Abstract
Bone Morphogenetic Proteins (BMPs) have diverse functions during development in vertebrates. We have recently shown that BMP2 signaling promotes venous specific angiogenesis in zebrafish embryos. However, factors that confer a context dependent pro-angiogenic function of BMP2 signaling within endothelial cells need to be identified. Here, we report that Disabled homolog 2 (Dab2), a cargo specific adaptor protein for Clathrin, is essential to mediate the pro-angiogenic function of BMP2 signaling. We find that inhibition of Dab2 attenuates internalization of BMP receptors and abrogates the pro-angiogenic effects of BMP signaling in endothelial cells. Moreover, inhibition of Dab2 decreases phosphorylation of SMAD-1, 5, and 8, indicating that Dab2 plays an essential role in determining the outcome of BMP signaling within endothelial cells, and may provide a molecular basis for a context dependent pro-angiogenic function of BMP2 signaling.
Introduction
Endocytosis is essential for a variety of cellular and physiological processes. In many cases, activated receptors undergo ‘receptor-mediated endocytosis’ upon ligand binding, which modifies the duration and strength of the response within cells allowing context dependent signaling (Dobrowolski and De Robertis, 2011; Le Roy and Wrana, 2005; Shilo and Schejter, 2011). This process is regulated by arrays of adaptor molecules that link membrane bound proteins (cargo) with Clathrin coated pit (CCP) (Reider and Wendland, 2011; Traub, 2009). Internalized receptors can be retained within endosomal compartments to prolong signaling, or can undergo rapid degradation which results in attenuation of the signaling (Di Guglielmo et al., 2003; Platta and Stenmark, 2011).
By selectively associating with distinct cargo specific adaptor molecules and co-receptors, activated receptors can change internalization and subsequent intracellular trafficking routes. This allows signal receiving cells to produce distinct context dependent responses to the same signal. For instance, activated Vascular Endothelial Growth Factor Receptor 2 (VEGFR2), the main receptor for VEGF-A, generates distinct responses in endothelial cells depending on the auxiliary molecules. While Synectin-MyosinVI complex dependent trafficking of VEGFR2 facilitates arteriogenesis in mice and zebrafish (Lanahan et al., 2010), EphrinB2 dependent internalization of VEGFR2 or R3 promotes developmental angiogenesis in mice (Sawamiphak et al., 2010; Wang et al., 2010).
Disabled homolog 2 (DAB2), which was first identified as the gene down-regulated in ovarian cancer and ovarian carcinoma cell lines (Mok et al., 1994), has been proposed as a cargo specific adaptor protein for Clathrin (Gallagher et al., 2004; Mishra et al., 2002). For instance, DAB2 can bind to Transforming Growth Factor beta (TGF-β) type I and type II receptors, and regulate TGF-β signaling activity (Di Guglielmo et al., 2003; Hocevar et al., 2001; Penheiter et al., 2010). In addition, DAB2 has been shown to interact with Megalin, Cublin, and Transferrin (Maurer and Cooper, 2005; Morris et al., 2002). During murine development, expression of Dab2 is detected in the primitive endoderm and contributes to the formation of the inner cell mass (Yang et al., 2002). In accordance with the early expression pattern, a targeted deletion of Dab2 causes embryonic lethality before E6.5 (Morris et al., 2002; Yang et al., 2002), indicating that DAB2 function is essential for early development. In addition, analyses with conditional knock-outs revealed that Dab2 function is essential for trafficking of Megalin (Morris et al., 2002). In zebrafish, dab2 is expressed within venous endothelial cells, otic vesicles, and pronephric ducts (Sprague et al., 2006). In addition, Morpholino (MO) mediated knock-down studies on dab2 show that Dab2 is essential for metabolite uptake within the pronephric duct (Anzenberger et al., 2006).
We have recently demonstrated that Bone Morphogenetic Protein 2 (Bmp2) signaling can function as a context dependent pro-angiogenic cue in zebrafish embryos. Activation of Bmp2b, a zebrafish ortholog of BMP2, induces ectopic angiogenesis only from venous endothelial cells (Wiley et al., 2011). To better understand the molecular basis of Bmp2b induced angiogenesis within venous endothelial cells, we screened for endothelial specific factors that facilitate venous specific responses to Bmp2b signaling and identified Dab2 as a key modulator. In this report, we show Dab2 is required for the formation of the caudal vein plexus (CVP), and is physically associated with BMP type II receptor (BMPRII). In addition, we demonstrate that DAB2 promotes internalization of the BMP2 ligand receptor complex, which in turn modulates downstream SMAD activation. Taken together, our data suggest that DAB2 mediated endocytosis of the BMP2 ligand-receptor complex is essential for the context dependent pro-angiogenic property of BMP2 signaling.
Results and Discussion
Dab2 is a venous specific modulator of Bmp2b signaling
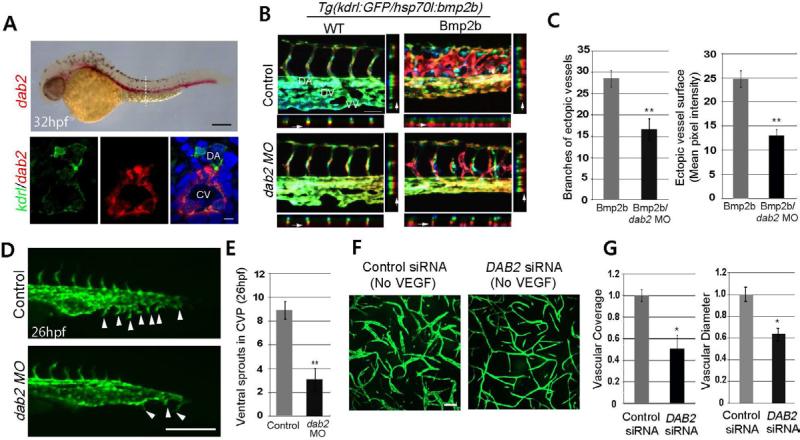
To identify factors that confer venous specificity of Bmp2b-mediated angiogenesis in zebrafish embryos, we first assessed the function of genes preferentially expressed within venous endothelial cells during the formation of the CVP by MO mediated gene knock down. One of the candidates, dab2, is selectively expressed within developing veins, pronephric ducts, and otic vesicles as previously reported (Anzenberger et al., 2006; Sprague et al., 2008) (Figure S1A). Considering the association of Dab2 with TGF-β signaling (Di Guglielmo et al., 2003), we chose to investigate the function of Dab2 in mediating Bmp2b signaling. Endothelial expression of dab2 becomes apparent at 24 hours post-fertilization (hpf) in both arterial and venous endothelial cells, and its expression is gradually restricted to venous endothelial cells between 32 and 48 hpf (Figures 1A and S1A).
Figure 1. Dab2 mediates venous specific pro-angiogenic function of Bmp2b.
(A) Fluorescence in situ for dab2 and immunostaining for kdrl:GFP and DAPI. Lateral view (top) and transverse section (bottom). Scale bar is 200μm (top) or 10μm (bottom). See also Figure S1A. Abbreviations: DA: dorsal aorta, CV: cardinal vein.
(B) Depth-coded images of control or dab2 MO injected 45hpf wild-type (WT) and Bmp2b over-expressing (Bmp2) embryos. XZ and YZ axis views of each image are also shown. Arrows point to ectopic vessels in the projection. Images were obtained between the16th and 20th somite. Scale bar is 100μm. See also Figure S1B-S1F. Abbreviations: DA: dorsal aorta, DV: dorsal vein, VV: ventral vein.
(C) Quantification. The number of branches (N=3, total number of embryos was 19 (Bmp2b) or 17 (Bmp2b/dab2 MO), **p<0.001) and vessel surface as assessed by mean GFP pixel intensity (N=3, total number of embryos was 21 (Bmp2b) or 19 (Bmp2b/dab2 MO), **p<0.001). Error bars represent standard error of the mean (s.e.m).
(D) Fluorescent images of 26hpf control (top) or dab2 (bottom) MO injected embryos. Arrowheads point to ventral sprouts. Scale bar is 100μm.
(E) Quantification of ventral sprouts in the CVP (N=3, total number of embryos was 25 (Control) or 20 (dab2 MO), **p<0.001). Error bars represent s.e.m.
(F) Fibrin gel assay of control and DAB2 siRNA treated HUVECs. Scale bar is 10μm. See also Figures S1G and S1H.
(G) Quantification of vascular coverage and diameter. Values were normalized to control siRNA treatment sample by PRISM program. N=3 and *p<0.05. Error bar is s.e.m.
To examine the function of Dab2 during vascular development, the phenotype of dab2 MO injected embryos was assessed. While the overall vascular structure of dab2 MO injected embryos was comparable to control MO injected embryos, the CVP failed to form, reminiscent of bmpr2a or bmpr2a MO injected embryos (Wiley et al., 2011) (Figures S1B, S1C and Movie S1). To determine whether Dab2 has any discernible function in Bmp2b signaling, we examined the extent of Bmp2b mediated angiogenesis in the presence and absence of functional Dab2. Over-expression of bmp2b induced robust angiogenesis from venous endothelial cells in control MO injected embryos as previously reported (Wiley et al., 2011) (Figure 1B). These ectopic vessels strongly expressed dab2 and stab2, indicating their venous identity (Wong et al., 2009) (Figure S1D). In contrast, the number of Bmp2b induced ectopic vessels was drastically reduced in dab2 MO injected embryos (Figures 1B, 1C and S1D), indicating that Dab2 function is essential for mediating the pro-angiogenic function of Bmp2b within venous endothelial cells.
Previously, factors that mediate BMP signaling in a tissue specific manner, such as Cysteine-rich motor neuron 1 protein (CRIM1) (James and Broihier, 2011) and Jiraiya (Aramaki et al., 2010), have been identified. Considering the expression pattern of dab2 in zebrafish and the mild phenotype of dab2 MO injected embryos (Figures S1B), Dab2 is likely to mediate Bmp2b signaling in a spatiotemporally restricted manner. To test this idea, we examined whether inactivation of Dab2 at earlier stages can ameliorate axis defects caused by excessive Bmp2b signaling (Figures S1E and S1F). In both control and dab2 MO injected embryos, an excessive level of BMP activity early on caused severe ventralization, suggesting that Dab2 is dispensable for Bmp2b signaling during axis formation. Therefore, Dab2 does not appear to be a generic modulator of Bmp2b signaling during development.
Dab2 is required for the angiogenesis of venous endothelial cells
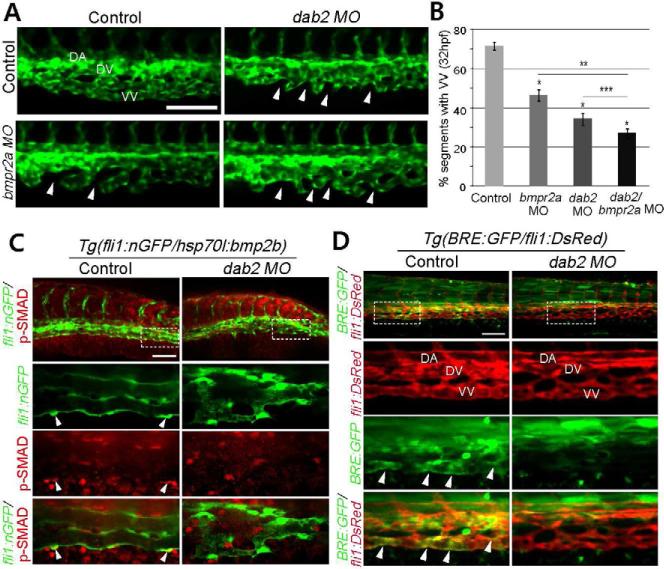
Next, the phenotype of dab2 MO injected embryos was examined at distinct development stages (Figure S2A). While control MO injected embryos displayed stereotypic angiogenesis in the CVP as previously reported (Wiley et al., 2011), the initial formation of ventral sprouts was severely disrupted in dab2 MO injected embryos, resulting in a drastically reduced number of ventral sprouts (Figures 1D and 1E). At 32 hpf, dab2 MO injected embryos failed to form a distinct dorsal and ventral veins (Figures 2A and S2A). Therefore, Dab2 appears to be essential for the formation of CVP.
Figure 2. Dab2 functionally interacts with Bmp2b signal to promote caudal vein plexus formation.
(A) Fluorescent images of 32hpf control, dab2, bmpr2a, and dab2/bmpr2a MO injected embryos. Arrowheads point to the regions which fail to connect to neighboring sprouts. Scale bar is 100μm. See also Figure S2A.
(B) Quantification of ventral vein (VV) defects. N= 3, and total number of embryos was 54 (control), 32 (bmpr2a MO), 26 (dab2 MO), or 35 (dab2/bmpr2a MO). p<0.001 in all cases except p value against dab2 MO injected embryos, where p=0.05. *: comparison with control MO injected embryos, **: comparison with bmpr2a MO injected embryos, and ***: comparison with dab2 MO injected embryos. Error bars represent s.e.m.
(C) Confocal micrographs of control or dab2 MO injected 45hpf Tg(hsp70l:bmp2b) embryos, showing p-Smad-1,5/8 (red) and endothelial cells (green). Arrowheads point to p-Smad-1,5/8 within venous endothelial cells. Scale bar is 100μm.
(D) Confocal micrographs of control or dab2 MO injected 45hpf Tg(BRE:GFP);Tg(fli1:DsRed) embryos. Arrowheads point to GFP expression by BRE. Scale bar is 100μm. Abbreviations: DA, dorsal aorta; DV, dorsal vein; VV, ventral vein.
In human umbilical vein endothelial cells (HUVECs), DAB2 appears to have a similar function in promoting angiogenesis. In Fibrin gel assay, vascular coverage and the diameter of the vessel were significantly decreased in DAB2 siRNA-treated HUVECs in the absence of VEGF-A (Figures 1F, 1G, and S1G). Upon VEGF-A stimulation, DAB2 siRNA-treated HUVECs displayed an increased vascular coverage, indicating that these cells were capable of responding to VEGF-A signaling (Figure S1H). The relative increase in vascular coverage and diameter by VEGF-A stimulation is comparable in control or DAB2 siRNA-treated HUVECs (Figure S1H).
Dab2 is essential for BMP2-mediated SMAD activity
The CVP phenotype observed in dab2 MO injected embryos is strikingly similar to those found in embryos with compromised Bmp2b signaling (Wiley et al., 2011) (Figure S2A), and more severe than the phenotype of bmpr2a MO injected embryos (Figures 2A and 2B), consistent with the idea that Bmpr2a and Bmpr2b have a partially redundant function in CVP formation (Wiley et al., 2011). Moreover, co-injection of dab2 and bmpr2a MO enhanced the defects (Figures 2A and 2B), suggesting that Dab2 and BMP Type II receptors may functionally interact to mediate BMP signaling in venous endothelial cells.
To elucidate the function of Dab2 in Bmp2b signaling, we examined the effects of Dab2 inhibition on SMAD activation within endothelial cells. The presence of phosphorylated SMADs can be detected in a subset of endothelial cells in Bmp2b over-expressing embryos. However, dab2 MO injection completely abolished phosphorylation of SMAD in endothelial cells (Figure 2C), indicating that Dab2 is an essential component of Bmp2b signaling. Similarly, the activity of the BMP Response Element (BRE) was abrogated in dab2 MO injected Tg(BRE:GFP)pt510 (Laux et al., 2011) embryos (Figure 2D).
DAB2 is essential for angiogenic activity of BMP6 in HUVECs
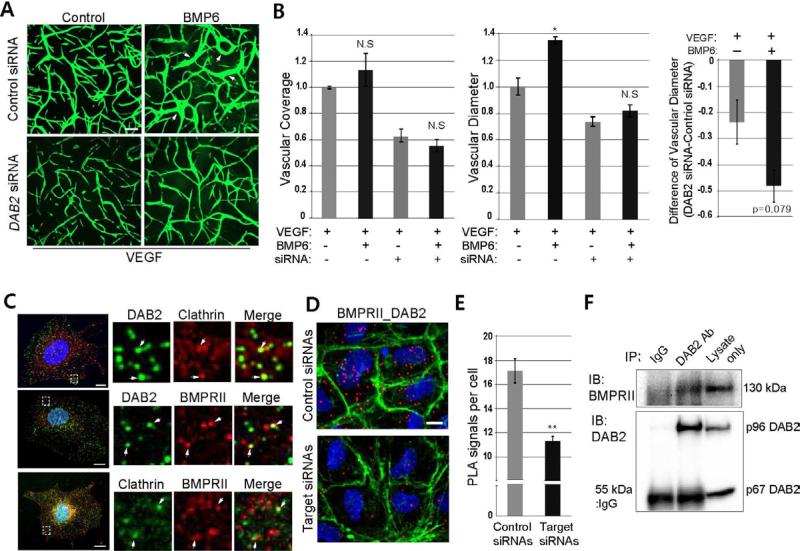
Considering knock-down of dab2 drastically decreased phosphorylation of SMADs and BRE activity, Dab2 function appears to be essential for BMP2 signaling in zebrafish. To examine whether DAB2 has a similar role in mammalian systems, we utilized HUVECs. As in mice (Maurer and Cooper, 2005; Xu et al., 1995), two isoforms of DAB2 are present in HUVECs, with the larger, predominantly expressed form corresponding to the p96 isoform found in mouse (Figure S1G). To interrogate the function of DAB2 in BMP signaling, we treated HUVECs with DAB2 siRNA that preferentially blocks the larger isoform (Figure S1G) and evaluated phenotypes of control and DAB2 siRNA-treated HUVECs on VEGF-A containing Fibrin gel in the presence and absence of BMP6. BMP6 was used since it elicits a robust response in Fibrin gel assay, and it activates the same sets of BMP Type I receptors as BMP2 (Morikawa et al., 2011; Valdimarsdottir et al., 2002). In control siRNA-treated HUVECs, BMP6 treatment increased vessel diameter without affecting the vascular coverage. However, the effect of BMP6 was abrogated in DAB2 siRNA-treated HUVECs (Figures 3A and 3B; arrows).
Figure 3. DAB2 interacts with BMP Type II receptor and Clathin.
(A) Representative images of control or DAB2 siRNA-treated HUVECs plated on Fibrin gel in the presence or absence of 50ng/ml of BMP6. Arrows point to vascular tube with an increased diameter. Scale bar is 200μm.
(B) Quantification of (A). Vascular coverage and diameter were evaluated by PRISM program. N=3 and *p<0.05. Error bar is s.e.m.
(C) Immunocytochemistry detecting DAB2, BMPRII, and Clathrin in HUVECs. Insets are taken from the areas within the rectangles in the left column. Arrows point to co-localization. Scale bar is 10μm. See also Figure S3A-S3C.
(D) Proximity Ligation Assay using antibodies against DAB2 and BMPRII in control (top) or DAB2/BMPRII siRNA-treated (bottom) HUVECs, counter-stained with DAPI (blue) and Phalloidin (green). Scale bar is 10μm. See also Figure S3D and S3E.
(E) Quantification of PLA signals of (D). N=3, and total number of images was 17 for control or target siRNA treatment. **p<0.005 and error bar presents s.e.m.
(F) Co-immunoprecipitation assay using DAB2 antibody. DAB2 antibody was able to pull down BMPRII from HUVEC lysate.
Considering the effects of BMP6 signaling on vessel morphology, it is likely that pro-angiogenic BMP and VEGF-A signaling may function in a distinct manner. For instance, BMP6 stimulation is only able to increase the vessel diameter, which is consistent with the recent finding that BMP signaling is pro-angiogenic without increasing cell proliferation (Finkenzeller et al., 2012). VEGF-A, in turn, only promotes an increase in the vascular coverage (Figures S1H, 3A and 3B).
DAB2, BMPRII and Clathrin are closely associated
Considering that DAB2 is a cargo specific adaptor protein for Clathrin, it is possible that DAB2 may mediate interactions between BMP Type II receptors and Clathrin on cell membranes. In the zebrafish genome, two clathrin heavy polypeptide genes, cltca and cltcb exist with cltca being preferentially expressed in endothelial cells and blood (Sprague et al., 2006; Trinh et al., 2011). In accordance with its expression pattern, MO mediated knock-down of cltca caused severe defects in the formation of blood vessels including intersegmental vessels (ISVs) and the CVP (Figures S3A and S3B). While the defects in ISVs are likely due to the decreased Vegf-A signaling (Lampugnani et al., 2006), the similarities between CVP phenotypes of cltca and dab2 MO injected embryos support the idea that Dab2 may mediate interactions between Clathrin and the Bmp2b signaling complex in endothelial cells (Figure S3C).
To further delineate the functional relationship among DAB2, Clathrin, and the BMP2 signaling complex, we examined the association of DAB2 with BMPRII and Clathrin. Since it has been suggested that BMPRII undergoes constant internalization (Hartung et al., 2006), we examined the interaction in un-stimulated HUVECs, and found that DAB2, BMPRII, and Clathrin co-localize with one another (Figure 3C), suggesting that these molecules may form a complex. Moreover, in situ proximity ligation assay (PLA), which detects co-localization of two proteins within a 40 nm distance of one another (Söderberg et al., 2006), showed a positive PLA signal between DAB2 and BMPRII, DAB2 and Clathrin, and BMPRII and Clathrin (Figures 3D and S3D). Although treatment with DAB2 or BMPRII siRNA significantly decreased the number of positive PLA signals per cell, treatment with BMP6 did not increase the interaction between DAB2 and BMPRII (Figures 3D, 3E and S3E), suggesting that constant internalization of BMPRII may be dependent on DAB2 function. Furthermore, antibodies against DAB2 pulled down endogenous BMPRII protein from HUVEC lysate, suggesting a physical association between these two proteins (Figure 3F). Taken together, our data indicate that DAB2 and BMPRII are likely to physically interact with each other within a Clathrin-coated pit in endothelial cells.
DAB2 promotes the internalization of BMP signaling complex in endothelial cells
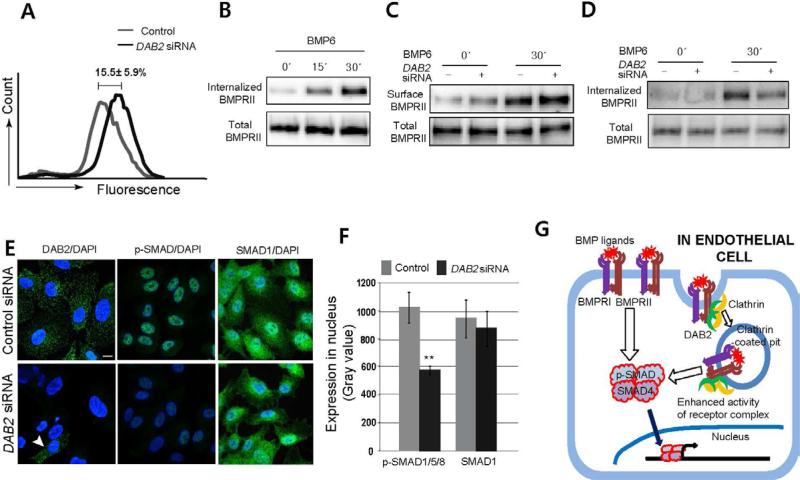
Our data suggest that DAB2 is likely to regulate BMP signaling by promoting the internalization of the BMP ligand receptor complex. To test this idea, we examined the effects of DAB2 knock down on the internalization of BMPRII by measuring the amount of membrane-retained BMPRII using fluorescence activated cell sorting (FACS). In un-stimulated HUVECs treated with DAB2 siRNA, we found that membrane retained BMPRII was significantly increased compared to control siRNA treated HUVECs, suggesting that DAB2 function may be essential for internalization of BMPRII (Figure 4A).
Figure 4. DAB2 promotes internalization of BMP2 signaling complex and modulates downstream SMAD phosphorylation.
(A) FACS analysis using antibody against the extracellular region of BMPRII on control or DAB2 siRNA-treated HUVECs. N=5.
(B) Internalization of BMPRII upon ligand stimulation. The amount of internalized BMPRII was measured by Biotinylation assay in BMP6-treated HUVECs at 0, 15, and 30 minutes after treatment (50ng/ml).
(C) Surface localized BMPRII in control or DAB2 siRNA-treated HUVECs upon BMP6 stimulation (50ng/ml).
(D) Internalized BMPRII in control or DAB2 siRNA-treated HUVECs upon BMP6 stimulation (50ng/ml). See also Figure S4A.
(E) Fluorescence micrographs of control or DAB2 siRNA-treated HUVECs, stained for DAB2 (left) and p-SMAD-1,5/8 (middle), and SMAD-1 (right). Arrowhead points to residual DAB2 expression cell in knock-down sample. Scale bar is 10μm. See also Figure S4B.
(F) Quantification. The gray value of p-SMAD-1, 5/8 and SMAD-1 within nuclei was measured. N=3, and total number of images was 14 (Control) or 16 (DAB2 siRNA) and **p<0.001.
(G) Schematic model. DAB2 is essential for the internalized of BMP signaling complex and subsequent phosphorylation of SMADs.
To test the contribution of BMPRII internalization to BMP signaling, we examine the effects of exogenous ligand stimulation on BMPRII internalization. In BMP6 stimulated HUVECs, Biotinylation assay showed that the amount of internalized BMPRII significantly increased within 30 minutes of BMP6 treatment (Figure 4B), indicating that the presence of ligand accelerates the internalization of BMPRII. In the absence of DAB2, the amount of BMPRII on the surface was significantly increased in both un-stimulated and BMP6-stimulated DAB2 siRNA-treated HUVECs (Figure 4C). Furthermore, the amount of internalized BMPRII and ALK3 (BMPRIA) upon ligand activation was drastically reduced in DAB2 siRNA treated HUVECs (Figure 4D and S4A). Combined, our data show that DAB2 is a critical regulator for the internalization of BMP receptors, suggesting that DAB2 is not only required for constant internalization of BMP receptors in un-stimulated cells, but is also essential for ligand activated internalization of BMP receptors.
Since DAB2 appears to be essential for the internalization of the BMP2 signaling complex, inhibition of DAB2 should abolish Clathrin mediated endocytosis of BMP receptors, therefore, attenuating phosphorylation of SMADs. To test this possibility, we examined the effects of DAB2 manipulation on the phosphorylation of SMADs in HUVECs. Consistent with our data, phosphorylation of SMAD-1, 5, and 8 was drastically decreased in DAB2 siRNA-treated HUVECs under un-stimulated and BMP6-stimulated conditions (Figure 4E, 4F and S4B), suggesting that internalization of the BMP signaling complex is required to promote phosphorylation of SMADs.
Our study demonstrates that Dab2 is an essential regulator of venous morphogenesis by mediating transduction of Bmp2b signaling. Moreover, we found that DAB2 is physically associated with BMPRII and Clathrin, and functions as a key regulator for the internalization of BMP receptors. By modulating internalization of the BMP signaling complex, DAB2 regulates outcomes of BMP signaling in HUVECs (Figure 4G). Since BMPRII is the obligatory component of the heterotetrameric signaling complex for all BMP ligands (Ehrlich et al., 2011; Little and Mullins, 2009), it is likely that DAB2 may mediate signaling of diverse BMP ligands. A recent report on the role of Fcho1/2, a Dab2 interacting protein, in the internalization of Alk2 (Umasankar et al., 2012), further supports this notion. Taken together, it is likely that interactions among DAB2, Clathrin, and BMPRII provide a molecular basis of the context dependent pro-angiogenic function of BMP2/6 signaling.
Experimental Procedures
Zebrafish husbandry, heatshock treatment, and MO injection
Wild-type zebrafish (Danio rerio) and their embryos were raised as previously described (Westerfield, M. 2000). Heat-shock treatment and MO injection were performed as previously described (Wiley et al., 2011). The efficacy of Bmp2b induction was verified by p-SMAD-1/5/8 antibody staining. List of the fish lines and the sequences of MO used in this study are listed in supplementary material.
In situ hybridization and immunohistochemistry
In situ hybridization and immunohistochemistry were performed as previously described (Wiley et al., 2011). The antibodies and recombinant proteins used for this study can be found in supplementary material.
siRNA Transfection and Fibrin gel assay
HUVECs were transfected with Human DAB2 or non-targeted control siRNA (Dharmacon) for DAB2 knock down and Hs_BMPR2_5 flexiTube siRNA (S100604996) and Allstars negative control (Qiagen) for BMPRII knock down. siRNA was used at a concentration of 50 nM for DAB2 and 20 nM for BMPRII using Oligofectamine (Invitrogen) transfectant. After 24 hours, HUVECs (250,000 cells/well in 24 well plates) were resuspended in 300 μl fibrinogen solution (2.5 mg/mL) in EBM-2 (Lonza) supplemented with 2% FBS and 50ug/mL aprotinin, and plated on top of a pre-coated fibrin layer (400 μl fibrinogen solution clotted with 1U thrombin for 20 minutes at 37°C). The secondary layer of fibrin was clotted for 1 hour at 37°C. C3H10T1/2 (250,000 cells/well), in EBM-2 supplemented with 2% FBS and 25 ng/mL VEGF-A, were then plated on top of the fibrin layers and cultures were incubated at 37°C and 5% CO2. Control cultures were grown with VEGF for 2–3 days, VEGF was then removed, and cultures were grown for another 2–3 days. VEGF and/or BMP6 were added after 2–3 days for the induction conditions. After 3-5 days, cultures were labeled with 4 ug/mL Calcein AM (Invitrogen) for 1 hour, and imaged by fluorescence microscopy using a standard FITC filter (Larrivée et al., 2012).
Western, immunopercipitation assay, and FACS analysis in HUVEC
HUVECs were cultured on 0.1% gelatin coated plates in complete endothelial cell growth media containing supplements (Lonza, CC-3162), and 100 unit/ml penicillin-streptomycin. Cells between passages 3 and 7 were used for analyses. For immunoprecipitation, Protein A/G PLUS-Agarose Immunoprecipitation Reagent (Santa Cruz Biotechnology) was used according to manufacturer's instruction. For FACS analyses, HUVECs were stained with FITC conjugated antibody that recognized the extracellular domain of BMPRII (Santa Cruz Biotechnology; SC-13704, 1:200) without a permeablization process and subsequently sorted for fluorescence.
In situ proximity ligation assay (PLA)
Cells were grown overnight on a 16 well glass slide (Lab-Tek) and were starved for 5-6 hours in serum free EBM, and stimulated with recombinant human BMP6 (50 ng/ml) for 30 minutes. In situ PLA was performed as manufacturer's protocol using in situ PLA using Duolink Detection Kit (Olink Bioscience).
Image acquisition, processing, and quantification
Image acquisition from zebrafish was performed using a Nikon confocal microscope and depth coded with ImageJ. For immunocytochemistry, fixed HUVECs cultured on an 8 well glass slide (Lab-Tek) were imaged using the 63X objective of a Nikon confocal microscope. Number of branches and mean pixel intensity of ectopic vessels were quantified in order to assess the severity of Bmp2b induced angiogenesis in Fig. 1C. To calculate mean pixel intensity, four to five focal planes that contained ectopic vessels from the entire z stack were flattened and adjusted by Adobe Photoshop and MBF ImageJ. To quantify ventral sprouts from the CVP shown in Fig. 1E, all sprouts projecting toward the ventral side of the anterior-posterior axis in 25 to 26 hpf embryos were counted. To assess the effects of MO injection on the formation of CVP in Fig. 2B, somites with anastomosed ventral sprouts were given the value of 1, while those without were given the value of 0, and the percentage of somites with the value 1 was calculated as previously shown (Wiley et al., 2011). To assess the amount of SMAD protein within the nucleus in Fig. 4F, six nuclei from each image were randomly selected and gray values of pixel intensity corresponding to the longer axis of nucleus were measured and quantified. For HUVEC Fibrin gel assay, images were analyzed by PRISM and Image J program. All graphs were generated by Microsoft Excel program.
Supplementary Material
Highlights.
Internalization of BMP signaling complex promotes phosphorylation of SMADs
DAB2 physically interacts with BMP Type II receptor
DAB2 promotes clathrin-mediated endocytosis of BMP signaling complex
Knock down of dab2 attenuates Bmp2b-induced angiogenesis in zebrafish
In Brief (eTOC blurb).
Kim et al. demonstrate that the clathrin adaptor DAB2 physically interacts with the Type II BMP receptor and mediates endocytosis of BMP signaling complexes in endothelial cells. Moreover, the internalization of BMP signaling complexes is important for robust endothelial SMAD phosphorylation and activity during vascular development.
Acknowledgements
The authors would like to thank Jose Cardona Costa for excellent fish care, Ty Lanahan and Hong Chen for helpful discussion, and Drs. Mike Simons, Bill Sessa, Hyung Chun, David Wiley, and Vicki Bautch for their discussion and critical reading on the manuscript. This work has been supported by a grant from the NIH (HL090960) to S.-W.J. and an American Heart Association post-doctoral fellowship to J.-D.K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anzenberger U, Bit-Avragim N, Rohr S, Rudolph F, Dehmel B, Willnow TE, Abdelilah-Seyfried S. Elucidation of megalin/LRP2-dependent endocytic transport processes in the larval zebrafish pronephros. J Cell Sci. 2006;119:2127–2137. doi: 10.1242/jcs.02954. [DOI] [PubMed] [Google Scholar]
- Aramaki T, Sasai N, Yakura R, Sasai Y. Jiraiya attenuates BMP signaling by interfering with type II BMP receptors in neuroectodermal patterning. Dev Cell. 2010;19:547–561. doi: 10.1016/j.devcel.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat Cell Biol. 2003;5:410–421. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- Dobrowolski R, De Robertis EM. Endocytic control of growth factor signalling: multivesicular bodies as signalling organelles. Nat Rev Mol Cell Biol. 2011 doi: 10.1038/nrm3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich M, Horbelt D, Marom B, Knaus P, Henis YI. Homomeric and heteromeric complexes among TGF-β and BMP receptors and their roles in signaling. Cell Signal. 2011;23:1424–1432. doi: 10.1016/j.cellsig.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Finkenzeller G, Hager S, Stark GB. Effects of bone morphogenetic protein 2 on human umbilical vein endothelial cells. Microvasc Res. 2012 doi: 10.1016/j.mvr.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Gallagher H, Oleinikov AV, Fenske C, Newman DJ. The adaptor disabled-2 binds to the third psi xNPxY sequence on the cytoplasmic tail of megalin. Biochimie. 2004;86:179–182. doi: 10.1016/j.biochi.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Hartung A, Bitton-Worms K, Rechtman MM, Wenzel V, Boergermann JH, Hassel S, Henis YI, Knaus P. Different routes of bone morphogenic protein (BMP) receptor endocytosis influence BMP signaling. Mol Cell Biol. 2006;26:7791–7805. doi: 10.1128/MCB.00022-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocevar BA, Smine A, Xu XX, Howe PH. The adaptor molecule Disabled-2 links the transforming growth factor beta receptors to the Smad pathway. EMBO J. 2001;20:2789–2801. doi: 10.1093/emboj/20.11.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James RE, Broihier HT. Crimpy inhibits the BMP homolog Gbb in motoneurons to enable proper growth control at the Drosophila neuromuscular junction. Development. 2011;138:3273–3286. doi: 10.1242/dev.066142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampugnani MG, Orsenigo F, Gagliani MC, Tacchetti C, Dejana E. Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J Cell Biol. 2006;174:593–604. doi: 10.1083/jcb.200602080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanahan AA, Hermans K, Claes F, Kerley-Hamilton JS, Zhuang ZW, Giordano FJ, Carmeliet P, Simons M. VEGF receptor 2 endocytic trafficking regulates arterial morphogenesis. Dev Cell. 2010;18:713–724. doi: 10.1016/j.devcel.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrivée B, Prahst C, Gordon E, del Toro R, Mathivet T, Duarte A, Simons M, Eichmann A. ALK1 signaling inhibits angiogenesis by cooperating with the Notch pathway. Dev Cell. 2012;22:489–500. doi: 10.1016/j.devcel.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laux DW, Febbo JA, Roman BL. Dynamic analysis of BMP-responsive smad activity in live zebrafish embryos. Dev Dyn. 2011;240:682–694. doi: 10.1002/dvdy.22558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roy C, Wrana JL. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat Rev Mol Cell Biol. 2005;6:112–126. doi: 10.1038/nrm1571. [DOI] [PubMed] [Google Scholar]
- Little SC, Mullins MC. Bone morphogenetic protein heterodimers assemble heteromeric type I receptor complexes to pattern the dorsoventral axis. Nat Cell Biol. 2009;11:637–643. doi: 10.1038/ncb1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer ME, Cooper JA. Endocytosis of megalin by visceral endoderm cells requires the Dab2 adaptor protein. J Cell Sci. 2005;118:5345–5355. doi: 10.1242/jcs.02650. [DOI] [PubMed] [Google Scholar]
- Mishra SK, Keyel PA, Hawryluk MJ, Agostinelli NR, Watkins SC, Traub LM. Disabled-2 exhibits the properties of a cargo-selective endocytic clathrin adaptor. EMBO J. 2002;21:4915–4926. doi: 10.1093/emboj/cdf487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok SC, Wong KK, Chan RK, Lau CC, Tsao SW, Knapp RC, Berkowitz RS. Molecular cloning of differentially expressed genes in human epithelial ovarian cancer. Gynecol Oncol. 1994;52:247–252. doi: 10.1006/gyno.1994.1040. [DOI] [PubMed] [Google Scholar]
- Morikawa M, Koinuma D, Tsutsumi S, Vasilaki E, Kanki Y, Heldin CH, Aburatani H, Miyazono K. ChIP-seq reveals cell type-specific binding patterns of BMP-specific Smads and a novel binding motif. Nucleic Acids Res. 2011;39:8712–8727. doi: 10.1093/nar/gkr572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SM, Tallquist MD, Rock CO, Cooper JA. Dual roles for the Dab2 adaptor protein in embryonic development and kidney transport. EMBO J. 2002;21:1555–1564. doi: 10.1093/emboj/21.7.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penheiter SG, Deep Singh R, Repellin CE, Wilkes MC, Edens M, Howe PH, Pagano RE, Leof EB. Type II transforming growth factor-beta receptor recycling is dependent upon the clathrin adaptor protein Dab2. Mol Biol Cell. 2010;21:4009–4019. doi: 10.1091/mbc.E09-12-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platta HW, Stenmark H. Endocytosis and signaling. Curr Opin Cell Biol. 2011;23:393–403. doi: 10.1016/j.ceb.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Reider A, Wendland B. Endocytic adaptors--social networking at the plasma membrane. J Cell Sci. 2011;124:1613–1622. doi: 10.1242/jcs.073395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamiphak S, Seidel S, Essmann CL, Wilkinson GA, Pitulescu ME, Acker T, Acker-Palmer A. Ephrin-B2 regulates VEGFR2 function in developmental and tumour angiogenesis. Nature. 2010;465:487–491. doi: 10.1038/nature08995. [DOI] [PubMed] [Google Scholar]
- Shilo BZ, Schejter ED. Regulation of developmental intercellular signalling by intracellular trafficking. EMBO J. 2011;30:3516–3526. doi: 10.1038/emboj.2011.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague J, Bayraktaroglu L, Bradford Y, Conlin T, Dunn N, Fashena D, Frazer K, Haendel M, Howe DG, Knight J, et al. The Zebrafish Information Network: the zebrafish model organism database provides expanded support for genotypes and phenotypes. Nucleic Acids Res. 2008;36:D768–772. doi: 10.1093/nar/gkm956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague J, Bayraktaroglu L, Clements D, Conlin T, Fashena D, Frazer K, Haendel M, Howe DG, Mani P, Ramachandran S, et al. The Zebrafish Information Network: the zebrafish model organism database. Nucleic Acids Res. 2006;34:D581–585. doi: 10.1093/nar/gkj086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderberg O, Gullberg M, Jarvius M, Ridderstråle K, Leuchowius KJ, Jarvius J, Wester K, Hydbring P, Bahram F, Larsson LG, et al. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Methods. 2006;3:995–1000. doi: 10.1038/nmeth947. [DOI] [PubMed] [Google Scholar]
- Traub LM. Tickets to ride: selecting cargo for clathrin-regulated internalization. Nat Rev Mol Cell Biol. 2009;10:583–596. doi: 10.1038/nrm2751. [DOI] [PubMed] [Google Scholar]
- Trinh l.A., Hochgreb T, Graham M, Wu D, Ruf-Zamojski F, Jayasena CS, Saxena A, Hawk R, Gonzalez-Serricchio A, Dixson A, et al. A versatile gene trap to visualize and interrogate the function of the vertebrate proteome. Genes Dev. 2011;25:2306–2320. doi: 10.1101/gad.174037.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umasankar PK, Sanker S, Thieman JR, Chakraborty S, Wendland B, Tsang M, Traub LM. Distinct and separable activities of the endocytic clathrin-coat components Fcho1/2 and AP-2 in developmental patterning. Nat Cell Biol. 2012 doi: 10.1038/ncb2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdimarsdottir G, Goumans MJ, Rosendahl A, Brugman M, Itoh S, Lebrin F, Sideras P, ten Dijke P. Stimulation of Id1 expression by bone morphogenetic protein is sufficient and necessary for bone morphogenetic protein-induced activation of endothelial cells. Circulation. 2002;106:2263–2270. doi: 10.1161/01.cir.0000033830.36431.46. [DOI] [PubMed] [Google Scholar]
- Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, Adams S, Davy A, Deutsch U, Lüthi U, et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature. 2010;465:483–486. doi: 10.1038/nature09002. [DOI] [PubMed] [Google Scholar]
- Wiley DM, Kim JD, Hao J, Hong CC, Bautch VL, Jin SW. Distinct signalling pathways regulate sprouting angiogenesis from the dorsal aorta and the axial vein. Nat Cell Biol. 2011;13:686–692. doi: 10.1038/ncb2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KS, Proulx K, Rost MS, Sumanas S. Identification of vasculature-specific genes by microarray analysis of Etsrp/Etv2 overexpressing zebrafish embryos. Dev Dyn. 2009;238:1836–1850. doi: 10.1002/dvdy.21990. [DOI] [PubMed] [Google Scholar]
- Xu XX, Yang W, Jackowski S, Rock CO. Cloning of a novel phosphoprotein regulated by colony-stimulating factor 1 shares a domain with the Drosophila disabled gene product. J Biol Chem. 1995;270:14184–14191. doi: 10.1074/jbc.270.23.14184. [DOI] [PubMed] [Google Scholar]
- Yang DH, Smith ER, Roland IH, Sheng Z, He J, Martin WD, Hamilton TC, Lambeth JD, Xu XX. Disabled-2 is essential for endodermal cell positioning and structure formation during mouse embryogenesis. Dev Biol. 2002;251:27–44. doi: 10.1006/dbio.2002.0810. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.