Abstract
Nerve growth factor (NGF) was reported to be increased in the serum and skin of atopic dermatitis (AD) patients, to the extent that serum nerve growth factor levels were proposed to serve as a marker of disease severity. We studied NGF levels in the serum and dermis using skin microdialysis and attempted to correlate them with disease severity. We also examined if potential differences between morning and evening levels of NGF can explain the phenomenon of nocturnal itch. In addition, neurogenic inflammation and itch were induced using histamine iontophoresis in lesional and non-lesional skin and the effect of experimental itch on dermal NGF concentration was examined. We found that systemic (serum) and eczematous skin levels of NGF in AD are significantly lower in comparison to healthy controls. Serum NGF decreases from morning to late afternoon in both groups. Interestingly, serum NGF levels were correlated to disease severity in the morning in AD, although the NGF concentration in AD were significantly lower than in the healthy group. The local itch and neurogenic inflammation induction via experimental histamine reduced local NGF levels in the eczema and non-lesional skin in atopics, but not in the healthy controls, where it was slightly increased. The higher the clinical severity of the eczema, a significantly less pronounced effect of neurogenic inflammation on the local levels of NGF was found. The availability of measurable NGF might be reduced by a higher expression of NGF receptors. The fluctuations of NGF levels during the day suggest a complex modulation of this neurotrophin, potentially linked to stress or to an altered neurophysiological mechanism.
Keywords: Nerve growth factor (NGF) levels, Atopic dermatitis, Itch, Histamine, Daily fluctuation
1. Introduction
Pruritus is known to be exacerbated at night in many systemic and inflammatory skin diseases (Patel et al., 2007). In particular, atopic dermatitis (AD), of which itch is an essential feature of the disease (Stander and Steinhoff, 2002; Koblenzer, 1999), is associated with significant nocturnal pruritus (Ebata et al., 1996, 1999). Patients with severe pruritus at night report a greatly diminished quality of life and impaired sleep (Yosipovitch et al., 2000, 2002a, 2002b). The mechanism of night-time itching, however, is not well understood. In previous studies, we found that the skin barrier function and skin blood flow (Yosipovitch et al., 1998, 2004) follows a circadian rhythm. Based on these observations, we advanced the hypothesis whether nocturnal pruritus may be related to an increase in the secretion of neuropeptides or neurotrophins, such as the nerve growth factor (NGF).
It has been reported that levels of NGF are increased in the serum and skin of AD patients (Pincelli et al., 1990; Toyoda et al., 2002) and serum NGF was proposed to be a marker of disease severity (Toyoda et al., 2002; Hodeib et al., 2010). However, a recent study did not concur with these findings (Schulte-Herbrüggen et al., 2007). Serum NGF level was also reported to be increased in the evening (at 8 PM) in healthy individuals (Bersani et al., 2004). However, it is known that systemic levels of NGF can be influenced by a variety of physiological, pathophysiological, neuroendocrine factors, such as gender, stress, psychiatric conditions/status (Levi-Montalcini et al., 1995; Joachim et al., 2007), and may very well be under homeostatic and circadian control.
The aims of our study were: (1) to investigate whether serum and dermal NGF levels are increased in AD and if there is a correlation with disease severity; (2) to investigate whether serum NGF levels are increased in the evening in AD patients; (3) to determine if histamine induces an increase in dermal NGF level.
2. Materials and methods
2.1. Study population
Adults between18and 55 years of age with moderate to severe AD and age-matched healthy volunteers were enrolled. The diagnosis of AD was based on the Hanifin and Rajka (1980) criteria and the subjects had to present with eczema involving their forearms. Study subjects were in good health with no other skin disease. Potential participants were excluded if they showed evidence of depression based on the Beck Depression Inventory (BDI > 10), or if they worked shifts between8PMand 6AM. The severity of AD was evaluated using the EASI score (Hanifin et al., 2001). The EASI score measures the severity of AD on a numerical scale from 0 (no involvement) to 72 (maximum extension and severity). Subjects were instructed to discontinue antihistamines, topical and oral corticosteroids, centrally acting drugs (neuroleptics, antidepressants, gabapentin or mirtazapine etc.) and opioids, at least 1 week prior to the experimental visit. Thirteen patients with AD (7 males, 6 females; mean age 30.4 ± 9.7, age range 19–55) and 17 healthy volunteers (8 females, 9 males: mean age 30.7 ± 8.9, range 19–52) participated in this study. The study was approved by the local Internal Review Board (IRB) of Wake Forest University Health Sciences and was conducted in accordance with the principles of the Declaration of Helsinki.
2.2. Sample collection
Dermal NGF levels were evaluated by skin microdialysis as described previously (Schmelz et al., 1997; Rukwied et al., 2000). Study subjects were accommodated comfortably in a climate controlled room with a temperature maintained at approximately 23 °C and 43% relative humidity. In AD subjects, a microdialysis probe was inserted into an area of eczema on the volar forearm and another probe into non-lesional skin, 4 cm away, in a parallel orientation. In healthy subjects, both probes were similarly inserted on the volar aspect of either forearm, 4 cm apart in a parallel fashion. In the first hour of skin microdialysis, baseline concentrations of NGF were assessed. In the next hour, histamine iontophoresis was applied to an area adjacent to the microdialysis catheters to induce itch and to assess dermal NGF levels following itch induction. The microdialysis probes were slowly and gently inserted superficially at the dermal– epidermal junction for a length of 1.5 cm, without the use of local anesthetics. Dual microdialysis was performed using an electronically-controlled dual pump (World Precision Instruments, Sarasota, Florida) running an isotonic 0.9% saline solution at a flow rate of 4 μl/min. The microdialysis catheters had a diameter of 0.4 mm and the semi-permeable membranes (Dermal Dialysis, Erlangen, Germany) had a pore size of 3 MDa (permitting free diffusion of macromolecules up to a molecular weight of 3 MDa). Dialysate samples were immediately frozen at −80 °C after collection and were thawed just before ELISA analysis.
Experimental itch was induced by histamine iontophoresis using a portable power DC source (Perimed, Sweden). The electrodes were applied to skin approximately 1 cm away from the place where the microdialysis catheters were inserted. Histamine 1% in a 2% methylcellulose gel was administered by applying a 200 μA current for 30 s. Blood samples were also collected by venipuncture from all subjects at 8.00 AM and 5.00 PM.
2.3. Measurement of free NGF levels
NGF levels in the skin microdialysate and serum samples were measured using the Promega NGF Emax ELISA kit (Promega, Madison, WI). In accordance with the protocol provided by the manufacturer, primary polyclonal antibodies were added to Nunc Immuno 96 MicroWell™ solid plates (Nalge Nunc International, Rochester, NY) and incubated for 24 h at 4 °C. Sample and block buffer was added to the wells for 1 h and then wells were washed 5 times with wash buffer.
2.3.1. Preparation of standard curve
NGF standard was diluted 1:4000 with the NGF block and sample buffer, and a volume of 200 μl was added to first row of columns 11 and 12 of a 96-well Nunc plate. Seven 1:2 serial dilutions were performed in these lanes. Samples were added at a 1/10 dilution for serum samples and undiluted for skin microdialysate. Each well contained 100 μl and was incubated with shaking for 6 h at room temperature. Subsequently, plates were washed 5 times. Secondary monoclonal anti-NGF antibody was added next and the plates were incubated overnight (12 h) at 4 °C. Samples were tested in triplicate. A standard curve using stock NGF (provided by the Emax kit) was generated every time ELISA was performed. The standard curve was constructed for every experiment and a linear curve fit was obtained. Samples of dialysate that contained NGF displayed values within the range of the standard curve.
2.4. Statistical analysis
Differences in NGF concentration between eczema, non-lesional skin in atopics and healthy skin, between morning and evening time points, before and after histamine administration were analyzed with a mixed model taking into account subject effects and multiple comparisons were Bonferroni adjusted using PASW 18.0 software (SPSS, Chicago, IL, USA). Overall daily fluctuations in dermal NGF were also examined as averages collapsed over histamine’s effect. A Spearman correlation analysis of serum and skin dialysate NGF values (baseline, post-histamine and % change after histamine) with disease severity expressed in the EASI scores was performed for both time points.
3. Results
3.1. Serum NGF levels
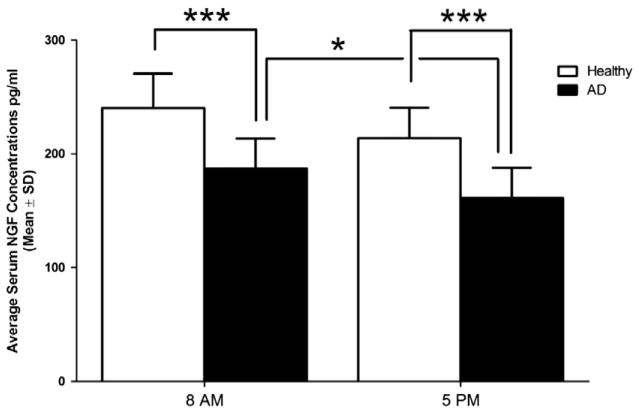
The average serum concentration (±S.D.) of free NGF was 236 ± 38 (at 8 AM), 211 ± 34 pg/ml (at 5 PM) in healthy subjects and 184 ± 28 (8 AM), 161 ± 24 (5 PM) pg/ml in AD subjects. NGF serum levels were significantly lower (p < 0.001) at both time points in AD subjects compared to healthy subjects (Table 1). A decrease of NGF serum concentrations from 8 AM to 5 PM was noted in both groups; more specifically, a significant decrease from 8 AM to 5 PM was found in the AD group (p = 0.027), but the change was not significant in the healthy group (Fig. 1).
Table 1.
NGF values measured in serum in healthy and AD subjects (n = 12), and the corresponding EASI scores of disease severity (pg/ml).
| AD # | EASI | Serum values
|
Healthy # | Serum values
|
||
|---|---|---|---|---|---|---|
| AM | PM | AM | PM | |||
| 1 | 3.2 | 220 | 192 | 1 | 286 | 244 |
| 2 | 2.2 | 162 | 142 | 2 | 218 | 231 |
| 3 | 13.4 | 226 | 150 | 3 | 288 | 189 |
| 4 | 2.4 | 144 | 136 | 4 | 216 | 218 |
| 5 | 2.2 | 148 | 128 | 5 | 208 | 255 |
| 6 | 14.6 | 226 | 216 | 6 | 268 | 210 |
| 7 | 11.9 | 190 | 155 | 7 | 236 | 234 |
| 8 | 3.7 | 166 | 156 | 8 | 202 | 155 |
| 9 | 9.6 | 186 | 175 | 9 | 287 | 164 |
| 10 | 23.5 | 200 | 167 | 10 | 201 | 245 |
| 11 | 38.8 | 184 | 158 | 11 | 196 | 180 |
| 12 | 5.7 | 192 | 159 | 12 | 278 | 240 |
Fig. 1.
Serum concentration (pg/ml ± S.D.) of NGF in the morning and early evening in healthy participants (n = 12) and atopic dermatitis patients (n = 12). ***p < 0.001 and *p < 0.05.
3.2. Dermal NGF levels
We were interested to answer the following questions, in regards to the skin levels of NGF in the skin: (1) Are these concentrations different in: eczematous areas of atopic skin in AD in comparison with non-lesional areas in AD and healthy skin? (2) Are dermal NGF levels changing from AM to PM? (3) We also investigated if: the levels of NGF were differentially affected by histamine administration in eczematous, non-lesional skin in AD or healthy skin, and if affirmative, if this response was different at two different time-points during the day (AM or PM). To answer the above questions, a mixed model analysis was performed to assess the effect the interaction terms (time = AM vs. PM, period = pre-histamine vs. post-histamine, group = healthy vs. eczema vs. non-lesional). We found a significant difference in the manner in which NGF levels fluctuated, across all groups overall (p < 0.001), from pre-histamine to post-histamine settings (by period, p = 0.007), by time of day (p = 0.026, AM vs. PM), by group and time (p < 0.001) and by group and period (p < 0.001). (Table 2).
Table 2.
ANOVA analysis of the multiple factors affecting dermal NGF levels: disease status, time of collecting samples during the day, histamine treatment and their combinations thereof. Legend: group = healthy or AD; period = before or after histamine application; time = AM or PM.
| Source | Type III tests of fixed effectsa
|
|||
|---|---|---|---|---|
| Numerator df | Denominator df | F | Sig. | |
| Intercept | 1 | 40 | 4866.975 | .000 |
| Group | 2 | 40 | 216.701 | .000 |
| Time | 1 | 122 | 5.095 | .026 |
| Period | 1 | 122 | 7.604 | .007 |
| Group × time | 2 | 122 | 13.086 | .000 |
| Group × period | 2 | 122 | 62.586 | .000 |
| Time × period | 1 | 122 | 2.832 | .095 |
Dependent variable: NGF levels in skin dialysate.
Dermal NGF levels in eczematous skin in AD subjects were consistently found to be significantly lower compared to non-eczematous skin in AD and the skin of healthy controls (both comparisons, p < 0.001) (Fig. 2, Table 3). There was no significant difference between morning and evening levels in dermal (free) NGF levels, within the same skin area type, in baseline conditions (Table 4). The NGF concentrations in non-lesional skin in AD subjects and healthy skin were similar, however there were significant differences revealed by the action of exogenous histamine. More precisely, administration of histamine increased measurable levels of NGF in healthy skin and decreased NGF levels in non-lesional skin in AD and in the eczematous areas (Fig. 3).
Fig. 2.
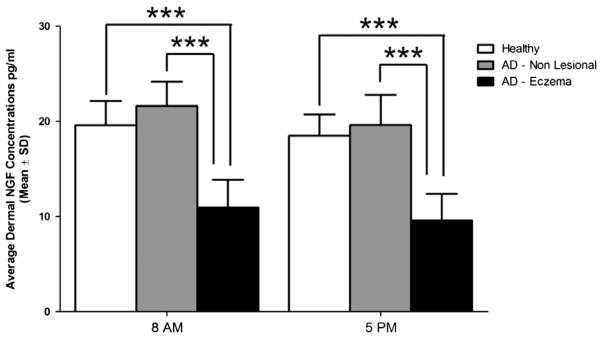
Dermal concentrations of NGF (pg/ml ± S.D.) in the morning and early evening in healthy participants (n = 17) in comparions with the eczematous areas and non-lesional areas of the skin in atopic dermatitis patients (n = 13). ***p < 0.001 in baseline conditions. Levels of the free NGF in healthy skin and in non-lesional skin of atopic subjects are both siginificantly higher than in the eczematous areas in AD.
Table 3.
NGF levels in skin microdialysate (pg/ml) in the healthy group (n = 17).
| Healthy skin # | AM
|
PM
|
||
|---|---|---|---|---|
| Pre his | Post his | Pre his | Post his | |
| 1 | 19.1 | 23.4 | 19.9 | 29.4 |
| 2 | 22.3 | 19.2 | 20.3 | 26.4 |
| 3 | 21.4 | 18.1 | 22 | 26.1 |
| 4 | 17.3 | 21.3 | 19.1 | 23.6 |
| 5 | 16.9 | 20.4 | 16.4 | 28.5 |
| 6 | 18.2 | 21.1 | 18.4 | 27.4 |
| 7 | 20.5 | 23.4 | 21.7 | 23.7 |
| 8 | 21.3 | 23.4 | 18.8 | 21.2 |
| 9 | 22.5 | 25.5 | 16.6 | 24.4 |
| 10 | 23.4 | 18.4 | 18.6 | 23.2 |
| 11 | 15.5 | 23.2 | 17.5 | 24.4 |
| 12 | 16.6 | 22.2 | 22.3 | 27.4 |
| 13 | 19.3 | 21.8 | 19.2 | 28.3 |
| 14 | 21.3 | 21.2 | 15.6 | 23.4 |
| 15 | 20.8 | 22.3 | 16.4 | 28.5 |
| 16 | 21.5 | 24.2 | 16.4 | 26.6 |
| 17 | 15.3 | 16.4 | 15.3 | 19.3 |
his = histamine.
Table 4.
NGF levels in skin dialysate (pg/ml) in the AD group (n = 13) and corresponding EASI scores.
| EASI score | AD non-lesional
|
AD eczema
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| # | AM
|
PM
|
AM
|
PM
|
|||||
| Pre his | Post his | Pre his | Post his | Pre his | Post his | Pre his | Post his | ||
| 11.9 | 1 | 21.2 | 16.3 | 18.7 | 13.4 | 11.5 | 7.55 | 10.55 | 11.9 |
| 3.2 | 2 | 18.4 | 14.4 | 20.4 | 9.2 | 11.8 | 10.1 | 7.2 | 7.2 |
| 13.4 | 3 | 24.4 | 12.2 | 25.1 | 13.4 | 8.7 | 8.2 | 7.4 | 7.3 |
| 2.2 | 4 | 20.1 | 14.2 | 14.6 | 11.2 | 13.4 | 9.2 | 13.3 | 14.4 |
| 5.7 | 5 | 19.3 | 16.6 | 22.5 | 9.8 | 7.3 | 7.3 | 14.4 | 7.2 |
| 23.5 | 6 | 22.1 | 18 | 17.7 | 9.8 | 11.6 | 8.06 | 7.46 | 8.7 |
| 5.6 | 7 | 24.3 | 17.2 | 18.3 | 10.8 | 8.2 | 15.1 | 7.9 | 11.6 |
| 3.7 | 8 | 26.4 | 16.1 | 18.6 | 14.4 | 14.2 | 8.1 | 12.9 | 8.1 |
| 38.8 | 9 | 19.8 | 23.3 | 23.4 | 7.7 | 14.7 | 7.6 | 7.8 | 7.7 |
| 14.6 | 10 | 24.6 | 21.5 | 19.2 | 7.9 | 7.9 | 7 | 7.6 | 7.1 |
| 2.2 | 11 | 18.8 | 15.5 | 17.6 | 9.4 | 15.8 | 15.2 | 7.2 | 10.8 |
| 2.4 | 12 | 21.2 | 14.4 | 15.5 | 11.4 | 9.4 | 7.5 | 13.1 | 14.6 |
| 9.6 | 13 | 20.4 | 13.8 | 23.5 | 12.2 | 7.8 | 7.9 | 7.7 | 7.5 |
his = histamine.
Fig. 3.
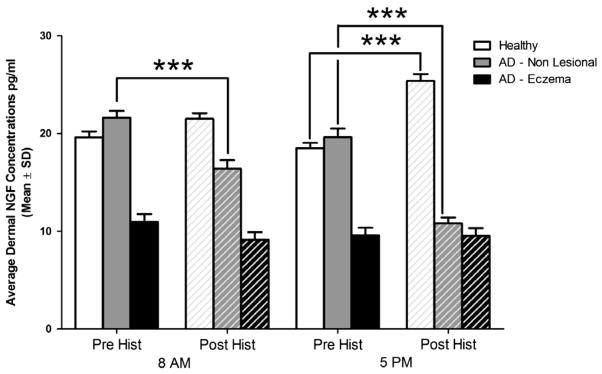
The effect of itch induction and neurogenic inflammation produced with histamine, on the dermal concentrations of NGF in the morning and early evening in healthy participants (n = 17) in comparison with eczematous and non-lesional areas of skin in AD (n = 13). ***p < 0.001. Levels of the free NGF in healthy skin increase in healthy skin (siginificantly in the PM) in contrast with non-eczematous areas in AD, where NGF levels drop significantly. (The levels of free NGF were so low in the eczematous areas to begin with that histamine did not seem induce any substantial effect.)
To further dissect the effect of itch and neurogenic inflammation induced experimentally with histamine on the local dermal NGF concentrations, we performed ANOVA to test whether % changes from pre-histamine (baseline) to post-histamine values contrasted significantly between these 3 groups, and separately, for AM and PM time points.
In the morning (8 AM) healthy skin levels of NGF increased following histamine administration and the change was significant vs. the change observed in the eczema (p = 0.027), but not significantly vs. non-lesional skin in AD. Non-lesional levels of NGF increased also significantly vs. eczema (p = 0.001). In the evening, the change in levels of NGF in eczematous skin was significantly different vs. both non-lesional skin in AD and healthy skin, while the change in AD non-lesional skin was significantly different vs. healthy skin (in other words, all contrasts were significant in a 3-way fashion at 5 PM (p < 0.001).
3.3. Correlations between NGF levels and disease severity
Serum values of NGF in the morning were significantly correlated with disease severity (Spearman rho coefficient: 0.611*, p = 0.035), while NGF values in the evening (5 PM) appeared not to be correlated with EASI scores. In other words, the higher the severity, the higher the serum NGF levels in atopics in the morning. However, AD patients had lower concentrations overall than healthy subjects (p = 0.027).
Correlations between dermal NGF levels and disease severity assessed when AM and PM average levels (pre-and post-histamine combined) were taken into account, showed that the morning levels of NGF in the non-lesional skin correlated with disease severity (Sperman’s rho: 0.631, p = 0.021), while they did not correlate in the eczema or in neither type of area at 5 PM. Subsequently, NGF levels were further analyzed for potential correlations with disease severity, in baseline conditions (pre-histamine) and post-histamine. The corresponding effect on NGF concentration following neurogenic inflammation induced by exogenous histamine was also explored in its relationship with the EASI scores Table 5). In the evening, in non-lesional skin of AD, baseline NGF levels were significantly correlated (p = 0.03) with disease severity.
Table 5.
Correlation of various NGF concentrations in systemic circulation or in the skin with disease severity (EASI scores), in the atopic dermatitis group (n = 13).
| NGF parameter | Correlation type | Statistical significance Spearman coeff. | p |
|---|---|---|---|
| AM serum NGF level | Direct | 0.61* | 0.037* |
| PM serum NGF level | Direct | 0.53 | 0.08 |
| AM non-lesional skin NGF pre-histamine | Direct | n.s. | n.s. |
| AM non-lesional skin NGF post-histamine | Direct | 0.53 | 0.059 |
| PM non-lesional skin NGF pre-histamine | Direct | 0.6* | 0.03* |
| PM non-lesional skin NGF post-histamine | Inverse | n.s. | n.s. |
| AM eczematous skin NGF pre-histamine | Inverse | n.s. | n.s. |
| AM eczematous skin NGF post-histamine | Inverse | n.s. | n.s. |
| PM eczematous skin NGF pre-histamine | Inverse | −0.49 | 0.088 |
| PM eczematous skin NGF post-histamine | Inverse | n.s. | n.s. |
n.s. = non-significant.
p<0.05.
4. Discussion
It was previously reported that increased plasma levels of NGF and substance P in AD patients correlated with disease severity and plasma NGF was thereby suggested to be a marker of disease severity. In this study, we sought to examine if NGF levels in the serum and dermis correlate with disease severity and nocturnal exacerbation of itch in AD (Patel et al., 2007). We report that the serum concentrations of NGF measured at 8 AM did in fact correlate with disease severity, although the overall values in the atopic group were lower than in the healthy participants. This correlation was not observed when NGF levels were measured at 5 PM.
NGF appears to have a clear role in the sprouting of nerve fibers and in the development of AD lesions. In AD lesions, higher levels of NGF were found in the keratinocytes in the epidermal basal and spinal layers, and an increased expression of NGF receptors (TrkA) was found in the epidermis and upper dermis (Dou et al., 2006). The level of NGF in the stratum corneum was reported to correlate with the severity of itching and eruptions in AD (Yamaguchi et al., 2009). In NC/Nga mice (an atopic mouse model), anti-NGF antibodies were also shown to significantly inhibit the development of skin lesions, epidermal innervation, and increase in scratching (Takano et al., 2005, 2007). Our investigations, however, found decreased levels of free NGF levels in the dermis or serum of AD subjects in comparison with healthy controls. We found that the serum NGF levels in AD subjects were in effect significantly lower than in controls at both time points (8 AM and 5 PM, p < 0.001).
In contrast to several other reports (Toyoda et al., 2002; Hodeib et al., 2010; Yamaguchi et al., 2009; Wang et al., 2008), the results of our study do not support the concept of NGF as a reliable marker for the severity of AD, since these correlation patterns seem to vary during the day, and overall, NGF (serum) levels in AD are lower in comparison with healthy volunteers. From this point of view, our results are in concordance with the study of Schulte-Herbrüggen et al. (2007) study, which did not find serum NGF levels to be increased in AD, however, in our experiment the concentrations of NGF at 8 AM appeared positively correlated with the EASI scores.
The drop in NGF levels – as measured by microdialysis – in the axon reflex area after histamine application, could be caused by an increase of perfusion and protein extravasation induced by the release of Substance P(SP) [similarly to the neurogenic protein extravasation seen in the complex regional pain syndrome, CRPS (Kingery, 2010)]. The underlying mechanism can be related to an amplified NGF-dependent signaling in AD (where lower measurable levels could still be masking an increased trkA expression). NGF upregulates SP expression in C-fibers, and thus, when an axon reflex erythema is induced by histamine, the microdialysis membranes become engulfed in the area of neurogenic inflammation. In healthy volunteers, a (neurogenic) vasodilation can occur with less protein extravasation. Due to a pre-existing upregulation of SP in AD patients, the histamine iontophoresis could elicit an increased release of SP in the axon reflex area, potentially sufficient to increase protein extravasation. This occurrence would dilute local NGF and therefore measurable NGF levels would appear to drop. In the eczema however, an inflammatory protein extravasation is present, thus, the increased release of SP might not affect the protein extravasation significantly, as it is already increased.
Histamine was reported to enhance the production of NGF in cultured human keratinocytes in vitro (Kanda and Watanabe, 2003). NGF, on the other hand, may promote the infiltration of mast cells into inflamed skin (Tam et al., 1997). Mast cells are abundant in the epidermis of AD lesional skin and can be triggered to release histamine and tryptase. This interaction between mast cells and NGF may create a vicious cycle, significantly contributing to the development of itch and skin lesions in AD. In this study, we found that histamine induced a slight elevation of dermal free NGF levels in healthy subjects, and a significant drop (decrease) in NGF levels in atopic subjects, both in non-lesional and eczematous skin. We cannot, however, exclude the possibility that NGF was released from keratinocytes upon histamine application, but was bound to its high-affinity receptors (TrkA), which has been found to be upregulated in atopic skin. On the other hand, histamine may not play the dominant role in triggering the release of NGF in skin lesions of AD, since it is known that administration of antihistamines alone does not result in improvement of AD lesions. Other stimuli, such as scratching (Yamaoka et al., 2007) and pro-inflammatory mediators, may have a more important contribution.
It was previously reported that serum NGF levels vary significantly during the day and evening hours, following a ‘V shaped’ pattern, being increased in the morning (9 AM) and evening (8 PM) and decreased at 1 PM (Bersani et al., 2004). The levels of another neurotrophin, the brain derived nerve factor (BDNF), were also found to vary following an ultradian rhythm (Raap et al., 2006; Hon et al., 2007). We measured NGF levels in the serum and dermis using 2 time points (8 AM and 5 PM), and we were able to find a decrease at 5 PM vs. 8 AM, significant in the serum of AD patients but not in healthy subjects. It therefore appears unlikely that free NGF could play a direct role in the exacerbation of itch in AD in the evening.
In conclusion, our results suggest that free NGF levels may not serve as a reliable marker for disease severity in AD and that moreover, free NGF most probably may not cause the exacerbation of pruritus in the evening. We also found that local free NGF in the skin, as measured by skin microdialysis, was decreased in atopic patients after the local itch induction and neurogenic inflammation induced by histamine, while the opposite effect occurred in the healthy skin.
Acknowledgments
This work was supported by an unrestricted grant from Connetics (Stiefel) Corporation to Dr. Gil Yosipovitch, who is also supported by NIAMS Grant 5R01AR055902.
Footnotes
Conflict of interest
The authors declare none.
References
- Bersani G, Iannitelli A, Massoni E, Garavini A, Grilli A, Di Giannantonio M, et al. Ultradian variation of nerve growth factor plasma levels in healthy and schizophrenic subjects. Int J Immunopathol Pharmacol. 2004;17 (3):367–372. doi: 10.1177/039463200401700316. [DOI] [PubMed] [Google Scholar]
- Dou YC, Hagströmer L, Emtestam L, Johansson O. Increased nerve growth factor and its receptors in atopic dermatitis: an immunohistochemical study. Arch Dermatol Res. 2006;298 (1):31–37. doi: 10.1007/s00403-006-0657-1. [DOI] [PubMed] [Google Scholar]
- Ebata T, Aizawa H, Kamide R. An infrared video camera system to observe nocturnal scratching in atopic dermatitis patients. J Dermatol. 1996;23:153–155. doi: 10.1111/j.1346-8138.1996.tb03990.x. [DOI] [PubMed] [Google Scholar]
- Ebata T, Aizawa H, Kamide R, Niimura M. The characteristics of nocturnal scratching in adults with atopic dermatitis. Br J Dermatol. 1999;141:82–86. doi: 10.1046/j.1365-2133.1999.02924.x. [DOI] [PubMed] [Google Scholar]
- Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol (Stockh) 1980;(Suppl 92):44–47. [Google Scholar]
- Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol. 2001;10 (1):11–18. doi: 10.1034/j.1600-0625.2001.100102.x. [DOI] [PubMed] [Google Scholar]
- Hodeib A, El-Samad ZA, Hanafy H, El-Latief AA, El-Bendary A, Abu-Raya A. Nerve growth factor, neuropeptides and cutaneous nerves in atopic dermatitis. Indian J Dermatol. 2010;55 (2):135–139. doi: 10.4103/0019-5154.62735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hon KL, Lam MC, Wong KY, Leung TF, Ng PC. Pathophysiology of nocturnal scratching in childhood atopic dermatitis: the role of brain-derived neurotrophic factor and substance P. Br J Dermatol. 2007;157 (5):922–925. doi: 10.1111/j.1365-2133.2007.08149.x. [DOI] [PubMed] [Google Scholar]
- Joachim RA, Kuhlmei A, Dinh QT, Handjiski B, Fischer T, Peters EM, et al. Neuronal plasticity of the brain–skin connection stress-triggered upregulation of neuropeptides in dorsal root ganglia and skin via nerve growth factor-dependent pathways. J Mol Med. 2007;85 (12):1369–1378. doi: 10.1007/s00109-007-0236-8. [DOI] [PubMed] [Google Scholar]
- Kanda N, Watanabe S. Histamine enhances the production of nerve growth factor in human keratinocytes. J Invest Dermatol. 2003;121:570–577. doi: 10.1046/j.1523-1747.2003.12428.x. [DOI] [PubMed] [Google Scholar]
- Kingery WS. Role of neuropeptide, cytokine, and growth factor signaling in complex regional pain syndrome. Pain Med. 2010;11 (8):1239–1250. doi: 10.1111/j.1526-4637.2010.00913.x. [DOI] [PubMed] [Google Scholar]
- Koblenzer CS. Itching and the atopic skin. J Allergy Clin Immunol. 1999;104:S109–S113. doi: 10.1016/s0091-6749(99)70052-7. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R, Dal Toso R, Della Valle F, Skaper SD, Alberta L. Update of the NGF saga. J Neurol Sci. 1995;130 (2):119–127. doi: 10.1016/0022-510x(95)00007-o. [DOI] [PubMed] [Google Scholar]
- Patel T, Ishiuji Y, Yosipovitch G. Nocturnal itch: why do we itch at night? Acta Derm Venereol. 2007;87 (4):295–298. doi: 10.2340/00015555-0280. [DOI] [PubMed] [Google Scholar]
- Pincelli C, Fantini F, Massimi P, Girolomoni G, Seidenari S, Giannetti A. Neuropeptides in skin from patients with atopic dermatitis: an immunohistochemical study. Br J Dermatol. 1990;122 (6):745–750. doi: 10.1111/j.1365-2133.1990.tb06261.x. [DOI] [PubMed] [Google Scholar]
- Raap U, Werfel T, Goltz C, Deneka N, Langer K, Bruder M, et al. Circulating levels of brain-derived neurotrophic factor correlate with disease severity in the intrinsic type of atopic dermatitis. Allergy. 2006;61 (12):1416–1418. doi: 10.1111/j.1398-9995.2006.01210.x. [DOI] [PubMed] [Google Scholar]
- Rukwied R, Lischetzki G, McGlone F, Heyer G, Schmelz M. Mast cell mediators other than histamine induce pruritus in atopic dermatitis patients: a dermal microdialysis study. Br J Dermatol. 2000;142 (6):1114–1120. doi: 10.1046/j.1365-2133.2000.03535.x. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Luz O, Averbeck B, Bickel A. Plasma extravasation and neuropeptide release in human skin as measured by intradermal microdialysis. Neurosci Lett. 1997;230:117–120. doi: 10.1016/s0304-3940(97)00494-1. [DOI] [PubMed] [Google Scholar]
- Schulte-Herbrüggen O, Fölster-Holst R, von Elstermann M, Augustin M, Hellweg R. Clinical relevance of nerve growth factor serum levels in patients with atopic dermatitis and psoriasis. Int Arch Allergy Immunol. 2007;144 (3):211–216. doi: 10.1159/000103994. [DOI] [PubMed] [Google Scholar]
- Stander S, Steinhoff M. Pathophysiology of pruritus in atopic dermatitis: an overview. Exp Dermatol. 2002;11:12–24. doi: 10.1034/j.1600-0625.2002.110102.x. [DOI] [PubMed] [Google Scholar]
- Takano N, Sakurai T, Kurachi M. Effects of anti-nerve growth factor antibody on symptoms in the NC/Nga mouse, an atopic dermatitis model. J Pharmacol Sci. 2005;99(3):277–286. doi: 10.1254/jphs.fp0050564. (Epub 2005 Nov. 8) [DOI] [PubMed] [Google Scholar]
- Takano N, Sakurai T, Ohashi Y, Kurachi M. Effects of high-affinity nerve growth factor receptor inhibitors on symptoms in the NC/Nga mouse atopic dermatitis model. Br J Dermatol. 2007;156 (2):241–246. doi: 10.1111/j.1365-2133.2006.07636.x. [DOI] [PubMed] [Google Scholar]
- Tam SY, Tsai M, Yamaguchi M, Yano K, Butterfield JH, Galli SJ. Expression of functional TrkA receptor tyrosine kinase in the HMC-1 human mast cell line and in human mast cells. Blood. 1997;90 (5):1807–1820. [PubMed] [Google Scholar]
- Toyoda M, Nakamura M, Makino T, Hino T, Kagoura M, Morohashi M. Nerve growth factor and substance P are useful plasma markers of disease activity in atopic dermatitis. Br J Dermatol. 2002;147 (1):71–79. doi: 10.1046/j.1365-2133.2002.04803.x. [DOI] [PubMed] [Google Scholar]
- Wang IJ, Hsieh WS, Guo YL, Jee SH, Hsieh CJ, Hwang YH, et al. Neuro-mediators as predictors of paediatric atopic dermatitis. Clin Exp Allergy. 2008;38(8):1302–1308. doi: 10.1111/j.1365-2222.2008.03026.x. (Epub 2008 May 28) [DOI] [PubMed] [Google Scholar]
- Yamaguchi J, Aihara M, Kobayashi Y, Kambara T, Ikezawa Z. Quantitative analysis of nerve growth factor (NGF) in the atopic dermatitis and psoriasis horny layer and effect of treatment on NGF in atopic dermatitis. J Dermatol Sci. 2009;53(1):48–54. doi: 10.1016/j.jdermsci.2008.08.011. (Epub 2008 Oct. 14) [DOI] [PubMed] [Google Scholar]
- Yamaoka J, Di ZH, Sun W, Kawana S. Changes in cutaneous sensory nerve fibers induced by skin-scratching in mice. J Dermatol Sci. 2007;46(1):41–51. doi: 10.1016/j.jdermsci.2006.12.007. (Epub 2007 Jan 18. Erratum in: J. Dermatol. Sci. 2007) [DOI] [PubMed] [Google Scholar]
- Yosipovitch G, Xiong GL, Haus E, Sackett-Lundeen L, Ashkenazi I, Maibach HI. Time-dependent variations of the skin barrier function in humans: transepidermal water loss, stratum corneum hydration, skin surface pH, and skin temperature. J Invest Dermatol. 1998;110:20–23. doi: 10.1046/j.1523-1747.1998.00069.x. [DOI] [PubMed] [Google Scholar]
- Yosipovitch G, Goon A, Wee J, Chan YH, Goh CL. The prevalence and clinical characteristics of pruritus among patients with extensive psoriasis. Br J Dermatol. 2000;143:969–973. doi: 10.1046/j.1365-2133.2000.03829.x. [DOI] [PubMed] [Google Scholar]
- Yosipovitch G, Ansari N, Goon A, Chan YH, Goh CL. Clinical characteristics of pruritus in chronic idiopathic urticaria. Br J Dermatol. 2002a;147:32–36. doi: 10.1046/j.1365-2133.2002.04758.x. [DOI] [PubMed] [Google Scholar]
- Yosipovitch G, Goon AT, Wee J, Chan YH, Zucker I, Goh CL. Itch characteristics in Chinese patients with atopic dermatitis using a new questionnaire for the assessment of pruritus. Int J Dermatol. 2002b;41:212–216. doi: 10.1046/j.1365-4362.2002.01460.x. [DOI] [PubMed] [Google Scholar]
- Yosipovitch G, Sackett-Lundeen L, Goon A, Yiong HC, Leok GC, Haus E. Circadian and ultradian (12 h) variations of skin blood flow and barrier function in non-irritated and irritated skin-effect of topical corticosteroids. J Invest Dermatol. 2004;122:824–829. doi: 10.1111/j.0022-202X.2004.22313.x. [DOI] [PubMed] [Google Scholar]