Abstract
Neurotoxicity is involved in various neurodegenerative diseases including Parkinson’s disease (PD), which affects mesencephalic dopaminergic neurons of the substantia nigra (SN). Positive α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor modulators (PARMs, a.k.a. Ampakines, such as CX614) increase brain-derived neurotrophic factor (BDNF) protein levels in vivo and in cultured hippocampal slices. BDNF is a survival factor for various neuronal cell types including mesencephalic dopaminergic neurons. Using cultured mesencephalic and hippocampal slices, we investigated whether preincubation with CX614 could provide neuroprotection against MPP+ toxicity and whether such neuroprotection was mediated by BDNF. Various treatment protocols were tested to demonstrate CX614-induced neuroprotection against MPP+. Pretreatment with CX614 significantly reduced MPP+-induced toxicity and increased BDNF levels in both hippocampal and mesencephalic cultured slices; CX614 pretreatment for 6 h in hippocampal slices and 24 h in mesencephalic slices was sufficient to produce significant neuroprotection as assessed with lactate dehydrogenase release in slice medium and propidium iodide uptake in slices. Both a BDNF scavenger and an inhibitor of the BDNF receptor TrkB, abrogated CX614-mediated reduction of MPP+-induced toxicity. Inhibition of Ca2+-activated proteases, calpains, was also protective against MPP+-induced toxicity. However, co-application of calpain inhibitor with CX614 abolished CX614-mediated protection, suggesting a dual action of calpains in this model. We conclude that CX614 is neuroprotective against MPP+-induced toxicity, an effect mediated by increased BDNF expression and activation of BDNF-dependent signaling pathways. Our results provide support for using PARMs as a new therapy for neurodegenerative disorders, including PD.
Keywords: Ampakine, BDNF, Dopamine, Hippocampus, Mesencephalon, Tyrosine hydroxylase, Calpain
1. Introduction
Parkinson’s disease (PD) and Alzheimer’s disease (AD) are progressive neurodegenerative disorders that affect different neuronal populations; AD mainly targets cortical and hippocampal neurons, and PD affects dopaminergic neurons of the substantia nigra leading to loss of dopamine release in the striatum (Dluzen et al., 2001; Fumagalli et al., 2006). 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and its active metabolite 1-methyl-4-phenylpyridinium (MPP+) cause PD-like symptoms in vivo and have been extensively used in rodents and primates to generate animal models of this disease (Langston and Irwin, 1986). This neurotoxin has also been applied to cultured mesencephalic slices to generate an in vitro model of PD (Madsen et al., 2003). At low concentrations, MPP+ has been shown to be specific for dopaminergic neurons because of its uptake by the dopamine transporter, but at higher concentrations it also causes neuronal death in other cell types, including hippocampal neurons, due to inhibition of mitochondrial complex I (Langston and Irwin, 1986).
Deregulation of brain-derived neurotrophic factor (BDNF) production and/or release has been implicated in many neurological and psychiatric disorders, including drug addiction, schizophrenia, PD, and AD. Many of these diseases involve dopaminergic and glutamatergic dysfunction (Harrison and Weinberger, 2005; Kelley, 2004; Toyooka et al., 2002). Although the etiology of PD is not well understood, the disease is primarily characterized by motor dysfunction as a result of decreased dopamine availability and reduced BDNF release in the striatum, both of which contribute to the exacerbation of PD (Kuipers and Bramham, 2006). BDNF has been suggested as possible therapy for PD because it exerts strong trophic effects on dopaminergic neurons, triggers dopamine release, and regulates dopamine receptor expression (for a review, see Fumagalli et al., 2006). Additionally, dopaminergic stimulation increases in vivo BDNF expression (Okazawa et al., 1992). Unfortunately however, systemic or direct in vivo application of BDNF does not provide neuroprotection for dopaminergic neurons (Fumagalli et al., 2006; Spina et al., 1992).
Positive AMPA receptor modulators (PARMs, a.k.a. Ampakines), such as CX614 [2H,3H,6aH-pyrrolidino[2″,1″-3′,2′]1,3-oxazino [6′,5′-5,4]-benzo[e]1,4-dioxan 10-one], constitute a new category of drugs that allosterically modulate AMPA receptors and increase BDNF mRNA and protein levels in vitro and in vivo (Arai and Kessler, 2007; Lauterborn et al., 2003; Lynch, 2007).
Although it is not yet known whether glutamatergic neurotransmission in mesencephalon could directly contribute to dopaminergic neuronal survival, it is plausible that BDNF could indirectly mediate glutamatergic modulation of the dopaminergic system. Accordingly, in the present study we investigated whether upregulation of BDNF levels with PARMs, such as CX614, could protect mesencephalic neurons from MPP+-induced cell death. We also examined whether CX614 was neuroprotective in hippocampal neurons against the same toxin. Our results indicate that CX614 pretreatment is neuroprotective against MPP+-induced toxicity in both cultured hippocampal and mesencephalic slices and that this effect is linked to the ability of CX614 to increase BDNF levels.
2. Methods
2.1. Animals
Neonatal Sprague-Dawley rat pups (Harlan, Indianapolis, IN) were anesthetized with 2-bromo 2-chloro-1,1,1-trifluorethane in a ventilated chamber according to the guidelines of the National Institutes of Health. All efforts were made to minimize animal distress and to reduce the numbers of animals used in this study.
2.2. Preparation of cultured hippocampal slices
Cultured hippocampal slices from 9 to 11 day postnatal rat pups were prepared as previously described (Jourdi et al., 2005a,b). Briefly, animals were decapitated and tissue containing hippocampus as well as parts of the entorhinal cortex and subiculum were bilaterally dissected and cut into 400 μm-thick slices using a McIllwain tissue slicer (Mickle Laboratory Co. Ltd., Guildford, UK). Slices from 4 to 6 pups were mixed and randomly explanted onto Millicel-CM membrane inserts (Millipore, Bedford, MA) at a density of 5 slices per insert. Inserts were placed in Falcon six-well culture plates (BD Biosciences, San Jose, CA) containing sterile medium (1 ml/well) VEM consisting of 50% basal Eagle’s medium, 25% Earl’s balanced salt solution, 136 mM NaCl, 2 mM CaCl2, 2.5 mM MgSO4, 5 mM NaHCO3, 3 mM glutamine, 40 mM glucose, 0.5 mM ascorbic acid, 20 mM acid-free HEPES buffer, 1 mg/l insulin, 10 U/ml penicillin, and 10 mg/l streptomycin (pH 7.3) and supplemented with 20% horse serum (all reagents from Sigma, St. Louis, MO). Cultures were maintained in a humidified incubator at 35 °C and with 5% CO2 for a minimum of 10 days and a maximum of 14 days before initiation of experimental procedures. Fresh medium changes were made thrice weekly.
2.3. Preparation of cultured mesencephalic slices
Slices containing the substantia nigra from the ventral mesencephalon were prepared with modifications to a previously described method (Madsen et al., 2003). Briefly, postnatal day 3 rat pups were decapitated and the brains removed and sagitally cut into two hemispheres. The cerebral cortex was dissected away and meninges were cleared from the midbrain and brainstem. Coronal slices (350 μm) were prepared with a McIllwain tissue slicer. Five slices per hemisphere centered around the substantia nigra were explanted on Millicel-CM biomembrane inserts (Millipore), maintained in six-well culture plates (BD Biosciences) containing the same medium (20% HS/VEM) used for hippocampal slices, and kept at 35 °C in a 5% CO2 humidified incubator for 5–7 days with three medium changes per week. Slices were then switched to serum-free Neurobasal Medium supplemented with B-27 (B27-NBM) and 0.5 μM glutamate (all reagents from Invitrogen) and kept in the same incubation conditions for an additional 5–7 day period prior to initiation of treatments.
2.4. Drugs and treatments
The positive AMPA receptor modulator CX614 (a generous gift from Cortex Pharmaceuticals, Irvine, CA) was used in this study because it is one of the best characterized PARMs in terms of its ability to upregulate BDNF in vivo and in vitro (Lauterborn et al., 2003). CX614 is highly selective for AMPA receptors and its effects on BDNF are not prevented by blockers of other types of glutamate receptors (Arai and Kessler, 2007; Hennegriff et al., 1997; Jourdi et al., 2005a,b; Lynch, 2007; Xia et al., 2005). CX614 was diluted 1:1000 from a 50 mM stock solution prepared in DMSO into 20% HS/VEM medium for cultured hippocampal slices and into B27-NBM for cultured mesencephalic slices. The former were treated for 6 h (unless otherwise noted). At the end of incubation, hippocampal slices were transferred to fresh medium (20% HS/VEM). Cultured mesencephalic slices were incubated with CX614 for 24 h after which the medium was replaced with fresh B27-NBM. Toxicity was induced with the addition of MPP+ (Sigma, St. Louis, MO). Fresh MPP+ was prepared in purified water as a 1000 × stock solution (250 mM) and filter-purified before use in tissue cultures. Preliminary experiments were conducted to determine optimal treatment protocols (time and concentrations of MPP+ and CX614) in OMSC and OHSC. The criteria used to select concentration and duration of treatments consisted of: 1) Incubation time with CX614 that produced significant BDNF upregulation; 2) MPP+ concentration and duration of treatment that caused toxicity by itself but did not induce irreversible damage to cultured slices; and 3) Time between CX614 and MPP+ treatments to prevent possible direct interactions between CX614 and MPP+. This third criterion was important to satisfy in light of our recent findings that acute application of CX614 increases BDNF release without increasing total BDNF expression (H. Jourdi and M. Baudry, unpublished results).
For hippocampal slices, MPP+ was diluted in serum-free VEM and the treatment was carried over 24 h in a humidified incubator (35 °C; 5% CO2) (final concentration 250 μM). For mesencephalic slices, MPP+ was dissolved in B27-NBM and treatment (20 μM, final concentration) was carried over 48 h in a humidified incubator (35 °C; 5% CO2). Samples were collected at the end of each experiment as indicated in Section 3. In experiments that used the tyrosine kinase inhibitor K252a {(9S,10R,12R)-2,3,9,10,11,12-Hexahydro-10-hydroxy-9-methyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3′,2′,1′-kl]pyrrolo[3,4-i][1,6]benzodiazocine-10-carboxylic acid methyl ester} (0.5 μM; Biosource, Camarillo, CA) or the BDNF scavenger TrkB-Fc (extracellular domain of human TrkB fused to the C-terminal histidine-tagged Fc region of human IgG1) (0.3 μg/ml; Sigma, St. Louis, MO), the medium containing these drugs was added 30–60 min before CX614 pretreatment and these compounds were maintained through every medium change until samples were collected.
2.5. Lactate dehydrogenase assay
Measurements of lactate dehydrogenase (LDH) released into the culture medium after MPP+ treatment were used as a measure of cellular damage as previously described (Jourdi et al., 2005a,b; Zhou and Baudry, 2006). Briefly, rate of nicotinamide adenine dinucleotide (NADH) depletion was measured using a SpectraMax Plus with data collected and analyzed by SOFTmax Pro software (Molecular Devices, Sunnyvale, CA). Each reaction consisted of 0.6 ml 0.1 M potassium phosphate buffer (10% KH2PO4 mixed with 10% K2HPO4; pH 7.5) and 0.4 ml sample medium. After 20 min, 0.5 ml of freshly made solution of sodium pyruvate and NADH solutions (20% NADH in 10% sodium pyruvate) was added to this solution and immediately followed by measuring absorbance at 340 nm (all reagents from Sigma). LDH activity was measured as units/ml, with one unit of activity representing the amount of LDH that causes a decrease of 0.001 absorbance unit of NADH per minute in the presence of sodium pyruvate. LDH release was then normalized to cultured tissue protein concentration and results were expressed as percent of controls.
2.6. Western blotting
Slices were collected into cold homogenizing buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 5 mM EDTA, 1 mM EGTA, 1 mM PMSF, 10 mM NaF, and 1 mM Na3VO4; all from Sigma–Aldrich, St. Louis, MO) supplemented with protease inhibitor cocktail [4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride, 2.08 μM; aprotinin, 1.6 μM; leupeptin, 40 μM; bestatin, 80 μM; pepstatin A, 30 μM; and trans-3-carboxyoxirane-2-carbonyl-l-leucylagmatine, 28 μM] (Sigma–Aldrich), sonicated five times using a Sonic Dismembrator (Fisher Scientific, Pittsburg, PA) set at 50% for 4- to 7-s intervals, and protein concentrations were determined. Equal amounts (20–40 μg) of sample proteins were separated according to their molecular weight on 8-10% SDS polyacrylamide gels and transferred onto polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA). After transfer, membranes were blocked with 5% milk in TBS-T (137 mM NaCl, 20 mM Tris base, and 0.1% Tween 20; pH 7.4) for 1.5 h. Membranes were probed with the primary antibody diluted with 5% milk (1:1000) and incubated overnight at 4 °C. Membranes were then washed with TBS-T and incubated with horseradish peroxidase-conjugated secondary antibodies (Amersham Biosciences Inc., Piscataway, NJ) diluted (1:10,000) with TBS-T for 1 h followed by washing in TBS-T. Membranes were exposed to enhanced chemiluminescence western blotting detection reagent (Amersham Biosciences Inc.) for 2 min, placed in an autoradiography cassette, and exposed to Hyperfilm (Amersham Biosciences Inc.) for different lengths of time. All membranes were stripped and reprobed with anti-actin antibodies (Sigma–Aldrich) for loading controls. Intensities of immunoreactive bands were quantified by densitometry, normalized to actin content, and data expressed as percent of controls.
2.7. Antibodies
The following primary antibodies were used at the indicated final concentrations: monoclonal mouse anti-actin (0.1 μg/ml; Sigma, St. Louis, MO), monoclonal mouse anti-spectrin (0.1 μg/ml; Millipore, Temecula, CA), polyclonal rabbit anti-BDNF (N-20 antibody) (0.5 μg/ml; Santa Cruz Biotech., Santa Cruz, CA), monoclonal mouse tyrosine hydroxylase (Millipore, Temecula, CA). Horseradish peroxidase-conjugated secondary antibodies were used at 1:10,000 dilution (Amersham Biosciences Inc).
2.8. Enzyme-linked immunosorbent assay
Hippocampal and mesencephalic slices were collected into cold homogenizing buffer (20 mM Tris pH 7.5; 137 mM NaCl,1% NP-40, 10% glycerol,1 mM PMSF, 0.5 mM Na3VO4; all from Sigma, St. Louis, MO) supplemented with the same protease inhibitor cocktail indicated earlier (Sigma–Aldrich), sonicated five times using a Sonic Dismembrator (Fischer Scientific) for 3–5 s intervals on ice, and protein concentrations were determined. BDNF levels were determined using BDNF EMAX ImmunoAssay System (Promega, Madison, WI) following kit instructions.
2.9. Propidium iodide staining and fluorescence microscopy
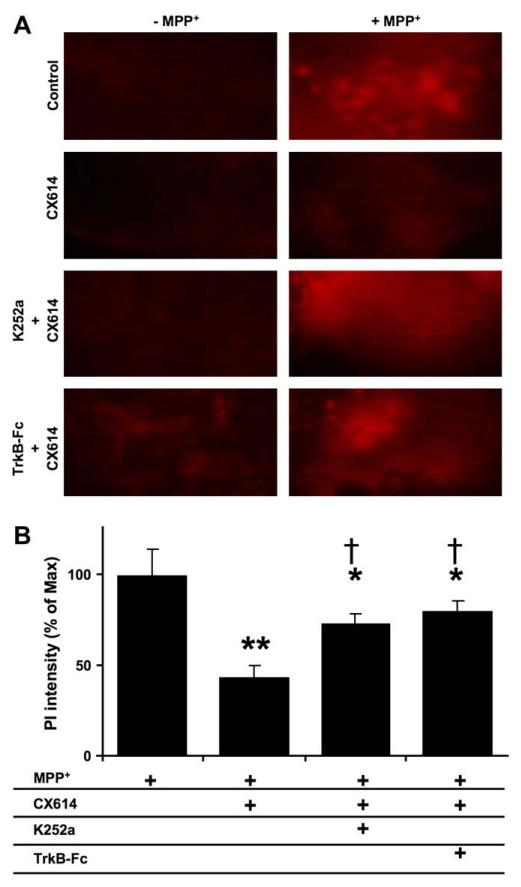
Propidium iodide (PI; 3 μM) (Calbiochem, San Diego, CA) was added during the last 24 h of treatment (i.e. preceding tissue collection). At the end of treatment, PI-stained cultured mesencephalic slices were imaged using an inverted Leica DMIRB epifluorescence microscope fitted with a 5 × phase contrast objective and a Spot RT slider color CCD camera (Roper Scientific, Tucson, AZ) with a 550LP filter. At this magnification, one picture included an entire mesencephalic slice. Viewed images were captured using Photoshop CS (Adobe Systems, San Jose, CA). For best intensity of images and to avoid saturation, all slices were exposed for 50 ms, and the camera gain was kept constant throughout each experiment. A threshold was applied to eliminate background fluorescence uniformly in all images. Fluorescence intensity was then estimated by the following method: first, images were adjusted to gray levels using Adobe Photoshop with the background of images in black and PI-stained structures in white; second, modified images were analyzed quantitatively by densitometry with ImageJ software. Finally, PI staining data were averaged for each condition and the means ± SEM values were expressed as percent of maximal PI staining (found in MPP+-treated slices) from the indicated number of independent experiments (Fig. 6B). Examples of raw PI imaging results were also shown (Fig. 6A).
Fig. 6.
Effects of TrkB-Fc and K252a on CX614-mediated protection against MPP+-induced toxicity in cultured mesencephalic slices. Slices were treated as in Fig. 5A, except that K252a (0.5 μM) of TrkB-Fc (0.3 μg/ml) treatments were initiated 30 min and 1 h, respectively, before CX614 (50 μM). Propidium iodide was added during the last day of the experiment. A. Fluorescent photomicrographs of representative fields of the substantia nigra from mesencephalic slices treated as indicated. Images were taken within 30 min of each other at the termination of all treatments. B. Quantification of data from the indicated experimental conditions (see Section2). Results are expressed as percent of maximum from 2 independent experiments with 8–12 mesencephalic slices in each group, and are means ± SEM. The results indicate significant differences between MPP+- and CX614 + MPP+-treated slices (**, p < 0.01; unpaired Student’s t-test) and between MPP+-, and CX614 + K252a + MPP+- or CX614 + TrkB-Fc + MPP+-treated cultures (*, p < 0.05; unpaired Student’s t-test). Results from CX614 + MPP+-treated cultures are also significantly different from CX614 + K252a + MPP+- and CX614 + TrkB-Fc + MPP+-treated cultures (†, p < 0.05; unpaired Student’s t-test).
3. Results
3.1. Treatment of cultured hippocampal slices with CX614 protects against MPP+-induced toxicity
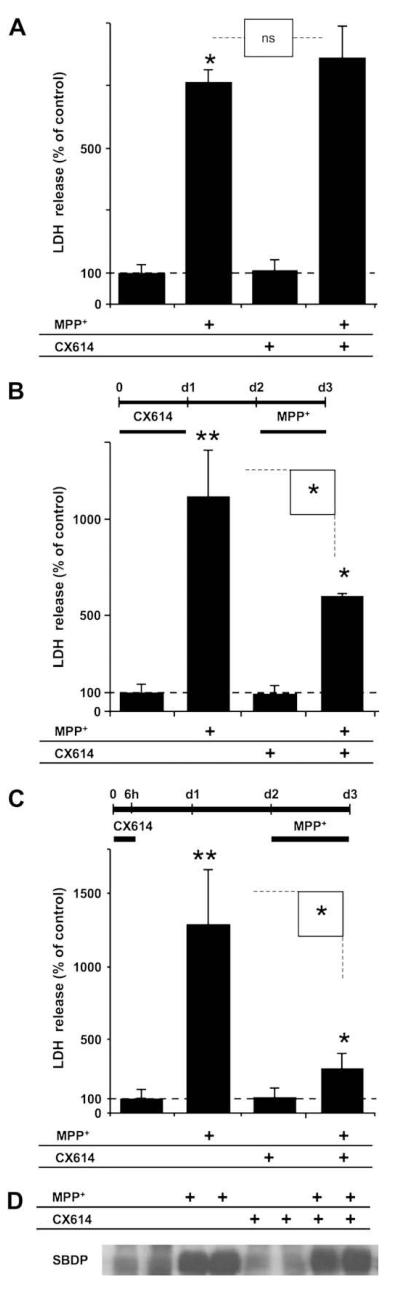
Cultured hippocampal slices were initially simultaneously treated for 24 h with CX614 (50 μM) and MPP+ (250 μM). At the end of treatment, culture medium was analyzed for lactate dehydrogenase (LDH) content as an indicator of cell membrane integrity following MPP+-induced toxicity (see Section 2). Co-treatment for 24 h did not provide protection against MPP+-induced toxicity (Fig. 1A). In fact, LDH release was not significantly different in samples that received CX614 and MPP+ together compared to MPP+ alone. These results indicated that CX614 could not provide protection from cell injury when applied simultaneously with the neurotoxin. Similar to our previous findings, treatment of cultured hippocampal slices with CX614 alone did not increase LDH release, indicating that CX614 was not inherently toxic (Jourdi et al., 2005a,b). We then determined whether pretreatment with CX614 could protect OHSC against MPP+-induced toxicity, and applied CX614 (50 μM) to cultured hippocampal slices for 6 h or for 24 h (Fig. 1B and C). At the end of CX614 treatment, slices were transferred to fresh medium and kept for 24–42 h. This was followed by incubation with MPP+ for one day. At the end of MPP+ treatment, slices and media were collected and analyzed for accumulation of spectrin breakdown products (SBDP; see below) and LDH release, respectively. These pretreatment protocols with CX614 resulted in 77% (Fig. 1C) and 50% (Fig. 1B) reduction in LDH release, respectively, when compared to slices treated with MPP+ alone. As the 6 h abbreviated pretreatment yielded highly significant decline in LDH release, this protocol was used in the remaining experiments for cultured hippocampal slices.
Fig. 1.
Effects of co-treatment or pre-treatment with CX614 on MPP+-induced LDH release and calpain activation in cultured hippocampal slices. A. Cultured hippocampal slices were treated simultaneously for 24 h with CX614 (50 μM) and MPP+ (250 μM). At the end of treatment, LDH release in the medium was assessed as described in Section2. B. Cultured hippocampal slices were pretreated with CX614 (50 μM) for 24 h (d1), transferred to CX614-free fresh medium for 1 day (d2) before being treated with MPP+ (250 μM) for 1 day (d3). Medium was collected at the end of treatment (d3) and LDH release in the medium was assessed. C. Treatment of cultured hippocampal slices was similar to (B) except that slices were pretreated with CX614 (50 μM) for 6 h only and incubated in CX614-free medium for 42 h before treatment with MPP+. D. Total protein samples from slices treated as in “C” were analyzed by western blotting for accumulation of spectrin breakdown products (SBDP) as a marker of calpain activation. Results are expressed as percent of control and are means ± SEM of 5 experiments with 3–6 samples per condition. Statistical significance was calculated with ANOVA followed by Tukey’s test; *, p < 0.05; **, p < 0.01; ns: not significant.
Activation of calcium-dependent cysteine proteases, a.k.a. calpains, has previously been implicated in MPP+-induced neuronal cell death. Spectrin is a cytoskeletal protein and one of the best characterized calpain substrates. Accumulation of calpain-generated spectrin breakdown products (SBDP; 145-150 kDa doublet bands) has been extensively used as a marker of calpain activation (Jourdi et al., 2005a,b). Accordingly, protein samples obtained from hippocampal slices treated as in Fig. 1C were analyzed by western blotting to determine SBDP accumulation (Fig. 1D). MPP+ treatment of slices resulted in calpain activation as evidenced by SBDP accumulation. However, calpain-mediated spectrin degradation was not reduced by preincubation of slices with CX614, indicating that calpain activation was independent from LDH release.
3.2. CX614-mediated neuroprotection against MPP+ in cultured hippocampal slices depends on brain-derived neurotrophic factor (BDNF)
The previous results suggested that CX614-dependent reduced LDH release required a mediator that was not available in the initial co-treatment protocol. As CX614 can increase mRNA and protein levels of BDNF, a survival-promoting factor, we evaluated the requirement of CX614-induced increase in BDNF for protection against MPP+-induced toxicity. To test this idea, cultured hippocampal slices were treated with K252a, an inhibitor of the BDNF receptor TrkB, or with TrkB-Fc, a scavenger of secreted BDNF. TrkB-Fc binds free BDNF with high affinity and prohibits it from binding to endogenous TrkB receptors expressed on neuronal cell surface.
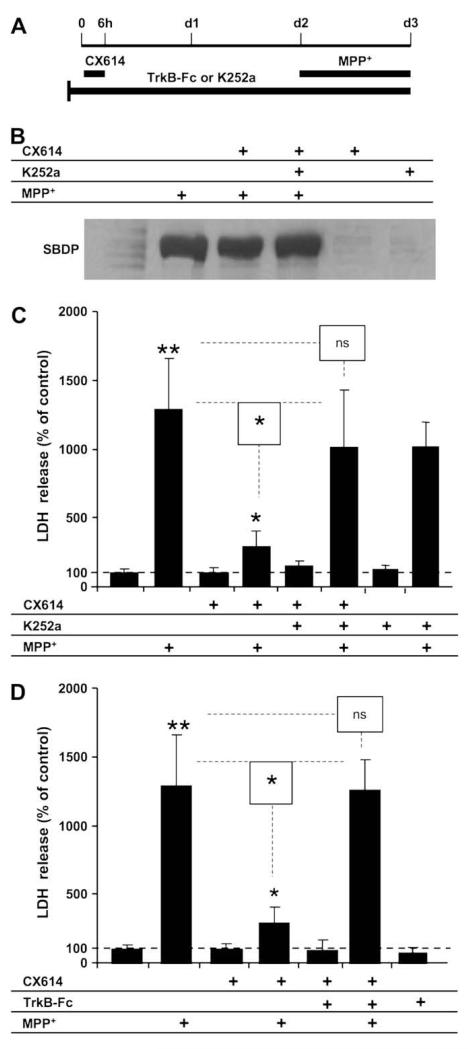
Slices were treated with CX614 (50 μM; 6 h), transferred to fresh CX614-free medium for 42 h and this was followed by incubation with the neurotoxin for 1 more day (Fig. 2A). K252a (0.3 μM) (Fig. 2B and C) or TrkB-Fc (0.5 μg/ml) (Fig. 2D) were applied throughout the experiment (Fig. 2A) and spectrin degradation (Fig. 2B) and LDH release (Fig. 2C and D) were measured. MPP+ was applied during the last day of culture and significantly increased SBDP accumulation compared to control. Treatment with K252a alone did not affect SBDP accumulation compared to control. In addition, pretreatment with CX614 or co-application of CX614 together with K252a did not reduce MPP+-induced accumulation of SBDP, again indicating that calpain activation was independent from LDH release (Fig. 2B).
Fig. 2.
Effects of K252a or TrkB-Fc and CX614 on MPP+-induced calpain activation and LDH release in cultured hippocampal slices. A. All slices were treated according to the protocol shown in the diagram; K252a (0.5 μM) or TrkB-Fc (0.3 μg/ml) treatment was initiated 30 min and 1 h, respectively, before CX614 addition (50 μM; 6 h). Slices were then transferred to CX614-free medium containing K252a or TrkB-Fc. MPP+ (250 μM) was added 42 h later and kept for 1 day (d3). SBDP accumulation was examined by western blotting as an indicator of calpain activation (B). LDH release into the medium (C,D), collected at the end of all treatments, was measured as described in Section2. B. MPP+-induced SBDP accumulation was not reduced by pretreatment with CX614 alone or CX614 + K252a. K252a and CX614 alone did not increase SBDP accumulation. C. K252a treatment eliminated CX614-mediated reduction of MPP+-induced increase in LDH release. D. TrkB-Fc treatment eliminated CX614-mediated reduction of MPP+-induced increase in LDH release. Results are expressed as percent of control and are means ± SEM of 3 experiments with 3–4 samples per condition. Statistical significance was calculated with ANOVA followed by Tukey’s test; *, p < 0.05; **, p < 0.01; ns, not significant.
Although continuous application of K252a alone did not affect LDH release, the protective effects of CX614 pretreatment were abolished when K252a was applied together with CX614 (Fig. 2C). Similarly, TrkB-Fc treatment did not affect LDH release compared to control cultures. However, co-application of TrkB-Fc with CX614 completely blocked CX614-mediated reduction of released LDH activity (Fig. 2D).
3.3. Calpain inhibition prevents CX614-mediated neuroprotection against MPP+-induced toxicity in cultured hippocampal slices
A previous report indicated that calpain inhibition was neuroprotective against MPP+-induced toxicity (Choi et al., 2001). Although our results indicated that calpain was activated following MPP+ treatment, they also indicated that pretreatment with CX614 did not block calpain activation (Figs. 1 and 2). As such, calpain activity was not necessarily linked with neurotoxicity.
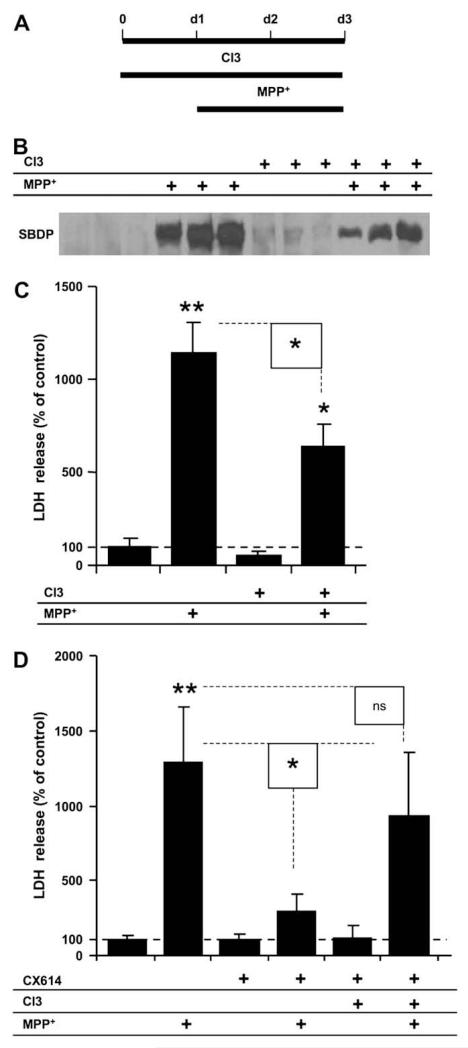
To better understand the role of calpain in MPP+-induced toxicity and its interaction with CX614 treatment, we compared LDH release (Fig. 3C) and SBDP accumulation (Fig. 3B) in cultured slices treated with MPP+ alone, calpain inhibitor III alone, or with both; we treated slices with calpain inhibitor III for 1 day before the addition of MPP+ and kept it throughout the treatment (Fig. 3A). SBDP accumulation was only partially reduced in slices treated with MPP+ in the presence of calpain inhibitor III (Fig. 3B). Similarly, LDH release results from cultured hippocampal slices treated with calpain inhibitor III with MPP+ indicated significant, but partial, reduction of LDH release compared to cultures treated with MPP+ alone (Fig. 3C).
Fig. 3.
Effects of calpain inhibition and CX614 on MPP+ toxicity in cultured hippocampal slices. A. Schematic representation of the protocol used in this experiment; Calpain Inhibitor III (CI3; 10 μM) was added in the culture medium 1 day (d1) before addition of MPP+ (250 μM), which was kept for 2 days (d3). At the end of treatment (d3), medium was collected for LDH analysis (C) and slices were collected for SBDP analysis (B). B. Western blot showing SBDP accumulation from three independent samples from each experimental condition. C. LDH activity in medium collected at the end of treatment (d3). D. CX614 (50 μM) was added at the same time as CI3 and hippocampal slices were treated as in (A) up to day 3 and medium was collected at the end of treatment for LDH analysis as indicated in Section2. Results are expressed as percent of control and are means ± SEM of 4 experiments with 3–6 samples per condition. Statistical significance was calculated with ANOVA followed by Tukey’s test; *, p < 0.05; **, p < 0.01; ns, not significant.
In order to investigate whether calpain was involved in CX614-mediated neuroprotection against MPP+-induced toxicity, slices were pretreated with CX614 together with calpain inhibitor III 1 day before MPP+ application (Fig. 3D). Medium was collected and LDH levels were assessed at the end of treatment. Surprisingly, co-application of calpain inhibitor III with CX614 abolished the protective effects of CX614 against MPP+ toxicity.
3.4. CX614 treatment of cultured hippocampal and mesencephalic slices increases their BDNF content
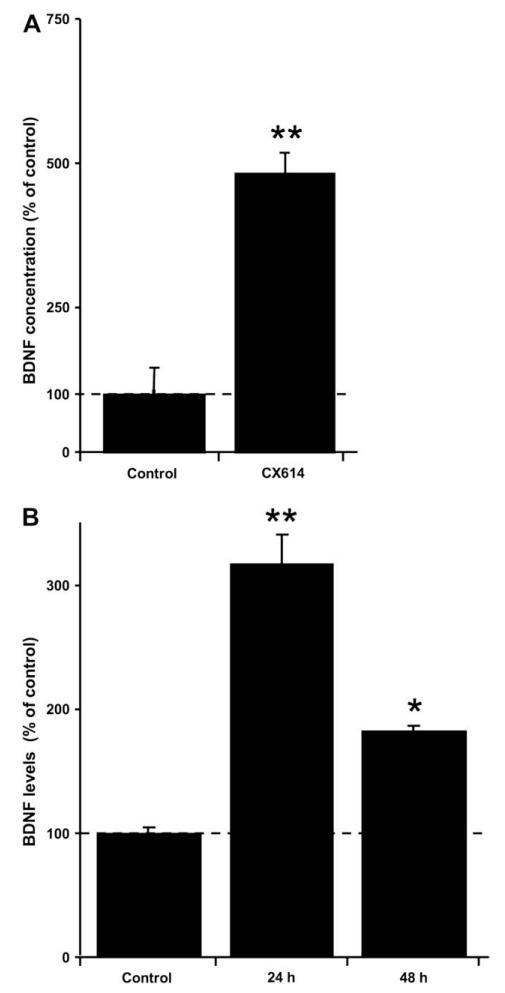
Since our current results indicated that the protective effects of CX614 were eliminated in the presence of TrkB-Fc and K252a, we first verified that CX614 could increase BDNF protein expression under our experimental conditions. When cultured hippocampal slices were incubated with CX614 for 24 h, BDNF protein levels measured by ELISA were increased 5 fold as compared to control (Fig. 4A).
Fig. 4.
Effects of CX614 treatment on BDNF levels in cultured hippocampal and mesencephalic slices. CX614 (50 μM) treatment of cultured hippocampal (A) and mesencephalic (B) slices was carried out for 6 h and 1 day, respectively. Slices were then transferred to and maintained in fresh medium for 1 day for hippocampal cultures and for 1 and 2 day(s) for mesencephalic cultures. BDNF tissue content was analyzed as indicated in Section 2. Results are expressed as percent of control and are means ± SEM of 6–8 experiments. Statistics calculated by unpaired Student’s t-test; *, p < 0.05; **, p < 0.01.
In order to investigate whether CX614 could also be protective in an in vitro model of Parkinson’s disease, we confirmed that BDNF levels could also be increased by CX614 in cultured mesencephalic slices. Preliminary experiments were conducted to determine the treatment protocol for maximal increase in BDNF levels following CX614 treatment. CX614 treatment was carried over various periods of time (1, 3, 6, 12, and 24 h) and the data indicated that 24 h incubation with CX614 (50 μM) resulted in maximal BDNF expression (data not shown). Slices were then incubated in CX614-free medium for 1 or 2 additional days, collected, and analyzed for BDNF content using western blotting (Fig. 4B). Western blotting was used for determination of BDNF contents in cultured mesencephalic slices because it requires less total protein amount per sample than ELISA. BDNF levels were the highest (300% of control) 1 day following the end of treatment of slices with CX614 for 24 h. In addition, 2 days after the end of CX614 treatment, BDNF levels were still significantly increased compared to control, suggesting that CX614 treatment results in a sustained increase of BDNF levels in cultured mesencephalic slices, similar to what has been reported for cultured hippocampal slices (Lauterborn et al., 2003).
3.5. CX614 pretreatment of cultured mesencephalic slices protects against MPP+ toxicity
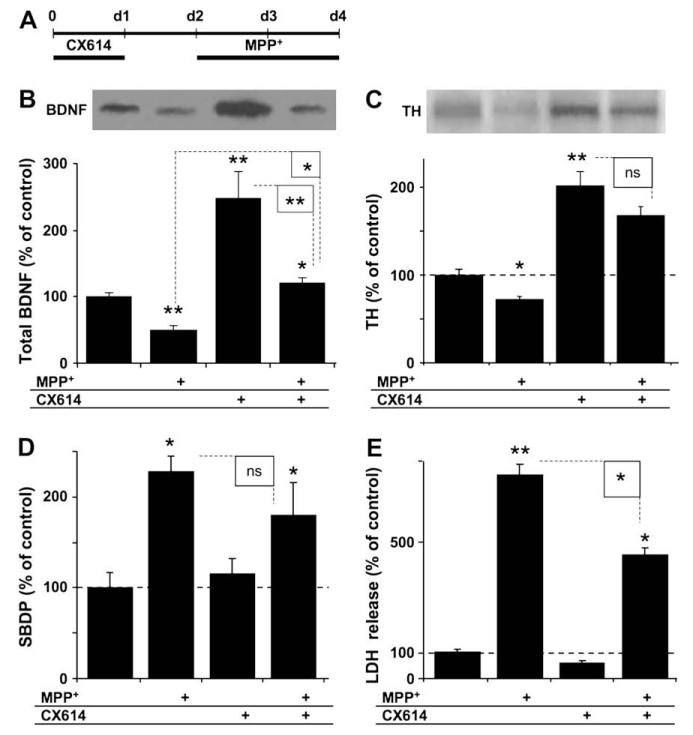
Cultured mesencephalic slices were treated with CX614 for 1 day, transferred to fresh medium for 1 day, and then incubated with MPP+ (20 μM) for 2 more days (Fig. 5A). At the end of treatment, slices were collected and analyzed for BDNF, tyrosine hydroxylase (TH), and SBDP contents (Fig. 5B–D) using western blotting. BDNF levels were decreased in cultured mesencephalic slices following MPP+ treatment in the absence of CX614 treatment (Fig. 5B). BDNF levels in cultures that were pretreated with CX614 and then treated with MPP+ were lower than in slices treated with CX614 (alone) and similar to those treated with the vehicle control. Tyrosine hydroxylase (TH) is a specific marker of dopaminergic neurons and its level of expression has been repeatedly used as an index of survival of dopaminergic cells. Accordingly, we estimated TH levels in the same samples and the results indicated significantly reduced TH levels following MPP+ (alone) treatment (Fig. 5C); TH levels were however significantly increased with CX614 treatment compared to vehicle-treated controls. Slices that received CX614 and then MPP+ had significantly higher TH levels, which were not statistically different from those found in CX614-treated slices. In addition, MPP+ treatment significantly increased SBDP levels while pretreatment with CX614 resulted in a slight but insignificant reduction of MPP+-induced calpain-mediated spectrin degradation (Fig. 5D). Slice media collected at the end of these experiments were analyzed for LDH activity (Fig. 5E). As was found in cultured hippocampal slices (Fig. 2), treatment of cultured mesencephalic slices with CX614 significantly (45%) reduced MPP+-induced LDH release, indicating that CX614 pretreatment was also protective against MPP+-induced toxicity.
Fig. 5.
Effects of CX614 on BDNF, TH and SBDP levels and LDH release following MPP+ treatment of cultured mesencephalic slices. A. All cultures were treated as in the displayed diagram; slices were pretreated with CX614 (50 μM, 1 day), transferred to fresh medium for 1 day, and then treated with MPP+ (20 μM) for 2 days. Tissues and media were collected for western blot analysis of BDNF (B), TH (C), and SBDP (D) contents and for LDH activity (E), respectively. B. BDNF western blot (upper panel) and quantification (lower panel) of total BDNF levels. C. Tyrosine hydroxylase (TH) western blot (upper panel) and quantification (lower panel) of total TH levels. D. Quantification of SBDP accumulation. E. LDH activity in media collected at the end of treatment (d4). Results are expressed as percent of control and are means ± SEM of 4 (B–D) and 6 (E) independent experiments. Statistics calculated with unpaired Student’s t-test (B–D) and with ANOVA followed by Tukey’s test (E); *, p < 0.05; **, p < 0.01; ns, not significant.
3.6. Blockade of CX614-mediated protection against MPP+-induced toxicity in cultured mesencephalic slices by a tyrosine kinase inhibitor and a BDNF scavenger
Cultured mesencephalic slices were treated with CX614 (50 μM) for 1 day, transferred to CX614-free medium for 1 day and then incubated with MPP+ for 2 days. As for cultured hippocampal slices, K252a (0.5 μM) and TrkB-Fc (0.3 μg/ml) were included in the culture medium throughout the experiment. During the last day in culture, propidium iodide was added to the culture medium to allow fluorescent microscopic visualization of dead cells. At the end of treatment, slices were photographed and data analyzed as indicated in Section 2. Incubation of cultured mesencephalic slices with MPP+ significantly increased PI staining while CX614 pretreatment reduced MPP+-induced PI increase (Fig. 6). In addition, untreated control slices and slices treated with CX614 alone, CX614 + K252a, or CX614 + TrkB-Fc had very little or no propidium iodide staining (Fig. 6A). Treatment with K252a or TrkB-Fc eliminated CX614-mediated reduction of MPP+-induced PI staining. Because PI staining was very low or undetectable in control, CX614 alone, CX614 + K252a, and in CX614 + TrkB-Fc conditions, the results from the remaining conditions were quantified and calculated (see Section 2) as percent of maximum PI staining observed in the MPP+ alone condition (Fig. 6B). PI staining in CX614, CX614 + K252a, or CX614 + TrkB-Fc conditions was not significantly different from values found in the vehicle control (unpaired Student’s t-test, p > 0.1 for all 3 conditions as compared to control). Quantification of the data confirmed the visual examination and indicated that both K252a and TrkB-Fc significantly reduced CX614-mediated protection against MPP+ toxicity (Fig. 6B).
4. Discussion
Our results indicate that ampakine pretreatment, but not co-treatment, significantly reduces MPP+-induced neurotoxicity in both cultured hippocampal and mesencephalic slices. The results also indicated that timing of treatment was critical for protection by CX614: co-incubation of CX614 with MPP+ failed to induce neuroprotection as assessed with LDH levels. While CX614 pretreatment resulted in neuroprotection in both types of slices, the length of CX614 pretreatment varied for slices from both regions. The requirement for CX614 pretreatment in both types of cultured slices suggests that protection was mediated by modulating the expression of some intermediate molecule(s). In agreement with previous studies, we found that CX614 increases BDNF levels in cultured hippocampal slices (Lauterborn et al., 2003). In addition, we observed that CX614 had similar effects in cultured mesencephalic slices, maintaining significantly elevated BDNF levels for 48 h. Moreover, TrkB-Fc and K252a, two compounds that alter BDNF availability and action respectively, eliminated CX614-induced protection in both types of cultures. These results indicate that CX614 treatment leads to upregulation of BDNF levels followed by its release and the subsequent activation of TrkB receptors. It is possible that AMPA receptors could signal through the tyrosine kinase Lyn and the mitogen-activated protein kinase (MAPK) pathway to increase BDNF expression (Hayashi et al., 1999). Alternatively, transactivation of TrkB following PARM treatment could mediate CX614-induced neuroprotection through the Fyn tyrosine kinase pathway (Rajagopal and Chao, 2006). Although our data with the TrkB-Fc reagent argue against this possibility, a partial contribution of TrkB transactivation to neuroprotective signaling cannot be completely ruled out. Future studies addressing TrkB transactivation and kinase signaling downstream of AMPA receptors should consolidate the role played by BDNF and other molecules, such as estrogen, in preventing dopaminergic and glutamatergic neuronal death in PD and in AD, respectively (Lee and Chao, 2001; Scharfman and MacLusky, 2006).
Cultured mesencephalic slices have presumably a lower relative density of glutamatergic neurons compared to cultured hippocampal slices. The CX614 pretreatment protocol we used was selected because it induced a robust BDNF protein upregulation (2-3 fold) (Figs. 4 and 5). As AMPA receptors are present on dopaminergic neurons in the substantia nigra (Blythe et al., 2007), it is possible that BDNF upregulation takes place in dopaminergic neurons. Future studies should use double immunostaining techniques to address this issue. Moreover, the relative lag time in BDNF protein expression observed in cultured mesencephalic as compared to hippocampal slices may be due to the differential action of CX614 on various subunits of AMPA receptors expressed in these two neuronal tissues (Chatha et al., 2000; Chen et al., 2001; O’Neill et al., 2005; Xia et al., 2005). In any event, our data validate the use of ampakines to upregulate BDNF and to provide neuroprotection in two models of neurotoxicity, including an in vitro model for PD.
Previous studies have also demonstrated dopaminergic neuronal protection against 6-hydroxy-dopamine and MPTP toxicity by BDNF and other modulators of AMPA receptors (LY404187 and LY503430) (Spina et al., 1992; Murray et al., 2003; O’Neill et al., 2004) in vitro as well as in vivo. Although O’Neill et al. (2004) postulated that in vivo LY404187-mediated neuroprotection might be due to a trophic factor, their results did not provide direct evidence to support that BDNF was involved in neuroprotection. In other reports, the same group showed only a mild increase in BDNF levels with LY503430 treatment (Murray et al., 2003; O’Neill et al., 2005). Our data clearly indicate notonly that CX614 pretreatment robustly upregulates (2–3 fold) BDNF levels in cultured mesencephalic slices, and provide conclusive evidence that CX614 neuroprotective properties are due to its ability to upregulate BDNF expression.
Interestingly, 6 h pretreatment of cultured hippocampal slices with CX614 was more protective than 24 h CX614 treatment (Fig. 1B and C). While this could suggest that prolonged treatment with CX614 could be toxic, our current findings as well as previously published results argue against this possibility. For example, LDH release in (CX614 + MPP+) and (MPP+ alone) conditions was not statistically different from each others (Fig. 1A). This was also well indicated by the results shown in Fig. 1A and B, since incubation of cultured slices with CX614 for 24 h did not alter LDH levels compared to the vehicle control. Moreover, continuous incubation of cultured hippocampal slices with CX614 for 48 h did not alter LDH levels in the supernatant culture medium as compared to the vehicle control (Jourdi et al., 2005a,b). The reduced neuroprotective efficacy of 24 h CX614 pretreatment could however be explained by our previously published results (Jourdi et al., 2005a,b); incubation of cultured slices with CX614 for 8 h or longer resulted in reduced AMPA receptor levels. This loss of AMPA receptors was found to be reversible, further indicating that slice condition was not compromised because of CX614 treatment. Therefore, it is likely that down-regulation of AMPA receptor expression caused by prolonged exposure of cultured slices to CX614 contributes to the lower efficacy of CX614 in inducing neuroprotection rather than to CX614 being inherently neurotoxic.
Furthermore, BDNF secretion was required to elicit protection in both types of cultured slices since the cell membrane-impermeable BDNF scavenger TrkB-Fc counteracted CX614-mediated protective effects. Whether this effect involves the regulated or constitutive BDNF secretory pathway (del Toro et al., 2006) will be evaluated in future studies.
In cultured mesencephalic slices, MPP+ was applied at a low concentration (20 μM) that primarily affects dopaminergic neurons and CX614 pretreatment was terminated 1 day before the start of prolonged (2 day) application of MPP+. This treatment protocol could still elicit neuroprotection (Fig. 5E) and maintain high TH expression levels (Fig. 5C) despite a relatively lower (close to vehicle-control) BDNF expression levels in CX614 + MPP+-treated slices (Fig. 5B). This apparent discrepancy between TH and BDNF expression levels could be due to a sustained increase in TH expression following the initial BDNF upregulation (Fumagalli et al., 2006; Kuipers and Bramham, 2006). Alternatively, CX614-dependent upregulation of BDNF (Lauterborn et al., 2003) could upregulate dopamine levels, which might lead to TrkB receptor transactivation (Iwakura et al., 2008), thus providing better neuroprotection from MPP+-induced toxicity and sustained upregulation of TH expression. Future studies should determine whether CX614 pretreatment could elicit BDNF-dependent dopamine upregulation and/or dopamine receptor-mediated transactivation of TrkB in mesencephalic slices and whether these effects are involved in protection of dopaminergic neurons from cell death.
Activation of calpains, a family of Ca2+-activated proteases, has been implicated in various models of neuronal cell death including in vitro and in vivo models of PD (Choi et al., 2001; Tsuji and Shimohama, 2002; Crocker et al., 2003; Higuchi et al., 2005). For instance, post-mortem tissues from various brain areas of AD and PD patients exhibit increased accumulation of calpain-truncated breakdown products of the cytoskeletal protein spectrin (SBDP), one of the best characterized calpain substrates (Jourdi et al., 2005a,b, and references therein). In agreement with previous reports, our results indicate that calpain is activated in both types of cultured slices following MPP+ treatment (Figs. 3B and 5C) and that calpain inhibition provides a small degree of protection against MPP+-induced toxicity, at least in cultured hippocampal slices (Fig. 3C).
We hypothesized that co-application of calpain inhibitor III with CX614 might have synergistic effects and lead to better protection against MPP+ toxicity. Surprisingly however, our results indicated that rather than providing additional protection from MPP+-induced toxicity, calpain inhibition eliminated CX614-mediated neuroprotection. Future studies should therefore investigate potential mechanisms that could explain how calpain inhibition abolishes CX614-mediated protection from MPP+ toxicity. Although these mechanisms are currently not understood, it is possible that calpain is involved in pathways linking AMPA receptors and BDNF expression, trafficking, and secretion (An et al., 2008; Bi et al., 1997; del Toro et al., 2006; Goffredo et al., 2002; Jourdi et al., 2003, 2005a; Lynch et al., 2007; Xiong et al., 2002). In this regard, it is noteworthy that huntingtin, a calpain substrate, promotes intracellular trafficking of BDNF-containing vesicles (del Toro et al., 2006; Gharami et al., 2008; Lynch et al., 2007). Mutant huntingtin has recently been shown to differentially affect the transport and release of two BDNF isoforms, implicating huntingtin in the regulated BDNF secretory pathway (del Toro et al., 2006).
In vivo, BDNF is secreted in the striatum where it is taken up and retrogradely transported in axons of nigrostriatal dopaminergic neurons. A previous study used adenovirus-mediated over-expression of calpastatin, the only known endogenous calpain inhibitor, and reported that its over-expression in mice protected dopaminergic neurons against MPTP neurotoxicity (Crocker et al., 2003). In this in vivo PD model, protection was also obtained by direct intraventricular administration of calpain inhibitor III for several days during and after MPTP treatment, or by local virus-mediated over-expression of calpastatin a few days before MPTP administration. According to these results, in vivo protection of mesencephalic dopaminergic neurons by calpain inhibition was provided locally at the level of the mesencephalon, but this protocol did not normalize striatal dopamine levels, and did not lead to significant protection of the striatal innervation by mesencephalic dopaminergic neurons. It is therefore tempting to speculate that lack of protection of striatal innervation by calpain inhibition could be due to the effects of these compounds on the expression or transport of survival-promoting factors such as BDNF. Importantly, our data demonstrated mesencephalic BDNF upregulation by CX614 treatment since the cultured mesencephalic slices were prepared without striatal connections.
Previous reports have shown dissociation between calpain activation and LDH release in cultured and acute hippocampal neuronal tissues (Jourdi et al., 2005a,b; Zhou and Baudry, 2006); even prolonged and continuous calpain activation does not necessarily result in increased LDH release or lead to neuronal death (Jourdi et al., 2005a,b). Our current results agree with these previous findings, as CX614 pretreatment reduced MPP+-induced release of LDH in both types of cultured slices without decreasing MPP+-induced calpain activation. These findings further support the notion that calpain activation could take place independently of MPP+-induced neuronal cell death.
The in vivo use of neurotrophic factors as therapy for PD has been highly controversial because these large molecules do not cross the blood-brain barrier and, thus, are difficult to administer (for a recent review see Dauer, 2007). Direct injection of BDNF in brain parenchyma did not provide clear evidence of protection and the potent neurotrophic activity of glial cell line-derived neurotrophic factor (GDNF) did not exclusively affect dopaminergic neurons. Despite initial promising results, GDNF treatment suffered from shortcomings caused by undesired side effects due to lack of specificity and by difficulties related to its route of administration leading to termination of clinical trials. Ciliary neurotrophic factor (CNTF) and mesencephalic astrocyte-derived neurotrophic factor (MANF) also support dopaminergic neuronal survival but there has been no indication yet as to whether they will constitute efficient treatments for PD (Dauer, 2007). In contrast, our results demonstrate a very promising approach that could be used to pharmacologically treat patients suffering from PD. In particular, our results demonstrate that CX614 is protective against MPP+-induced neuronal damage in two different models of neurotoxicity. Considering the magnitude of BDNF upregulation by CX614, it will be important to validate our results in an in vivo model of PD. Preliminary trials have shown that PARMs can be safely used in humans and that they enhance cognitive functions (Arai and Kessler, 2007; Lynch, 2007; Ingvar et al., 1997). Accordingly, our current results have direct implications for treatment of neurodegenerative diseases, including PD, as PARM treatment of patients may serve multiple roles to mitigate neuronal cell death and improve behavioral outcome and neuronal survival in various areas of the brain.
Acknowledgements
This work was supported by NINDS grants P01NS045260-01 (PI: Dr. C.M. Gall) and NS048521-02 (Dr. M. Baudry).
References
- An JJ, Gharami K, Liao GY, Woo NH, Lau AG, Vanevski F, Torre ER, Jones KR, Feng Y, Lu B, Xu B. Distinct role of long 3′ UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell. 2008;134(1):175–187. doi: 10.1016/j.cell.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai AC, Kessler M. Pharmacology of ampakine modulators: from AMPA receptors to synapses and behavior. Curr. Drug Targets. 2007;8(5):583–602. doi: 10.2174/138945007780618490. [DOI] [PubMed] [Google Scholar]
- Bi X, Chen J, Baudry M. Developmental changes in calpain activity, GluR1 receptors and in the effect of kainic acid treatment in rat brain. Neuroscience. 1997;81(4):1123–1135. doi: 10.1016/s0306-4522(97)00218-2. [DOI] [PubMed] [Google Scholar]
- Blythe SN, Atherton JF, Bevan MD. Synaptic activation of dendritic AMPA and NMDA receptors generates transient high-frequency firing in substantia nigra dopamine neurons in vitro. J. Neurophysiol. 2007;97(4):2837–2850. doi: 10.1152/jn.01157.2006. [DOI] [PubMed] [Google Scholar]
- Chatha BT, Bernard V, Streit P, Bolam JP. Synaptic localization of ionotropic glutamate receptors in the rat substantia nigra. Neuroscience. 2000;101(4):1037–1051. doi: 10.1016/s0306-4522(00)00432-2. [DOI] [PubMed] [Google Scholar]
- Chen LW, Wei LC, Lang B, Ju G, Chan YS. Differential expression of AMPA receptor subunits in dopamine neurons of the rat brain: a double immunocytochemical study. Neuroscience. 2001;106(1):149–160. doi: 10.1016/s0306-4522(01)00255-x. [DOI] [PubMed] [Google Scholar]
- Choi WS, Lee EH, Chung CW, Jung YK, Jin BK, Kim SU, Oh TH, Saido TC, Oh YJ. Cleavage of Bax is mediated by caspase-dependent or -independent calpain activation in dopaminergic neuronal cells: protective role of Bcl-2. J. Neurochem. 2001;77:1531–1541. doi: 10.1046/j.1471-4159.2001.00368.x. [DOI] [PubMed] [Google Scholar]
- Crocker SJ, Smith PD, Jackson-Lewis V, Lamba WR, Hayley SP, Grimm E, Callaghan SM, Slack RS, Melloni E, Przedborski S, Robertson GS, Anisman H, Merali Z, Park DS. Inhibition of calpains prevents neuronal and behavioral deficits in an MPTP mouse model of Parkinson’s disease. J. Neurosci. 2003;23(10):4081–4091. doi: 10.1523/JNEUROSCI.23-10-04081.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer W. Neurotrophic factors and Parkinson’s disease: the emergence of a new player? Sci. STKE. 2007;411:pe60. doi: 10.1126/stke.4112007pe60. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Gao X, Story GM, Anderson LI, Kucera J, Walro JM. Evaluation of nigrostriatal dopaminergic function in adult +/+ and +/− BDNC mutant mice. Exp. Neurol. 2001;170:121–128. doi: 10.1006/exnr.2001.7698. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Racagni G, Riva MA. Shedding light into the role of BDNF in the pharmacotherapy of Parkinson’s disease. Pharmacogenomics J. 2006;6:95–104. doi: 10.1038/sj.tpj.6500360. [DOI] [PubMed] [Google Scholar]
- Gharami K, Xie Y, An JJ, Tonegawa S, Xu B. Brain-derived neurotrophic factor over-expression in the forebrain ameliorates Huntington’s disease phenotypes in mice. J. Neurochem. 2008;105(2):369–379. doi: 10.1111/j.1471-4159.2007.05137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffredo D, Rigamonti D, Tartari M, De Micheli A, Verderio C, Matteoli M, Zuccato C, Cattaneo E. Calcium-dependent cleavage of endogenous wild-type huntingtin in primary cortical neurons. J. Biol. Chem. 2002;277(42):39594–39598. doi: 10.1074/jbc.C200353200. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol. Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Umemori H, Mishina M, Yamamoto T. The AMPA receptor interacts with and signals through the protein tyrosine kinase Lyn. Nature. 1999;397:72–76. doi: 10.1038/16269. [DOI] [PubMed] [Google Scholar]
- Hennegriff M, Arai A, Kessler M, Vanderklish P, Mutneja MS, Rogers G, Neve RL, Lynch G. Stable expression of recombinant AMPA receptor subunits: binding affinities and effects of allosteric modulators. J. Neurochem. 1997;68:2424–2434. doi: 10.1046/j.1471-4159.1997.68062424.x. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Tomioka M, Takano J, Shirotani K, Iwata N, Masumoto H, Maki M, Itohara S, Saido TC. Distinct mechanistic roles of calpain and caspase activation in neurodegeneration as revealed in mice overexpressing their specific inhibitors. J. Biol. Chem. 2005;280(15):15229–15237. doi: 10.1074/jbc.M500939200. [DOI] [PubMed] [Google Scholar]
- Ingvar M, Ambros-Ingerson J, Davis M, Granger R, Kessler M, Rogers GA, Schehr RS, Lynch G. Enhancement by an ampakine of memory encoding in humans. Exp. Neurol. 1997;146:553–559. doi: 10.1006/exnr.1997.6581. [DOI] [PubMed] [Google Scholar]
- Iwakura Y, Nawa H, Sora I, Chao MV. Dopamine D1 receptor-induced signaling through TrkB receptors in striatal neurons. J. Biol. Chem. 2008;283(23):15799–15806. doi: 10.1074/jbc.M801553200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdi H, Iwakura Y, Narisawa-Saito M, Ibaraki K, Xiong H, Watanabe M, Hayashi Y, Takei N, Nawa H. Brain-derived neurotrophic factor signal enhances and maintains the expression of AMPA receptor-associated PDZ proteins in developing cortical neurons. Dev. Biol. 2003;263(2):216–230. doi: 10.1016/j.ydbio.2003.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdi H, Lu X, Yanagihara T, Lauterborn JC, Bi X, Gall CM, Baudry M. Prolonged positive modulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors induces calpain-mediated PSD-95/Dlg/ZO-1 protein degradation and AMPA receptor down-regulation in cultured hippocampal slices. J. Pharmacol. Exp. Ther. 2005a;314(1):16–26. doi: 10.1124/jpet.105.083873. [DOI] [PubMed] [Google Scholar]
- Jourdi H, Yanagihara T, Martinez U, Bi X, Lynch G, Baudry M. Effects of positive AMPA receptor modulators on calpain-mediated spectrin degradation in cultured hippocampal slices. Neurochem. Int. 2005b;46:31–40. doi: 10.1016/j.neuint.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kuipers SD, Bramham CR. Brain-derived neurotrophic factor mechanisms and function in adult synaptic plasticity: new insights and implications for therapy. Curr. Opin. Drug Discov. Devel. 2006;9:580–586. [PubMed] [Google Scholar]
- Langston JW, Irwin I. MPTP: current concepts and controversies. Clin. Neuropharmacol. 1986;9:485–507. [PubMed] [Google Scholar]
- Lauterborn JC, Truong GS, Baudry M, Bi X, Lynch G, Gall CM. Chronic elevation of brain-derived neurotrophic factor by ampakines. J. Pharmacol. Exp. Ther. 2003;307(1):297–305. doi: 10.1124/jpet.103.053694. [DOI] [PubMed] [Google Scholar]
- Lee FS, Chao MV. Activation of Trk neurotrophin receptors in the absence of neurotrophins. Proc. Natl. Acad. Sci. U.S.A. 2001;98(6):3555–3560. doi: 10.1073/pnas.061020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G. Glutamate-based therapeutic approaches: ampakines. Curr. Opin. Pharmacol. 2007;6(1):82–88. doi: 10.1016/j.coph.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Lynch G, Kramar EA, Rex CS, Jia Y, Chappas D, Gall CM, Simmons DA. Brain-derived neurotrophic factor restores synaptic plasticity in a knock-in mouse model of Huntington’s disease. J. Neurosci. 2007;27(16):4424–4434. doi: 10.1523/JNEUROSCI.5113-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen JT, Jansen P, Hesslinger C, Meyer M, Zimmer J, Gramsbergen JB. Tetrahydrobiopterin precursor sepiapterin provides protection against neurotoxicity of 1-methyl-4-phenylpyridinium in nigral slice cultures. J. Neurochem. 2003;85:214–223. doi: 10.1046/j.1471-4159.2003.01666.x. [DOI] [PubMed] [Google Scholar]
- Murray TK, Whalley K, Robinson CS, Ward MA, Hicks CA, Lodge D, Vandergriff JL, Baumbarger P, Siuda E, Gates M, Ogden AM, Skolnick P, Zimmerman DM, Nisenbaum ES, Bleakman D, O’Neill MJ. LY503430, a novel alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor potentiator with functional, neuroprotective and neurotrophic effects in rodent models of Parkinson’s disease. J. Pharmacol. Exp. Ther. 2003;306(2):752–762. doi: 10.1124/jpet.103.049445. [DOI] [PubMed] [Google Scholar]
- O’Neill MJ, Murray TK, Whalley K, Ward MA, Hicks CA, Woodhouse S, Osborne DJ, Skolnick P. Neurotrophic actions of the novel AMPA receptor potentiator, LY404187, in rodent models of Parkinson’s disease. Eur. J. Pharmacol. 2004;486(2):163–174. doi: 10.1016/j.ejphar.2003.12.023. [DOI] [PubMed] [Google Scholar]
- O’Neill MJ, Murray TK, Clay MP, Lindstrom T, Yang CR, Nisenbaum ES. LY503430: pharmacology, pharmacokinetics, and effects in rodent models of Parkinson’s disease. CNS Drug. Rev. 2005;11(1):77–96. doi: 10.1111/j.1527-3458.2005.tb00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazawa H, Murata M, Watanabe M, Kamei M, Kanazawa I. Dopaminergic stimulation up-regulates the in vivo expression of brain-derived neurotrophic factor (BDNF) in the striatum. FEBS Lett. 1992;313:138–142. doi: 10.1016/0014-5793(92)81430-t. [DOI] [PubMed] [Google Scholar]
- Rajagopal R, Chao MV. A role for Fyn in Trk receptor transactivation by G-protein-coupled receptor signaling. Mol. Cell. Neurosci. 2006;33(1):36–46. doi: 10.1016/j.mcn.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. The influence of gonadal hormones on neuronal excitability, seizures, and epilepsy in the female. Epilepsia. 2006;47(9):1423–1440. doi: 10.1111/j.1528-1167.2006.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spina MB, Squinto SP, Miller J, Lindsay RM, Hyman C. Brain-derived neurotrophic factor protects dopamine neurons against 6-hydroxydopamine and N-methyl-4-phenylpyridinium ion toxicity: involvement of the glutathione system. J. Neurochem. 1992;59:99–106. doi: 10.1111/j.1471-4159.1992.tb08880.x. [DOI] [PubMed] [Google Scholar]
- del Toro D, Canals JM, Ginés S, Kojima M, Egea G, Alberch J. Mutant huntingtin impairs the post-Golgi trafficking of brain-derived neurotrophic factor but not its Val66Met polymorphism. J. Neurosci. 2006;26(49):12748–12757. doi: 10.1523/JNEUROSCI.3873-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyooka K, Iritani S, Makifuch T, Shirakawa O, Kitamura N, Maeda K, Nakamura R, Niizato K, Watanabe M, Kakita A, Takahashi H, Someya T, Nawa H. Selective reduction of a PDZ protein, SAP-97, in the prefrontal cortex of patients with chronic schizophrenia. J. Neurochem. 2002;83:797–806. doi: 10.1046/j.1471-4159.2002.01181.x. [DOI] [PubMed] [Google Scholar]
- Tsuji T, Shimohama S. Protein degradation in Alzheimer’s disease and aging of the brain. Prog. Mol. Subcell. Biol. 2002;29:43–60. doi: 10.1007/978-3-642-56373-7_4. [DOI] [PubMed] [Google Scholar]
- Xia YF, Kessler M, Arai AC. Positive alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor modulators have different impact on synaptic transmission in the thalamus and hippocampus. J. Pharmacol. Exp. Ther. 2005;313(1):277–285. doi: 10.1124/jpet.104.078196. [DOI] [PubMed] [Google Scholar]
- Xiong H, Futamura T, Jourdi H, Zhou H, Takei N, Diverse-Pierluissi M, Plevy S, Nawa H. Neurotrophins induce BDNF expression through the glutamate receptor pathway in neocortical neurons. Neuropharmacology. 2002;42(7):903–912. doi: 10.1016/s0028-3908(02)00043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Baudry M. Developmental changes in NMDA neurotoxicity reflect developmental changes in subunit composition of NMDA receptors. J. Neurosci. 2006;26(11):2956–2963. doi: 10.1523/JNEUROSCI.4299-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]