Abstract
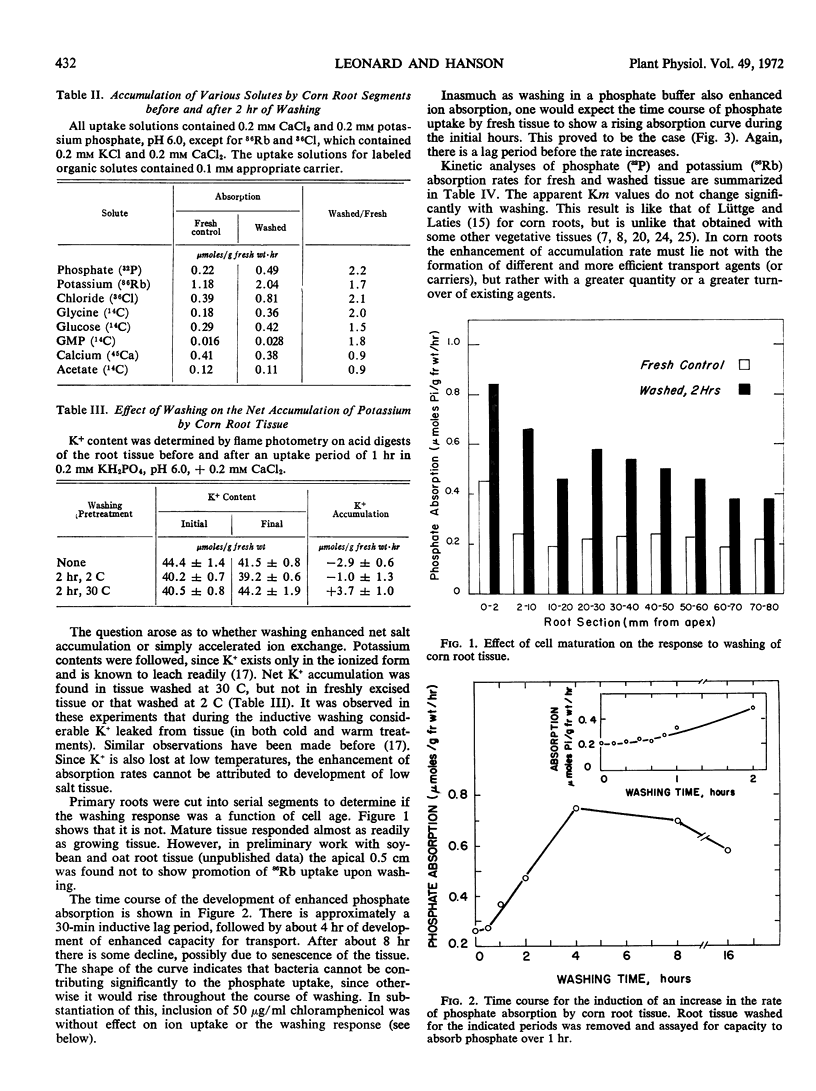
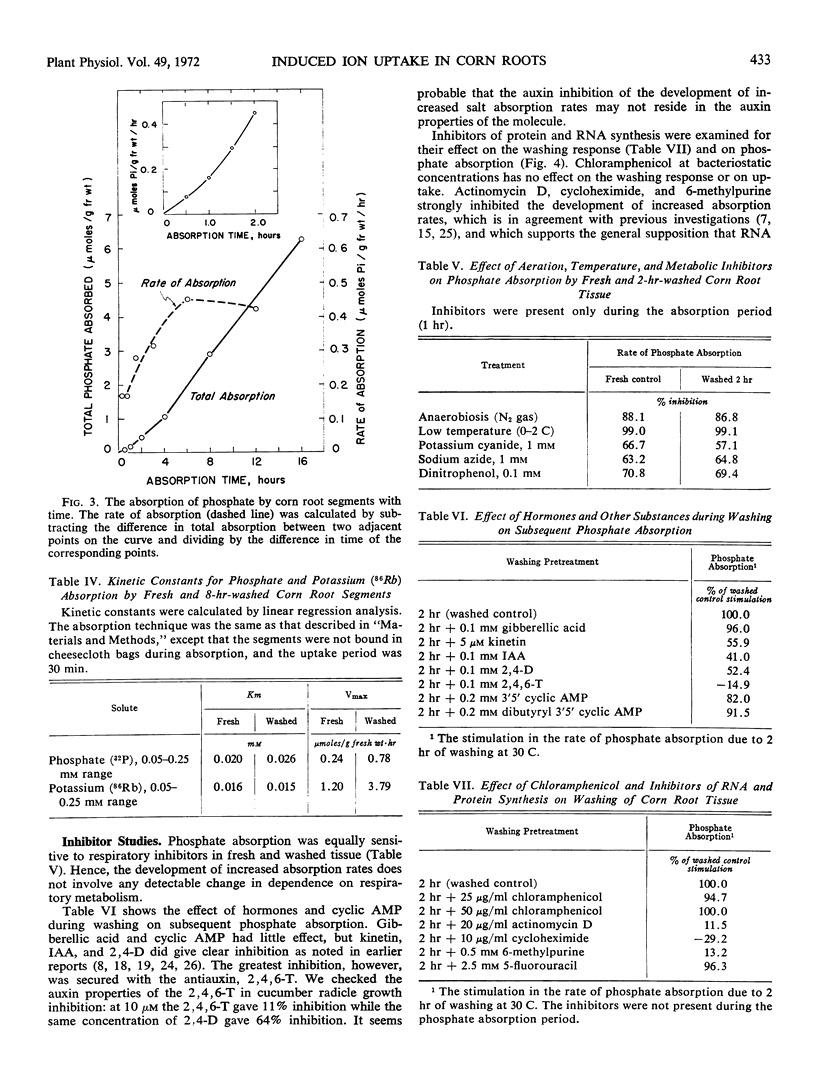
Washing excised or intact primary roots of corn (Zea mays L., WF9 × M14) in aerated distilled water or dilute salt solutions for 2 hours induced doubling of the rate of accumulation of various nutrient ions and solutes. This response to washing depended upon aerobic metabolism, but involved no increase in aerobic respiration. Excision of root tissue was not required as the effect could be obtained with intact root systems. Increased phosphate absorption followed after a lag period of 30 to 40 minutes and continued for 6 hours before leveling off at about 3.5 times the initial rate. Chloramphenicol was not inhibitory to the development of increased absorption, while inhibitors of RNA and protein synthesis were. Auxins and kinetin were also inhibitory, but so was the antiauxin, 2,4,6-trichlorophenoxyacetic acid.
Kinetic analyses of phosphate and potassium (86Rb) absorption showed no change in apparent Michaelis constant. Electron microscopic examination and analysis for membrane protein and lipid-phosphate content after washing showed no proliferation of membrane or other changes in cell structure and composition. It appears that washing augments or activates existing membrane transport mechanisms by processes involving protein synthesis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ellis R. J., Macdonald I. R. Specificity of cycloheximide in higher plant systems. Plant Physiol. 1970 Aug;46(2):227–232. doi: 10.1104/pp.46.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E. The essential role of calcium in selective cation transport by plant cells. Plant Physiol. 1961 Jul;36(4):437–444. doi: 10.1104/pp.36.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. R., Hodges T. K. Phosphorus metabolism of germinating oat seeds. Plant Physiol. 1966 Nov;41(9):1459–1464. doi: 10.1104/pp.41.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock J. G. Uptake of 3-o-Methylglucose by Healthy and Hypomyces-infected Squash Hypocotyls. Plant Physiol. 1969 Sep;44(9):1267–1272. doi: 10.1104/pp.44.9.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key J. L. Effect of purine and pyrimidine analogues on growth and RNA metabolism in the soybean hypocotyl-the selective action of 5-fluorouracil. Plant Physiol. 1966 Oct;41(8):1257–1264. doi: 10.1104/pp.41.8.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laties G. G., Budd K. THE DEVELOPMENT OF DIFFERENTIAL PERMEABILITY IN ISOLATED STELES OF CORN ROOTS. Proc Natl Acad Sci U S A. 1964 Aug;52(2):462–469. doi: 10.1073/pnas.52.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macklon A. E., Higinbotham N. Electropotential in excised pea epicotyls. Plant Physiol. 1968 Jun;43(6):888–892. doi: 10.1104/pp.43.6.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner H., Handley R., Overstreet R. Potassium Loss and Changes in the Fine Structure of Corn Root Tips Induced by H-ion. Plant Physiol. 1966 Dec;41(10):1725–1735. doi: 10.1104/pp.41.10.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmura T., Howell R. W. Inhibitory Effect of Water on Oxygen Consumption by Plant Materials. Plant Physiol. 1960 Mar;35(2):184–188. doi: 10.1104/pp.35.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. M. The influence of growth regulating substances on the development of enhanced metabolic rates in thin slices of beetroot storage tissue. Plant Physiol. 1966 Sep;41(7):1173–1178. doi: 10.1104/pp.41.7.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman M. G. Adaptation of barley roots to low oxygen supply and its relation to potassium and sodium uptake. Plant Physiol. 1969 Sep;44(9):1233–1240. doi: 10.1104/pp.44.9.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman M. G., Mertz S. M., Graves J. S., Pierce W. S., Higinbotham N. Electrical potential differences in cells of barley roots and their relation to ion uptake. Plant Physiol. 1971 Jan;47(1):76–80. doi: 10.1104/pp.47.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rains D. W., Floyd R. A. Influence of calcium on sodium and potassium absorption by fresh and aged bean stem slices. Plant Physiol. 1970 Jul;46(1):93–98. doi: 10.1104/pp.46.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rains D. W. Sodium and potassium absorption by bean stem tissue. Plant Physiol. 1969 Apr;44(4):547–554. doi: 10.1104/pp.44.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G. H., Kramer P. J. Radial salt transport in corn roots. Plant Physiol. 1967 Jul;42(7):985–990. doi: 10.1104/pp.42.7.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G. H., Kramer P. J. Radial transport of ions in roots. Plant Physiol. 1969 Aug;44(8):1095–1100. doi: 10.1104/pp.44.8.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]


