Abstract
Background
Clinical trials of cardiac resynchronization therapy (CRT) have enrolled a select group of patients, with few patients in subgroups such as right bundle-branch block (RBBB). Analysis of population-based outcomes provides a method to identify real-world predictors of CRT outcomes.
Methods and Results
Medicare Implantable Cardioverter-Defibrillator Registry (2005 to 2006) data were merged with patient outcomes data. Cox proportional-hazards models assessed death and death/heart failure hospitalization outcomes in patients with CRT and an implantable cardioverter-defibrillator (CRT-D). The 14 946 registry patients with CRT-D (median follow-up, 40 months) had 1-year, 3-year, and overall mortality rates of 12%, 32%, and 37%, respectively. New York Heart Association class IV heart failure status (1-year hazard ratio [HR], 2.23; 3-year HR, 1.98; P<0.001) and age ≥80 years (1-year HR, 1.74; 3-year HR, 1.75; P<0.001) were associated with increased mortality both early and late after CRT-D. RBBB (1-year HR, 1.44; 3-year HR, 1.37; P<0.001) and ischemic cardiomyopathy (1-year HR, 1.39; 3-year HR, 1.44; P<0.001) were the next strongest adjusted predictors of both early and late mortality. RBBB and ischemic cardiomyopathy together had twice the adjusted hazard for death (HR, 1.99; P<0.001) as left BBB and nonischemic cardiomyopathy. QRS duration of at least 150 ms predicted more favorable outcomes in left BBB but had no impact in RBBB. A secondary analysis showed lower hazards for CRT-D compared with standard implantable cardioverter-defibrillators in left BBB compared with RBBB.
Conclusions
In Medicare patients, RBBB, ischemic cardiomyopathy, New York Heart Association class IV status, and advanced age were powerful adjusted predictors of poor outcome after CRT-D. Real-world mortality rates 3 to 4 years after CRT-D appear higher than previously recognized.
Keywords: bundle-branch block, heart failure, outcomes, registries
Clinical trials have established that cardiac resynchronization therapy (CRT), either alone or in combination with an implantable cardioverter-defibrillator (ICD) (CRT-D), improves survival and heart failure (HF) symptoms in appropriately selected patients.1–4 Although clinical trials have provided the basis for the current use of CRT, these clinical trials are selective in the patients they enroll and in the treatment offered, so the treatment provided in clinical trials differs meaningfully from what patients typically receive in practice.5 In addition, subgroup analysis is limited in these trials by the small number of patients with select characteristics. As a result, uncertainty remains about the clinical determinants of CRT effectiveness in clinical practice in typical target populations such as older patients with Medicare coverage.
Factors likely to influence clinical outcomes after CRT include surface ECG findings such as bundle-branch block (BBB) morphology, coexisting ischemic heart disease, age, and the presence of arrhythmias such as atrial fibrillation. Relative to BBB morphology, current guidelines specify that patients should have a QRS duration (QRSd) ≥120 ms but make no additional specifications in this regard.6 Even so, it is reasonable to expect different outcomes with right BBB (RBBB) and left BBB (LBBB) because RBBB is associated with less left ventricular mechanical dyssynchrony than LBBB.7,8 Clinical trials have not definitively addressed this issue because subgroup analysis from clinical trials of CRT based on BBB morphology is difficult as a result of the limited number of patients with RBBB enrolled in these trials. Recent nonrandomized series with limited numbers of patients with RBBB have reached conflicting conclusions on this issue.9–11
We used the data from the Medicare ICD Registry to characterize prognosis after CRT-D and to assess the relationship between clinical factors measured at the time of implantation and outcomes after CRT-D in ≈15 000 Medicare patients. In particular, we tested the hypothesis that patients with RBBB have significantly worse outcomes after CRT-D implantation than those with LBBB.
Methods
Characteristics of the ICD Registry, Medicare Claims, and Medicare Eligibility Records
Beginning in January 2005, the Center for Medicare and Medicaid Services (CMS) required providers implanting ICDs for certain indications to enter demographic information, clinical history, clinical characteristics, and device information for Medicare-funded procedures into a patient registry initially maintained by the Iowa Foundation for Medical Care. CMS later transferred responsibility for maintaining the registry to the American College of Cardiology (National Cardiovascular Data Registry ICD) for procedures performed after April 2006. For this study, we obtained data for 44 878 Medicare patients who received ICDs during the 16-month period from January 2005 to April 2006 and merged these data with Medicare data on postimplantation survival and HF hospitalizations. In total, 42 194 of the 44 878 (>94%) of the patients in the original ICD Registry were successfully matched with Medicare outcomes data based on the beneficiary identification. Approximately one third of these registry patients (14 946) received a CRT-D device. ICD Registry data fields used in the analysis are listed in Table 1.
Table 1.
Demographics for All CRT-D Patients (n=14 946)
| All (n=14 946) | LBBB(n=10 356) | RBBB(n=1638) | IVCD (n=2952) | P | |
|---|---|---|---|---|---|
| Age, mean±SD, y | 73.02±10.50 | 73.01±10.35 | 73.38±10.21 | 72.85±11.16 | 0.550 |
| Duration HF, mean±SD, mo | 24.72±25.39 | 24.73±25.06 | 23.44±25.51 | 25.41±26.41 | 0.001 |
| LVEF, mean±SD, % | 23.13±6.34 | 22.89±6.31 | 23.81±6.28 | 23.56±6.40 | <0.001 |
| QRSd, mean±SD, ms | 156.93±25.03 | 157.66±24.27 | 154.30±21.95 | 155.83±28.83 | <0.001 |
| SBP, mean±SD, mm Hg | 126.45±22.38 | 126.69±22.49 | 125.69±22.02 | 126.02±22.16 | 0.150 |
| DBP, mean±SD, mm Hg | 70.16±13.72 | 70.10±13.98 | 70.05±13.18 | 70.41±13.08 | 0.430 |
| Heart rate, mean±SD, bpm | 72.03±18.02 | 72.11±16.84 | 71.65±15.99 | 71.99±22.55 | 0.008 |
| Gender, n (%) | <0.001 | ||||
| Female | 4080 (27.30) | 3194 (30.84) | 266 (16.24) | 620 (21.00) | |
| Male | 10 866 (72.70) | 7162 (69.16) | 1372 (83.76) | 2332 (79.00) | |
| NYHA class,* n (%) | <0.001 | ||||
| I | 181 (1.21) | 98 (0.95) | 26 (1.59) | 57 (1.93) | |
| II | 1643 (10.99) | 1030 (9.95) | 196 (11.97) | 417 (14.13) | |
| III | 11 070 (74.07) | 7796 (75.28) | 1193 (72.83) | 2081 (70.49) | |
| IV | 2052 (13.73) | 1432 (13.83) | 223 (13.61) | 397 (13.45) | |
| Ischemic CM, n (%) | 10 340 (69.18) | 6858 (66.22) | 1274 (77.78) | 2208 (74.80) | <0.001 |
| Prior CABG, n (%) | 6271 (41.96) | 3977 (38.40) | 849 (51.83) | 1445 (41.96) | <0.001 |
| Atrial fibrillation, n (%) | 5190 (34.73) | 3252 (31.40) | 599 (36.57) | 45.36 (45.32) | 0.008 |
| No anticoagulation for AF, n (%) | 2089 (13.98) | 1368 (13.21) | 231 (14.10) | 490 (16.60) | 0.580 |
| Ventricular tachycardia, n (%) | 2927 (19.58) | 1856 (17.92) | 353 (21.55) | 718 (24.32) | <0.001 |
| Sudden cardiac arrest, n (%) | 256 (1.71) | 165 (1.59) | 34 (2.08) | 57 (1.93) | 0.006 |
| Diabetes mellitus, n (%) | 5343 (35.75) | 3596 (34.72) | 656 (40.05) | 1091 (36.96) | <0.001 |
| Prior MI, n (%) | 7601 (50.86) | 4998 (48.26) | 994 (60.68) | 1609 (54.51) | <0.001 |
| Smoker status, n (%) | <0.001 | ||||
| Never | 6317 (42.27) | 4528 (43.72) | 606 (37.00) | 1183 (40.07) | |
| Former smoker | 7270 (48.64) | 4899 (47.31) | 877 (53.54) | 1494 (50.61) | |
| Current smoker | 1359 (9.09) | 929 (8.97) | 155 (9.46) | 275 (9.32) | |
| Medications, n (%) | |||||
| β-blocker | 11 793 (78.90) | 8203 (79.21) | 1287 (78.57) | 2303 (78.01) | 0.010 |
| ACEI or ARB | 11 089 (74.19) | 7830 (75.61) | 1198 (73.14) | 2061 (69.82) | <0.001 |
| Digoxin | 6228 (41.67) | 4335 (41.86) | 628 (38.34) | 1265 (42.85) | <0.001 |
| Diuretic | 11 760 (78.68 | 8183 (79.02) | 1281 (78.21) | 2296 (77.78) | <0.001 |
| Amiodarone | 2030 (13.58) | 1347 (13.01) | 216 (13.19) | 467 (15.82) | 0.740 |
| Coumadin | 4758 (31.83) | 3035 (29.31) | 532 (32.48) | 1191 (40.35) | 0.290 |
CM indicates cardiomyopathy; CABG, coronary artery bypass graft surgery; MI, myocardial infarction; and ACEI, angiotensin-converting enzyme inhibitor.
NYHA class was recorded at the time of implantation and may not reflect recent worsening of NYHA status (see text).
Medicare hospital claims files and program eligibility records were obtained from CMS for all patients with records in the ICD Registry. These files contained data for ICD Registry patients on all inpatient hospital admissions through December 2008 and dates of death through June 2009. Hospital claims data included dates of hospital admission; dates of in-hospital death; length of stay; International Classification of Diseases, ninth clinical modification (ICD-9-CM) diagnosis codes identifying the primary cause of the hospitalization and secondary conditions; diagnosis-related group codes; and total charges for all services provided to the beneficiary during the stay. HF hospitalizations were defined as inpatient admissions associated with a primary ICD-9-CM diagnosis code of 428.x.
Linkage of the ICD implantation registry data with Medicare beneficiary enrollment and use data files maintained by the Research Data Distribution Center was conducted for the purpose of this research by CMS. All data files produced for this research were stored in encrypted format and maintained on secured-access computers. Analyses of all data were approved by the CMS Privacy Board.
Patients included all had a QRSd of at least 120 ms and a left ventricular ejection fraction (LVEF) ≤0.35. Patients with New York Heart Association (NYHA) HF class less than III or IV at the time of implantation were included because the NYHA classification provides a limited assessment12 and class scores significantly fluctuate over time in patients. In addition, CRT has been shown to result in beneficial remodeling in selected class I and II patients on the basis of the Multicenter Automatic Defibrillator Implantation Trial–CRT (MADIT-CRT).13
Statistical Analysis
Two-sided t tests were used to compare unadjusted means for continuous variables between the RBBB and LBBB groups, and Fisher exact tests were used to compare unadjusted proportions for categorical variables between these groups. Kaplan-Meier curves with death alone and death plus HF hospitalizations as the outcomes of interest were calculated to assess unadjusted comparisons of time to event for HF patients with RBBB and LBBB having received CRT-D. The log-rank test statistic was used to determine the statistical significance of unadjusted differences in time to event. A Cox proportional hazards model was used to assess the statistical significance of BBB morphology as a predictor of survival, adjusted for the concurrent effects of differences in patient demographics, clinical history, clinical characteristics, device information, and the other previously described covariates. The effect of BBB morphology (LBBB, RBBB, and intraventricular conduction delay [IVCD]) on survival was represented as a hazard ratio (HR) and 95% confidence interval (CI), with the effects referent to patients with LBBB. The same methodology was used to calculate adjusted HRs for the events of interest (death and the combined outcome of death or hospitalization) censored at 1 year and separately for events censored at 3 years using the identical set of covariates. Wald χ2 tests were used to assess the significance of individual covariates. Events were assessed at both 1 and 3 years to determine whether there were any meaningful differences in the effects of the covariates at these follow-up points and to facilitate comparison with published clinical trial data.
In the larger ICD registry cohort (n=24 486) with an LVEF ≤0.35 and QRSd ≥120 ms receiving either CRT-D (n=14 946) or standard ICDs (n=9540), the interaction between BBB morphology and whether the patient received CRT-D or a standard ICD (BBB×CRT) was also tested in similar Cox proportional-hazards models with death or death/HF hospitalization as the outcomes. The Wald χ2 test was used to assess the significance of the interaction, and specific HRs were determined for CRT based on BBB morphology. This study was reviewed and approved as exempt human subjects research by the Institutional Review Board of the University of Virginia.
Results
The frequency and distributional characteristics measured for the study population are listed in Table 1. Among 14 946 patients who received CRT-D and met standard QRS and LVEF criteria for CRT implantation, 10 356 (69%) had an LBBB, 1638 (11%) had an RBBB, and 2952 (20%) had a nonspecific IVCD. Most patients were on appropriate medical therapy with angiotensin-converting enzyme (ACE) inhibitors/angiotensin receptor blockers (ARBs) and β-blockers.
Patient mortality and hospitalization outcomes at 30 days and at 1, 2, and 3 years of follow-up are presented in Table 2. During the available follow-up period, 5557 (37.2%) died, 5088 (34.0%) had an HF hospitalization, and 7821 (52.3%) experienced the composite outcome of death or HF hospitalization. The median available follow-up period was 40 months. Of the 37.2% of patients who died during the entire follow-up period, 12.6% died during the first year after CRT-D, after which ≈10% died during each of the second and third years of follow-up, resulting in a 3-year mortality rate of 31.8%. Approximately half of the patients (48.2%) either died or were hospitalized for HF by the end of the third year of follow-up, with many of these reaching this composite end point during the first year after CRT-D implantation.
Table 2.
Summary Outcomes for Patients Receiving CRT-D (n=14 946)
| At 30 d | At 1 y | At 2 y | At 3 y | Total* | |
|---|---|---|---|---|---|
| Death, % (n) | 1.4 (213) | 12.6 (1880) | 22.2 (3320) | 31.8 (4753) | 37.2 (5557) |
| HF hospitalization, % (n) | 4.6 (694) | 19.2 (2870) | 26.5 (3966) | 32.2 (4819) | 34.0 (5088) |
| Composite, % (n) | 5.8 (873) | 26.3 (3936) | 38.2 (5716) | 48.2 (7209) | 52.3 (7821) |
Over a median follow-up of 40 months.
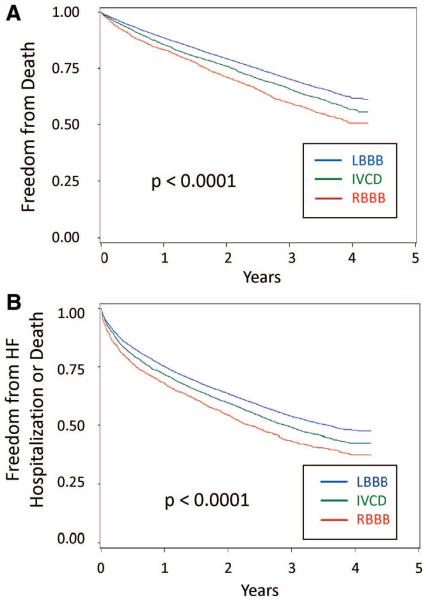
Death alone and the composite end point were more common in patients with RBBB than in those with LBBB, and those with a nonspecific IVCD had intermediate outcomes. As shown in Figure 1A, 45.8% of patients with RBBB compared with 35.0% of those with LBBB died during follow-up. As shown in Figure 1B, death or HF hospitalization occurred in 60.2% of patients with RBBB compared with 50.0% of patients with LBBB during follow-up. The survival curves continue to separate during the 4 years, and the log-rank test statistic was highly significant (P<0.0001) for both models. Unadjusted rates of death and the composite outcome at 1 and 3 years differed significantly by bundle-branch morphology, cardiomyopathy type, and QRSd, as shown in Table 3. This table also shows poor outcomes for patients with baseline NYHA class IV HF, with a 21.6% mortality rate during the first year and 44.3% dying over 3 years.
Figure 1.
Kaplan-Meier curves for BBB morphology. Kaplan-Meier plots are shown for freedom from death alone (A) and freedom from death or heart failure hospitalization (B) in patients with CRT-D. Patients with RBBB had higher rates of both outcomes than those with LBBB, and those with a nonspecific IVCD had an intermediate prognosis.
Table 3.
Unadjusted Outcomes in Patients With CRT-D by BBB Type, QRSd, Cardiomyopathy Type, and HF Class
| Early (1-y) Death, % | Late (3-y) Death, % | Early (1-y) Composite, % | Late (3-y) Composite, % | |
|---|---|---|---|---|
| LBBB | 11.3 | 29.7 | 24.8 | 46.1 |
| IVCD | 14.5 | 34.2 | 28.3 | 50.9 |
| RBBB | 16.7 | 40.3 | 31.9 | 56.5 |
| QRSd ≥150 ms | 11.5 | 30.7 | 24.2 | 46.2 |
| QRSd 120–149 ms | 14.1 | 33.3 | 29.3 | 51.2 |
| Nonischemic CM | 9.5 | 24.2 | 22.7 | 40.2 |
| Ischemic CM | 13.9 | 35.5 | 27.9 | 51.8 |
| NYHA class I–II | 8.0 | 24.6 | 18.3 | 38.6 |
| NYHA class III | 11.6 | 30.7 | 25.4 | 47.4 |
| NYHA class IV | 21.6 | 44.3 | 38.0 | 61.0 |
CM indicates cardiomyopathy. P<0.001 for all comparisons (χ2).
Results for the Cox proportional-hazards model assessing adjusted predictors of death are presented in Table 4. This includes adjustment for all covariates, including QRSd, which had the distribution shown in Figure 2. After NYHA class IV HF and age ≥80 years, RBBB (1-year HR, 1.44 [95% CI,1.26 to 1.65]; 3-year HR, 1.37 [95% CI, 1.26 to 1.49]) and ischemic cardiomyopathy (1-year HR, 1.39 [95% CI,1.21 to 1.59]; 3-year HR, 1.44 [95% CI,1.33 to 1.57]) were the next strongest adjusted predictors of both early and late mortality. Those with QRSd of at least 150 ms (cutoff used in CRT trials for inclusion4 or for subgroup analysis14) had a decreased hazard compared with those with QRSd of 120 to 149 ms, with an HR of 0.77 (95% CI, 0.70 to 0.84) at 1 year and 0.86 (95% CI, 0.81 to 0.91) at 3 years. Diabetes mellitus and atrial fibrillation were associated with an increased hazard, whereas ACE inhibitors/ARBs and β-blockers were associated with a decreased hazard. These covariates were also important predictors in the Cox proportional-hazards model for the composite end point of death or HF hospitalization, as presented in Table 5.
Table 4.
Short- and Long-Term Adjusted Predictors of Death in CRT-D Patients
| Early (1-y) HR (95% CI) | P | Late (3-y) HR (95% CI) | P | |
|---|---|---|---|---|
| Age, y | <0.001 | <0.001 | ||
| 50–59 | 1.00 | 1.00 | ||
| 60–69 | 1.01 (0.79–1.28) | 0.97 (0.83–1.13) | ||
| 70–79 | 1.18 (0.94–1.49) | 1.23 (1.07–1.42) | ||
| ≥80 | 1.74 (1.37–2.21) | 1.75 (1.51–2.04) | ||
| Female gender | 0.92 (0.82–1.03) | 0.155 | 0.87 (0.81–0.94) | <0.001 |
| Conduction delay | <0.001 | <0.001 | ||
| LBBB | 1.00 | 1.00 | ||
| RBBB | 1.44 (1.26–1.65) | 1.37 (1.26–1.49) | ||
| IVCD | 1.18 (1.05–1.32) | 1.08 (1.00–1.16) | ||
| QRSd, ms | <0.001 | <0.001 | ||
| 120–149 | 1.00 | 1.00 | ||
| ≥150 | 0.77 (0.70–0.84) | 0.86 (0.81–0.91) | ||
| Cardiomyopathy origin | <0.001 | <0.001 | ||
| Nonischemic | 1.00 | 1.00 | ||
| Ischemic | 1.39 (1.21–1.59) | 1.44 (1.33–1.57) | ||
| NYHA HF class | <0.001 | <0.001 | ||
| I | 1.00 | 1.00 | ||
| II | 0.86 (0.53–1.41) | 1.04 (0.77–1.42) | ||
| III | 1.25 (0.78–1.99) | 1.29 (0.96–1.73) | ||
| IV | 2.23 (1.39–3.57) | 1.98 (1.46–2.67) | ||
| Diabetes mellitus | 1.38 (1.25–1.51) | <0.001 | 1.32 (1.25–1.40) | <0.001 |
| Atrial fibrillation | 1.27 (1.14–1.41) | <0.001 | 1.21 (1.14–1.30) | <0.001 |
| Ventricular tachycardia | 1.11 (0.99–1.24) | 0.066 | 1.11 (1.03–1.19) | 0.005 |
| Prior CABG | 1.04 (0.94–1.16) | 0.425 | 1.06 (0.99–1.13) | 0.075 |
| Cigarette smoker status | 0.144 | 0.001 | ||
| Never | 1.00 | 1.00 | ||
| Former | 1.10 (1.00–1.22) | 1.10 (1.04–1.17) | ||
| Current | 1.03 (0.87–1.24) | 1.18 (1.06–1.31) | ||
| LVEF | 0.97 (0.96–0.98) | <0.001 | 0.98 (0.98–0.99) | <0.001 |
| Systolic BP | 0.991 (0.988–0.994) | <0.001 | 0.994 (0.993–0.996) | <0.001 |
| Diastolic BP | 0.994 (0.989–0.998) | 0.062 | 0.994 (0.992–0.997) | <0.001 |
| Heart rate | 1.003 (1.002–1.004) | <0.001 | 1.002 (1.001–1.003) | <0.001 |
| Medications | ||||
| β-blocker | 0.90 (0.81–1.00) | 0.053 | 0.88 (0.83–0.95) | <0.001 |
| ACE inhibitor or ARB | 0.65 (0.59–0.72) | <0.001 | 0.72 (0.68–0.77) | <0.001 |
| Diuretic | 1.13 (1.00–1.28) | 0.045 | 1.22 (1.13–1.31) | <0.001 |
| Amiodarone | 1.35 (1.20–1.52) | <0.001 | 1.14 (1.06–1.24) | 0.001 |
CABG indicates coronary artery bypass graft surgery; BP, blood pressure.
Figure 2.
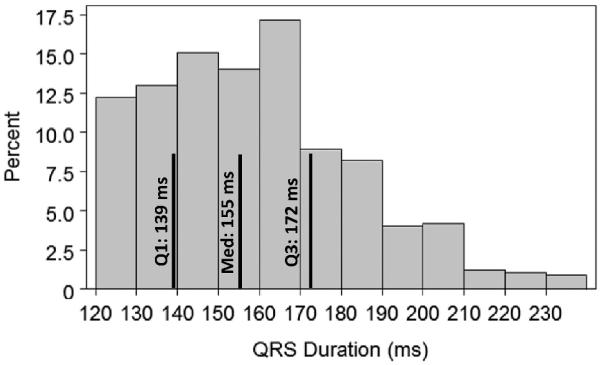
Distribution of QRSd in Medicare patients with CRT-D. The distribution of QRSd in the 14 946 receiving CRT-D is shown, with the quartiles highlighted on the histogram.
Table 5.
Short- and Long-Term Adjusted Predictors of Composite Outcome in CRT-D Patients
| HR (95% CI) | ||||
|---|---|---|---|---|
|
|
||||
| Early (1 y) | P | Late (3 y) | P | |
| Age, y | <0.001 | <0.001 | ||
| 50–59 | 1.00 | 1.00 | ||
| 60–69 | 0.90 (0.77–1.04) | 0.84 (0.75–0.94) | ||
| 70–79 | 0.95 (0.82–1.10) | 0.96 (0.86–1.07) | ||
| ≥80 | 1.15 (0.99–1.34) | 1.24 (1.10–1.38) | ||
| Female gender | 1.08 (1.00–1.16) | 0.065 | 1.00 (0.94–1.05) | 0.872 |
| Conduction delay | <0.001 | <0.001 | ||
| LBBB | 1.00 | 1.00 | ||
| RBBB | 1.32 (1.20–1.45) | 1.28 (1.20–1.38) | ||
| IVCD | 1.08 (1.00–1.17) | 1.05 (0.99–1.12) | ||
| QRSd, ms | <0.001 | <0.001 | ||
| 120–149 | 1.00 | 1.00 | ||
| ≥150 | 0.78 (0.73–0.83) | 0.83 (0.79–0.87) | ||
| Cardiomyopathy origin | <0.001 | <0.001 | ||
| Nonischemic | 1.00 | 1.00 | ||
| Ischemic | 1.24 (1.13–1.35) | 1.32 (1.22–1.40) | ||
| NYHA HF class | <0.001 | <0.001 | ||
| I | 1.00 | 1.00 | ||
| II | 0.90 (0.64–1.26) | 0.97 (0.76–1.24) | ||
| III | 1.29 (0.93–1.77) | 1.25 (0.99–1.57) | ||
| IV | 1.93 (1.40–2.68) | 1.75 (1.38–2.22) | ||
| Diabetes mellitus | 1.35 (1.27–1.44) | <0.001 | 1.37 (1.30–1.43) | <0.001 |
| Atrial fibrillation | 1.27 (1.18–1.37) | <0.001 | 1.22 (1.15–1.28) | <0.001 |
| Ventricular tachycardia | 1.13 (1.05–1.22) | 0.001 | 1.10 (1.04–1.17) | <0.001 |
| Prior CABG | 1.13 (1.05–1.22) | <0.001 | 1.11 (1.06–1.18) | <0.001 |
| Cigarette smoker status | 0.010 | <0.001 | ||
| Never | 1.00 | 1.00 | ||
| Former | 1.07 (1.00–1.14) | 1.07 (1.01–1.12) | ||
| Current | 1.19 (1.06–1.33) | 1.17 (1.08–1.28) | ||
| LVEF | 0.98 (0.97–0.98) | <0.001 | 0.98 (0.98–0.99) | <0.001 |
| Systolic BP | 0.994 (0.993–0.996) | <0.001 | 0.996 (0.995–0.997) | <0.001 |
| Diastolic BP | 0.997 (0.995–1.000) | 0.085 | 0.998 (0.995–1.000) | 0.037 |
| Heart rate | 1.002 (1.001–1.003) | <0.001 | 1.002 (1.001–1.002) | <0.001 |
| Medications | ||||
| β-blocker | 0.94 (0.87–1.01) | 0.086 | 0.90 (0.85–0.95) | <0.001 |
| ACE inhibitor or ARB | 0.75 (0.70–0.80) | <0.001 | 0.81 (0.77–0.85) | <0.001 |
| Diuretic | 1.21 (1.11–1.31) | <0.001 | 1.27 (1.20–1.36) | <0.001 |
| Amiodarone | 1.29 (1.18–1.40) | <0.001 | 1.21 (1.13–1.29) | <0.001 |
Figure 3 provides a summary of absolute death rates based on BBB morphology and cardiomyopathy type. The combination of RBBB and ischemic cardiomyopathy was associated with nearly double the unadjusted mortality rate at 1, 2, and 3 years compared with patients with LBBB and nonischemic cardiomyopathy (3-year mortality comparison: 41.9% versus 22.4%; P<0.0001), whereas RBBB with nonischemic cardiomyopathy or LBBB with ischemic cardiomyopathy resulted in intermediate death rates. These effects were consistent at all follow-up intervals.
Figure 3.
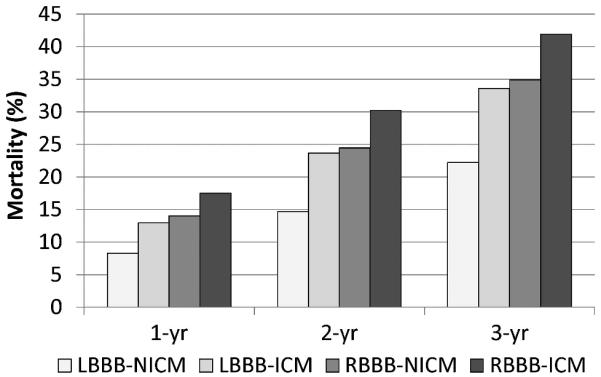
Mortality rates based on BBB morphology and cardiomyopathy type. The effects of BB morphology and cardiomyopathy type on mortality are consistent over time. ICM indicates ischemic cardiomyopathy; NICM, nonischemic cardiomyopathy.
For comparison, Figure 4 shows both unadjusted (Figure 4A) and adjusted (Figure 4B) HRs from the Cox proportional-hazards model for death. As shown in Figure 3, RBBB/ischemic cardiomyopathy was associated with approximately twice the hazard (HR, 1.99; 95% CI, 1.75 to 2.26) of LBBB/nonischemic cardiomyopathy for both the unadjusted and adjusted analyses.
Figure 4.
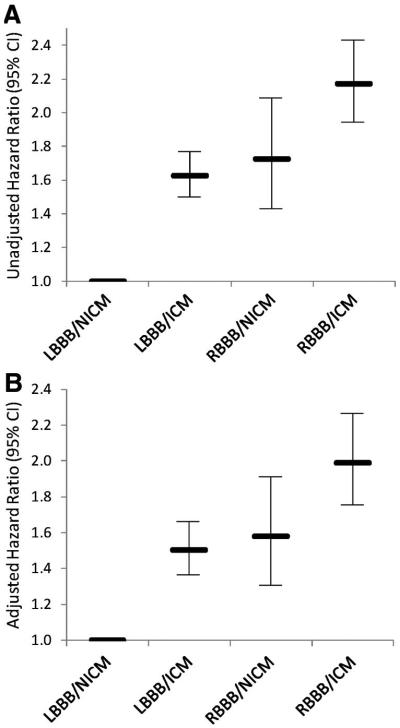
Hazards associated with BBB morphology and cardiomyopathy type. Both unadjusted (A) and adjusted (B) hazards from the Cox proportional-hazards model are shown. Of note, the adjusted hazard for RBBB and ischemic cardiomyopathy (ICM) is about twice that for LBBB and nonischemic cardiomyopathy (NICM).
The adjusted hazards in Table 6 with patients parsed into groups based on BBB morphology and QRSd show a significant decrease in the hazard for either outcome in LBBB when the QRSd is at least 150 ms. In contrast, QRSd did not have any significant effects on outcomes with RBBB present.
Table 6.
Hazards for Death and Composite Outcome in Cox CRT-D Models: BBB Morphology and QRSd
| HR (95% CI) | ||||
|---|---|---|---|---|
|
|
||||
| Early Death | Late Death | Early Composite | Late Composite | |
| LBBB | ||||
| QRSd ≥150 ms | 0.73 (0.65–0.82) | 0.85 (0.79–0.91) | 0.74 (0.68–0.80) | 0.82 (0.77–0.86) |
| IVCD | ||||
| QRSd ≥150 ms | 0.79 (0.65–0.95) | 0.86 (0.76–0.98) | 0.78 (0.68–0.89) | 0.82 (0.74–0.91) |
| RBBB | ||||
| QRSd ≥150 ms | 0.94 (0.74–1.20) | 0.93 (0.80–1.08) | 1.03 (0.87–1.23) | 0.96 (0.84–1.09) |
For the 24 496 registry patients (includes the original 14 946 patients) with a wide QRS and confirmed LVEF of ≤0.35 who received either CRT-D or a standard ICD, similar Cox proportional-hazards models with the addition of interaction terms for BBB morphology and CRT implantation showed significant interaction terms for both models (P<0.05), as shown in Tables I and II in the online-only Data Supplement.
Discussion
This large population-based study demonstrates that even with optimal medical therapy (nearly 80% of patients receiving β-blockers and ACE inhibitors or ARBs) and state-of-the-art CRT-D device therapy, approximately one third of patients died during the first 3 years of follow-up after CRT-D, and about half either died or were hospitalized for HF. Among all patients receiving CRT-D, BBB morphology was among the most powerful predictors of outcome, even after adjustment for QRS width15,16 and other covariates. This study also demonstrated a significant influence on prognosis after CRT-D for other clinical variables, including NYHA class IV HF, age ≥80 years, ischemic cardiomyopathy (consistent with the deleterious effects of increased scar burden17,18 and ischemia19 in CRT), diabetes mellitus, and atrial fibrillation (consistent with known effects of atrial arrhythmias in patients with CRT20). Medications such as ACE inhibitors and β-blockers were associated with favorable outcomes, whereas diuretic use was associated with a worse prognosis, consistent with other studies of HF.21,22
Although clinical trials of CRT1–4 are the foundation for current recommendations for CRT implantation, patients in clinical trials are highly selected, and treatment may differ from clinical practice.5,23 As a result, less is known about factors influencing CRT outcomes in clinical practice, particularly in patients with Medicare, who make up a large proportion of patients referred for CRT-D. The ICD Registry presents an excellent data repository for answering this question, and the merge with Medicare outcomes data provided a 40-month median follow-up (versus 16 months in the Comparison of Medical, Pacing, and Defibrillation Therapies in Heart Failure [COMPANION]).1 Other studies of mortality after ICDs in Medicare patients used the Medicare data set only and had limited information available about predictors and long-term prognosis after CRT-D.24,25 Ongoing studies such as the European CRT survey26 may provide additional insights with respect to other populations.
Both the COMPANION1 and Cardiac Resynchronization Heart Failure (CARE-HF)2 trials were published in 2004 and 2005, respectively, and data collection for the ICD Registry occurred in 2005 and 2006. The 30-day and 1-year CRT-D death rates in COMPANION (1.8% and 12%, respectively) were similar to those in registry patients. COMPANION showed a 17.6% CRT-D death rate during the median 16 months of follow-up, but the death rate in registry patients over 40 months was 37.2%. This finding highlights the fact that although CRT-D improves symptoms and survival for HF patients, HF is a progressive disease, and real-world mortality rates over 3 to 4 years are high and difficult to infer from clinical trials with shorter follow-up periods.
This death rate also reflects the fact that registry patients were ≈7 years older than patients in the COMPANION trial (73 versus 66 years). The present study also extends the observations of the MADIT-227 and other investigators28 relative to age and ICD outcomes in patients with CRT-D in that patients >80 years of age had a significantly higher death rate in our cohort. Even so, the risk factors discussed in the present analysis were still strong predictors even after adjustment for age. With regard to gender, although ICDs are underused in women,29 female patients did no worse than their male counterparts in this cohort.
Both RBBB and shorter QRSd had significantly higher short-term and long-term adjusted hazards for death among all patients with CRT-D. We note that these differences among CRT-D patients do not address differences in treatment efficacy, and in the absence of a control group for comparison, potential systematic differences in baseline disease related to treatment allocation cannot be separated from potential differences in response to therapy. Furthermore, it is not possible to determine whether an increased hazard indicates decreased benefit relative to the reference or harm. With these caveats, it is important to note that HF with RBBB is nevertheless different from HF with LBBB (less circumferential mechanical dyssynchrony,8,30 normal activation of the left ventricle in the absence of other disease,7 and possible association with right ventricular dysfunction). In addition, pump failure was the most common cause of death in COMPANION,31 and mortality rates after CRT-D may reflect different effects of CRT on pump function in different HF states. Of note, CRT-D device parameters may also not be optimized properly in routine practice for patients with RBBB.
The secondary analysis in patients with both CRT-D and standard ICDs, all with QRSd >120 ms and LVEF ≤0.35, was consistent with a CRT-specific influence of RBBB on outcomes. Because this analysis is limited by the fact that we were unable to control for additional factors that may have determined why certain patients received CRT-D and others received standard ICDs, we have based our primary analysis on the 14 946 patients who actually received CRT-D. Nevertheless, the consistency between the primary and secondary analyses is reassuring.
Limitations
The ICD Registry contained a reasonably complete characterization of the patients included; however, some potentially important characteristics were unavailable. For example, B-type natriuretic peptide and creatinine were not available; however, end-stage renal disease was rare (0.03%) in the study population. Characterization of right ventricular function may have an effect on CRT outcomes, but this information was not available. Although the existing registry data have inherent limitations, the registry provides the most clinically detailed record available for the broad population of older Americans who have received these devices. Death dates and HF hospitalizations are accurately reported in the Medicare database; however, because the primary ICD-9-CM code was used to determine whether a patient was hospitalized for HF, it was not possible to adjudicate further whether these admissions were truly for acute decompen-sated HF. Even so, the major findings of this study were related to death, and the COMPANION trial has shown that the majority of deaths in patients with CRT-D are cardiovascular.31
Conclusions
We have shown that Medicare patients, who are frequently referred for ICDs and CRT-D, had a persistent 10% to 12% annual mortality rate over >3 years of follow-up after CRT-D and that RBBB, ischemic cardiomyopathy, NYHA class IV HF, and age ≥80 years were the most powerful covariates predicting poor early and late outcomes after CRT-D. These data offer a realistic picture of HF as a progressive disease and show that outcomes vary significantly on the basis of BBB morphology and other covariates. In light of the increased complexity, procedure time, and follow-up requirements associated with CRT-D relative to standard ICD implantation, these factors influencing real-world outcomes after CRT-D should be considered when referring patients for CRT-D in clinical practice.
Supplementary Material
CLINICAL PERSPECTIVE.
Clinical trials of cardiac resynchronization therapy with or without an implantable cardioverter defibrillator (CRT/CRT-D) have enrolled a select group of patients in a controlled setting with limited numbers in subgroups such as right bundle-branch block (RBBB). As a result, uncertainty remains about determinants of short- and long-term CRT outcomes in clinical practice in typical target populations such as patients enrolled in Medicare. Using an analysis of 14 946 Medicare patients with mean age of 73 years who had CRT-D implanted in 2005 or 2006, we found a steady 10% to 12% death rate per year after the procedure during a median follow-up of 40 months. RBBB, ischemic cardiomyopathy, New York Heart Association class IV status, and advanced age were powerful adjusted predictors of poor outcome after CRT-D. The combination of RBBB (associated with less left ventricular mechanical dyssynchrony than left BBB) and ischemic cardiomyopathy was associated with doubling of the adjusted hazard for death after CRT-D relative to left BBB with nonischemic cardiomyopathy. A wider QRS duration was predictive of better outcomes in left BBB but not in RBBB. These data offer a realistic picture of heart failure as a progressive disease even with CRT-D, with significant adjusted hazards for RBBB and other covariates both early and late after CRT-D. The increased complexity, procedure time, and follow-up requirements associated with CRT-D relative to standard cardioverter-defibrillator implantation highlight the need for improved CRT-D candidate selection. Based on these data, the impact of factors such as BBB morphology on CRT-D outcomes may help inform the choice of CRT-D versus standard implantable cardioverter-defibrillators in clinical practice.
Acknowledgments
We wish to thank Tami Swenson, MA, technical advisor at the Research Data Assistance Center (School of Public Health, University of Minnesota), for her assistance in preparing the research proposal for CMS. We also thank Rosemarie Hakim, PhD, and Daniel Babish, PhD, from the CMS Office of Clinical Standards and Quality, for their help in preparing the ICD registry data for analysis.
Source of Funding Funding was provided by an internal grant from the University of Virginia.
Dr Bilchick has research grant support from the National Institutes of Health (1 K23 HL094761-01) and from St. Jude Medical. Dr DiMarco has received research grant support from Boston Scientific, has served as a consultant for Medtronic and St. Jude Medical, and has received honoraria from St. Jude Medical.
Footnotes
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/cgi/content/full/CIRCULATIONAHA.110.956011/DC1.
Disclosures The other authors report no conflicts.
References
- 1.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 2.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 3.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, Packer M, Clavell AL, Hayes DL, Ellestad M, Trupp RJ, Underwood J, Pickering F, Truex C, McAtee P, Messenger J, MIRACLE Study Group Multicenter InSync Randomized Clinical Evaluation. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–1853. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 4.Cazeau S, Leclercq C, Lavergne T, Walker S, Varma C, Linde C, Garrigue S, Kappenberger L, Haywood GA, Santini M, Bailleul C, Daubert JC. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001;344:873–880. doi: 10.1056/NEJM200103223441202. [DOI] [PubMed] [Google Scholar]
- 5.Ting HH, Shojania KG, Montori VM, Bradley EH. Quality improvement: science and action. Circulation. 2009;119:1962–1974. doi: 10.1161/CIRCULATIONAHA.108.768895. [DOI] [PubMed] [Google Scholar]
- 6.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NAM, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51:2085–2105. doi: 10.1016/j.jacc.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 7.Bilchick KC, Helm RH, Kass A. Physiology of biventricular pacing. Curr Cardiol Rep. 2007;9:358–365. doi: 10.1007/BF02938362. [DOI] [PubMed] [Google Scholar]
- 8.Byrne MJ, Helm RH, Daya S, Osman NF, Halperin HR, Berger RD, Kass DA, Lardo AC. Diminished left ventricular dyssynchrony and impact of resynchronization in failing hearts with right versus left bundle branch block. J Am Coll Cardiol. 2007;50:1484–1490. doi: 10.1016/j.jacc.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Rickard J, Kumbhani DJ, Gorodeski EZ, Baranowski B, Wazni O, Martin DO, Grimm R, Wilkoff BL. Cardiac resynchronization therapy in non-left bundle branch block morphologies. Pacing Clin Electrophysiol. 2009;33:590–595. doi: 10.1111/j.1540-8159.2009.02649.x. [DOI] [PubMed] [Google Scholar]
- 10.Adelstein EC, Saba S. Usefulness of baseline electrocardiographic QRS complex pattern to predict response to cardiac resynchronization. Am J Cardiol. 2009;103:238–242. doi: 10.1016/j.amjcard.2008.08.069. [DOI] [PubMed] [Google Scholar]
- 11.Wokhlu A, Rea RF, Asirvatham SJ, Webster T, Brooke K, Hodge DO, Wiste HJ, Dong Y, Hayes DL, Cha YM. Upgrade and de novo cardiac resynchronization therapy: impact of paced or intrinsic QRS morphology on outcomes and survival. Heart Rhythm. 2009;6:1439–1447. doi: 10.1016/j.hrthm.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Raphael C, Briscoe C, Davies J, Whinnett ZI, Manisty C, Sutton R, Mayet J, Francis DP. Limitations of the New York Heart Association functional classification system and self-reported walking distances in chronic heart failure. Heart. 2007;93:476–482. doi: 10.1136/hrt.2006.089656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, Estes NA, III, Foster E, Greenberg H, Higgins SL, Pfeffer MA, Solomon SD, Wilber D, Zareba W, MADIT-CRT Trial Investigators Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–1338. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 14.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews M. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 15.Gottipaty VK, Krelis SP, Fei Lu K. The resting electrocardiogram provides a sensitive and inexpensive marker of prognosis in patients with chronic congestive heart failure [abstract] J Am Coll Cardiol. 1999;33:145A. [Google Scholar]
- 16.Padeletti L, Giaccardi M, Turreni F, Musilli N, Colella A, Pieragnoli P, Michelucci A, Ricciardi G, Porciani MC. Influence of QRS prolongation on the natural history of CHF. Eur Heart J Supp. 2004;6:D79–D82. [Google Scholar]
- 17.White JA, Yee R, Yuan X, Krahn A, Skanes A, Parker M, Klein G, Drangova M. Delayed enhancement magnetic resonance imaging predicts response to cardiac resynchronization therapy in patients with intraventricular dyssynchrony. J Am Coll Cardiol. 2006;48:1953–1960. doi: 10.1016/j.jacc.2006.07.046. [DOI] [PubMed] [Google Scholar]
- 18.Bleeker GB, Schalij MJ, van der Wall EE, Bax JJ. Postero-lateral scar tissue resulting in non-response to cardiac resynchronization therapy. J Cardiovasc Electrophysiol. 2006;17:899–901. doi: 10.1111/j.1540-8167.2006.00499.x. [DOI] [PubMed] [Google Scholar]
- 19.Orlov MV, Maysky M, Akrivakis ST, Ujhelyi MR, Hoffmeister P, Shukla G, McAllister S, Kotler G, Almasry I, Chaudhry GM, Haffajee CI. Baseline myocardial perfusion predicts response to cardiac resynchronization therapy: a prospective observational study. J Interv Card Electrophysiol. 2008;23:127–133. doi: 10.1007/s10840-008-9285-3. [DOI] [PubMed] [Google Scholar]
- 20.Pelosi F, Jr, Morady F. CRT-D therapy in patients with left ventricular dysfunction and atrial fibrillation. Ann Noninvasive Electrocardiol. 2005;10:55–58. doi: 10.1111/j.1542-474X.2005.00072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eshaghian S, Horwich TB, Fonarow GC. Relation of loop diuretic dose to mortality in advanced heart failure. Am J Cardiol. 2006;97:1759–1764. doi: 10.1016/j.amjcard.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed A, Husain A, Love TE, Gambassi G, Dell'Italia LJ, Francis GS, Gheorghiade M, Allman RM, Meleth S, Bourge RC. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27:1431–1439. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaul S, Diamond GA. Trial and error: how to avoid commonly encountered limitations of published clinical trials. J Am Coll Cardiol. 2010;55:415–427. doi: 10.1016/j.jacc.2009.06.065. [DOI] [PubMed] [Google Scholar]
- 24.Groeneveld PW, Farmer SA, Suh JJ, Matta MA, Yang F. Outcomes and costs of implantable cardioverter-defibrillators for primary prevention of sudden cardiac death among the elderly. Heart Rhythm. 2008;5:646–653. doi: 10.1016/j.hrthm.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 25.Al-Khatib SM, Greiner MA, Peterson ED, Hernandez AF, Schulman KA, Curtis LH. Patient and implanting physician factors associated with mortality and complications after implantable cardioverter-defibrillator implantation, 2002–2005. Circ Arrhythm Electrophysiol. 2008;1:240–249. doi: 10.1161/CIRCEP.108.777888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dickstein K, Bogale N, Priori S, Auricchio A, Cleland JG, Gitt A, Limbourg T, Linde C, van Veldhuisen DJ, Brugada J. The European cardiac resynchronization therapy survey. Eur Heart J. 2009;30:2450–2460. doi: 10.1093/eurheartj/ehp359. [DOI] [PubMed] [Google Scholar]
- 27.Goldenberg I, Vyas AK, Hall WJ, Moss AJ, Wang H, He H, Zareba W, McNitt S, Andrews ML. Risk stratification for primary implantation of a cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol. 2008;51:288–296. doi: 10.1016/j.jacc.2007.08.058. [DOI] [PubMed] [Google Scholar]
- 28.Pellegrini CN, Lee K, Olgin JE, Turakhia MP, Tseng ZH, Lee R, Badhwar N, Lee B, Varosy PD. Impact of advanced age on survival in patients with implantable cardioverter defibrillators. Europace. 2008;10:1296–1301. doi: 10.1093/europace/eun253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernandez AF, Fonarow GC, Liang L, Al-Khatib SM, Curtis LH, LaBresh KA, Yancy CW, Albert NM, Peterson ED. Sex and racial differences in the use of implantable cardioverter-defibrillators among patients hospitalized with heart failure. JAMA. 2007;298:1525–1532. doi: 10.1001/jama.298.13.1525. [DOI] [PubMed] [Google Scholar]
- 30.Bilchick KC, Dimaano V, Wu KC, Helm RH, Weiss RG, Lima JA, Berger RD, Tomaselli GF, Bluemke DA, Halperin HR, Abraham T, Kass DA, Lardo AC. Cardiac magnetic resonance assessment of dyssynchrony and myocardial scar predicts function class improvement following cardiac resynchronization therapy. J Am Coll Cardiol Cardiovasc Imaging. 2008;1:561–568. doi: 10.1016/j.jcmg.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carson P, Anand I, O'Connor C, Jaski B, Steinberg J, Lwin A, Lindenfeld J, Ghali J, Barnet JH, Feldman AM, Bristow MR. Mode of death in advanced heart failure: the Comparison of Medical, Pacing, and Defibrillation Therapies in Heart Failure (COMPANION) trial. J Am Coll Cardiol. 2005;46:2329–2334. doi: 10.1016/j.jacc.2005.09.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.