Abstract
Scaffolding proteins containing postsynaptic density-95/discs large/zone occludens-1 (PDZ) domains interact with synaptic receptors and cytoskeletal components and are therefore implicated in synaptic development and plasticity. Little is known, however, about what regulates the expression of PDZ proteins and how the levels of these proteins influence synaptic development. Here, we show that ligands for epidermal growth factor receptors (ErbB1) decrease a particular set of PDZ proteins and negatively influence synaptic formation or maturation. In short-term neocortical cultures, concentrations of epidermal growth factor and amphiregulin (2–9 pM) decreased the expression of glutamate receptor interacting protein 1 (GRIP1) and synapse-associated protein 97 kDa (SAP97) without affecting postsynaptic density-95 (PSD-95) levels and glial proliferation. In long-term cultures, epidermal growth factor treatment resulted in a decrease in the frequency of pan-PDZ-immunoreactive aggregates on dendritic processes. A similar activity on the same PDZ proteins was observed in the developing neocortex following epidermal growth factor administration to rat neonates. Immunoblotting revealed that administered epidermal growth factor from the periphery activated brain ErbB1 receptors and decreased GRIP1 and SAP97 protein levels in the neocortex. Laser-confocal imaging indicated that epidermal growth factor administration suppressed the formation of pan-PDZ-immunoreactive puncta and dispersed those structures in vivo as well. These findings revealed a novel negative activity of ErbB1 receptor ligands that attenuates the expression of the PDZ proteins and inhibits postsynaptic maturation in developing neocortex.
Keywords: EGFR, HER1, amphiregulin, synaptic elimination, cerebral cortex, postsynaptic, schizophrenia
Proteins carrying PDZ (postsynaptic density-95/discs large/zone occludens-1) domains are referred to as PDZ proteins, which have a modular organization and often function as scaffolding proteins. Recent studies have indicated that these proteins interact with various types of synaptic proteins, such as ion channels, signal transducers, and cytoskeletal components, at the postsynaptic density (PSD), and regulate neural transmission (Kim and Sheng, 2004). The family of PDZ proteins includes PSD-95, PSD-93/chapsyn-110, synapse-associated protein 97 kDa (SAP97), SAP102, glutamate receptor interacting protein (GRIP), and α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor (AMPAR) binding protein (ABP/GRIP2) (Nagano et al., 1998). A number of studies have shown that PSD-95 binds directly to N-methyl-d-aspartate receptors (NMDARs) and Shaker-type K+ channels, thereby playing an important role in the functional localization of these proteins by linking the receptors to the cytoskeleton (Valtschanoff and Weinberg, 2001; Lei et al., 2001; Petersen et al., 2003; Lin et al., 2004). SAP97 binds to AMPARs and similarly modulates their subcellular dynamics (Leonard et al., 1998; Valtschanoff et al., 2000; Wu et al., 2002; Ko et al., 2003). Thus, PDZ proteins perform key roles in synaptic function and plasticity. Little is known, however, about the molecular regulators of PDZ protein expression during synaptic development. Growth factors and neurotrophins, such as brain-derived neurotrophic factor (BDNF) and neuregulin-1 (NRG1), influence synaptic formation and maturation in part by regulating the expression of glutamate and acetylcholine receptors and controlling their subcellular localizations (Ozaki et al., 1997; Narisawa-Saito et al., 1999a, 2002; Cotrufo et al., 2003). We previously reported that endogenous and exogenous BDNF enhances AMPAR function by increasing PDZ protein levels and their interactions with AMPARs in developing neocortical neurons (Jourdi et al., 2003). Cotrufo et al. (2003) reported that nerve growth factor up-regulates the expression of PSD-95 and GRIP in developing neocortex.
In contrast to BDNF, we observed that epidermal growth factor (EGF) suppresses AMPAR expression in cultured neocortical neurons, implying that EGF negatively affects synaptic development and function (Narisawa-Saito et al., 1999a). In the present study, we analyzed the negative role of ErbB1 receptor ligands in the regulation of the expression of PDZ proteins. EGF binds to an ErbB1 receptor tyrosine kinase that belongs to the vertebrate ErbB family. This receptor also interacts with a variety of receptor ligands including transforming growth factor α, amphiregulin, heparin-binding EGF-like growth factor, betacellulin, epiregulin and epigen (Harris et al., 2003). These ErbB1 receptor ligands regulate the proliferation of astrocytes and neuronal progenitor cells in the CNS (Yamada et al., 1997). In addition, ErbB1 receptor ligands have a trophic effect on midbrain dopaminergic neurons (Ferrari et al., 1991; Casper et al., 1994; Iwakura et al., 2005). Although ErbB1 receptors are known to be distributed in a variety of postmitotic neurons (Werner et al., 1988), the biological role of ErbB1 receptor ligands in the brain remains to be characterized. In the present study, we investigated the attenuation of the expression of PDZ proteins by ErbB1 receptor ligands both in neuron-enriched cultures and in vivo. The potential contribution of this activity to synaptic formation or maturation in the developing neocortex is discussed.
EXPERIMENTAL PROCEDURES
Animals
Sprague–Dawley rats were purchased with dams from SLC Ltd. (Shizuoka, Japan), and maintained under a 12-h light/dark cycle with free access to food and water. All of the experiments described were performed in accordance with the local and international guidelines on the ethical use of laboratory animals. Efforts were made to minimize the number of animals and their suffering.
Cell culture
The neocortices of day-19 rat embryos were dissected. The tissues were digested with papain (1 mg/ml), and mechanically dissociated with the aid of a plastic pipette. The dissociated cells were plated onto poly-d-lysine-coated dishes at relatively low cell densities (100–200 cells/mm2). Neocortical neurons were grown in serum-free Dulbecco’s modified Eagle medium containing nutrient mixture N2 (Narisawa-Saito et al., 1999a). This procedure reduced astroglial contamination to less than 5% of the total cell population until 7 days in vitro (DIV) (Narisawa-Saito et al., 1999a). For biochemical assay, cultures were supplemented daily with purified recombinant human EGF (20 pg/ml) or amphiregulin (200 pg/ml) for 6 days. An ErbB1 receptor inhibitor, PD153035 (100 nM, Tocris Cookson Inc., St. Louis, MO, USA) was added 2 h before each EGF treatment. Alternatively, EGF treatment was extended until DIV12 for immunocytochemistry (see below).
Immunoblotting
Tissues or cells were lysed in SDS lysis buffer containing 1% SDS, 20 mM Tris–HCl (pH 7.4), 5 mM EDTA, 10 mM NaF, 2 mM Na3VO4, 0.5 mM phenylarsine oxide, and 1 mM phenylmethylsulfonyl fluoride. The lysates were boiled for 3 min, and then clarified by centrifugation. The protein concentrations of the supernatants were determined using a BCA protein assay kit (Pierce, Rockford, IL, USA). Equal amounts of protein were then subjected to electrophoresis on 7.5% or 10% SDS–polyacrylamide gels. Proteins were transferred onto nitrocellulose membranes (Millipore, Bedford, MA, USA) in 0.1 M Tris base, 0.192 M glycine and 20% methanol using a semi-dry electrophoretic transfer system. The membranes were blocked overnight at 4 °C with 0.1% Tween 20 in Tris-buffered saline (T-TBS) containing 5% bovine serum albumin. Membranes were then probed with the following primary antibodies: anti-SAP97 (1:800 dilution, StressGen, Victoria, BC, Canada), anti-GRIP1 (1:1000, BD Transduction Laboratory, San Diego, CA, USA), anti-pan-PDZ (1:1000, Upstate Biotechnology, Waltham, MA, USA), anti-PSD-95 (1:2000, Upstate Biotechnology), anti-β-actin (1:1000, Chemicon Int., Temecula, CA, USA), anti-neuron specific enolase (NSE; 1:1000, Polysciences, Inc., Warrington, PA, USA), anti-glial fibrillary acidic protein (GFAP; 1:4000, DakoCytomation, Glostrup, Denmark), anti-phospho-ErbB1 receptor (1:500, Santa Cruz Biotechnology, Santa Cruz, CA, USA), or anti-ErbB1 receptor (1:500, Santa Cruz Biotechnology) antibodies. The membranes were incubated with these antibodies diluted in T-TBS containing 5% bovine serum albumin at room temperature for 1 h. After several washes with T-TBS, the membranes were incubated with horseradish peroxidase-conjugated donkey anti-mouse IgG or goat anti-rabbit IgG secondary antibodies (1:10,000, DakoCytomation) at room temperature for 1 h. The membranes were then washed at least four times with T-TBS and target proteins were visualized using an ECL chemiluminescence system (Promega, Madison, WI, USA). To quantify the amount of protein, we determined liner ranges of individual immunoblots with serial dilution of control samples; 1.0–40 μg/ lane for GFAP, β-actin; 2.5–20 μg/ lane for GRIP1; 5–20 μg protein/lane for SAP97, PSD95, NSE. Using immunoblots carrying an appropriate amount of protein, we measured the signal density from immunoblots using NIH Image analysis software. The changes in protein levels were expressed as a percentage of their respective controls. In parallel, data were confirmed by the immunoblot analysis with serial dilution of samples.
Reverse transcriptase–polymerase chain reaction (RT-PCR)
Using an RT-PCR High kit (Toyobo, Osaka, Japan), cDNA fragments for SAP97, GRIP1 and β-actin were directly synthesized from total RNA and amplified within the linear range of amplification using a Light Cycler (Roche Diagnostics, Indianapolis, IN, USA). The oligonucleotide primers were designed as follows: 5′-TCCAGCAGTGTGAAGACCTG-3′ and 5′-CAGCTTCTTGGGACTTGAGG-3′ generating a 371-bp fragment for GRIP1, 5′-AAATGCCATCAAGAGGTTGC-3′ and 5′-CAGGGCAGAGAGATGAGACC-3′ generating a 328-bp fragment for SAP97, and 5′-GGCATCCTGACCCTGAAGTA-3′ and 5′-GGGGTGTTGAAGGTCTCAAA-3′ generating a 203-bp product for β-actin. PCR primers were designed from DNA sequences of neighboring exons for SAP97 and GRIP1 not to allow genomic amplification. The relative abundance of the mRNAs was estimated with individual Cot values and logarithmic amplification curves by the Fit Point program (Roche Diagnostics). Final PCR products were electrophoresed in a 2% agarose gel, stained with ethidium bromide, and imaged with a CCD camera (Cosmicar; Pentax, Tokyo, Japan).
Immunocytochemistry
Cultured cells were washed with phosphate-buffered saline and fixed for 20 min with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.3). Fixed cells were immunostained with either anti-MAP2 antibodies (1:100, Chemicon Int.) or anti-GFAP antibodies (1:1000, DakoCytomation). Alternatively, the culture period was extended until DIV12 to allow synaptic formation (Jourdi et al., 2003). Cultures were immunostained with anti-PSD-95 family/pan-PDZ (mAb K28/86.2, 1:500, Upstate Biotechnology) antibodies. Their immunoreactivity was revealed using the ABC-diaminobenzidine method (Vector Laboratories Inc., Burlingame, CA, USA) and visualized with the aid of a microscope (Axioskop; Carl Zeiss Oberkochen, Germany) fitted with an LCD color camera (DP50-CU; Olympus, Tokyo, Japan). All pictures were taken with a 20× or 60× objective, at 1/300 s shutter speed using Studio Lite software (Pixera Corporation Osaka, Japan).
EGF administration to neonatal rats
Recombinant human EGF or cytochrome c (Sigma Chemical Co., St. Louis, MO, USA or Higeta-Syoyu Co., Chiba, Japan) was dissolved in physiologic saline and administered s.c. at the nape of the neck of newborn rats daily from postnatal days 2–10 at a dose of 875 ng/g of body weight (injection volume 0.0125 ml/g). Peripherally administered EGF penetrates the blood–brain barrier of neonatal rats and affects neurochemical markers in the brain (Futamura et al., 2003). Cytochrome c was used for control injections because it has similar chemical characteristics as EGF including its molecular weight and isoelectric point, but lacks the biological activity of EGF (Calamandrei and Alleva, 1989). Brains were removed from EGF- or cytochrome c-injected rats and used in the following experiments.
Immunohistochemistry
On postnatal day 11, rats were anesthetized by inhalation of diethyl ether (Wako Pure Chemical, Osaka, Japan), and killed by transcardial perfusion with 4% paraformaldehyde and 0.1% glutaraldehyde in a 0.1 M phosphate-buffered solution (pH 7.4). The brains were removed and immersed in the same fixative for 24 h. Tissues were dehydrated in a graded ethanol series and embedded in paraffin wax. Serial thin slices (4 μm thick) were cut from the blocked samples, deparaffinized with xylene and ethanol, and stained with anti-PSD-95 family/pan-PDZ antibodies (1:100, Upstate Biotechnology). Immunoreactivity was visualized with goat anti-mouse IgG antibodies conjugated to Alexa Fluor 546 (Invitrogen, Carlsbad, CA, USA). Brain slices were optically sectioned further with a laser-scanning confocal microscope (FV500 with 0.6-μm optical depth, Olympus).
RESULTS
Attenuation of GRIP1 and SAP97 expression in neocortical cultures
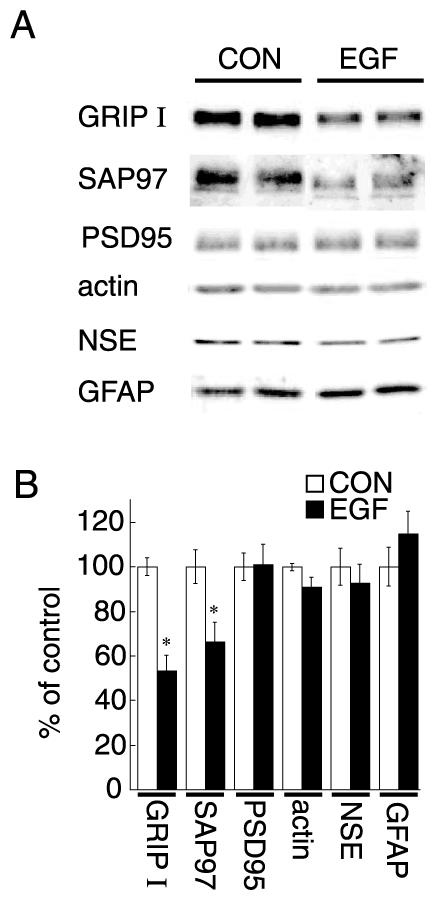
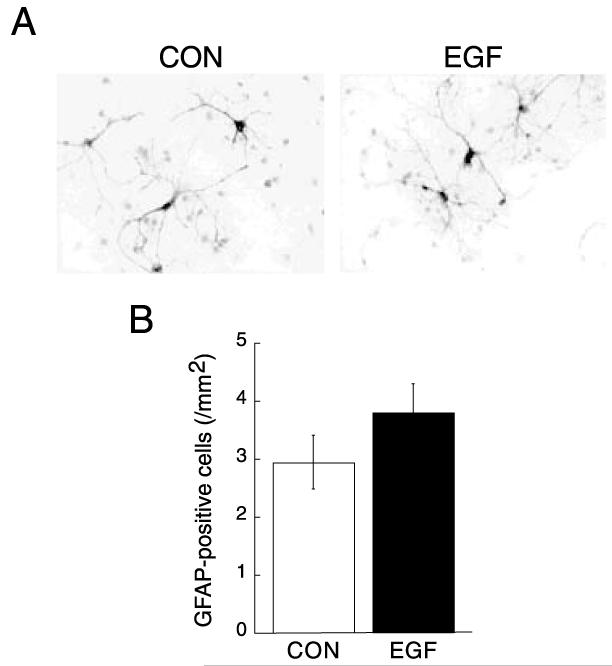
We examined the effects of ErbB1 receptor ligands on various PDZ proteins in neocortical cultures. Low-density cultures were prepared from embryonic rat neocortices and maintained in serum-free conditions for 7 days to minimize glial proliferation. Chronic treatment with EGF (20 pg/ml/day) significantly decreased GRIP1 and SAP97 protein levels (Fig. 1). In contrast, EGF treatment did not alter protein levels of PSD-95. β-actin, NSE (a neuronal marker), and GFAP (an astroglial marker) levels were not affected by the treatment, suggesting that EGF treatment decreased the expression of the specific set of PDZ proteins without affecting the size or the composition of cellular population in culture. We confirmed the suppressive effects of EGF treatment on GRIP1 and SAP97 proteins by immunoblotting with serial dilution of samples (data not shown). In the present culture condition, EGF treatment failed to increase the frequency of GFAP-positive cells (Fig. 2). There was no apparent influence on neuronal survival as monitored with MAP2 immunostaining (Fig. 1 legend).
Fig. 1.
Effects of EGF on the expression of GRIP1 and SAP97 proteins in neocortical cultures. Neocortical cultures were prepared from day-19 rat embryos and maintained in the presence or absence of EGF for 7 days (20 pg/ml, n=5 sister cultures for each condition). (A) Protein was extracted from sister cultures and subjected to immunoblotting with antibodies specific for GRIP1, SAP97, PSD-95, β-actin, NSE, and GFAP. Typical immunoblots are shown for display. (B) The intensity of immunoreactivity was quantified by densitometry. Data represent the mean±S.E.M. (n=5). * P<0.05 vs. control (Student’s t test). CON, control. Note that there were no significant differences in MAP2-positive cell densities at 7 DIV (108±10 cells/mm2 for control cultures and 100±10 cells/mm2 for EGF-treated cultures, n=4) or in protein yields (176±14 μg/dish for control cultures and 189±4 μg/dish for EGF-treated cultures, n=5). The densitometric analysis was re-evaluated by independent immunoblotting with serial dilution of samples (data not shown).
Fig. 2.
Effects of EGF on astrocytes in neocortical cultures. (A) Neocortical cultures were similarly prepared from rat embryos and grown in the presence or absence of EGF (20 pg/ml). (B) After fixation, cultures were subjected to immunostaining for GFAP. Density of GFAP-positive cells is 3.0±0.5 cells/mm2 in control cultures and 3.8±0.5 cells/mm2 for EGF-treated cultures, n=10).
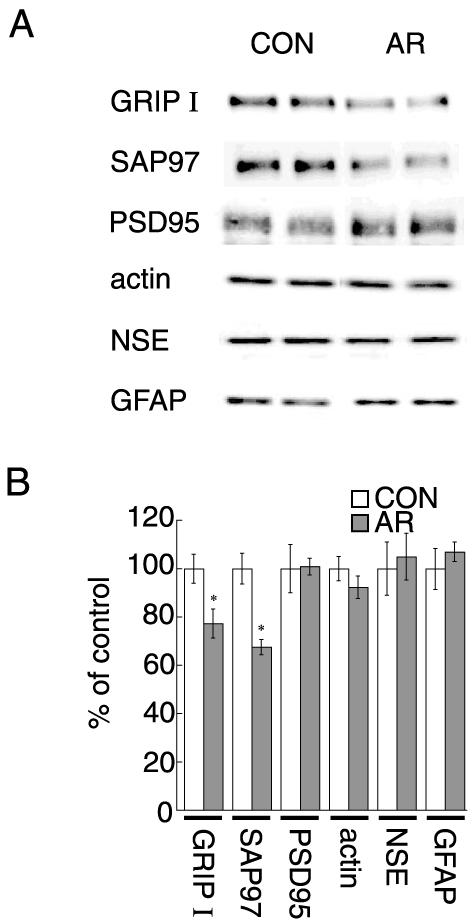
Amphiregulin, another member of the EGF family, similarly decreased protein levels of GRIP1 and SAP97 (Fig. 3). As previous studies indicate the lower affinity of amphiregulin to EGF (ErbB1) receptors (Adam et al., 1995), a higher concentration of amphiregulin was required to exert the suppressive effects.
Fig. 3.
Effects of amphiregulin on protein levels of GRIP1 and SAP97. Neocortical cultures were maintained with or without daily supplements of amphiregulin (AR, 200 pg/ml) for 7 days (n=5 sister cultures for each condition). (A) Protein was extracted from sister cultures and subjected to immunoblotting with antibodies specific for GRIP1, SAP97, PSD-95, β-actin, NSE and GFAP. Typical immunoblots are shown for display. (B) The intensity of immunoreactivity was quantified by densitometry. Data represent the mean±S.E.M. (n=5). * P<0.05 vs. control (Student’s t-test).
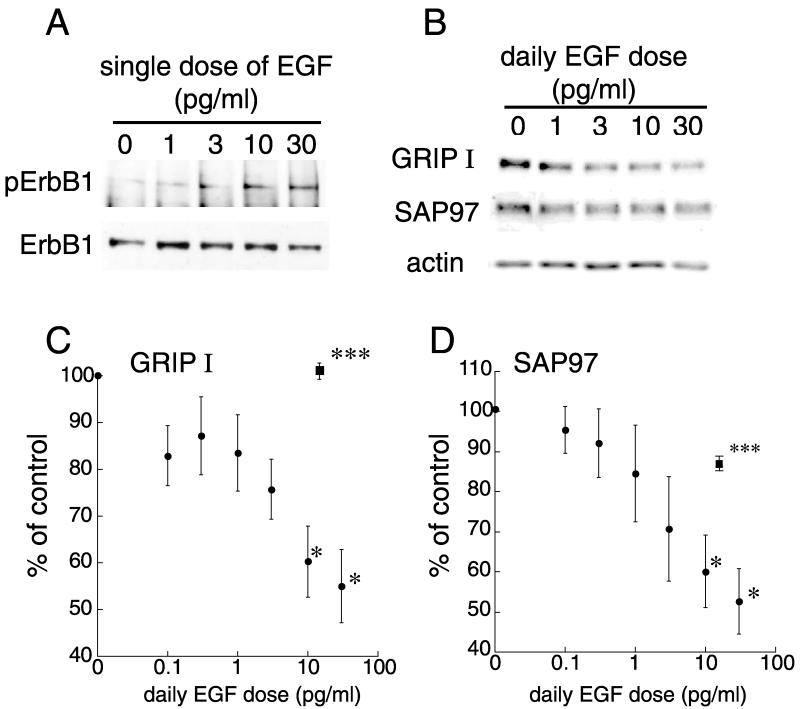
Because the required concentrations of EGF were relatively low in comparison with the dissociation constant of EGF receptor (ErbB1; Kd=10−10 M) (Berkers et al., 1991), we tested the EGF dose dependency of the regulation of PDZ protein levels (Fig. 4). Lower concentrations (10 and 30 pg/ml) of EGF can phosphorylate ErbB1 receptors in cultured neocortical neurons (Fig. 4A). The dose response curve indicated that a sub-picomolar range of EGF (10–30 pg/ml) maximally decreased the expression of GRIP1 and SAP97 (Fig. 4B–D). The co-application of the ErbB1 receptor inhibitor, PD153035, with EGF fully blocked the EGF-dependent decrease in GRIP1 and SAP97 (Fig. 4C, D), suggesting that the action of EGF and amphiregulin involves ErbB1 receptor signaling. The EGF dose was below the level that stimulated glial-cell proliferation in the present culture conditions (Fig. 2). These results suggest that ErbB1 receptor ligands attenuate the expression of PDZ proteins in cultured neocortical neurons.
Fig. 4.
The EGF dose dependency of the regulation of GRIP1 and SAP97 protein levels and effects of an ErbB1 receptor inhibitor (A) Phospho-ErbB1 and ErbB1 receptor levels were monitored in neocortical cultures. The concentrations of EGF (10 and 30 pg/ml) can phosphorylate ErbB1 receptors. (B–D) Neocortical cultures were treated daily with various concentrations of EGF (0, 0.1, 0.3, 1.0, 3.0, 10 and 30 pg/ml) from 1 to 7 DIV, and subjected to immunoblotting with antibodies specific for GRIP1, SAP97 and β-actin. (B) Typical immunoblots for GRIP1, SAP97, and β-actin are shown. (C, D) Immunoblots from five independent cultures were quantified by densitometry and averaged. Subchronic treatment with 10 and 30 pg/ml EGF significantly decreased the protein levels of GRIP1 (C) and SAP97 (D). Data represent the mean±S.E.M. (n=5 independent cultures). * P<0.05, *** P<0.001 vs. control (Student’s t-test). Closed squares represent effects of the combination of the ErbB1 receptor inhibitor (100 nM PD153035) and 20 pg/ml EGF.
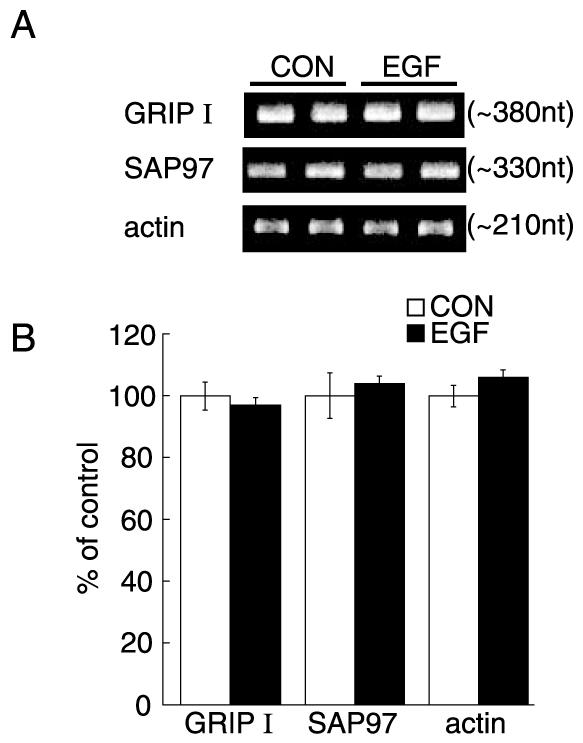
EGF effects on GRIP1 and SAP97 mRNA levels
We examined whether or not EGF regulates the GRIP1 and SAP97 mRNA levels by inhibiting the transcription of their respective genes. RNA was extracted from the above low-density neocortical cultures treated with or without EGF. Real-time quantitative PCR revealed that EGF treatment did not alter GRIP1 or SAP97 mRNA levels (Fig. 5). The internal control of β-actin mRNA was also not altered. These results indicated that an ErbB1 receptor ligand, EGF, influences the expression of the PDZ proteins at a translational or post-translational level.
Fig. 5.
GRIP1 and SAP97 mRNA levels in cultured neocortical neurons. GRIP1, SAP97 and β-actin mRNA levels were quantified by the real-time RT-PCR using SYBR-Green as an indicator. Total RNA was extracted from EGF-treated and non-treated cultures (n=5 sister cultures for each condition) and subjected to a real-time PCR analysis (see details in Experimental Procedures). (A) Typical PCR products were separated on an agarose gel and are presented for display. PCR products for GRIP1 and SAP97 mRNAs appeared as a single band in a gel. The nucleotide sizes of the products were estimated using the calibration curve of DNA size markers and are shown in parentheses, which almost matched the DNA sizes calculated from their mRNA sequences. (B) Relative mRNA levels were calculated by the Fit Point program based on Cot values and slopes of logarithmic amplification. Data represent the mean±S.E.M. (n=5).
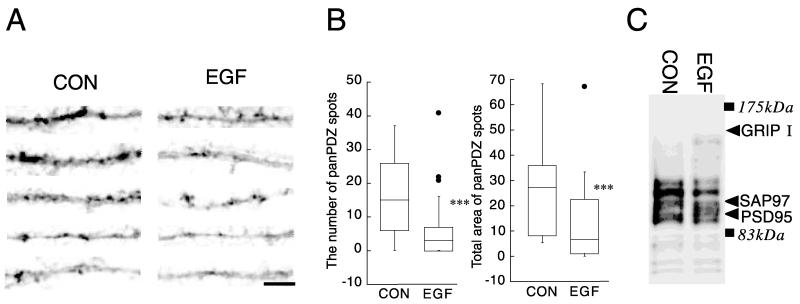
Reduction in pan-PDZ immunoreactivity in EGF-treated long-term cultures
The effects of EGF on proteins carrying a PDZ domain(s) were also evaluated in neocortical cultures having more developed synapses (Fig. 6). Neocortical cultures were maintained for 12 days in the presence or absence of EGF. There was significant proliferation of non-neuronal cells in EGF-treated culture after DIV7 (data not shown). Our previous study indicates that a BDNF-triggered increase in total PDZ protein content results in an increase in pan-PDZ-positive postsynaptic sites on dendrites (Jourdi et al., 2003). The expression of PDZ proteins was, therefore, assessed by immunocytochemistry using the anti-pan-PDZ antibody (mAb K28/86.2) that is suggested to recognize multiple members of the PSD-95 family including PSD-95, SAP97 and Chapsyn 110/PSD-93 (Honjo et al., 2000). EGF treatment influenced pan-PDZ immunoreactivity along the dendrites. Punctate staining for the pan-PDZ antibody was weaker in EGF-treated culture and less frequent along the dendrites of EGF-treated neurons. This trend was confirmed by calculating the number of pan-PDZ immunoreactive spots as well as the total size of the areas immunoreactive for this antibody (Fig. 6B). These results reveal the negative influence of EGF treatment on post-synaptic development of neocortical neurons.
Fig. 6.
Effects of EGF on the immunoreactivity for pan-PDZ proteins in long-term cultures. Lower density neocortical cultures were grown for 12 days with or without EGF. After fixation, immunoreactivity for the anti-pan-PDZ antibody was visualized with the peroxidase-conjugated avidin–biotin complexes. (A) Pictures of dendrites were randomly chosen for display and arranged in the order of intensity of the immunoreactivity from the top. Dendritic processes including the above samples were randomly pictured (n=20 each). (B) The top 30% margin of the whole dynamic range of the immunoreactivity was defined as a positive threshold with an aid of Photoshop software (Adobe Systems Inc., Tokyo, Japan). The number of the marked areas above the threshold was counted along dendritic processes and normalized the length of dendrites (left). Alternatively, total size of the marked areas was calculated from 20 dendrites and plotted (right). *** P<0.005 vs. control (Mann-Whitney U test). Scale bar=5 μm. Note: Almost all spots immunoreactive for the anti-pan-PDZ antibody presumably represent post-synaptic structures as those matched the sites carrying a presynaptic marker in previous studies (Jourdi et al., 2003; Rameau et al., 2004). (C) Immunoreactivity for the pan-PDZ antibody was characterized by immunoblotting. Protein was similarly extracted from the EGF-treated and control cultures and subjected to immunoblotting with the pan-PDZ antibody. The blot was re-probed with antibodies against PSD95, GRIP1 and SAP97 to show their sizes in the blot.
We also characterized the immunoreactivity of the pan-PDZ antibody (mAb K28/86.2). This antibody is originally raised against a PDZ domain of PSD95/SAP90 (Honjo et al., 2000; Jourdi et al., 2003). Our immunoblotting revealed that this antibody recognizes, at least, more than 10 molecules with different sizes, some of which corresponded to PSD-95 and SAP97 (Fig. 6C). This antibody failed to recognize GRIP1, as there was no immunoreactivity at the size of GRIP1. However, there were also multiple molecules whose molecular sizes did not match these identified PDZ proteins and whose levels were increased or decreased by EGF. The result suggests that effects of EGF were not limited to the PDZ molecules, GRIP1 and SAP97.
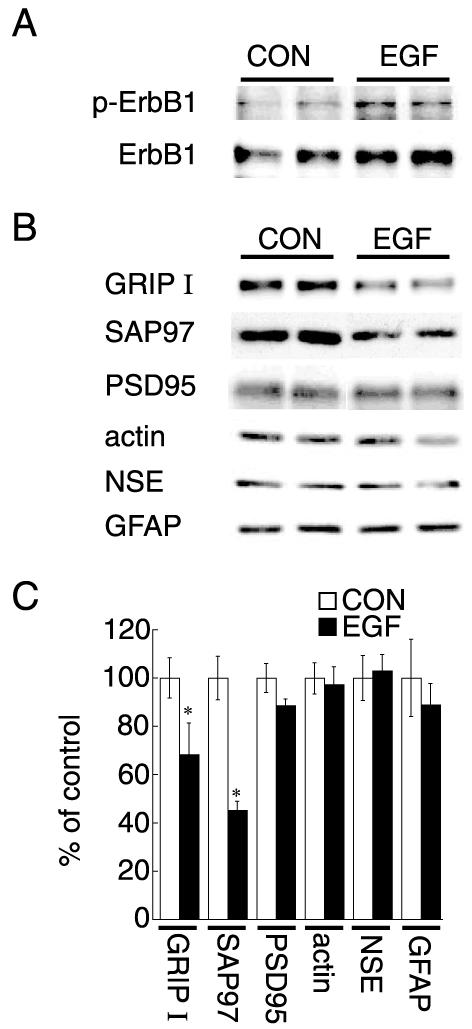
Decreases in GRIP1 and SAP97 expression following in vivo EGF administration
We next tested whether in vivo ErbB1 receptor stimulation influences the expression of GRIP1 and SAP97. EGF was administered s.c. to neonatal rats. Administered EGF penetrated blood–brain barrier, leading to the phosphorylation of ErbB1 receptors in rat neocortex as indicated previously (Futamura et al., 2003) (Fig. 7A). Repeated administration of EGF decreased the protein expression of GRIP1 and SAP97 in rat neocortex (Fig. 7B, C). In contrast, there was no significant alteration in PSD-95 levels. Additionally, protein levels of β-actin, NSE or GFAP were not influenced significantly in vivo. These results show that the ErbB1 receptor ligand, EGF, negatively regulates the protein expression of GRIP1 and SAP97 in vivo.
Fig. 7.
In vivo EGF administration and the expression of PDZ proteins in developing neocortex to rat neonates. (A) Phospho-ErbB1 and ErbB1 receptor levels were monitored for 1 h after s.c. EGF administration. Subsequently, the brain was removed and lysed for immunoblotting with anti-phospho-ErbB1 and anti-ErbB1 receptor antibodies. (B) EGF or cytochrome c (control) was repeatedly administered s.c. to rat neonates on postnatal days 2-10. On postnatal day 11, protein was extracted from the neocortex and subjected to immunoblotting with antibodies specific for GRIP1, SAP97, PSD-95, β-actin, NSE and GFAP. (C) Immunoreactive bands were quantified by densitometry. Data represent mean±S.E.M. (n=5 rats for each condition). * P<0.05 vs. control (Student’s t-test). The densitometric analysis was re-evaluated by independent immunoblotting with serial dilution of samples (data not shown).
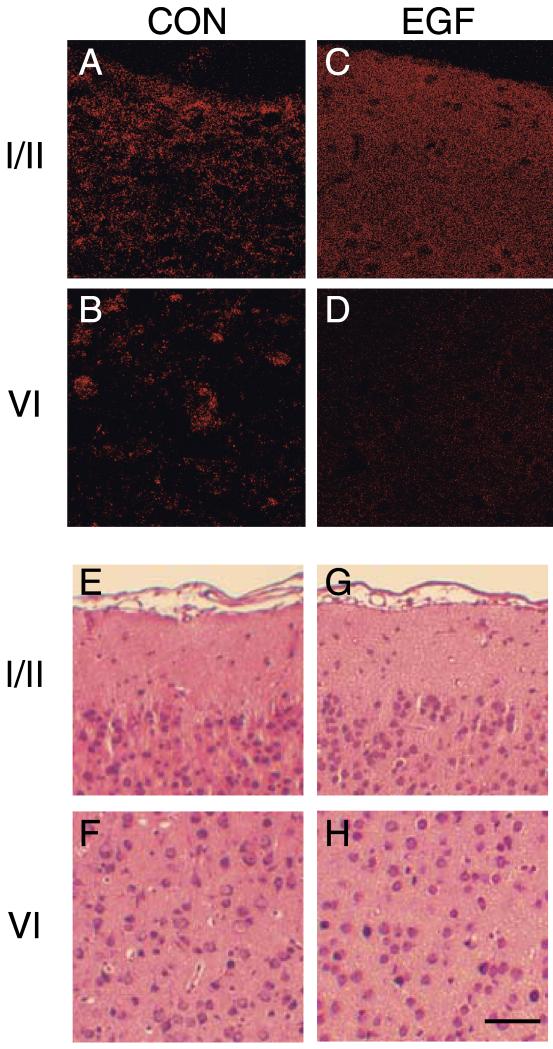
Altered distributions of pan-PDZ-immunoreactivity in developing neocortex
To estimate potential post-synaptic effects of the decrease in GRIP1 and SAP97 proteins, we used immunohistochemistry to investigate whether EGF treatment altered the distributions of PDZ proteins. Neonatal EGF treatment changed the localization of puncta labeled by anti-pan PDZ antibodies (Fig. 8A–D). PDZ-immunoreactive puncta accumulated or aggregated in the neocortex of control rats, and were not uniformly distributed in all cortical layers. In contrast, the puncta in EGF-treated rats were smaller or dispersed, and the PDZ immunoreactivity was more uniformly distributed in the upper cortical layers. The strength of the pan-PDZ immunoreactivity was diminished in the lower cortical layers by EGF administration. On the other hand, there were no apparent signs of EGF-induced neuronal degeneration in the examined neocortex as shown by the conventional hematoxylin and eosin staining (Fig. 8E–H). These results indicate that EGF changes the levels and distributions of the PDZ proteins in rat neocortex.
Fig. 8.
In vivo effects of neonatal EGF treatment on pan-PDZ-immunoreactivity in the neocortex. Confocal microscopic images were taken from paraffin-embedded sections stained with anti-pan-PDZ antibodies (A–D). In parallel, adjacent sections were counterstained with hematoxylin and eosin (E–H). Sections were prepared from the neocortex of control rats (A, B, E, F) or EGF-treated rats (C, D, G, H). Effects of EGF on pan-PDZ-immunoreactivity were most apparent in layer I/II (A, C, E, G) and layer VI (B, D, F, H) of the neocortex. Hematoxylin and eosin staining reveals that EGF administration had no apparent effects on the gross neocortical structure. Scale bar=150 μm.
DISCUSSION
EGF/ErbB1 receptor effects on PDZ proteins in vitro and in vivo
The present results demonstrate that, in low-density neocortical cultures, ErbB1 receptor ligands attenuate the expression of the PDZ proteins, which are most enriched in post-synaptic sites. Among the PDZ proteins examined, GRIP1 and SAP97 protein levels were most markedly affected by EGF and amphiregulin. As there was an apparent overall decrease in pan-PDZ immunoreactivity in EGF- or amphiregulin-treated culture, we cannot rule out the possibility that the expression of several other PDZ proteins was also down-regulated in parallel. The negative influences were also observed at a synaptic level. EGF-triggered ErbB1 activation led to a decrease in pan-PDZ immunoreactive puncta both in culture and in vivo. These results revealed a novel negative activity of ErbB1 receptor ligands that attenuates the expression and development of the post-synaptic components in developing neocortex.
In the combination with the insulin-rich serum-free medium, there were no apparent differences in neuronal survival, glial contamination, and neurite extension between EGF-treated and control cultures. Higher-density cultures and higher EGF concentrations, however, allowed non-neuronal cells to proliferate significantly and made it difficult to evaluate the direct effects of EGF on synaptic components with quantitative molecular techniques (data not shown). As far as neocortical cultures were harvested on 7 DIV before synaptic maturation and analyzed in biochemistry, accordingly, biochemical data presumably represent direct effects of EGF on PDZ protein expression. When the culture period was extended until 12 DIV to allow synaptic formation and maturation (Jourdi et al., 2003; Rameau et al., 2004), there were a number of non-neuronal cells grown in EGF-treated culture (data not shown). The culture condition still allowed us to examine the EGF effects by culture staining. Immunocytochemistry revealed a significant reduction in the number of pan-PDZ aggregates in EGF-treated neurons. The given results from short-term cultures indicate that the reduction in postsynaptic structures is likely to represent the suppressive effect of EGF on PDZ proteins. It is noteworthy that a concentration of EGF in a sub-picomolar range (approximately 2 pM) was enough to reduce the PDZ protein levels. The concentration is much lower than those conventionally required for the high-affinity binding of EGF receptors (ErbB1; 100 pM) (Futamura et al., 2003). This suggests that particular heteromeric compositions of the ErbB receptors might contribute to this sensitivity (Jaulin-Bastard et al., 2001; Wong and Guillaud, 2004).
To further evaluate the effects of EGF on PDZ proteins in vivo, we administered EGF to rat neonates. Since the blood–brain barrier has not yet been established in rat neonates, EGF penetrated into the brain and acted on developing neurons without affecting cell proliferation (Kleshcheva, 1988). Animals treated with EGF as neonates develop normally and acquire normal learning abilities, ruling out the possibility that EGF treatment grossly impairs normal brain development and functions (Kornblum et al., 1995; Futamura et al., 2003). Repeated daily administration of EGF specifically suppressed the expression of the PDZ proteins in the rat neocortex. Additionally, immunohistochemical staining with anti-pan-PDZ antibodies revealed that EGF treatment inhibited the accumulation of anti-pan-PDZ immunoreactive puncta in vivo as observed in primary neocortical cultures, presumably suggesting the negative effects on postsynaptic development. Previous histologic examination verified normal brain structures in EGF-treated neonates and revealed that there is no apparent effect on astrocytes as determined by GFAP staining (Futamura et al., 2003). Given the basal levels of ErbB1 receptor phosphorylation in the neocortex, these results presumably implicate endogenous ErbB1 receptor ligands in selective development of post-synaptic structures containing various PDZ proteins. More elaborate analyses are, however, needed to support this hypothesis.
Comparison with BDNF, a positive regulator for synaptic development
Previous studies have shown that BDNF positively regulates the expression of SAP97 and GRIP1 protein during the prenatal and early postnatal stages of development (Jourdi et al., 2003). SAP97 and GRIP1 interact with AM-PARs subunits, GluR1, GluR2 and GluR3 (Dong et al., 1997; Leonard et al., 1998; Wyszynski et al., 1999; Valtschanoff et al., 2000), and modulate AMPARs trafficking (Hirai, 2001; Klocker et al., 2002). Thus, the up-regulation of PDZ proteins by BDNF results in their enhanced interaction with AMPAR, leading to their mutual stabilization (Jourdi et al., 2003). These findings might explain a peculiar phenomenon whereby the BDNF-triggered increases in AMPARs do not involve changes in AMPAR mRNA levels (Narisawa-Saito et al., 1999b). BDNF rather alters the protein stability of AMPAR and the PDZ proteins (Jourdi et al., 2003). In the present study, similarly, ErbB1 receptor ligands also regulate the levels of these AMPAR-associated PDZ proteins without influencing their mRNA levels, suggesting potential effects on protein stability. It is, however, noteworthy that the effects of EGF were opposite to those of BDNF. The fact that molecular interactions between these PDZ proteins and AMPARs can regulate their synaptic stability suggests that EGF-triggered downregulation of the PDZ proteins might result in a decrease in AMPAR protein expression as well. This explanation has been supported by the finding from our laboratory that EGF decreases the expression of GluR1 protein in cultured neocortical neurons (Narisawa-Saito et al., 1999a). Taken together, these observations imply that ErbB1 receptor signals might influence the synaptic functions of AMPARs by modulating the expression of PDZ proteins.
EGF and BDNF, which produce the opposite biological activities on developing cortical neurons, recruit the same sets of signal transducers such as the Ras/MAP kinase cascade, the PI3 kinase/Akt cascade and the PLC gamma/PKC cascade (Yamada et al., 1997; Wong and Guillaud, 2004). In agreement, both factors are suggested to promote neuronal survival and differentiation, which involve the common signal cascades (Yamada et al., 1997). What is the intracellular signal that plays a critical role in distinction of the biological reactions with EGF and BDNF? Unlike BDNF, EGF triggers other signal pathways such as STAT3 signaling and NFkappaB cascade (Anest et al., 2004; Wong and Guillaud, 2004). It is known that EGF exerts a similar de-differentiation activity on chondrocytes (Huh et al., 2003). Future studies will address the question and illuminate the molecular nature of signal cascade(s) attenuating post-synaptic development.
Positive and negative regulation of PDZ protein expression and synaptic development
There are several diffusible regulators that influence synaptic formation and maturation including acetylcholine receptor-inducing activity (ARIA) (NRG1), BDNF and basic fibroblast growth factor (bFGF) (Sandrock et al., 1997; Alsina et al., 2001; Jourdi and Nawa, 2002). The soluble protein factors of ARIA and BDNF increase the levels of acetylcholine receptors, AMPARs and NMDARs, and influence their subcellular distributions to promote selective postsynaptic development (Sandrock et al., 1997; Alsina et al., 2001). A negative activity on synaptic development is also reported on bFGF. Treatment of primary cortical neurons with bFGF selectively down-regulated protein expression of the PDZ proteins and decreased their interaction to AMPAR (Jourdi and Nawa, 2002). The concept of selective neural connections was introduced in the classic studies on the development of neuro-muscular synapses or on the innervation of the superior cervical ganglion (English and Schwartz, 1995; Smith et al., 2002). These intercellular factors contribute to selective synaptic maturation and elimination during the development of functional neural circuits. Several factors are also implicated in the consolidation and pruning of distinct subsets of synapses depending on neural activity (English and Schwartz, 1995; Smith et al., 2002). The present results showed that ErbB1 receptor ligands act as a negative regulator for the expression and accumulation of PDZ proteins and that they might be involve in selective synaptic development or elimination.
There are several examples of positive and negative regulators that contribute to homeostatic balance and stabilization of their respective systems. For example, follistatin and inhibin specifically suppress the secretion of follicle stimulating hormone, whereas activin enhances it (Ying, 1988). In addition to this regulation, activin and follistatin act as competitors during the regulation of hepatocyte growth (Schwall et al., 1993). We propose that ErbB1 receptor ligands might act as negative regulators of homeostatic control during postsynaptic development and suggest that impairment of ErbB1 receptor signaling might contribute to the aberrant synaptic organization observed in neurodevelopmental disorders (Toyooka et al., 2002; Futamura et al., 2002).
Acknowledgments
This work was supported by a grant-in-aid for Creative Scientific Research, Grand-in Aid for Scientific Research (B), and Grant for Promotion of Niigata University Research Projects.
Abbreviations
- AMPAR
α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor
- ARIA
acetylcholine receptor-inducing activity
- BDNF
brain-derived neurotrophic factor
- bFGF
basic fibroblast growth factor
- DIV
days in vitro
- EGF
epidermal growth factor
- GFAP
glial fibrillary acidic protein
- GRIP
glutamate receptor interacting protein
- NMDAR
N-methyl-d-aspartate receptor
- NRG1
neuregulin-1
- NSE
neuron specific enolase
- PDZ
postsynaptic density-95/discs large/zone occludens-1
- PSD
postsynaptic density
- RT-PCR
reverse transcriptase–polymerase chain reaction
- SAP97
synapse-associated protein 97 kDa
- T-TBS
Tween 20 in Tris-buffered saline
REFERENCES
- Adam R, Drummond DR, Solic N, Holt SJ, Sharma RP, Chamberlin SG, Davies DE. Modulation of the receptor binding affinity of amphiregulin by modification of its carboxyl terminal tail. Biochim Biophys Acta. 1995;1266:83–90. doi: 10.1016/0167-4889(94)00224-3. [DOI] [PubMed] [Google Scholar]
- Alsina B, Vu T, Cohen-Cory S. Visualizing synapse formation in arborizing optic axons in vivo: dynamics and modulation by BDNF. Nat Neurosci. 2001;4:1093–1101. doi: 10.1038/nn735. [DOI] [PubMed] [Google Scholar]
- Anest V, Cogswell PC, Baldwin AS., Jr IkappaB kinase alpha and p65/RelA contribute to optimal epidermal growth factor-induced c-fos gene expression independent of IkappaBalpha degradation. J Biol Chem. 2004;279:31183–31189. doi: 10.1074/jbc.M404380200. [DOI] [PubMed] [Google Scholar]
- Berkers JA, van Bergen en Henegouwen PM, Boonstra J. Three classes of epidermal growth factor receptors on HeLa cells. J Biol Chem. 1991;266:922–927. [PubMed] [Google Scholar]
- Calamandrei G, Alleva E. Epidermal growth factor has both growth-promoting and growth-inhibiting effects on physical and neurobehavioral development of neonatal mice. Brain Res. 1989;477:1–6. doi: 10.1016/0006-8993(89)91387-5. [DOI] [PubMed] [Google Scholar]
- Casper D, Roboz GJ, Blum M. Epidermal growth factor and basic fibroblast growth factor have independent actions on mesencephalic dopamine neurons in culture. J Neurochem. 1994;62:2166–2177. doi: 10.1046/j.1471-4159.1994.62062166.x. [DOI] [PubMed] [Google Scholar]
- Cotrufo T, Viegi A, Berardi N, Bozzi Y, Mascia L, Maffei L. Effects of neurotrophins on synaptic protein expression in the visual cortex of dark-reared rats. J Neurosci. 2003;23:3566–3571. doi: 10.1523/JNEUROSCI.23-09-03566.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, O’Brien RJ, Fung ET, Lanahan AA, Worley PF, Huganir RL. GRIP: a synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature. 1997;386:279–284. doi: 10.1038/386279a0. [DOI] [PubMed] [Google Scholar]
- English AW, Schwartz G. Both basic fibroblast growth factor and ciliary neurotrophic factor promote the retention of polyneuronal innervation of developing skeletal muscle fibers. Dev Biol. 1995;169:57–64. doi: 10.1006/dbio.1995.1126. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Toffano G, Skaper SD. Epidermal growth factor exerts neuronotrophic effects on dopaminergic and GABAergic CNS neurons: comparison with basic fibroblast growth factor. J Neurosci Res. 1991;30:493–497. doi: 10.1002/jnr.490300306. [DOI] [PubMed] [Google Scholar]
- Futamura T, Toyooka K, Iritani S, Niizato K, Nakamura R, Tsuchiya K, Someya T, Kakita A, Takahashi H, Nawa H. Abnormal expression of epidermal growth factor and its receptor in the fore-brain and serum of schizophrenic patients. Mol Psychiatry. 2002;7:673–682. doi: 10.1038/sj.mp.4001081. [DOI] [PubMed] [Google Scholar]
- Futamura T, Kakita A, Tohmi M, Sotoyama H, Takahashi H, Nawa H. Neonatal perturbation of neurotrophic signaling results in abnormal sensorimotor gating and social interaction in adults: implication for epidermal growth factor in cognitive development. Mol Psychiatry. 2003;8:19–29. doi: 10.1038/sj.mp.4001138. [DOI] [PubMed] [Google Scholar]
- Harris RC, Chung E, Coffey RJ. EGF receptor ligands. Exp Cell Res. 2003;284:2–13. doi: 10.1016/s0014-4827(02)00105-2. [DOI] [PubMed] [Google Scholar]
- Hirai H. Modification of AMPA receptor clustering regulates cerebellar synaptic plasticity. Neurosci Res. 2001;39:261–267. doi: 10.1016/s0168-0102(00)00237-6. [DOI] [PubMed] [Google Scholar]
- Honjo Y, Nakagawa S, Takeichi M. Blockade of cadherin-6B activity perturbs the distribution of PSD-95 family proteins in retinal neurones. Genes Cells. 2000;5 doi: 10.1046/j.1365-2443.2000.00327.x. [DOI] [PubMed] [Google Scholar]
- Huh YH, Kim SH, Kim SJ, Chun JS. Differentiation status-dependent regulation of cyclooxygenase-2 expression and prosta-glandin E2 production by epidermal growth factor via mitogen-activated protein kinase in articular chondrocytes. J Biol Chem. 2003;278:9691–9697. doi: 10.1074/jbc.M211360200. [DOI] [PubMed] [Google Scholar]
- Iwakura Y, Piao YS, Mizuno M, Takei N, Kakita A, Takahashi H, Nawa H. Influences of dopaminergic lesion on epidermal growth factor-ErbB signals in Parkinson’s disease and its model: neurotrophic implication in nigrostriatal neurons. J Nueochem. 2005;93:974–983. doi: 10.1111/j.1471-4159.2005.03073.x. [DOI] [PubMed] [Google Scholar]
- Jaulin-Bastard F, Saito H, Le Bivic A, Ollendorff V, Marchetto S, Birnbaum D, Borg JP. The ERBB2/HER2 receptor differentially interacts with ERBIN and PICK1 PSD-95/DLG/ZO-1 domain proteins. J Biol Chem. 2001;276:15256–15263. doi: 10.1074/jbc.M010032200. [DOI] [PubMed] [Google Scholar]
- Jourdi H, Nawa H. Basic fibroblast growth factor modulates the expression of PDZ domain-containing proteins in cultured cortical neurons. Acta Med Biol. 2002;50:107–115. [PMC free article] [PubMed] [Google Scholar]
- Jourdi H, Iwakura Y, Narisawa-Saito M, Ibaraki K, Xiong H, Watanabe M, Hayashi Y, Takei N, Nawa H. Brain-derived neurotrophic factor signal enhances and maintains the expression of AMPA receptor-associated PDZ proteins in developing cortical neurons. Dev Biol. 2003;263:216–230. doi: 10.1016/j.ydbio.2003.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- Kleshcheva RP. The development of components of the blood-brain barrier in the neocortex of the white rat. Arkh Anat Gistol Embriol. 1988;95:22–26. [PubMed] [Google Scholar]
- Klocker N, Bunn RC, Schnell E, Caruana G, Bernstein A, Nicoll RA, Bredt DS. Synaptic glutamate receptor clustering in mice lacking the SH3 and GK domains of SAP97. Eur J Neurosci. 2002;16:1517–1522. doi: 10.1046/j.1460-9568.2002.02228.x. [DOI] [PubMed] [Google Scholar]
- Ko J, Kim S, Valtschanoff JG, Shin H, Lee JR, Sheng M, Premont RT, Weinberg RJ, Kim E. Interaction between liprin-alpha and GIT1 is required for AMPA receptor targeting. J Neurosci. 2003;23:1667–1677. doi: 10.1523/JNEUROSCI.23-05-01667.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblum HI, Gall CM, Seroogy KB, Lauterborn JC. A subpopulation of striatal gabaergic neurons expresses the epidermal growth factor receptor. Neuroscience. 1995;69:1025–1029. doi: 10.1016/0306-4522(95)00392-v. [DOI] [PubMed] [Google Scholar]
- Lei S, Czerwinska E, Czerwinski W, Walsh MP, MacDonald JF. Regulation of NMDA receptor activity by F-actin and myosin light chain kinase. J Neurosci. 2001;21:8464–8472. doi: 10.1523/JNEUROSCI.21-21-08464.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard AS, Davare MA, Horne MC, Garner CC, Hell JW. SAP97 is associated with the alpha-amino-3-hydroxy-5-methylis-oxazole-4-propionic acid receptor GluR1 subunit. J Biol Chem. 1998;273:19518–19524. doi: 10.1074/jbc.273.31.19518. [DOI] [PubMed] [Google Scholar]
- Lin Y, Skeberdis VA, Francesconi A, Bennett MV, Zukin RS. Postsynaptic density protein-95 regulates NMDA channel gating and surface expression. J Neurosci. 2004;24:10138–10148. doi: 10.1523/JNEUROSCI.3159-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano T, Jourdi H, Nawa H. Emerging roles of Dlg-like PDZ proteins in the organization of the NMDA-type glutamatergic synapse. J Biochem (Tokyo) 1998;124:869–875. doi: 10.1093/oxfordjournals.jbchem.a022200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narisawa-Saito M, Silva AJ, Yamaguchi T, Hayashi T, Yamamoto T, Nawa H. Growth factor-mediated Fyn signaling regulates alpha-amino-3- hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor expression in rodent neocortical neurons. Proc Natl Acad Sci U S A. 1999a;96:2461–2466. doi: 10.1073/pnas.96.5.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narisawa-Saito M, Carnahan J, Araki K, Yamaguchi T, Nawa H. Brain-derived neurotrophic factor regulates the expression of AMPA receptor proteins in neocortical neurons. Neuroscience. 1999b;88:1009–1014. doi: 10.1016/s0306-4522(98)00496-5. [DOI] [PubMed] [Google Scholar]
- Narisawa-Saito M, Iwakura Y, Kawamura M, Araki K, Kozaki S, Takei N, Nawa H. Brain-derived neurotrophic factor regulates surface expression of alpha-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid receptors by enhancing the N-ethylmaleimide-sensitive factor/GluR2 interaction in developing neocortical neurons. J Biol Chem. 2002;277:40901–40910. doi: 10.1074/jbc.M202158200. [DOI] [PubMed] [Google Scholar]
- Ozaki M, Sasner M, Yano R, Lu HS, Buonanno A. Neuregulin-beta induces expression of an NMDA-receptor subunit. Nature. 1997;390:691–694. doi: 10.1038/37795. [DOI] [PubMed] [Google Scholar]
- Petersen JD, Chen X, Vinade L, Dosemeci A, Lisman JE, Reese TS. Distribution of postsynaptic density (PSD)-95 and Ca2+/calmodulin-dependent protein kinase II at the PSD. J Neurosci. 2003;23:11270–11278. doi: 10.1523/JNEUROSCI.23-35-11270.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameau GA, Chiu LY, Ziff EB. Bidirectional regulation of neuronal nitric-oxide synthase phosphorylation at serine 847 by the N-methyl-D-aspartate receptor. J Biol Chem. 2004;279 doi: 10.1074/jbc.M311103200. [DOI] [PubMed] [Google Scholar]
- Sandrock AW, Jr, Dryer SE, Rosen KM, Gozani SN, Kramer R, Theill LE, Fischbach GD. Maintenance of acetylcholine receptor number by neuregulins at the neuromuscular junction. Science. 1997;276:599–603. doi: 10.1126/science.276.5312.599. [DOI] [PubMed] [Google Scholar]
- Schwall RH, Robbins K, Jardieu P, Chang L, Lai C, Terrell TG. Activin induces cell death in hepatocytes in vivo and in vitro. Hepatology. 1993;18:347–356. doi: 10.1016/0270-9139(93)90018-i. [DOI] [PubMed] [Google Scholar]
- Smith PG, Warn JD, Steinle JJ, Krizsan-Agbas D, Hasan W. Modulation of parasympathetic neuron phenotype and function by sympathetic innervation. Auton Neurosci. 2002;96:33–42. doi: 10.1016/s1566-0702(01)00371-x. [DOI] [PubMed] [Google Scholar]
- Toyooka K, Iritani S, Makifuchi T, Shirakawa O, Kitamura N, Maeda K, Nakamura R, Niizato K, Watanabe M, Kakita A, Takahashi H, Someya T, Nawa H. Selective reduction of a PDZ protein, SAP-97, in the prefrontal cortex of patients with chronic schizophrenia. J Neurochem. 2002;83:797–806. doi: 10.1046/j.1471-4159.2002.01181.x. [DOI] [PubMed] [Google Scholar]
- Valtschanoff JG, Burette A, Davare MA, Leonard AS, Hell JW, Weinberg RJ. SAP97 concentrates at the postsynaptic density in cerebral cortex. Eur J Neurosci. 2000;12:3605–3614. doi: 10.1046/j.1460-9568.2000.00256.x. [DOI] [PubMed] [Google Scholar]
- Valtschanoff JG, Weinberg RJ. Laminar organization of the NMDA receptor complex within the postsynaptic density. J Neurosci. 2001;21:1211–1217. doi: 10.1523/JNEUROSCI.21-04-01211.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner MH, Nanney LB, Stoscheck CM, King LE. Localization of immunoreactive epidermal growth factor receptors in human nervous system. J Histochem Cytochem. 1988;36:81–86. doi: 10.1177/36.1.3275713. [DOI] [PubMed] [Google Scholar]
- Wong RW, Guillaud L. The role of epidermal growth factor and its receptors in mammalian CNS. Cytokine Growth Factor Rev. 2004;15:147–156. doi: 10.1016/j.cytogfr.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Wu H, Nash JE, Zamorano P, Garner CC. Interaction of SAP97 with minus-end-directed actin motor myosin VI. Implications for AMPA receptor trafficking. J Biol Chem. 2002;277:30928–30934. doi: 10.1074/jbc.M203735200. [DOI] [PubMed] [Google Scholar]
- Wyszynski M, Valtschanoff JG, Naisbitt S, Dunah AW, Kim E, Standaert DG, Weinberg R, Sheng M. Association of AMPA receptors with a subset of glutamate receptor-interacting protein in vivo. J Neurosci. 1999;19:6528–6537. doi: 10.1523/JNEUROSCI.19-15-06528.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Ikeuchi T, Hatanaka H. The neurotrophic action and signalling of epidermal growth factor. Prog Neurobiol. 1997;51:19–37. doi: 10.1016/s0301-0082(96)00046-9. [DOI] [PubMed] [Google Scholar]
- Ying SY. Inhibins, activins, and follistatins: gonadal proteins modulating the secretion of follicle-stimulating hormone. Endocr Rev. 1988;9:267–293. doi: 10.1210/edrv-9-2-267. [DOI] [PubMed] [Google Scholar]