluoro-3′-deoxythymidine with positron emission tomography in predicting breast cancer response to therapy. Mol Imaging Biol. 2006;8(1):36–42. doi: 10.1007/s11307-005-0029-9. [DOI] [PubMed] [Google Scholar]
- 18.Chen W, Cloughesy T, Kamdar N, Satyamurthy N, Bergsneider M, Liau L, et al. Imaging proliferation in brain tumors with 18F-FLT PET: comparison with 18F-FDG. J Nucl Med. 2005;46(6):945–52. [PubMed] [Google Scholar]
- 19.Wright JD, Dehdashti F, Herzog TJ, Mutch DG, Huettner PC, Rader JS, et al. Preoperative lymph node staging of early-stage cervical carcinoma by [18F]-fluoro-2-deoxy-D-glucose-positron emission tomography. Cancer. 2005;104(11):2484–91. doi: 10.1002/cncr.21527. [DOI] [PubMed] [Google Scholar]
- 20.Edge SB, Fritz AG, Byrd DR, Compton CC, Greene FL, Trotti A. Cancer staging manual. 7. New York, NY: Springer; 2010. [Google Scholar]
- 21.Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73(11):2680–6. doi: 10.1002/1097-0142(19940601)73:11<2680::aid-cncr2820731105>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 22.Bouzourene H, Bosman FT, Seelentag W, Matter M, Coucke P. Importance of tumor regression assessment in predicting the outcome in patients with locally advanced rectal carcinoma who are treated with preoperative radiotherapy. Cancer. 2002;94(4):1121–30. [PubMed] [Google Scholar]
- 23.Rau B, Hunerbein M, Barth C, Wust P, Haensch W, Riess H, et al. Accuracy of endorectal ultrasound after preoperative radiochemotherapy in locally advanced rectal cancer. Surg Endosc. 1999;13(10):980–4. doi: 10.1007/s004649901151. [DOI] [PubMed] [Google Scholar]
- 24.Ryan R, Gibbons D, Hyland JM, Treanor D, White A, Mulcahy HE, et al. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology. 2005;47(2):141–6. doi: 10.1111/j.1365-2559.2005.02176.x. [DOI] [PubMed] [Google Scholar]
- 25.Yun M, Oh SJ, Ha HJ, Ryu JS, Moon DH. High radiochemical yield synthesis of 3′-deoxy-3′-[18F]fluorothymidine using (5′-O-dimethoxytrityl-2′-deoxy-3′-O-nosyl-beta-D-threo pentofuranosyl)thymine and its 3-N-BOC-protected analogue as a labeling precursor. Nucl Med Biol. 2003;30(2):151–7. doi: 10.1016/s0969-8051(02)00409-2. [DOI] [PubMed] [Google Scholar]
- 26.Suehiro M, Vallabhajosula S, Goldsmith SJ, Ballon DJ. Investigation of the role of the base in the synthesis of [18F]FLT. Appl Radiat Isot. 2007;65(12):1350–8. doi: 10.1016/j.apradiso.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 27.Benz MR, Evilevitch V, Allen-Auerbach MS, Eilber FC, Phelps ME, Czernin J, et al. Treatment monitoring by 18F-FDG PET/CT in patients with sarcomas: interobserver variability of quantitative parameters in treatment-induced changes in histopathologically responding and nonresponding tumors. J Nucl Med. 2008;49(7):1038–46. doi: 10.2967/jnumed.107.050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 29.Glimelius B, Oliveira J. Rectal cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2008;19(Suppl 2):ii31–2. doi: 10.1093/annonc/mdn078. [DOI] [PubMed] [Google Scholar]
- 30.Vriens D, de Geus-Oei LF, van der Graaf WT, Oyen WJ. Tailoring therapy in colorectal cancer by PET-CT. Q J Nucl Med Mol Imaging. 2009;53(2):224–44. [PubMed] [Google Scholar]
- 31.Huh JW, Min JJ, Lee JH, Kim HR, Kim YJ. The predictive role of sequential FDG-PET/CT in response of locally advanced rectal cancer to neoadjuvant chemoradiation. Am J Clin Oncol. 2011 doi: 10.1097/COC.0b013e3182118e7d. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Hur H, Kim NK, Yun M, Min BS, Lee KY, Keum KC, et al. 18Fluoro-deoxy-glucose positron emission tomography in assessing tumor response to preoperative chemoradiation therapy for locally advanced rectal cancer. J Surg Oncol. 2011;103(1):17–24. doi: 10.1002/jso.21736. [DOI] [PubMed] [Google Scholar]
- 33.de Geus-Oei LF, Vriens D, van Laarhoven HW, van der Graaf WT, Oyen WJ. Monitoring and predicting response to therapy with 18F-FDG PET in colorectal cancer: a systematic review. J Nucl Med. 2009;50 (Suppl 1):43S–54S. doi: 10.2967/jnumed.108.057224. [DOI] [PubMed] [Google Scholar]
- 34.Rosenberg R, Herrmann K, Gertler R, Kunzli B, Essler M, Lordick F, et al. The predictive value of metabolic response to preoperative radiochemotherapy in locally advanced rectal cancer measured by PET/CT. Int J Colorectal Dis. 2009;24(2):191–200. doi: 10.1007/s00384-008-0616-8. [DOI] [PubMed] [Google Scholar]
- 35.Guerra L, Niespolo R, Di Pisa G, Ippolito D, De Ponti E, Terrevazzi S, et al. Change in glucose metabolism measured by 18F-FDG PET/CT as a predictor of histopathologic response to neoadjuvant treatment in rectal cancer. Abdom Imaging. 2011;36(1):38–45. doi: 10.1007/s00261-009-9594-8. [DOI] [PubMed] [Google Scholar]
- 36.Herrmann K, Bundschuh RA, Rosenberg R, Schmidt S, Praus C, Souvatzoglou M, et al. Comparison of different SUV-based methods for response prediction to neoadjuvant radiochemotherapy in locally advanced rectal cancer by FDG-PET and MRI. Mol Imaging Biol. 2011;13(5):1011–9. doi: 10.1007/s11307-010-0383-0. [DOI] [PubMed] [Google Scholar]
- 37.Cascini GL, Avallone A, Delrio P, Guida C, Tatangelo F, Marone P, et al. 18F-FDG PET is an early predictor of pathologic tumor response to preoperative radiochemotherapy in locally advanced rectal cancer. J Nucl Med. 2006;47(8):1241–8. [PubMed] [Google Scholar]
- 38.Guillem JG, Moore HG, Akhurst T, Klimstra DS, Ruo L, Mazumdar M, et al. Sequential preoperative fluorodeoxyglucose-positron emission tomography assessment of response to preoperative chemoradiation: a means for determining longterm outcomes of rectal cancer. J Am Coll Surg. 2004;199(1):1–7. doi: 10.1016/j.jamcollsurg.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 39.van Waarde A, Cobben DC, Suurmeijer AJ, Maas B, Vaalburg W, de Vries EF, et al. Selectivity of 18F-FLT and 18F-FDG for differentiating tumor from inflammation in a rodent model. J Nucl Med. 2004;45(4):695–700. [PubMed] [Google Scholar]
- 40.Kenny L, Coombes RC, Vigushin DM, Al-Nahhas A, Shousha S, Aboagye EO. Imaging early changes in proliferation at 1 week post chemotherapy: a pilot study in breast cancer patients with 3′-deoxy-3′-[18F]fluorothymidine positron emission tomography. Eur J Nucl Med Mol Imaging. 2007;34(9):1339–47. doi: 10.1007/s00259-007-0379-4. [DOI] [PubMed] [Google Scholar]
- 41.Sohn HJ, Yang YJ, Ryu JS, Oh SJ, Im KC, Moon DH, et al. [18F]Fluorothymidine positron emission tomography before and 7 days after gefitinib treatment predicts response in patients with advanced adenocarcinoma of the lung. Clin Cancer Res. 2008;14(22):7423–9. doi: 10.1158/1078-0432.CCR-08-0312. [DOI] [PubMed] [Google Scholar]
- 42.Chen W, Delaloye S, Silverman DH, Geist C, Czernin J, Sayre J, et al. Predicting treatment response of malignant gliomas to bevacizumab and irinotecan by imaging proliferation with [18F] fluorothymidine positron emission tomography: a pilot study. J Clin Oncol. 2007;25(30):4714–21. doi: 10.1200/JCO.2006.10.5825. [DOI] [PubMed] [Google Scholar]
- 43.Wieder HA, Geinitz H, Rosenberg R, Lordick F, Becker K, Stahl A, et al. PET imaging with [18F]3′-deoxy-3′-fluorothymidine for prediction of response to neoadjuvant treatment in patients with rectal cancer. Eur J Nucl Med Mol Imaging. 2007;34(6):878–83. doi: 10.1007/s00259-006-0292-2. [DOI] [PubMed] [Google Scholar]
- 44.Herrmann K, Ott K, Buck AK, Lordick F, Wilhelm D, Souvatzoglou M, et al. Imaging gastric cancer with PET and the radiotracers 18F-FLT and 18F-FDG: a comparative analysis. J Nucl Med. 2007;48(12):1945–50. doi: 10.2967/jnumed.107.044867. [DOI] [PubMed] [Google Scholar]