Summary
B cell receptor (BCR) ligation generates reactive oxygen intermediates (ROI) that play a role in cellular responses. Although ROI can oxidize all macromolecules, it was unclear which modifications control B cell responses. In this study, we demonstrate the importance of the first oxidation product of cysteine, sulfenic acid, and its reversible formation in B cell activation. Upon BCR crosslinking, B cells increase ROI levels with maximal production occurring within 15 minutes. Increased ROI preceded elevated cysteine sulfenic acid, which localized to the cytoplasm and nucleus. Analysis of individual proteins revealed that the protein tyrosine phosphatases (PTPs) SHP-1, SHP-2, and PTEN, as well as actin, were modified to sulfenic acid following BCR ligation. Additionally, we used 5,5-dimethyl-1,3-cyclohexanedione (dimedone), a compound that covalently reacts with sulfenic acid to prevent its further oxidation or reduction, to determine the role of reversible cysteine sulfenic acid formation in regulating B cell responses. Dimedone incubation resulted in a concentration dependent block in anti-IgM induced cell division, accompanied by a failure to induce capacitative calcium entry (CCE), and maintain tyrosine phosphorylation. These studies illustrate that reversible cysteine sulfenic acid formation is a mechanism by which B cells modulate pathways critical for activation and proliferation.
Keywords: reactive oxygen intermediates, cysteine sulfenic acid, B cell activation, B cell proliferation
Introduction
B cell activation begins with recognition of antigen by the B cell receptor (BCR) initiating a signal transduction cascade through the phosphorylation of Igα and Igβ heterodimers, B cell linker (BLNK), Bruton's tyrosine kinase (Btk), phospholipase Cγ2 (PLCγ2), and phosphoinositide-3-kinase (PI3K) [1]. Signals are further propagated through a rise in intracellular calcium [2]. These signals culminate in a new program of gene expression allowing differentiation into memory and plasma cells. Recently, several studies suggest that a combination of post-translational modifications regulate B cell activation and fate [3]. For instance, it is well documented that phosphorylation is a key post-translational modification in BCR activation [4]. Recently, Infantino et al. [5] demonstrated that arginine methylation of the BCR negatively regulates signaling pathways essential for B cell activation while positively regulating differentiation. Therefore, determining additional modifications and the mechanisms by which they regulate B cell signaling events is critical not only for understanding B cell activation but also for developing new vaccines and autoimmunity therapies.
It is well documented that reactive oxygen intermediates (ROI) are necessary for the innate immune system's defense against microorganisms. Neutrophils and macrophages kill invading pathogens by activating the NADPH oxidase enzyme complex to produce superoxide (O2•−), hydrogen peroxide (H2O2), and hydroxyl radicals (•OH) [6, 7]. Recently, studies have begun to elucidate the role of ROI in humoral immune responses. For instance, Capasso et al. [8] and Richards and Clark [9] demonstrated that murine B cells increase ROI levels following BCR ligation. These reports are consistent with an earlier study documenting that the A20 murine B cell lymphoma line increased ROI levels upon anti-IgG stimulation [10]. Additionally, in vivo studies found that mice with B cells deficient in ROI generating proteins have decreased antibody responses to T-cell dependent antigens, suggesting that ROI act as positive regulators in B cell responses [8]. However, Richards and Clark [9] determined that BCR induced ROI negatively regulated B cell proliferation and antibody responses to T-cell independent type 2 antigens. Together, these studies demonstrate that the role of ROI in B cell biology is complex and warrants further investigation. A particularly important unanswered question is the mechanisms by which ROI affect B cell activation.
While ROI can modify all macromolecules, reversible oxidation of cysteine is a mechanism to modulate signal transduction pathways. In the presence of ROI, thiols (-SH) can be oxidized to cysteine sulfenic acid (-SOH) [11, 12]. This intermediate can be stabilized to a sulfenamide, form a disulfide bond with other protein thiols, undergo reduction, or be further oxidized to sulfinic (-SO2H) or sulfonic (-SO3H) acid [12]. These post-translational modifications of cysteine act as a sensor for altering protein-protein interactions and function [13]. A recent study by Michalek and colleagues [14] documented that reversible cysteine sulfenic acid formation is necessary for naive CD8+T cell activation, proliferation, and function. However, it was unknown whether this post-translational modification was necessary for B cell activation.
Here we demonstrate that following antibody and antigen-mediated activation, B cells increase ROI levels. Using an antibody that recognizes proteins derivatized with 5,5-dimethyl-1,3-cyclohexanedione (dimedone), a compound that covalently reacts with cysteine sulfenic acid [15], we show that cysteine sulfenic acid levels increase following BCR ligation, and localize to both the cytoplasm and nucleus. We demonstrate that incubation of cells with dimedone resulted in a concentration dependent block in anti-IgM induced proliferation. This decrease resulted from an inability of the cells in the presence of dimedone to sustain early tyrosine phosphorylation events and initiate capacitative calcium entry (CCE). These findings demonstrate the important role of ROI and reversible cysteine sulfenic acid formation in signaling pathways that promote B cell activation and proliferation.
Results
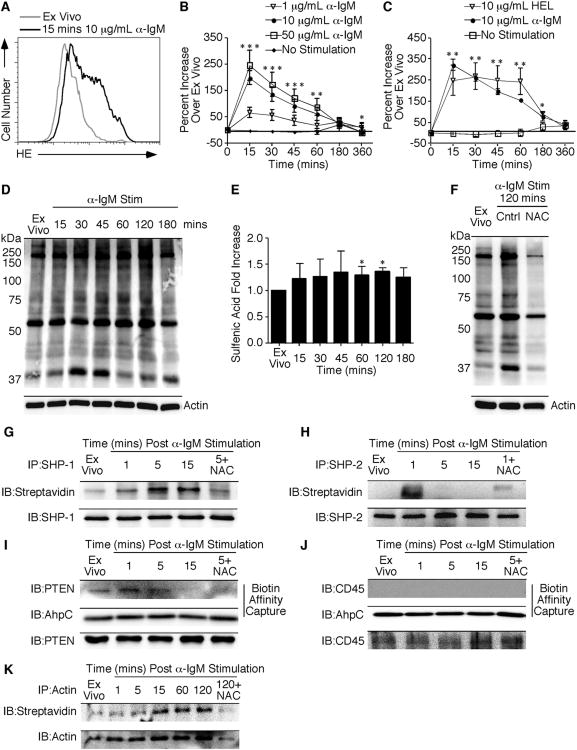
Primary B Cells Increase HE Oxidation and Steady State Sulfenic Acid Levels After Receptor Crosslinking
Previous studies have demonstrated that A20, a murine B cell lymphoma line, increased ROI levels following anti-IgG stimulation [10]. To determine the ROI production by primary B cells after stimulation with anti-IgM, we measured superoxide levels using the dye dihydroethidium (DHE). DHE is an indicator of superoxide and emits a blue fluorescence in the cytosol of the cell until it is oxidized. Following oxidation, the dye intercalates into the DNA of the cell and emits a red fluorescence, which can be recorded by flow cytometry. Primary B cells increased HE fluorescence within 15 minutes of 10μg/mL anti-IgM stimulation (Figure 1A). By 6 hours of stimulation, superoxide production had decreased to ex vivo levels (Figure 1B). ROI production correlated with anti-IgM concentration. Cells stimulated with the lowest concentration of anti-IgM produced the least amount of ROI. Regardless of anti-IgM concentration, similar ROI kinetics were observed. To determine ROI production following B cell activation with their cognate antigen, the kinetics of ROI production were measured in hen egg lysozyme (HEL) stimulated MD4 transgenic B cells. Figure 1C demonstrates an increase in HE oxidation within 15 minutes of 10 μg/mL HEL stimulation. This increased level of oxidation remained elevated for 1 hour. When MD4 B cells were stimulated with anti-IgM alone there was a comparable increase and similar kinetics in HE fluorescence compared to purified B cells from naïve C57BL/6 mice. Thus, purified B cells produce ROI in response to antibody and antigen-mediated BCR stimulation.
Figure 1.
Purified B cells increase HE oxidation and cysteine sulfenic acid levels following receptor ligation.
Purified B cells from naïve C57BL/6 mice were stimulated with the indicated concentrations ofanti-IgM followed by incubation with HE. (A) Histogram of HE fluorescence at 15 minutes after 10 μg/mL anti-IgM stimulation compared to unstimulated cells. (B) Percent increase in HE MFI compared to unstimulated cells over a 6-hour time course was determined and the average and standard deviation are plotted. (C) Purified B cells from MD4 mice were stimulated for 6 hours with 10 μg/mL HEL or anti-IgM followed by HE incubation. Results are presented as in (B). Purified B cells were stimulated with 10 μg/mL anti-IgM for the indicated timepoints before lysis in the presence of 1 mM dimedone. (D) Representative blot indicating total cysteine sulfenic acid in the proteome. Actin was used as a loading control. (E) Quantification of fold increase in cysteine sulfenic acid levels relative to ex vivo samples following normalization to the actin signal. The average and standard deviation are plotted. (F) Representative blot of samples treated with 20 mM NAC prior to stimulation. Three to six mice were examined at each time point in a minimum of two independent experiments with a two-tailed Student's t test comparison. *, p ≤ 0.05; significant difference between unstimulated and stimulated samples. (G) SHP-1 and (H) SHP-2 were immunoprecipitated after DCP-Bio1 labeling. Blots were probed for sulfenic acid using streptavidin-HRP. The blot was stripped and probed for total protein as a loading control. (I) Following affinity capture of biotinylated proteins, blots were probed for PTEN or (J) CD45. AhpC was used as a procedural control for the capture and total protein is shown. (K) Actin was immunoprecipitated following DCP-Bio1 labeling as described above. Results are representative of two independent experiments.
Increased ROI production has been associated with cellular signaling in response to T cell receptor, insulin, and growth factor stimulation [14, 16-20]. To determine if increased ROI production following B cell stimulation led to increased cysteine sulfenic acid formation, an anti-dimedone antibody was used. This antibody recognizes proteins derivatized with dimedone, thus allowing the detection of cysteine sulfenic acid [21]. Within 15 minutes of BCR stimulation, global cysteine sulfenic acid levels increased slightly (Figure 1D). However, after 15 minutes, the sulfenic acid levels remained elevated until 1-2 hours post stimulation, where levels reached a maximum (Figure 1E). BCR stimulation resulted in a modest 36% increase in sulfenic acid levels at the maximum time point. To verify the increase in cysteine sulfenic acid levels was due to ROI production, B cells were pretreated with N-acetyl-cysteine (NAC) prior to stimulation (Figure 1F). Cysteine sulfenic acid levels were decreased in B cells stimulated in the presence of the antioxidant. Thus, B cell activation is accompanied by an increase in ROI production and steady state levels of cysteine sulfenic acid.
Since we detected modest changes in total levels of cysteine sulfenic acid following BCR activation, we next evaluated specific proteins that underwent this modification. To understand the contribution of this process to B cell activation, we evaluated the kinetics of sulfenic acid formation in the protein tyrosine phosphatases critical to B cell activation: SHP-1, SHP-2, PTEN, and CD45. Following SHP-1 immunoprecipitation, we observed an increase in sulfenic acid levels within 5 minutes of BCR ligation (Figure 1G). This increase remained elevated for 15 minutes and was dependent upon ROI production as evidenced by NAC inhibition. In contrast, SHP-2 was oxidized to sulfenic acid within 1 minute of BCR stimulation and the labeling quickly declined by 5 minutes (Figure 1H). Sulfenic acid kinetics in PTEN were similar to SHP-1, with maximal labeling at 5 minutes (Figure 1I). The AhpC in Figure 1I serves as a procedural control for the biotin-based affinity capture, while PTEN controls for total protein levels. Given its critical role in the initiation of BCR signaling, we measured the oxidation of CD45 [22]. In contrast to the intracellular PTPs, CD45 was not oxidized to sulfenic acid following B cell activation (Figure 1J). Additionally, we also measured the oxidation of actin following BCR stimulation since glutathionylation has been shown to be important for cytoskeleton reorganization [23]. Sulfenic acid levels in actin peaked at 15 minutes and remained elevated for 120 minutes after B cell activation (Figure 1K). Taken together, these results demonstrate that the increase in ROI following BCR ligation is accompanied by changes in cysteine oxidation in proteins critical to B cell activation.
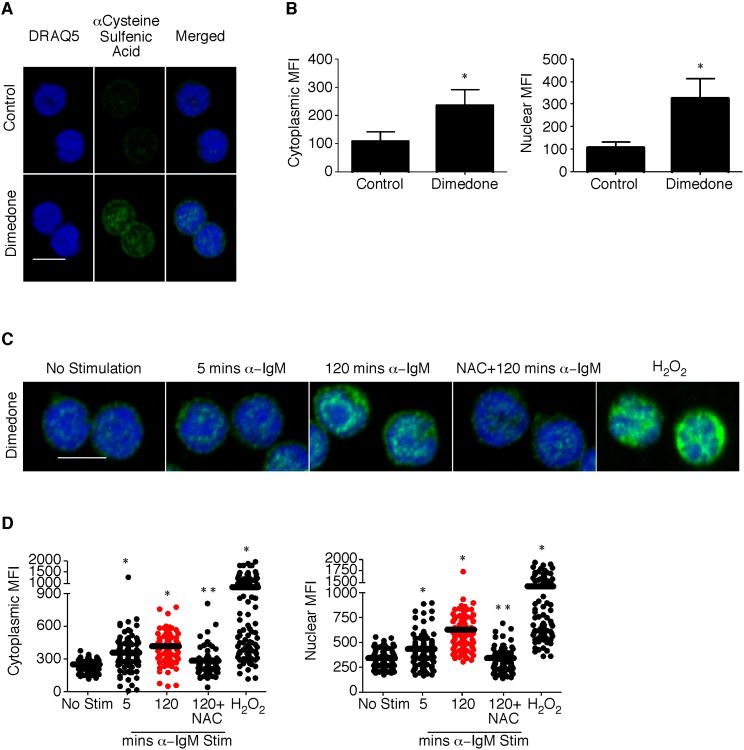
Cysteine Sulfenic Acid Localizes to the Cytoplasm and Nucleus Following BCR Activation
Multiple studies have determined sulfenic acid localization in various cell types [24, 25]. However, to better understand the localization in B cells, we performed immunofluorescence staining and confocal microscopy. Control samples in vehicle (media alone) show little background fluorescent staining, indicating the specificity of the antibody for dimedone-derivatized proteins (Figure 2A and B). Within 5 minutes of BCR activation total levels of cysteine sulfenic acid, which localized to the cytoplasm and nucleus, increased (Figure 2C and D). However, after 120 minutes of BCR stimulation, the mean fluorescent intensity of cysteine sulfenic acid was greaterin the nucleus compared to the cytoplasm. Hydrogen peroxide was used as a positive control for detecting sulfenic acid formation. Both the increase and localization in sulfenic acid were dependent upon ROI production as determined by NAC treatment. Thus, cysteine sulfenic acid localizes to multiple cellular compartments during B cell activation.
Figure 2.
Cysteine sulfenic acid localizes to distinct cytosolic and nuclear puncta following B cell activation.
B cells were purified from the spleens of naïve C57BL/6 mice using CD43 negative magnetic microbeads. Cells were seeded into a well containing a poly-L-lysine coated glass coverslip and allowed to adhere. (A) Samples were treated with vehicle or 10 mM dimedone for 15 minutes at 37°C. All samples were stained with an antibody against dimedone and with an Alexa-Fluor 488 conjugated secondary antibody. (B) Quantification of cytoplasmic and nuclear cysteine sulfenic acid staining in the presence of vehicle or dimedone. (C) Seeded B cells were stimulated in the absence or presence of 10 μg/mL anti-IgM or 0. 2 mM H2O2 for the indicated timepoints prior to vehicle or dimedone treatment. All samples were processed and stained as in (A). (D) Quantification of cytoplasmic and nuclear cysteine sulfenic acid staining in the absence or presence of stimulation in six fields of view. The average and standard deviation are plotted. Two mice were examined at each time point in a minimum of two independent experiments with a two-tailed Student's t test comparison. *, p ≤ 0.05; significant difference between control and dimedone treated samples or unstimulated and stimulated samples. **, p ≤ 0.05; significant difference between 2 hour anti-IgM and N-acetyl-cysteine pretreated sample. Merged images through the center of the cell are shown. Magnification, 63×. Scale, 5 μm.
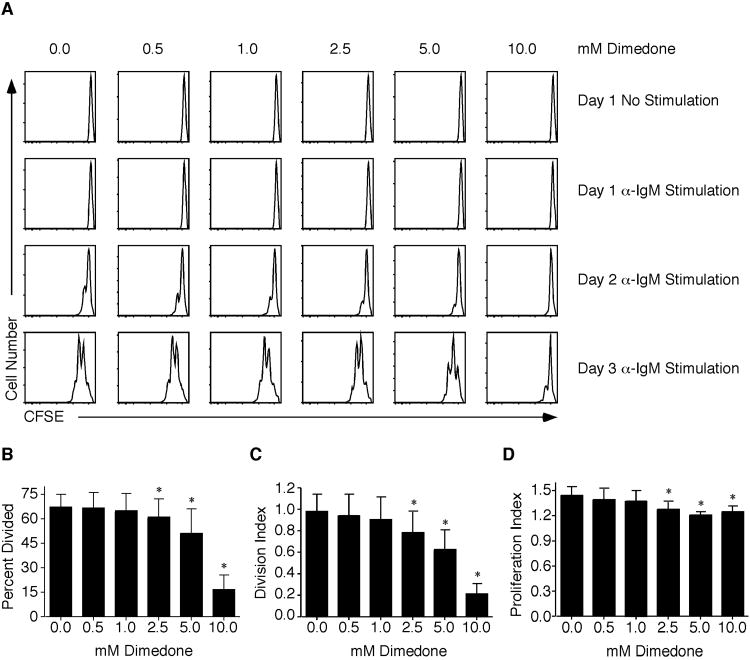
Dimedone Incubation Decreases Anti-IgM Induced B Cell Proliferation
To determine whether the reversible cysteine sulfenic acid formation is required for B cell proliferation, purified B cells were incubated in the presence of anti-IgM and increasing concentrations of dimedone. Dimedone is a compound that covalently reacts with cysteine sulfenic acid to prevent its further oxidation or reduction. Proliferation of B cells was assessed by the loss of CFSE fluorescence. The division index is the average number of divisions that a cell has undergone, while the proliferation index is the average number of divisions that those cells that divided underwent. After 24 hours of 10μg/mL anti-IgM stimulation, no division occurred regardless of dimedone pretreatment (Figure 3A). Following 72 hours of stimulation, vehicle samples had divided 1 to 2 times. At 0. 5 mM and 1. 0 mM dimedone, there were little effects on proliferation. However, increasing the concentration from 2. 5 mM to 10. 0 mM decreased B cell proliferation. Analyzing the percent divided, proliferation, and division indices on day 3 after anti-IgM stimulation revealed a significant decrease in B cell proliferation at 2. 5 mM to 10. 0 mM dimedone (Figure 3B-D). NAC pretreatment, which decreases overall ROI production and subsequent sulfenic acid formation, reduces B cell proliferation similar to dimedone incubation (Supplemental Figure 1). Taken together, these data demonstrate that reversible cysteine sulfenic acid formation is an oxidative modification critical to B cell proliferation.
Figure 3.
Dimedone incubation decreases anti-IgM stimulated B cell proliferation.
Purified B cells were isolated from naïve splenocytes and labeled with CFSE. Prior to stimulation with 10 μg/mL anti-IgM, cells were pretreated in vehicle or increasing concentrations of dimedone for 1 hour. (A) Proliferation was assessed by the loss of CFSE fluorescence at various time points post stimulation. (B) The percent divided, (C) division, and (D) proliferation indices were calculated on day 3 post stimulation. The average and standard deviations are plotted. Five to ten mice were examined at each time point in a minimum of five independent experiments with a two-tailed Student's t test comparison. *, p ≤ 0.05; significant difference between vehicle and dimedone treated samples.
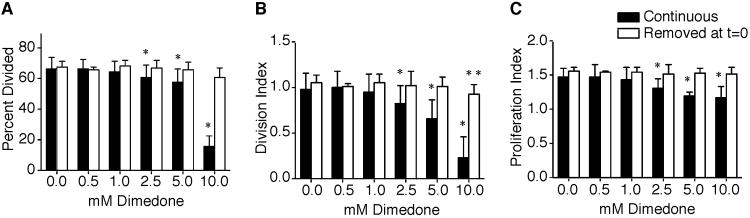
Cysteine Sulfenic Acid Formation Following Activation Is Required For B Cell Proliferation
To determine if the decrease in proliferation was due to the reaction of dimedone with cysteine sulfenic acid proteins in unactivated B cells, two sets of purified cells were pretreated in vehicle or dimedone for 1 hour. One pretreated set was stimulated with anti-IgM in the continuous presence of dimedone. A duplicate set of pretreated samples had dimedone removed prior to anti-IgM stimulation. B cells continuously incubated with dimedone and stimulated with anti-IgM exhibited reduced percent divided, division, and proliferation indices (Figure 4A-C). The division and proliferation indices of samples in which dimedone had been removed prior to stimulation were not significantly different from the control samples. Thus, cysteine sulfenic acid formation following activation is critical in regulating proliferation.
Figure 4.
Cysteine sulfenic acid formation following B cell activation is necessary for proliferation.
B cells were purified from naïve splenocytes by magnetic microbeads and labeled with CFSE. One set of cells was pretreated in vehicle or 10 mM of dimedone for 1 hour prior to stimulation. A duplicate set was also pretreated in the absence or presence dimedone for 1 hour and then dimedone was removed by washing the cells three times before stimulation. On day 3 post stimulation, the (A) percent divided, (B) division, and (C) proliferation indices were calculated with the average and standard deviation shown. Five mice were examined at each time point in 3 independent experiments with a two-tailed Student's t test comparison. *, p ≤ 0.05; significant difference between continuously incubated dimedone and vehicle treated samples. **, p ≤ 0.05; significant difference between dimedone sample with removal at t=0 and the vehicle treated control.
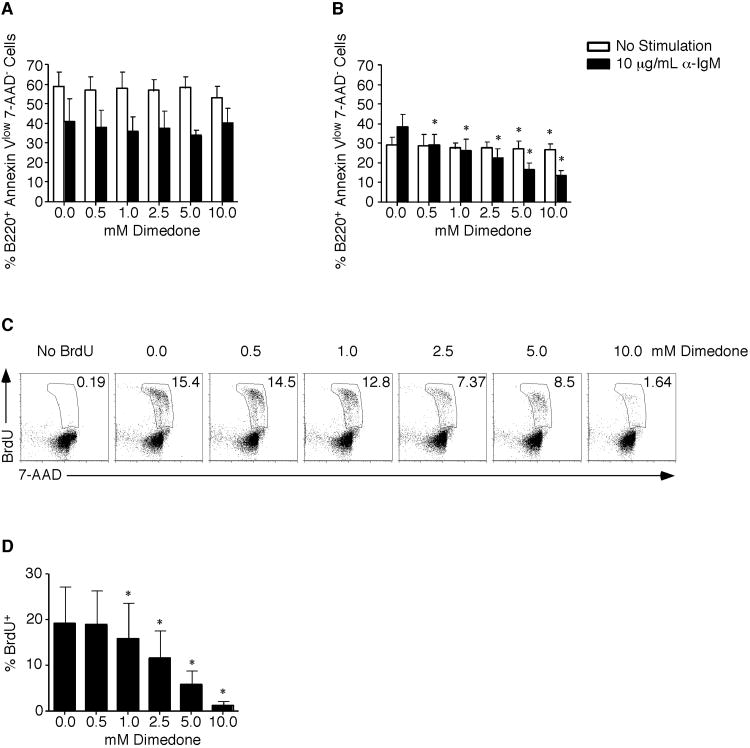
Dimedone Incubation Decreases BCR-Mediated Survival and Cell Cycle Progression
BCR-induced proliferation requires both prosurvival and cell cycle progression signals. To determine if dimedone affected survival, purified B cells were incubated for either 24 (Figure 5A) or 48 hours (Figure 5B) in vehicle or dimedone. At 24 hours there was no effect on survival regardless of whether cells were unstimulated or stimulated with anti-IgM (Figure 5A). By 48 hours, the survival of unstimulated cells was not affected demonstrating dimedone is not inherently cytotoxic (Figure 5B). This contrasted with anti-IgM stimulated cells where viable cells were decreased (38% (vehicle) versus 13% (10 mM dimedone)). Thus, dimedone incubation blocks BCR-induced survival signals. To determine whether dimedone also blocked BCR-induced cell cycle progression, S phase entry was analyzed by measuring BrdU and 7-AAD incorporation. When B cells were activated in the absence of dimedone, 15. 4% of cells were in S phase by 48 hours (Figure 5C and D). However, following 10 mM dimedone incubation only 1. 6% of cells were in S phase. Taken together, these data demonstrate that although the initial viability was not affected, prolonged incubation with dimedone decreases BCR-induced survival and cell cycle progression signals.
Figure 5.
Dimedone incubation decreases BCR-mediated survival and cell cycle progression.
Purified B cells were isolated from splenocytes using magnetic microbeads. Cells were pretreated for 1 hour in vehicle or dimedone. After 24 (A) or 48 hours(B) with or without 10 μg/mL anti-IgM stimulation, the percentage of viable cells was determined by Annexin-V and 7-AAD staining. The average and standard deviation are plotted. At 45 hours post 10 μg/mL anti-IgM stimulation, BrdU was added to the cultures. Cells were stained with anti-BrdU and 7-AAD. (C) Representative dot plots of BrdU and 7-AAD staining are shown. (D) The percentage of cells in S phase is shown with the average and standard deviation plotted. Three to four mice were examined at each time point in 2 independent experiments with a two-tailed Student's t test comparison. *, p ≤ 0.05; significant difference between dimedone and vehicle treated samples for each timepoint.
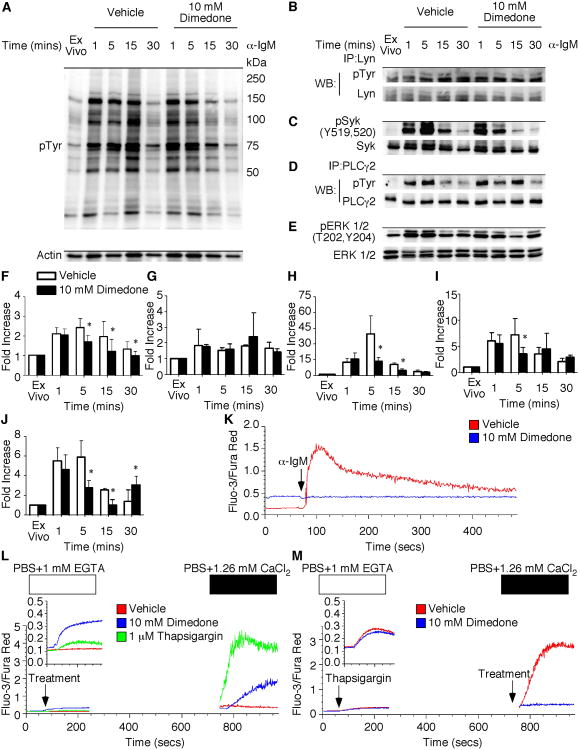
Dimedone Incubation Fails to Maintain Early Tyrosine Phosphorylation Events and Decreases Calcium Flux Following Activation
To determine the mechanisms by which dimedone decreases prosurvival and cell cycle progression signals, we examined signaling processes that require reversible cysteine sulfenic acid formation. Global tyrosine, Lyn, Syk, PLCγ2, and ERK 1/2 phosphorylation were determined in the presence of vehicle or dimedone. Immunoblot analysis of global tyrosine phosphorylation revealed a ∼2. 0 fold increase in phosphorylation within 1 minute of BCR stimulation (Figure 6A and F). Dimedone treatment did not decrease the global tyrosine phosphorylation at 1 minute. However, after 5 and 15 minutes of BCR stimulation, dimedone treatment decreased tyrosine phosphorylation compared to vehicle treated samples. Thus, reversible cysteine sulfenic acid formation plays a role in the maintenance of global tyrosine phosphorylation.
Figure 6.
Dimedone incubation decreases sustained total tyrosine phosphorylation and calcium flux.
Purified B cells were isolated from splenocytes using magnetic microbeads. Cells were pretreated for 1 hour in vehicle or dimedone prior to stimulation. At various times post stimulation, cells were lysed and resolved by SDS-PAGE. (A) Representative blot of total tyrosine phosphorylation in the presence of vehicle or dimedone. Actin was used as a loading control. (B) Lyn was immunoprecipitated following stimulation. Samples were probed for total tyrosine phosphorylation. The blot was then stripped and total protein was probed as a control. (C) Western blot analysis of Syk phosphorylation at Y519 and Y520. Syk was used as a loading control. (D) PLCγ2 was immunoprecipitated following stimulation as described above. (E) Phospho-ERK 1/2 (T202, Y204) western blot of cells stimulated in the presence of vehicle or dimedone. ERK 1/2 was used for the loading control. (F-J) Quantification of total tyrosine, Lyn, Syk, PLCγ2, and ERK 1/2 phosphorylation was determined as fold increase over unstimulated cells. The average and standard deviation are plotted. At each time point, 3-6 mice were examined in a minimum of 3 independent experiments with a two-tailed Student's t test comparison. *, p≤0.05; significant difference between dimedone and vehicle treated samples. (K) Purified cells were incubated with 5 μM Fura-Red-AM and 2. 5 μMFluo-3-AM in the presence of DMSO control or dimedone for 30 minutes. After washing, cells were stimulated with 10 μg/mL anti-IgM at the flow cytometer. (L) B cells were incubated with Fura Red AM and Fluo-3-AM. After washing, cells were resuspended in PBS supplemented with 1 mM EGTA and were acquired on the cytometer for 1 minute prior to incubation with DMSO control, dimedone, or thapsigargin. Samples were placed back on the cytometer and acquired for an additional 5 minutes and 30 seconds. Following centrifugation for 3 minutes, the EGTA was removed and the cells were resuspended in PBS supplemented with 1. 26 mM CaCl2 in the (M) presence of DMSO control or dimedone. Samples were placed on the cytometer and acquired for a total of 1024 seconds. The arrows indicate addition of stimuli, dimedone, or vehicle. In all plots, the ratio of Fluo-3 to Fura-Red fluorescence was recorded as a function of time. The histograms are representative of three to four mice in a minimum of 3 independent experiments.
Because we observed a decrease in global tyrosine phosphorylation, we wanted to determine if specific tyrosine phosphorylation events following BCR ligation were altered in the presence of dimedone. Immunoblot analysis of Lyn phosphorylation identified similar phosphorylation levels in the vehicle and dimedone treated samples at all time points (Figure 6B and G). Phospho-Syk analysis by Western blot demonstrated a ∼12 fold increase in phosphorylation after 1 minute of BCR stimulation in the absence of dimedone (Figure 6C and H). By 5 minutes, the phosphorylation of Syk had increased ∼39 fold over ex vivo. However, treatment of cells with dimedone significantly decreased Syk phosphorylation at 5 and 15 minutes. Similar results were detected with PLCγ2 (Figure 6D and I) and ERK1/2 (Figure 6E and J) phosphorylation in the presence of dimedone. Therefore, reversible cysteine sulfenic acid formation is necessary for the maintenance of global tyrosine, Syk, PLCγ2, and ERK 1/2, but not Lyn, phosphorylation during BCR activation.
Since the early tyrosine phosphorylation events were inhibited by dimedone pretreatment, we wanted to determine whether sulfenic acid modification of proteins was altered. To address this, purified B cells were pretreated with vehicle or dimedone prior to measuring sulfenic acid formation in the total proteome and individual candidates. Although somewhat elevated cysteine sulfenic acid levels following dimedone pretreatment were observed, no increase in sulfenic acid levels following B cell activation were observed in the presence of dimedone (Supplemental Figure 2A). Furthermore, when individual proteins were analyzed, dimedone pretreatment decreased (SHP-1 and PTEN) or blocked (SHP-2) sulfenic acid formation following B cell activation when compared to vehicle (Supplemental Figure 2B-D).
To determine whether calcium flux, which is critical for B cell activation, requires reversible cysteine sulfenic acid formation, purified cells were incubated with Fura-Red-AM and Fluo-3-AM to detect intracellular calcium levels. Unstimulated cells incubated with the DMSO control had a basal level of calcium, which increased upon 10μg/mL anti-IgM incubation (Figure 6K). However, B cells in the presence of 10 mM dimedone did not increase intracellular calcium levels following BCR crosslinking. To determine the specific steps during store operated calcium influx that require reversible cysteine sulfenic formation, we measured ER calcium release by incubating B cells in PBS supplemented with 1 mM EGTA. ER calcium release was initiated when B cells were incubated with 10 mM dimedone, but not the DMSO control, in the absence of stimulation (Figure 6L). However, when extracellular calcium was added to the cells, CCE was slightly decreased in the dimedone samples compared to the control thapsigargin treatment. To directly assess whether CCE requires reversible cysteine sulfenic acid formation, B cells were stimulated with thapsigargin in calcium free buffer and then supplemented with CaCl2containingDMSO control or dimedone. Thapsigargin treatment initiated similar levels of ER calcium release in both samples. However, compared to the DMSO control, cells in the presence of CaCl2 and dimedone did not exhibit an increase in CCE (Figure 6M). Interestingly, NAC treatment had similar effects on ER calcium release and CCE in B cells (Supplemental Figure 3A and B). Taken together, these results indicate that ROI and the reversible cysteine sulfenic acid formation regulate sustained tyrosine phosphorylation, ER calcium release, and CCE mobilization in B cells.
Discussion
In this study, we examined the role of reversible cysteine sulfenic acid formation during B cell activation and proliferation. Here we report six novel observations. First, compared to antibody mediated BCR ligation, we demonstrate cognate antigen stimulation elicits similar kinetics of ROI production. Second, the ROI generated during BCR ligation are associated with increased sulfenic acid levels in the total proteome. Third, the global increase in cysteine sulfenic acid following B cell activation is localized to both the cytosol and nucleus. Fourth, SHP-1, SHP-2, and PTEN are modified to cysteine sulfenic acid following BCR ligation. Fifth, B cell proliferation requires reversible cysteine sulfenic acid formation. Sixth, both ER calcium release and CCE require reversible cysteine sulfenic acid formation. Taken together, these results demonstrate that ROI generated during BCR ligation function as secondary messengers by oxidizing cysteine residues in signaling proteins that promote activation and proliferation.
The observations made here and elsewhere strongly support ROI and reversible cysteine sulfenic acid as positive regulators of BCR signaling. First, a prior study by Capasso et al. [8] has shown that ROI are necessary for maintaining oxidized SHP-1 to facilitate proper BCR signaling. We extended upon this study and identified several PTPs including SHP-1, SHP-2, and PTEN as targets for cysteine oxidation, suggesting that multiple signaling pathways following B cell activation are redox regulated. Second, Singh and colleagues [10] demonstrated that a rise in ROI and intracellular calcium are necessary to amplify early BCR induced phosphorylation signals. We have expanded upon their study and determined that both ER calcium release and CCE are redox regulated, suggesting that multiple calcium regulators are sensitive to oxidation and reduction and these changes control their function. Additionally, we have also identified reversible cysteine sulfenic acid formation as an oxidative modification necessary for both CCE and the signal transduction amplification loop following B cell activation. Third, our CFSE experiment in the presence of dimedone clearly shows that reversible cysteine sulfenic acid formation is necessary for B cell proliferation. This finding provides evidence that proteins necessary for B cell proliferation transition through cysteine sulfenic acid in order to exert their functions. Moreover, our data provides a mechanism by which antioxidant treatment decreases B cell proliferation (Supplemental Figure 1) [26, 27]. Together, these observations provide a model in which ROI positively regulate pathways in B cell activation and proliferation through the reversible oxidation of cysteine residues in signaling proteins. This is a critical finding as it demonstrates that manipulation of ROI and target pathways may improve B cell responses following vaccination or alternatively, dampen responses during autoimmunity.
A previous study by Richards and Clark [9] demonstrated that BCR induced ROI limits proliferation. However, we demonstrate that B cell proliferation requires the production of ROI for the reversible formation of cysteine sulfenic acid. How can the discrepancy between our studies be reconciled? There are many sources of ROI including ER stress, mitochondrial electron transport chain (ETC), and NADPH oxidase enzyme complex (NOX) [28]. Using pharmacological inhibitors of ROI sources, Vené et al. [29]determined that the majority of ROI is produced from complex I of the ETC and NOX following B cell activation. The study by Richards and Clark [9] eliminates only one major ROI source, which functions to limit B cell proliferation. Together, these studies suggest the source of ROI could govern which proteins and pathways are targeted to either limit or promote B cell responses. It is well documented that cysteine sulfenic acid formation in target proteins can either activate or inhibit protein function [13]. We clearly observe a global requirement for reversible cysteine sulfenic acid formation in B cell proliferation, however; eliminating a particular ROI source could be driving an aberrant cysteine oxidation profile in target proteins, which could explain the altered B cell proliferation kinetics.
Sulfenic acid modified proteins have been detected in multiple organisms and cell types including endothelial, cardiac, and CD8+ T cells [14, 21, 30-37]. How does cysteine sulfenic acid formation in B cells differ from these other cell types? Our observations revealed a modest increase in total cysteine sulfenic acid following B cell activation. In contrast, Michalek et al. [14] observed that CD8+ T cells increase cysteine sulfenic acid levels 2-fold following activation. This increase was comparable to a study where rat hearts were perfused with H2O2prior to sulfenic acid detection [36]. Under physiological ROI production, such as those following antigen receptor crosslinking, changes in total sulfenic acid formation are likely to be less. However when compared to B cells, CD8+ T cells have a longer duration of ROI production following physiological stimulation, possibly accounting for the differences in sulfenic acid [14]. The range of global protein oxidation that is consistent with survival is probably narrower in B cells compared to other cell types. Aside from measuring total cysteine sulfenic acid levels, we determined that sulfenic acid localizes to distinct cytosolic puncta following B cell activation. These cytosolic puncta could be composed of sulfenic acid modified proteins we identified clustered in signaling complexes near highly compact lipid rafts and BCRs [38]. However, the nuclear puncta could contain sulfenic acid modified proteins such histone deacetylases or heterochromatin protein 1 that have been shown to be redox sensitive [39, 40]. Previous work using HeLa cells reported diffuse cytosolic sulfenic acid localization following H2O2 treatment [25]. Another study using endothelial cells demonstrated sulfenic acid localization on the leading edge of the lamellipodia following VEGF stimulation [24]. The difference in sulfenic acid localization could be explained by the cytoplasmic to nuclear ratio between the cell types. Compared to lymphocytes, epithelial and endothelial cells have a greater cytoplasmic to nuclear ratio. Because the cytoplasm is smaller in lymphocytes, the ROI generated during activation could more readily diffuse into the nucleus. Furthermore, our studies also demonstrate different kinetics of sulfenic acid formation in PTPs and actin following B cell activation. Unlike CD8+ T cells, SHP-2 cysteine oxidation occurs within 1 minute of B cell activation [14]. It is possible that receptor crosslinking, internalization, and NOX activation occurs more quickly in B cells than CD8+T cells due to the method of stimulation. Compared to CD8+ T cells, we detected cysteine oxidation in actin earlier following receptor ligation. A previous study using mouse fibroblasts showed that cysteine 374 of actin is sensitive to oxidation, and is required for glutathionylation of actin and cytoskeleton spreading [23]. Following TCR stimulation, actin is reorganized to form the immunological synapse between the T cell and APC [41]. This contact is required for 2 hours to facilitate proper T cell activation [41]. However, in B cells, receptor internalization occurs within 15 minutes [9, 42]. The differential kinetics in actin oxidation between the cell types could control the differences in actin reorganization following activation. Interestingly, in B cells, SHP-1 maximal oxidation occurred at 5 minutes and was similar to CD8+ T cells [8]. Previous work has shown that recruitment of SHP-1 to CD22 is necessary to down-regulate BCR signals [43]. Docking of SHP-1 to CD22 could explain the delay in oxidation, ensuring that SHP-1 activity is decreased when it is recruited to the plasma membrane to allow full signal through the BCR. Furthermore, we are the first to document that PTEN is oxidized following B cell activation. Like SHP-1, cysteine oxidation of PTEN and its subsequent inactivation could be delayed allowing the opposing kinase, PI3K, to dock at CD19 [44]. Interestingly, we could not detect sulfenic acid formation in CD45 following B cell activation. It is possible that CD45 could be in a disulfide bond with glutathione, sulfenamide, sulfinic, or sulfonic acid species, which may account for our inability to detect sulfenic acid. Together, our results demonstrate that B cells exhibit a unique cysteine oxidation profile following activation compared to other cell types and it is tightly regulated to facilitate proper signal transduction and activation.
In this study, we demonstrate that the reversible oxidation of cysteine is a mechanism by which ROI modifies proteins to promote B cell activation and proliferation. The goal of autoimmune therapies and vaccination is to dampen or enhance the immune response, respectively. By identifying proteins in signaling pathways that are regulated by oxidation, it may be possible to design targeted therapeutics to modulate B cell responses.
Materials and Methods
Mice and Cell Isolation
Spleens were removed from six to eight week old C57BL/6 mice after cervical dislocation. After teasing apart the spleen on a wire mesh screen, red blood cells were osmotically lysed using ACK Lysis Buffer (Lonza). Splenocytes were resuspended in complete media composed of RPMI 1640 supplemented with 10% fetal calf serum (FCS, HyClone), L-glutamine (HyClone), penicillin-streptomycin (Cellgro), non-essential amino acids (GIBCO), and 2-mercaptoethanol (GIBCO).
B Cell Isolation
B cells were isolated from spleens of C57BL/6 mice using MiltenyiBiotecCD43 negative selection magnetic bead separation according to the manufacturer's protocol. Purity was routinely >96% B220+ cells as determined by acquisition on FACSCalibur instrument.
In Vitro Stimulation and Dimedone Pretreatment
For all stimulations, with the exception of the calcium flux experiments, purified cells were pretreated for 1 hour at 37°C with complete media alone (vehicle) or media containing 5,5-dimethyl-1,3-cyclohexanedione (dimedone) (Sigma-Aldrich). Purified B cells were cultured at 5×105 cells/mL in 48 well plates and were stimulated with F(ab′)2 goat anti-mouse IgM (Jackson Immuno Research) or hen egg lysozyme (HEL) (Sigma-Aldrich).
ROI Detection by Dihydroethidium (DHE)
Following stimulation in a 96 well flat bottom plate, purified B cells were incubated with 4μM dihydroethidium (DHE, Molecular Probes) as previously described by Laniewski and Grayson [45].
Surface Staining
Surface staining was performed by incubating the cells in a 1:100 dilution of rat anti-mouse B220-APC (BD Pharmingen) in 2% FACS Buffer (phosphate buffered saline plus 2% FCS) for 30 minutes at on ice. Cells were washed three times and fixed in 2% paraformaldehyde (Sigma-Aldrich).
Confocal Microscopy
Purified (1. 5 × 106) B cells were seeded into wells containing an air-dried, poly-L-lysine (0. 01% solution, Sigma-Aldrich) coated coverslip for 30 minutes at room temperature. After washing with PBS, cells were stimulated in the presence or absence of 10 μg/mL anti-IgM or 0. 2 mM hydrogen peroxide at 37°C. Additionally, one sample was pretreated with 20 mM N-acetyl-cysteine (NAC) for 1 hour prior to stimulation. At the end of each timepoint, samples were washed, incubated in vehicle or dimedone, and processed for confocal microscopy according to Seo and Carroll [25] using a 1:500 dilution of anti-dimedone antibody (Millipore) and a secondary goat anti-rabbit Alexa-Fluor 488 (Invitrogen). Following sulfenic acid staining, cells were stained with DRAQ5 (Cell Signaling) and mounted with ProLong Gold anti-fade reagent (Invitrogen) according to manufacturer's protocol. 12-Bit images were acquired using a Zeiss LSM 510 confocal laser scanning microscope with a 63× magnification objective lens. For each experiment, exposure settings were determined to avoid saturation and were used for all samples in order to compare intensities.
The open source software ImageJ (National Institutes of Health) was used to quantify cysteine sulfenic acid levels within the nucleus and cytoplasm. The mean fluorescent intensity within the borders of the cell and nucleus was determined. To determine the cytoplasmic fluorescence, the nuclear value was subtracted from the whole cell value. Six fields of view were analyzed for each condition.
Sulfenic Acid Labeling
Purified (2×106) B cells were stimulated with 10 μg/mL anti-IgM, washed one time with PBS, and lysed in the presence of 50 mM Tris-HCl, 100 mM NaCl, 20 mM β-glycerophosphate, 0. 1% SDS, 0. 5% sodium deoxycholate, 0. 5% Igepal, 0. 5% Triton-X-100, 1 mM Na3VO4, 20 mM NaF, 1 mM PMSF, 10 μg/mL aprotinin, 10 μg/mL leupeptin, and 1 mM dimedone. After incubating on ice for 30 minutes, samples were stored at −80°C.
For biotin-based affinity capture experiments, purified B cells (4×106) were stimulated with 10 μg/mL anti-IgM, washed one time with PBS, and lysed in the presence of 50 mM Tris-HCl, 100 mM NaCl, 100 μM DTPA, 20 mM β-glycerophosphate, 0. 1% SDS, 0. 5% sodium deoxycholate, 0. 5% Igepal, 0. 5% Triton-X-100, 1 mM Na3VO4, 20 mM NaF, 1 mM PMSF, 10 μg/mL aprotinin, 10 μg/mL leupeptin, 200 units of catalase, 10 mM N-ethyl-maleimide, and 5 mM DCP-Bio1 [46]. Cell lysates were sonicated for 10 seconds and incubated on ice for 1 hour prior to biotin-based affinity capture. Additionally, one set of samples was pretreated with vehicle or 10 mM dimedone for 1 hour prior to stimulation and sulfenic acid labeling.
For immunoprecipitation experiments, 2-4 × 106 purified B cells were stimulated with 10 μg/mL anti-IgM and lysates were prepared as previously described by Michalek et al. [14]. Briefly, cells were washed with PBS prior to lysis in the presence of DCP-Bio1 and lysates were precleared for 1 hour at 4°C with protein G beads (Dynal). Following magnetic bead removal, lysates were incubated with 2. 5 μg/mL anti-SHP-2 (BD Pharmingen), anti-SHP-1, or anti-actin (Santa Cruz Biotechnology) at 4°C with constant rotation overnight. The following day, protein G beads were added and the lysates were rotated at 4°C for 3 hours. After discarding the supernatant, the magnetic beads were washed three times, resuspended in lysis buffer, and protein was eluted by boiling in reducing sample buffer (Pierce).
Biotin-Based Affinity Capture
Affinity capture of biotinylated proteins was performed according to Nelson et al. [47]. Samples were boiled with reducing sample buffer, separated on a 10% pre-cast SDS denaturing gel, and transferred to a PVDF membrane. The membrane was blocked and probed with anti-PTEN (Cell Signaling) or anti-CD45 (Santa Cruz Biotechnology) according to the manufacturer's protocol.
Sulfenic Acid Detection
For sulfenic acid detection, samples lysed in the presence of 1 mM dimedone were separated on a 10-12% pre-cast SDS denaturing gel and transferred to a PVDF membrane. The membrane was blocked and incubated with anti-dimedone antibody (Millipore) according to the manufacturer's protocol. Proteins were visualized as previously described [14]. The blot was stripped and probed with anti-actin. To quantify sulfenic acid, actin and cysteine sulfenic acid levels were normalized between samples using a Kodak Image Station 4000R and Carestream Molecular Imaging Software. The entire length of the gel lane was used to determine sulfenic acid levels. Only the protein band was used for actin. The sulfenic acid signal was then normalized to actin protein levels.
Detection of sulfenic acid during immunoprecipitation experiments was performed as previously described [14]. Briefly, samples lysed in the presence of 5 mM DCP-Bio1 were separated on a 7. 5-15% SDS denaturing gel and transferred to a PVDF membrane. The membrane was blocked overnight at 4°C with 5% FCS in tris buffered saline supplemented with 0. 1% Tween-20 (TBS-T). The following day, the membrane was washed three times and incubated with 1:50, 000 dilution Streptavidin-HRP (SouthernBiotech) in 5% FCS in TBS-T for 1 hour at room temperature. After washing, the membrane was developed as previously described [14].
CFSE Labeling
CFSE (5-6-carboxyfluorescein diacetate, succinimidyl ester, Molecular Probes) was resuspended in DMSO at a 5 mM stock and stored at −20°C. Purified B cells were washed with cold PBS three times and resuspended in PBS at 20×106 cells/mL. The CFSE stock solution was diluted in PBS to 6. 67 μM and was mixed 1:1 (v/v) with cells for a final concentration of 3. 33 μM. After 3 minutes of incubation, cells were vortexed at room temperature and incubation continued for 2 additional minutes. One tenth of the volume of FCS was added and the cells were vortexed and incubated for 1 minute. Samples were washed three times in complete media and used in experiments. Percent divided, division, and proliferation indices were determined by FlowJo software.
Cell Viability Assay
Purified B cells (5×105) were used ex vivo or after 24-48 hours of stimulation with media or 10 μg/mL anti-IgM in the presence of vehicle or dimedone. Cells were harvested and surface stained with B220-APC as described above. After surface staining, cells were resuspended in 7-amino-actinomycin D (7-AAD) and Annexin-V FITC (BD Pharmingen) for 15 minutes at room temperature according to the manufacturer's protocol. Cells were acquired immediately on a FACSCalibur Instrument. All samples were analyzed using FlowJo Software.
BrdU Labeling
Purified (5×105) B cells were stimulated with 10 μg/mL anti-IgM in the presence of vehicle or dimedone. At 45 hours, samples were pulsed with 10 μM BrdU (Sigma-Aldrich) for 3 hours and labeling was performed as described previously [14]. Briefly, cells were harvested and resuspended in 1% paraformaldehyde with 0. 05% Igepal (Sigma-Aldrich), vortexed, and incubated at 4°C overnight. Samples were washed at room temperature two times with PBS at 1200 rpm for 6 minutes, resuspended in 1 mL PBS and 4. 2 mM MgCl2 containing 50 Kunitz U/mL DNase I (Sigma-Aldrich), and incubated at 37°C for 30 minutes. Following two washes in wash buffer (PBS supplemented with 5% FCS and 0. 5% Igepal) at 1200 rpm for 6 minutes at 4°C, samples were resuspended in the same buffer containing 2% mouse serum and a 1/5 dilution of anti-BrdU FITC (BD Pharmingen). Samples were incubated on ice for 45 minutes. After two washes, cells were resuspended in 10 μL 7-AAD (BD Pharmingen) plus FACS buffer for 15 minutes on ice. Cells were acquired immediately on a FACSCalibur Instrument.
Detection of Phosphorylated Proteins by Western Blot
Purified B cells (2×106) were stimulated in the presence or absence of dimedone with 10 μg/mL anti-IgM. After stimulation, cells were pelleted, washed with PBS, and lysed in buffer described previously [14]. Samples were boiled in the presence of reducing sample buffer, ran on a 7. 5% pre-cast SDS-PAGE gel (Bio-Rad), transferred to a PVDF membrane, and probed for phospho-Syk (Y525/526) (C87C1), Syk, phospho-p44/p42 MAPK (T202/Y204), or p44/p42 (Cell Signaling) according to the manufacturer's protocol.
For phospho-tyrosine detection, 2. 5×106 purified B cells were stimulated in the presence or absence of dimedone with 10 μg/mL anti-IgM. Samples were lysed, ran on a SDS-PAGE, transferred to a membrane, and probed for tyrosine phosphorylated proteins (4G10-HRP, Millipore) as previously described by Fujimoto et al. [48]. After the blot was developed and imaged, it was stripped and probed with anti-actin as previously described [14].
Immunoprecipitation of Lyn and PLCγ2 was performed by stimulating 2×106 purified B cells in the presence or absence of dimedone with 10 μg/mL anti-IgM. Following stimulation, cells were pelleted, washed, lysed, and immunoprecipitation was performed as described previously [14] using 2. 5 μg/mL anti-Lyn or anti-PLCγ2 (Santa Cruz Biotechnology). Samples were run on a 7. 5 or 12% pre-cast SDS-PAGE gel and transferred to a PVDF membrane. Prior to phosphotyrosine detection, the membrane was blocked and probed with anti-Lyn according to manufacturer's protocol using a HRP conjugated light chain specific mouse anti-rabbit IgG (Jackson ImmunoResearch). After the blot was imaged and developed, the membrane was stripped and probed with the anti-phosphotyrosine antibody described previously. For phospho-PLCγ2 detection, the blot was probed for phospho-tyrosine followed by total protein.
To determine the fold-increase in phosphorylation for all proteins, the entire protein lane or the protein band was normalized to the total protein. The fold increase in phosphorylation was calculated by multiplying the fold difference in the normalized total protein value by the phosphorylated signal.
Calcium Flux Assay
Fura-Red acetoxymethyl (AM) ester and Fluo-3-AM were purchased from Molecular Probes and dissolved in DMSO as a 1 mM and 1. 25 mM stock, respectively. Purified B cells were incubated with 5 μM Fura-Red AM and 2. 5 μM Fluo-3-AMin PBS containing 5% FCS for 30 minutes at 37°C in the presence of DMSO control or 10 mM dimedone (dissolved in DMSO). Samples were washed two times with PBS supplemented with 5% FCS and resuspended in the same media containing 10 mM dimedone or DMSO control. Cells were acquired for 60 seconds on the FACSCalibur Flow Cytometer and then 10 μg/mL anti-IgM was added to the samples and recording was resumed on the instrument. Endoplasmic reticulum (ER) calcium release and CCE was measured as described by Jia et al. [49].
Supplementary Material
Supplemental Figure 1. NAC treatment decreases anti-IgM induced B cell proliferation. B cells were purified from naïve splenocytes and labeled with CFSE. Prior to stimulation with 10 μg/mL anti-IgM, cells were pretreated in PBS control or 20 mM NAC for 1 hour. Proliferation was assessed by the loss of CFSE fluorescence at various days post stimulation. Results are representative of four mice in two independent experiments.
Supplemental Figure 2. Dimedone pretreatment decreases cysteine sulfenic acid formation in the total proteome and effector molecules following BCR ligation. Purified B cells from naïve C57BL/6 mice were pretreated with vehicle or 10 mM dimedone prior to stimulation with 10 μg/mL anti-IgMfor the indicated timepoints. Cells were harvested, washed in PBS, and lysed in the presence of DCP-Bio1. (A) Total protein was precipitated following DCP-Bio1 labeling. Blots were probed for sulfenic acid using streptavidin-HRP. The blot was stripped and probed for actin as a loading control. (B) SHP-1 and (C) SHP-2 were immunoprecipitated after DCP-Bio1 labeling as previously described. (D) PTEN was probed following affinity capture of biotinylated proteins as previously described. Results are representative of two independent experiments.
Supplemental Figure 3. NAC treatment initiates ER calcium release and inhibits CCE in B cells.
Purified B cells were incubated with 5 μM Fura-Red-AM and 2. 5 μM Fluo-3-AM for 30 minutes. (A) After washing, cells were resuspended in PBS supplemented with 1 mM EGTA and were acquired on the cytometer for 1 minute prior to incubation with PBS control, NAC, or thapsigargin. Samples were placed back on the cytometer and acquired for an additional 5 minutes and 30 seconds. Following centrifugation for 3 minutes, the EGTA was removed and the cells were resuspended in PBS supplemented with 1. 26 mM CaCl2 in the (B) presence of PBS control or NAC. Samples were placed on the cytometer and acquired for a total of 1024 seconds. The arrows indicate addition of stimuli, dimedone, or PBS control. In all plots, the ratio of Fluo-3 to Fura-Red fluorescence was recorded as a function of time. The histograms are representative of three mice in a minimum of three independent experiments.
Acknowledgments
We thank David Ornelles and Kenneth Grant for their helpful input with the confocal microscopy experiments.
This work was supported by NIAID grants RO1-AI068952 and R56-AI073571 to J. M. G and NCI grant R33 CA126659 to L. B. P. K. E. C. was supported by NIAID grant 5T32AI007401-20.
Abbreviations used in this paper
- ROI
reactive oxygen intermediates
- DHE/HE
dihydroethidium
- dimedone
5,5-dimethyl-1,3-cyclohexanedione
- Fluo-3-AM
Fluo-3 acetoxymethyl ester
- Fura-Red-AM
Fura-Red-acetoxymethyl ester
- HEL
hen egg lysozyme
- NAC
N-acetyl-cysteine
- PVDF
polyvinylidene fluoride
- PLCγ2
phospholipase c gamma 2
- Syk
spleen tyrosine kinase
References
- 1.Dal Porto JM, Gauld SB, Merrell KT, Mills D, Pugh-Bernard AE, Cambier J. B cell antigen receptor signaling 101. Mol Immunol. 2004;41:599–613. doi: 10.1016/j.molimm.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Scharenberg AM, Humphries LA, Rawlings DJ. Calcium signalling and cell-fate choice in B cells. Nat Rev Immunol. 2007;7:778–789. doi: 10.1038/nri2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurosaki T, Shinohara H, Baba Y. B cell signaling and fate decision. Annual review of immunology. 2010;28:21–55. doi: 10.1146/annurev.immunol.021908.132541. [DOI] [PubMed] [Google Scholar]
- 4.Shinohara H, Kurosaki T. Genetic analysis of B cell signaling. Sub-cellular biochemistry. 2006;40:145–187. doi: 10.1007/978-1-4020-4896-8_10. [DOI] [PubMed] [Google Scholar]
- 5.Infantino S, Benz B, Waldmann T, Jung M, Schneider R, Reth M. Arginine methylation of the B cell antigen receptor promotes differentiation. The Journal of experimental medicine. 2010;207:711–719. doi: 10.1084/jem.20091303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fialkow L, Wang Y, Downey GP. Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radic Biol Med. 2007;42:153–164. doi: 10.1016/j.freeradbiomed.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 7.Iles KE, Forman HJ. Macrophage signaling and respiratory burst. Immunol Res. 2002;26:95–105. doi: 10.1385/IR:26:1-3:095. [DOI] [PubMed] [Google Scholar]
- 8.Capasso M, Bhamrah MK, Henley T, Boyd RS, Langlais C, Cain K, Dinsdale D, et al. HVCN1 modulates BCR signal strength via regulation of BCR-dependent generation of reactive oxygen species. Nat Immunol. 11:265–272. doi: 10.1038/ni.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richards SM, Clark EA. BCR-induced superoxide negatively regulates B-cell proliferation and T-cell-independent type 2 Ab responses. Eur J Immunol. 2009;39:3395–3403. doi: 10.1002/eji.200939587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh DK, Kumar D, Siddiqui Z, Basu SK, Kumar V, Rao KV. The strength of receptor signaling is centrally controlled through a cooperative loop between Ca2+ and an oxidant signal. Cell. 2005;121:281–293. doi: 10.1016/j.cell.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 11.Poole LB, Karplus PA, Claiborne A. Protein sulfenic acids in redox signaling. Annu Rev Pharmacol Toxicol. 2004;44:325–347. doi: 10.1146/annurev.pharmtox.44.101802.121735. [DOI] [PubMed] [Google Scholar]
- 12.Poole LB, Nelson KJ. Discovering mechanisms of signaling-mediated cysteine oxidation. Curr Opin Chem Biol. 2008;12:18–24. doi: 10.1016/j.cbpa.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paulsen CE, Carroll KS. Orchestrating redox signaling networks through regulatory cysteine switches. ACS Chem Biol. 5:47–62. doi: 10.1021/cb900258z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michalek RD, Nelson KJ, Holbrook BC, Yi JS, Stridiron D, Daniel LW, Fetrow JS, et al. The requirement of reversible cysteine sulfenic acid formation for T cell activation and function. J Immunol. 2007;179:6456–6467. doi: 10.4049/jimmunol.179.10.6456. [DOI] [PubMed] [Google Scholar]
- 15.Allison WS. Formation and Reactions of Sulfenic Acids in Proteins. Accounts of Chemical Research. 1976;9:293–299. [Google Scholar]
- 16.May JM, de Haen C. Insulin-stimulated intracellular hydrogen peroxide production in rat epididymal fat cells. J Biol Chem. 1979;254:2214–2220. [PubMed] [Google Scholar]
- 17.Mahadev K, Wu X, Zilbering A, Zhu L, Lawrence JT, Goldstein BJ. Hydrogen peroxide generated during cellular insulin stimulation is integral to activation of the distal insulin signaling cascade in 3T3-L1 adipocytes. J Biol Chem. 2001;276:48662–48669. doi: 10.1074/jbc.M105061200. [DOI] [PubMed] [Google Scholar]
- 18.Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 19.Bae YS, Kang SW, Seo MS, Baines IC, Tekle E, Chock PB, Rhee SG. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J Biol Chem. 1997;272:217–221. [PubMed] [Google Scholar]
- 20.Wani R, Qian J, Yin L, Bechtold E, King SB, Poole LB, Paek E, et al. Isoform-specific regulation of Akt by PDGF-induced reactive oxygen species; Proceedings of the National Academy of Sciences of the United States of America; 2011; pp. 10550–10555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maller C, Schroder E, Eaton P. Glyceraldehyde 3-phosphate dehydrogenase is unlikely to mediate hydrogen peroxide signaling: studies with a novel anti-dimedone sulfenic acid antibody. Antioxidants & redox signaling. 2011;14:49–60. doi: 10.1089/ars.2010.3149. [DOI] [PubMed] [Google Scholar]
- 22.Mustelin T, Vang T, Bottini N. Protein tyrosine phosphatases and the immune response. Nature reviews Immunology. 2005;5:43–57. doi: 10.1038/nri1530. [DOI] [PubMed] [Google Scholar]
- 23.Fiaschi T, Cozzi G, Raugei G, Formigli L, Ramponi G, Chiarugi P. Redox regulation of beta-actin during integrin-mediated cell adhesion. The Journal of biological chemistry. 2006;281:22983–22991. doi: 10.1074/jbc.M603040200. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan N, Urao N, Furuta E, Kim SJ, Razvi M, Nakamura Y, McKinney RD, et al. Localized cysteine sulfenic acid formation by vascular endothelial growth factor: role in endothelial cell migration and angiogenesis. Free radical research. 2011;45:1124–1135. doi: 10.3109/10715762.2011.602073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seo YH, Carroll KS. Profiling protein thiol oxidation in tumor cells using sulfenic acid-specific antibodies; Proceedings of the National Academy of Sciences of the United States of America; 2009; pp. 16163–16168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunt NH, Cook EP, Fragonas JC. Interference with oxidative processes inhibits proliferation of human peripheral blood lymphocytes and murine B-lymphocytes. Int J Immunopharmacol. 1991;13:1019–1026. doi: 10.1016/0192-0561(91)90056-d. [DOI] [PubMed] [Google Scholar]
- 27.Fedyk ER, Borrello MA, Brown DM, Phipps RP. Regulation of B cell tolerance and triggering by immune complexes. Chem Immunol. 1994;58:67–91. [PubMed] [Google Scholar]
- 28.Winterbourn CC, Hampton MB. Thiol chemistry and specificity in redox signaling. Free radical biology & medicine. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Vene R, Delfino L, Castellani P, Balza E, Bertolotti M, Sitia R, Rubartelli A. Redox remodeling allows and controls B-cell activation and differentiation. Antioxidants & redox signaling. 2010;13:1145–1155. doi: 10.1089/ars.2009.3078. [DOI] [PubMed] [Google Scholar]
- 30.Poole LB, Klomsiri C, Knaggs SA, Furdui CM, Nelson KJ, Thomas MJ, Fetrow JS, et al. Fluorescent and affinity-based tools to detect cysteine sulfenic acid formation in proteins. Bioconjug Chem. 2007;18:2004–2017. doi: 10.1021/bc700257a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poole LB, Zeng BB, Knaggs SA, Yakubu M, King SB. Synthesis of chemical probes to map sulfenic acid modifications on proteins. Bioconjug Chem. 2005;16:1624–1628. doi: 10.1021/bc050257s. [DOI] [PubMed] [Google Scholar]
- 32.Choi H, Kim S, Mukhopadhyay P, Cho S, Woo J, Storz G, Ryu SE. Structural basis of the redox switch in the OxyR transcription factor. Cell. 2001;105:103–113. doi: 10.1016/s0092-8674(01)00300-2. [DOI] [PubMed] [Google Scholar]
- 33.Zheng M, Aslund F, Storz G. Activation of the OxyR transcription factor by reversible disulfide bond formation. Science. 1998;279:1718–1721. doi: 10.1126/science.279.5357.1718. [DOI] [PubMed] [Google Scholar]
- 34.Delaunay A, Pflieger D, Barrault MB, Vinh J, Toledano MB. A thiol peroxidase is an H2O2 receptor and redox-transducer in gene activation. Cell. 2002;111:471–481. doi: 10.1016/s0092-8674(02)01048-6. [DOI] [PubMed] [Google Scholar]
- 35.Oshikawa J, Urao N, Kim HW, Kaplan N, Razvi M, McKinney R, Poole LB, et al. Extracellular SOD-derived H2O2 promotes VEGF signaling in caveolae/lipid rafts and post-ischemic angiogenesis in mice. PLoS One. 5:e10189. doi: 10.1371/journal.pone.0010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saurin AT, Neubert H, Brennan JP, Eaton P. Widespread sulfenic acid formation in tissues in response to hydrogen peroxide. Proc Natl Acad Sci U S A. 2004;101:17982–17987. doi: 10.1073/pnas.0404762101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carballal S, Radi R, Kirk MC, Barnes S, Freeman BA, Alvarez B. Sulfenic acid formation in human serum albumin by hydrogen peroxide and peroxynitrite. Biochemistry. 2003;42:9906–9914. doi: 10.1021/bi027434m. [DOI] [PubMed] [Google Scholar]
- 38.Pierce SK. Lipid rafts and B-cell activation. Nature reviews Immunology. 2002;2:96–105. doi: 10.1038/nri726. [DOI] [PubMed] [Google Scholar]
- 39.Doyle K, Fitzpatrick FA. Redox signaling, alkylation (carbonylation) of conserved cysteines inactivates class I histone deacetylases 1, 2, and 3 and antagonizes their transcriptional repressor function. The Journal of biological chemistry. 2010;285:17417–17424. doi: 10.1074/jbc.M109.089250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Higo S, Asano Y, Kato H, Yamazaki S, Nakano A, Tsukamoto O, Seguchi O, et al. Isoform-specific intermolecular disulfide bond formation of heterochromatin protein 1 (HP1) The Journal of biological chemistry. 2010;285:31337–31347. doi: 10.1074/jbc.M110.155788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee KH, Holdorf AD, Dustin ML, Chan AC, Allen PM, Shaw AS. T cell receptor signaling precedes immunological synapse formation. Science. 2002;295:1539–1542. doi: 10.1126/science.1067710. [DOI] [PubMed] [Google Scholar]
- 42.Fleire SJ, Goldman JP, Carrasco YR, Weber M, Bray D, Batista FD. B cell ligand discrimination through a spreading and contraction response. Science. 2006;312:738–741. doi: 10.1126/science.1123940. [DOI] [PubMed] [Google Scholar]
- 43.Doody GM, Justement LB, Delibrias CC, Matthews RJ, Lin J, Thomas ML, Fearon DT. A role in B cell activation for CD22 and the protein tyrosine phosphatase SHP. Science. 1995;269:242–244. doi: 10.1126/science.7618087. [DOI] [PubMed] [Google Scholar]
- 44.Aiba Y, Kameyama M, Yamazaki T, Tedder TF, Kurosaki T. Regulation of B-cell development by BCAP and CD19 through their binding to phosphoinositide 3-kinase. Blood. 2008;111:1497–1503. doi: 10.1182/blood-2007-08-109769. [DOI] [PubMed] [Google Scholar]
- 45.Laniewski NG, Grayson JM. Antioxidant treatment reduces expansion and contraction of antigen-specific CD8+ T cells during primary but not secondary viral infection. J Virol. 2004;78:11246–11257. doi: 10.1128/JVI.78.20.11246-11257.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klomsiri C, Nelson KJ, Bechtold E, Soito L, Johnson LC, Lowther WT, Ryu SE, et al. Use of dimedone-based chemical probes for sulfenic acid detection evaluation of conditions affecting probe incorporation into redox-sensitive proteins. Methods Enzymol. 473:77–94. doi: 10.1016/S0076-6879(10)73003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nelson KJ, Klomsiri C, Codreanu SG, Soito L, Liebler DC, Rogers LC, Daniel LW, et al. Use of dimedone-based chemical probes for sulfenic acid detection methods to visualize and identify labeled proteins. Methods Enzymol. 473:95–115. doi: 10.1016/S0076-6879(10)73004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fujimoto M, Poe JC, Jansen PJ, Sato S, Tedder TF. CD19 amplifies B lymphocyte signal transduction by regulating Src-family protein tyrosine kinase activation. Journal of immunology. 1999;162:7088–7094. [PubMed] [Google Scholar]
- 49.Jia W, Pua HH, Li QJ, He YW. Autophagy regulates endoplasmic reticulum homeostasis and calcium mobilization in T lymphocytes. Journal of immunology. 2011;186:1564–1574. doi: 10.4049/jimmunol.1001822. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. NAC treatment decreases anti-IgM induced B cell proliferation. B cells were purified from naïve splenocytes and labeled with CFSE. Prior to stimulation with 10 μg/mL anti-IgM, cells were pretreated in PBS control or 20 mM NAC for 1 hour. Proliferation was assessed by the loss of CFSE fluorescence at various days post stimulation. Results are representative of four mice in two independent experiments.
Supplemental Figure 2. Dimedone pretreatment decreases cysteine sulfenic acid formation in the total proteome and effector molecules following BCR ligation. Purified B cells from naïve C57BL/6 mice were pretreated with vehicle or 10 mM dimedone prior to stimulation with 10 μg/mL anti-IgMfor the indicated timepoints. Cells were harvested, washed in PBS, and lysed in the presence of DCP-Bio1. (A) Total protein was precipitated following DCP-Bio1 labeling. Blots were probed for sulfenic acid using streptavidin-HRP. The blot was stripped and probed for actin as a loading control. (B) SHP-1 and (C) SHP-2 were immunoprecipitated after DCP-Bio1 labeling as previously described. (D) PTEN was probed following affinity capture of biotinylated proteins as previously described. Results are representative of two independent experiments.
Supplemental Figure 3. NAC treatment initiates ER calcium release and inhibits CCE in B cells.
Purified B cells were incubated with 5 μM Fura-Red-AM and 2. 5 μM Fluo-3-AM for 30 minutes. (A) After washing, cells were resuspended in PBS supplemented with 1 mM EGTA and were acquired on the cytometer for 1 minute prior to incubation with PBS control, NAC, or thapsigargin. Samples were placed back on the cytometer and acquired for an additional 5 minutes and 30 seconds. Following centrifugation for 3 minutes, the EGTA was removed and the cells were resuspended in PBS supplemented with 1. 26 mM CaCl2 in the (B) presence of PBS control or NAC. Samples were placed on the cytometer and acquired for a total of 1024 seconds. The arrows indicate addition of stimuli, dimedone, or PBS control. In all plots, the ratio of Fluo-3 to Fura-Red fluorescence was recorded as a function of time. The histograms are representative of three mice in a minimum of three independent experiments.