Summary
Human skin and mucosal surfaces are in constant contact with resident and invasive microbes. Recognition of microbial products by receptors of the innate immune system triggers rapid innate defense and transduces signals necessary for initiating and maintaining the adaptive immune responses. Microbial sensing by innate pattern recognition receptors is not restricted to pathogens. Rather, proper development, function, and maintenance of innate and adaptive immunity rely on continuous recognition of products derived from the microorganisms indigenous to the internal and external surfaces of mammalian host. Tonic immune activation by the resident microbiota governs host susceptibility to intestinal and extra-intestinal infections including those caused by viruses. This review highlights recent developments in innate viral recognition leading to adaptive immunity, and discusses potential link between viruses, microbiota and the host immune system. Further, we discuss the possible roles of microbiome in chronic viral infection and pathogenesis of autoimmune disease, and speculate on the benefit for probiotic therapies against such diseases.
Keywords: dendritic cells, T cells, viral, inflammation, AIDS, Toll-like receptors/PRR
Introduction
Mucosal surfaces are continuously exposed to an enormous number of microorganisms. Some of them, such as the gut-resident commensal bacteria, co-exist with the host in a mutually beneficial relationship, whereas others cause pathological infections. The mammalian host relies on both the innate and adaptive immunity to combat pathogen invasion, including infection by viruses. During viral infections, the innate immune system uses pattern-recognition receptors (PRRs) to trigger a rapid defense program to eliminate viruses, and at the same time direct appropriate adaptive immune responses. A growing body of literature demonstrates that the resident microbiota play a significant role in shaping the development and function of the innate and adaptive immune responses. Consequently, interactions between the immune system and the resident microbiota govern host susceptibility to infections and disease pathogenesis. Here we first review recent literature on the role of various innate recognition receptors in modulating adaptive immunity during viral infections. Next, we present evidence implicating the vital role of the resident microbiota in shaping antiviral defense and modulating the outcome of virus infections. Finally, we explore the possibility of using probiotic therapies to boost immunity against human viral pathogens.
Antiviral defense depends on innate and adaptive immune responses
The innate immune system is equipped with a number of germline-encoded microbial sensors, called pattern-recognition receptors (PRRs). PRRs detect the presence of invariant molecular structures, or pathogen-associated molecular patterns (PAMPs), found in most microorganisms (1). However, PRRs do not distinguish between microorganisms that cause disease (pathogens) and those that do not. Viral sensing by innate recognition receptors is either cell-intrinsic or cell-extrinsic depending on whether it is mediated by infected or uninfected cells (2, 3). Cell-intrinsic recognition relies on intracellular recognition of viral nucleic acids or virus-inflicted damage by PRRs expressed in the cytosol of infected cells. These include the retinoic acid inducible gene I (RIG-I)-like receptors (RLRs), DNA sensors, and the nucleotide binding and oligomerization domain (NOD)-like receptors (also known as nucleotide-binding domain, leucine-rich repeat containing proteins or NLRs), which are ubiquitously expressed in both immune and non-immune cell types (3). In cell-extrinsic recognition, the presence of viral components, usually viral nucleic acids, is detected by receptors expressed in endosomes without direct infection. This pathway is utilized by sentinel cells of the immune system such as plasmacytoid dendritic cells (pDCs), which detect viral nucleic acids by expressing Toll-like receptors (TLRs) in the endosomes without necessarily becoming infected by viruses (3). In some cases, however, infected pDCs recognize RNA viruses through TLR7 after delivery of viral RNA from the cytosol to the endosome via autophagy (4, 5).
Activation of TLRs and RLRs triggers a cascade of signals leading to the activation of NF-κB dependent production of proinflammatory cytokines including IL-6 and TNF-α, and the activation of IRF3 and/or IRF7-dependent transcription of type I interferon (IFN) genes (6). Type I interferons, represented by IFN-α family members and IFN-β, bind to and activate IFN-αβ receptor (IFNAR) to signal through the JAK-STAT pathway. Signaling through IFNAR induces over 300 genes, collectively known as IFN-stimulated genes (ISGs), which cooperate to control virus infection and spread by interfering with multiple stages of virus infection cycle. Type I IFNs also enable neighboring uninfected cells to increase resistance to viral infection (7). IFNAR signaling further induces genes, such as IRF7, resulting in positive-feed back amplification of the IFN response (8). In addition to providing antiviral resistance, type I IFNs also activate the cytotoxic activity of natural killer (NK) cells and cytotoxic T lymphocytes (CTLs) to kill virus-infected cells and induce cytokines and chemokines important for the initiation of adaptive immunity (7, 9).
In contrast to the limited number of PRRs mediating the innate immune response, the adaptive immune response depends on clonal antigen-specific T and B cells with a virtually unlimited number of receptor diversity. Adaptive immune defense is necessary for efficient clearance of the virus, survival of the host and the establishment of immunological memory, which provides a protective response upon secondary infections. Activation of PRRs upon sensing viral nucleic acids or virus-inflicted damage is essential for triggering an innate immune response and shaping the ensuing adaptive antiviral immunity (reviewed in (3, 10–12)).
Cell extrinsic viral recognition
Endosomal TLRs
TLRs are transmembrane PRRs that comprise an ectodomain containing leucine-rich repeats for PAMP recognition, a transmembrane domain, and a cytosolic Toll-IL-1 receptor (TIR) domain. TLRs are expressed either on the cell surface or inside intracellular vesicles where they recognize PAMPs in the extracellular space or in the phagosome or endosomes. There are 10 and 12 functional TLR members in humans and mice, respectively, each recognizing specific molecular patterns associated with bacteria, mycobacteria, viruses, parasites and fungi (reviewed in (6)). The TLRs involved in recognizing virus infections are TLR3, TLR7, TLR8 and TLR9, which are expressed in the endosomes to sense double-stranded RNA (dsRNA) (TLR3), single-stranded RNA (ssRNA) (TLR7 and TLR8), and unmethylated CpG motifs in dsDNA (TLR9), respectively. Other TLRs, including TLR2 and TLR4, have also been shown to recognize virus-encoded products, but such recognition is specific to certain viruses. It is interesting to note that inflammatory monocytes use TLR2 to recognize DNA viruses and produce large amounts of type I IFNs (13). Even though TLR2 is not considered an endosomal TLR, in inflammatory monocytes, endocytosis of TLR2 was required for IFN induction following virus recognition. TLR7, TLR8 and TLR9 induce signals through the adaptor, myeloid differentiation primary response gene 88 (MyD88), whereas TLR3 utilizes TIR-domain-containing adapter-inducing interferon-β (TRIF). In plasmacytoid dendritic cells (pDCs), which are specialized viral sentinel cells, TLR7 and TLR9 recruit IRF7 and induce robust levels of type I IFNs.
TLR-mediated control of adaptive immune responses is the best-studied example of how viral sensing by PRRs provides the necessary signals for instructing adaptive immune responses. The generation of adaptive immunity begins when dendritic cells (DCs) present viral antigen to naïve T cells in the secondary lymphoid organs. Tissue-resident DCs at mucosal surfaces continuously survey the environment for incoming pathogens. Several distinct subsets of DCs exist in the mammalian host, each of which express distinct set of TLRs (14). Thus, a given subset of DCs will only respond to the PAMPs for which they have the appropriate TLRs. For instance, both human and mouse pDCs do not express TLR3. Instead, they express TLR7 and TLR9 and respond to the respective agonists to rapidly induce type I IFNs during viral infections (11). In response to TLR activation, DCs increase the expression of costimulatory molecules on their surface and produce proinflammatory cytokines. In addition, following pathogen uptake in peripheral tissues, DCs upregulate the chemokine receptor CCR7 and migrate to the draining lymph node where they present the processed antigens to naïve antigen-specific T cells in the form of peptide-major histocompatibility complexes (pMHCs) (reviewed in (11)).
For priming of CD4 T cells, direct recognition of PAMPs by TLR in DCs is critical for their activation and effector differentiation. DCs can be indirectly activated by inflammatory mediators without engaging PRR signaling to increase their surface expression of costimulatory molecules and MHC-II, but not the production of IL-12 (15). Consequently, bystander activation by inflammatory mediators alone can only support clonal expansion of CD4 T cells but not the development of effector function (15). In addition, physical association of PAMP and antigen allows DCs to preferentially present the foreign antigen as pMHC complex on cell surface (16), thereby using TLR recognition as a means to distinguish self from non-self antigen. For CD8 T cells to become activated during virus infection, DCs must present microbial antigens on MHC class I. This can be mediated either by the infected DCs through conventional MHC I antigen processing pathway, or by uninfected DCs through a process known as cross-presentation. The latter pathway has the advantage over the direct presentation in that the antigen presenting DCs are not subject to viral manipulation. TLR signaling in DCs has been shown to promote cross-presentation to CD8 T cells (17). Cross-presentation is not restricted to microbial antigens, since autoantigens can be cross-presented at steady state (18). In this case, autoantigens are presumably devoid of PAMPs, and CD8 T cells become tolerized. With respect to cross-priming of viral antigens, it remains unclear how DCs target self vs. non-self antigens for MHC I presentation, and how coupling of PAMP and antigen regulates DCs’ ability to cross-prime CD8 T cells in vivo (3).
Cell intrinsic viral recognition
Cytosolic RLRs
RLRs belong to a family of DExD/H box RNA helicases that function as cytoplasmic sensors for RNA. Three members of the RLR family have been identified: RIG-I, melanoma differentiation associated factor 5 (MDA5), and laboratory of genetics and physiology 2 and a homolog of mouse D11lgp2 (LGP2) (19). RIG-I and MDA-5 bind to the adaptor protein IFN-beta promoter stimulator 1 (IPS-1) (8) also known as MAVS (9), Cardif (10), or VISA (11,12), through the caspase recruitment domain (CARD), resulting in activation of downstream transcription factors for the induction of type I interferons and proinflammatory cytokines. RIG-I is essential for the production of type I IFNs following recognition of short double-stranded RNA (dsRNA) or 5′-triphosphate RNA present in RNA viruses, whereas MDA5 detects viral RNA from picornaviruses and long dsRNAs (more than 2 kb) such as polyinosinic polycytidylic acid (poly I:C) in the cytosol (20–22). Some viruses such as West Nile virus and reovirus are recognized by both RIG-I and MDA-5 (23, 24). Thus, mice lacking RIG-I or MDA-5 or the shared signaling adaptor, IPS-1, are highly susceptible to certain virus infections. LGP2 was originally considered to be a negative regulator of RLR signaling, owing to the lack of CARD domain (25, 26). However, studies using Lgp2−/− mice indicate that LGP2 is involved in the production of type I IFNs after infections with a number of viruses sensed by RIG-I and MDA-5, with the exception of influenza virus (27).
Cytosolic NLRs
The nucleotide-binding domain and leucine-rich-repeat containing family of proteins, generally referred to as the Nod-like receptors or NLRs sense microbial invaders including viruses and activate the inflammasome. NLRs contain three major domains: an N-terminal protein-interacting effector domain, a central NACHT nucleotide-binding domain, and a C-terminal leucine-rich repeat (LRR) domain. NLR proteins can be subdivided into four subfamilies depending on the structure of N-terminal domains – acidic transactivation domain-containing NLRA subgroup (CIITA), caspase recruitment domain (CARD)-containing subfamily (NOD1, NOD2, NLRCs), pyrin domain (PYD)-containing subfamily (NLRPs) and baculoviral inhibitory repeat (BIR) domain containing subgroup (NAIPs) (28). One of the best-studied groups of NLR proteins is pyrin-domain containing NLRP family of proteins, most notably NLRP3, which recruits the adaptor protein ASC to form a cytosolic multi-molecular complex called the inflammasome, leading to the activation of caspase-1 (29). The inflammasomes are multiprotein complexes that act as a platform for caspase-1 activation, which in turn cleaves immature (‘pro’) forms of cytokines such as pro-interleukin-1β (IL-1β) and pro-IL-18, resulting in their secretion into the extracellular space (29, 30). The critical role of NLRs and inflammasomes in antiviral defense was recently reviewed (31, 32). The NLRP3 inflammasomes recognize both DNA and RNA viruses including adenoviruses, Sendai virus, influenza virus, vaccinia virus and encephalomyocarditis virus (32). In addition to the NLRP3 inflammasome, the RIG-I-containing inflammasome (33) and the absent in melanoma 2 (AIM2)-inflammasome (34) are also involved in antiviral immunity to RNA and DNA viruses, respectively.
Influenza virus infection provides a good model to study the function of NLRPs in antiviral defense, because this virus infection results in robust inflammasome activation compared to other viruses (35). Two distinct signals are required to trigger inflammasome mediated cytokine production (36, 37). The first signal is required to induce transcriptional and translational activation of genes encoding pro-IL-1β, pro-IL-18 and the NLRP3, which can be induced by PAMPs or proinflammatory cytokines including TNF-α and IL-1β (36). The second signal is triggered in response to stress or damage, leading to the activation of caspase-1 that results in the processing and secretion of mature cytokines, IL-1β and IL-18 (36, 37). Infection with influenza virus is sufficient to trigger both signal 1 and signal 2 for NLRP3 inflammasome activation and downstream cytokine production (35). Viral genomic RNA stimulates signal 1 through TLR7 in bone-marrow derived macrophages (BMMs) and bone-marrow derived dendritic cells (BMDCs) to induce transcriptional activation of pro-IL-1β (35). M2 viral protein, a proton channel encoded by influenza viruses important for virus infection and replication, is required and sufficient to trigger signal 2 for NLRP3 inflammasome activation in BMMs and BMDCs. M2 is expressed in the secretory pathway and localized to the acidic trans-Golgi network (TGN) shortly after influenza virus infection. Neutralizing the pH of the TGN mimics the activity of M2 and activates the inflammasome in cultured BMMs, indicating that perturbation of ionic concentration in the TGN by M2 triggers NLRP3 inflammasome activation. Interestingly, other viruses are known to encode ion channels (38), raising the possibility that this type of ionic perturbation may be a more general mechanism for sensing virus infection through the NLR complex.
PRR-mediated control of adaptive antiviral responses
Numerous studies have focused on the requirement for signals from specific PRR in linking innate recognition to adaptive immune responses. However, the picture is far from clear, in part because different viruses are detected by more than one PRR. Importantly, compensatory and/or redundant pathways are also in place to ensure that the host mounts an effective adaptive immune response against invading viruses even when one pathway is blocked or impaired (reviewed in (39)). Furthermore, it appears that there are different requirements for PRRs to trigger distinct sets of adaptive immune responses. Mice deficient in one particular PRR may exhibit cell-type specific defects in one aspect of adaptive immunity but not the others following virus infections. Below, we discuss recent evidence for the requirement of PRR-mediated control of adaptive immune responses during virus infections.
Differential requirements for TLR, and RLR signaling in adaptive antiviral defense
The contribution of TLRs versus RLRs has been studied in infections with many viruses. Here, we highlight findings from mouse infections with lymphocytic choriomeningitis (LCMV), respiratory syncytial virus (RSV), respiratory influenza A virus (IAV) and West Nile virus (WNV). One study showed that pDCs were the major source of type I interferons during LCMV infection (40). Although Ips-1−/− mice showed a defective IFN response, MyD88−/− but not Ips-1−/− mice were more susceptible to LCMV-induced mortality. Furthermore, MyD88-dependent signaling was critical for the induction of virus-specific CD8 cytotoxic T lymphocytes (CTLs) (40). In contrast, another study showed that antiviral defense against RSV was mediated by pathways involving IPS-1 and MyD88. RSV induced an early innate antiviral defense via an IPS-1-dependent pathway. IPS-1- and MyD88-dependent signaling also cooperated to generate a B cell antibody response against the virus. However, MyD88−/− Ips-1−/− double knock-out (DKO) mice were able to induce RSV-specific CTL response, indicating that the adaptive CD8 T cell response depends on a recognition pathway independent of TLRs or RLRs (41). More recently, antiviral defense against RSV was found to be dependent on a member of the NLR family called NOD2, which could interact with viral ssRNA to induce type I interferons in an IPS-1-dependent manner (42). Furthermore, Nod2−/− mice were highly susceptible to RSV-induced death compared to WT infected mice (42). However, whether adaptive immune responses against RSV depend on NOD2 mediated recognition of ssRNA was not examined.
In response to influenza A infection, Lopez et al. initially reported that following aerosolized influenza virus infection, neither T cell nor virus-specific antibody (Ab) responses were dependent on MyD88 (43). In contrast, Heer et al. demonstrated that MyD88, but not TLR7, was required for virus-specific IgG2a, IgG2c responses. Yet, MyD88 and TLR7 were dispensable for CD4 and CD8 T cell activation and effector function (44). In contrast, TLR7 and MyD88 negatively regulated IgG1 responses to IAV (44). Furthermore, by using an in vitro system, this study showed that MyD88-dependent signaling together with IFN-α could act directly on B cells to support the magnitude of Ab response and fine-tune the anti-influenza immunoglobulin (Ig) isotypes. In addition, Koyama et al. reported that MyD88 signaling was required for the induction of CD4 T cells and B cell anti-hemagglutinin Ab responses, but not Ab responses to whole influenza virions following intranasal IAV infection. This study reported no defect of Myd88−/− mice in CTL responses (45). Another study by Seo et al. has recently shown that the Ab response to influenza virions was intact in Myd88−/− × Trif−/− mice. However, proliferation of CD4 T cells and production of Th1 cytokines relied on MyD88, but not TRIF (46). Together, these studies, albeit with inconsistencies, indicate that activation of primary CD8 T cells during influenza infection depends on mechanisms other than MyD88 signaling, whereas the CD4 T cell response depends on TLR7-independent MyD88-dependent signaling. Furthermore, MyD88 signaling contributes to virus-specific antibody isotype switching and the magnitude of specific Ig response. In contrast to the roles of MyD88-dependent pathway, TLR-3/TRIF- and RIG-I/IPS-1-dependent signaling are not required for the induction of CD4, CD8 or Ab responses following IAV infection (44, 45). In this regard, it is interesting to note that Tlr3−/− mice actually exhibit increased survival despite the higher viral titers in the lung, suggesting a pathological role of TLR3 during IAV infection (47). Discrepancy between the TLR7 vs. the MyD88 requirement for adaptive immunological outcomes following live IAV infection (44, 45) can be at least in part explained by the involvement of other receptors upstream of MyD88, such as IL-1R. This possibility is discussed in detail below.
While it appears that RLR signaling through IPS-1 is not required for shaping adaptive immune defense against LCMV, RSV or IAV, a recent study has provided a glimpse of how RLR signaling could regulate the activity of the adaptive immune system during virus infections. Following West Nile virus (WNV) infection, Ips-1−/− mice exhibit uncontrolled inflammation marked by elevated systemic levels of type I IFN and proinflammatory cytokines and chemokines (48). Ips-1−/− mice were highly susceptible to WNV-induced mortality due to uncontrolled viral replication in specific organs and viral invasion into the CNS. Interestingly, Ips-1−/− mice displayed increased levels of virus-specific T and B cell response despite a complete loss of antibody neutralization capacity. This dysregulated response in the absence of IPS-1 was associated with a reduction in regulatory T cells (Tregs), a group of CD4+ T lymphocytes with suppressive functions. This study illustrates that although RLR-mediated signaling is not required for the generation of T and B cell response in WNV, it can nonetheless modulate the balance of the adaptive immune responses during virus infections.
In addition to IPS-1, other PRRs induce proinflammatory cytokines and IFNs following WNV infection, including TLR3 and TLR7 (49–51). The role of TLR3 in anti-WNV response is controversial, as one report showed a detrimental role of TLR3, while another showed requirement for TLR3 in host defense. In the first study, Tlr3−/− mice had improved survival rates after WNV infection (50). This is likely due to a decreased inflammatory cytokine response in Tlr3−/− mice, which leads to protection of CNS from inflammation and neurovirulence. A second study showed the opposite result, in that Tlr3−/− had decreased survival after WNV infection, elevated viral titers in peripheral tissues, and early viral entry in the CNS (49). The nature of the discrepancy between these studies is unknown. In contrast, TLR7 appears to have a protective role during WNV infection. TLR7-dependent signaling directs proper immune cell trafficking to sites of WNV infection during the adaptive immune response in vivo (51). Similar findings were reported in Myd88−/− mice, which suffered from increased WNV replication in the brain and decreased cellular recruitment to the site of infection (52). Altogether, these studies indicate important contributions of TLR- and RLR-mediated signaling to host defense that can vary among different viruses.
NLR-mediated control of adaptive immunity
A number of studies have revealed a role for NLRs in inducing the requisite signals for T and B cell activation (reviewed in (53)). In response to virus infections, inflammasome activation and downstream cytokine production play a key role in innate (54, 55) and adaptive immunity (56) during influenza virus infection (reviewed in (57)). Increased production of IL-1β and IL-18 was detected in the bronchoalveolar lavage (BAL) after IAV infection in mice in vivo (54–56). Mice deficient in NLRP3, apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) or caspase-1 failed to secrete IL-1β or IL-18 into the alveolar space in response to IAV infection. Even though NLRP3-ASC inflammasomes generate IL-1β in the alveolar space, following sublethal challenge with a mouse-adapted A/Puerto Rico 8 (PR8) influenza virus, mice deficient in caspase-1 or ASC, but not NLRP3, were found to have diminished levels of virus-specific CD4 and CD8 T cells and impaired B-cell antibody responses marked by a significant reduction in virion-specific nasal IgA and systemic IgG2a isotypes. The requirement for inflammasomes to generate adaptive immune responses was attributed to impaired secretion of IL-1β, since Il1r1−/− and Myd88−/− mice suffered from a similar lack of adaptive immune responses and virus-induced mortality. These data indicated that ASC, but not NLRP3, is required for in vivo source of IL-1β, which is required for the generation of adaptive immune responses to IAV. As discussed above, previous studies indicated that the induction of adaptive CD4 and CD8 T cell response against influenza virus is unaffected in mice deficient in TLR- or RLR-mediated signaling. A trivial explanation is that TLR signaling and RLR signaling act as redundant pathways for the priming and activation of virus-specific T cells. Alternatively, it is possible that activation of influenza virus-specific T cells does not depend on signals through viral PAMP sensing mediated by TLRs or RLRs. Instead, endogenous innate damage signals triggered by virus infection are sufficient for inducing adaptive T cell responses during influenza virus infection. In this scenario, inflammasome-mediated cytokine production induced by influenza virus infection could be a form of “pathogen-induced molecular pattern” or PIMP (58) that is capable of instructing the development of a specific arm of adaptive immune defense. In support of the latter possibility, our data demonstrated that NLR-dependent recognition is required for antiviral immune responses to IAV (56). Future studies are needed to distinguish among these possibilities.
Immune recognition of the intestinal microbiota contributes to host defense
Microbial products are not only produced by pathogens that cause diseases, but also by the indigenous microorganisms residing in the gastrointestinal tract, which are detected by PRRs in the steady state. Interaction between the host and the gut microbiota is highly dynamic and has a profound impact on the immune system locally and systemically. On one hand, multiple mechanisms are in place to keep the immune system in check by establishing tolerance and curtailing overzealous responses to the microorganisms in the gut (recently reviewed in (59–61)). On the other hand, cross-talk between commensal bacteria and PRR-mediated signaling in the steady state is crucial to the development and function of the immune system and the maintenance of intestinal homeostasis. Consequently, the immune system has been described as a rheostat, with the intestinal microbiota playing a significant role in modulating the set-point (62). Below, we review recent evidence that PRR-mediated sensing of resident commensal microbiota in the steady state regulates the development and function of innate and adaptive immune systems in the intestine and extra-intestinal sites, and prepares the host to defend against intrusion by pathogenic microorganisms.
The intestinal microbiota in health and disease
Humans are sometimes described as supraorganisms, serving host to a collection of microorganisms termed the “microbiota” in body sites exposed to the environment, with the majority of them residing in the gastrointestinal tract. The dynamic interaction between humans and their microbiota and the impact of host-microbe mutualism on health and disease have been extensively reviewed elsewhere (63, 64). Large-scale metagenomic sequencing analysis provides a culture-independent way of characterizing the structure, function and dynamics of the microbial communities residing in the gastrointestinal tract (65). Even with more than 50 bacterial phyla on earth, the human microbial communities are dominated by four phyla including the Firmicutes, Bacteroidetes, Actinobacteria and Proteobacteria (66). Within each of these phyla, an abundance of bacterial species exists. At least 500 –1000 species of bacteria are estimated to inhabit in the human gut, and these are estimated to contain 100 times more genes than the human genome (67). Although there is a large amount of diversity at the organismal level in our microbiota, each individual person shares a core set of genes (a core microbiome) that carry out functions common to the human intestine (68). This functional core microbiome shared among individuals is enriched in genes involved in microbial adaptation to the host environment, as well as metabolism of biochemical products such as amino acids and glycans and biosynthesis of vitamins and organic compounds (69, 70).
The use of germfree mice, derived and maintained in sterile isolators has, provided an excellent tool to study the role of microbiota in contributing to pathological conditions. However, the maintenance of germfree animals is costly and requires special facilities in order to avoid contamination. Furthermore, the numbers of animal strains or genetically engineered animals that have been re-derived germfree remain limited at present and these animals do not have a well-developed intestinal architecture and a fully matured immune system compared to conventionally housed specific-pathogen free (SPF) mice (71). Administration of oral antibiotics that target a broad-spectrum of bacteria has provided a complementary approach to study host-microbe interactions. Following antibiotic treatment, the density and composition of the intestinal microbiota are dramatically affected in both human and animal studies (72, 73).
It is well known that the microbiota contributes to mucosal immune defense by preventing colonization or expansion of potentially pathogenic microbes in the gastrointestinal tract. Antibiotic treatment in mice permits the colonization by enteric pathogens including vancomycin-resistant Enterococcus (VRE) (74) and Salmonella typhimurium (75). Pathogen clearance can be restored by reintroduction of the normal microbiota or bacterial-derived products in antibiotic-treated mice. In addition, germfree animals are more susceptible to a wide variety of intestinal infectious pathogens including Shigella flexneri and Listeria monocytogenes (reviewed in (71)). Moreover, there is ample evidence from human and animal studies that microbiota can modulate the outcome of infections and pathogenesis of diseases outside of the gastrointestinal tract. Aberrant changes in the intestinal microbiome as a result of host genetics or antibiotic therapy in humans are associated with an increasing number of pathological conditions including obesity (76), inflammatory bowel disease (77), diabetes (78) and atopic diseases (79). In mice, recognition of peptidoglycan from the microbiota by Nod-1 primes systemic innate immunity by enhancing the cytotoxcitiy of bone-marrow derived neutrophils in response to systemic infection with the bacterial pathogens Streptococcus pneumoniae and Staphylococcus aureus (80). Furthermore, microbiota and dietary habits contribute to the risks of cardiovascular diseases (81), obesity and type 2 diabetes (82) in mice. Disruption of the gut microbiota by antibiotic treatment and increased levels of fungal species in the intestinal tract can also predispose mice to allergic airway diseases (83). In addition, a recent study suggested that bacterial products from the gut microbiota protected the central nervous system against experimental autoimmune encephalomyelitis (EAE), a widely used animal model for multiple sclerosis in humans (84).
Microbiota in the development of intestinal and extraintestinal lymphoid tissues
The gut microbiota are essential for the proper development of both mucosal and systemic immune organs (reviewed in (71, 85)). Germ-free mice have an underdeveloped lymphatic system, reduced number and size of gut-associated lymphoid tissues (GALT) including Peyer’s patches and isolated lymphoid follicles (ILFs), and reduced number of intraepithelial lymphocytes. PRR signaling can induce intestinal lymphoid tissue genesis, as one study showed that recognition of peptidoglygan from gut-residing Gram-negative bacteria by Nod1 in intestinal stromal cells was both necessary and sufficient to induce the generation of ILFs in the small intestine, whereas maturation of ILFs required subsequent signaling via TLRs (86). Furthermore, there is reduced production of intestinal IgA in germ-free mice compared to conventionally reared mice. Germ-free animals also have smaller intestine-draining mesenteric lymph nodes (MLN) with reduced cellularity and fewer germinal centers, as well as poorly-formed high-endothelial venules with reduced lymphocyte binding activity in the MLN.
Absence of commensal bacteria also has a systemic effect on the immune system. The spleens and secondary lymphoid organs of germ-free mice possess poorly structured T- and B-cell zones. These mice have fewer germinal centers and plasma cells, which correlates with reduced levels of immunoglobulins systemically. Germ-free mice have reduced systemic immune responses to some types of antigenic stimulation including the delayed hypersensitivity reaction to sheep red blood cells and the antibody response against heat-killed E. coli. Other responses are not affected, such as T cell-mediated tolerance to ovalbumin (OVA) and immunoglobulin responses to the haptenated protein, phosphorylcholine, 2,4-dinitrophenyl--keyhole limpet hemocyanin (DNP-KLH) in the spleen. Furthermore, germ-free mice have systemic CD4 T cell deficiency in the spleen and aberrant Th1/Th2 cytokine production in response to in vitro stimulation, which can be restored by the injection of purified bacteria product from specific commensal bacteria (87).
Innate sensing of microbiota modulates innate host defense
Commensal bacteria play many vital roles in preparing the host immune system for battling against pathogen invasion. Almost all TLRs have been found at the mRNA level in human colon and small intestines, but our knowledge about their function in different physiological locations and their cell type-specific expression remains incomplete (88). Growing evidence supports the role of microbial sensing by PRRs in the development of intestinal homeostasis and maintenance of host immune function at steady state. A seminal discovery in this regard was the finding that TLR signaling via MyD88 plays a critical role in maintaining epithelial homeostasis and protection from epithelial injury (89). Using a model of chemical-induced intestinal injury, Rakoff-Nahoum et al. showed that Myd88−/− mice displayed an exacerbated inflammatory phenotype with severe colonic epithelial damage. Further, mice depleted of the gut microbiota were also highly susceptible to epithelial damage-induced inflammation, suggesting that innate recognition of commensal microbiota through TLRs provides a tonic tissue-repair signals critical in responding to inflammatory stimuli (89). In addition, both TLR and NLR signaling have been shown to fortify epithelial barrier by enhancing expression of tight junction molecules (90) and inducing lymphoid tissue genesis (86) in the intestine. Together, these studies indicate that PRR-mediated recognition of commensal bacteria mediates protection against breach of epithelial integrity.
Microbial colonization in the gastrointestinal tract is also associated with the induction of a number of antimicrobial peptides produced by Paneth cells at the base of the crypts in the small intestines (91, 92). These proteins act as first line of defense against incoming enteric pathogens. Mice treated with broad-spectrum antibiotics have reduced expression of RegIIIγ, rendering them highly susceptible to infection with antibiotic-resistant bacteria (74). However, oral administration of lipopolysaccharide (LPS), a TLR4 ligand, boosts innate immune defense against the bacteria (74), suggesting that antibiotic treatment decreases the number of microbial ligands available for constitutive activation of innate effectors in the intestine. It is likely that multiple PRRs can be engaged by the gut microbiota to stimulate innate host defense through the induction of antimicrobial peptides. Studies using Nod2−/− and Myd88−/− mice indicate that NOD2-dependent detection of the bacterial ligand, muramyl-dipeptide, is required for the induction of cryptdins (α-defensins in humans) (93) whereas MyD88-dependent signaling regulates the expression of RegIIIγ (94). Decrease in the expression of antimicrobial peptides in Myd88−/− and Nod2−/− mice is associated with increased susceptibility to intestinal bacterial infections (93–95).
In addition to influencing the level of antimicrobial peptides, recent studies indicate that commensal microbiota can modulate the expression of PRRs. In vitro experiments with human primary macrophages showed that the commensal bacteria Lactobacillus rhamnosus GG enhanced TLR2 expression (96). Furthermore, depletion of gut microbiota by antibiotics in mice can result in reduced surface expressions of TLR2 and TLR4 in peritoneal macrophages and less inflammation following intraperitoneal LPS injection in vivo (97), indicating that intestinal microbiota can constitutively prime peritoneal macrophages in preparation for pathogen invasion. Moreover, another study demonstrated that butyrate, a short-chain fatty acid produced by the intestinal bacteria during fermentation of food products, induces the expression of NOD2, resulting in enhanced activation following stimulation by peptidoglycan in intestinal epithelial cells (98), indicating that bacteria-dependent secretion of metabolic byproducts may also influence the expression of PRR in intestinal epithelial cells. This is a growing area of study in the field, and future research is expected to reveal how bacterial metabolites influence host immunity both locally and systemically.
Microbiota regulation of Th17 and Treg cells
As described above, microbiota play a pivotal function in the development of lymphoid organs and the regulation of innate host defense. In addition, certain commensal bacteria appear to directly stimulate the development of adaptive immune cell lineages. The homeostasis of effector T cell populations in the gut is dependent on the composition of intestinal bacteria. The intestinal mucosa contains large numbers of CD4 T cells including T helper 17 (Th17) cells and Foxp3+ regulatory T cells (Tregs). Th17 cells secrete IL-17, IL-21 and IL-22 and are important in controlling inflammatory responses against bacterial and fungal invasions whereas Tregs actively suppress excessive immune responses in the intestine. Both Tregs and Th17 cells depend on the cytokine TGF-β for their differentiation (reviewed in (99, 100)). Recent studies have indicated that signals derived from the gut microbiota act as adjuvants to constitutively prime the adaptive immune system against oral pathogens by regulating the differentiation of Tregs and Th17 cells.
Microbiota regulation of Tregs
Recent studies have demonstrated the ability of gut microbiota to impact the balance between Treg and other effector T cell populations. Bacterial polysaccharide (PSA) from the gut microorganism Bacteroides fragilis expanded CD4 T cells and corrected the systemic CD4 T cell deficiency and imbalance in T cell cytokine production in germ-free mice (87). PSA from B. fragilis protected against inflammatory colitis induced by an opportunistic bacteria in immunocompromised host through a mechanism dependent on the induction of IL-10-producing CD4 T cells (101). Recently, PSA from B. fragilis has been shown to directly stimulate Tregs through TLR-2 to mediate their suppressive functions (102, 103).
In a separate study, suppressive role of commensal bacteria on Treg conversion was demonstrated to depend on TLR9. Tlr9−/− mice showed an increased level of Treg cells and reduced levels of IL-17 and IFN-γ-producing effector CD4 T cells. Further, this was mediated by TLR9-dependent recognition of microbiota-derived DNA limiting Treg conversion and regulating the balance between Treg and T effector (Teff) cells in the lamina propria (104). Treg versus Teff cell disequilibrium in Tlr9−/− mice led to impaired immune responses to oral infection with the pathogen Encephalitozoon cuniculi (104).
A more recent study identified Clostridium spp., Gram-positive and spore-forming bacteria indigenous to the mouse gastrointestinal tract, as major inducers of Tregs in the colon. Colonization of germ-free mice by a mix of Clostridium strains promoted TGF-β expression and increased Treg number and function in the colon (105). Clostridium spp.-mediated Treg induction in the colonic lamina propia is independent of PRR signalings via MyD88, RIP2 (signaling adaptor for NOD1 and NOD2) or Card9 (involved in Dectin-1 anti-fungal response) (105). Enrichment in Clostridium spp. following oral feeding of SPF mice resulted in improved resistance to DSS colitis and reduced systemic IgE response to ovalbumin immunization in alum. Collectively, these studies highlight the importance of microbiota and their components in negatively or positively regulating Treg number and function.
Microbiota drives Th17 responses
Commensal-derived signals also modulate the development of Th17 cells, which do not develop in the small intestine of germ-free mice in the absence of commensal bacteria (106, 107). One mechanism is through commensal-mediated production of ATP, which can induce the differentiation of Th17 cells (107). Another mechanism is through the induction of Th17 cells in the small intestine by a specific class of commensal bacteria, the segmented filamentous bacteria (SFB) (108, 109). SFB colonization in germ-free mice is important for the induction of Th17 cells to protect against the intestinal pathogen, Citrobacter rodentium (108). In contrast, it has been shown that the frequency of Th17 cells is significantly elevated in the large intestine the absence of commensal bacteria (110). Furthermore, commensal bacteria-dependent expression of IL-25 by intestinal epithelial cells limits the expansion of Th17 cells by inhibiting the production of IL-23, suggesting that the mechanism by which the gut microbiota regulates Th17 cell development differ between the large intestine and the small intestine (110).
It is important to note that the ability of bacteria to induce Th17 or Tregs is not restricted to SFB and Clostridium, respectively. Colonization of germ-free mice with altered Schaedler flora (ASF), containing a small consortium of bacteria, induced both Th17 and Tregs (111). This study found that the increase in Tregs in ASF-colonized mice depended on TLR signaling, as Myd88−/− × Ticam−/− mice, which were unable to respond through all TLRs, failed to increase Treg frequency in the colon. Therefore, innate recognition of microbiota by TLRs leads to both positive (111) and negative (104) regulation of Treg cells in the intestine.
Microbiota and virus infections
Several studies published a few decades ago have shown that microbiota had the potential to modulate the outcome of certain virus infections. Germ-free mice are more susceptible to influenza virus, Coxsackie virus and Friend leukemia virus (reviewed in (62, 71)). However, these earlier studies did not determine the mechanism by which commensal microbiota contribute to host defense against these viruses. With recent expansion in our knowledge on the crosstalk between the microbiota and the immune system, there is considerable interest in understanding how microbiota can increase host resistance to viral infections by induction of the innate and adaptive immune system.
The influence on the microbiota in immune functions and host defense against murine cytomegalovirus (MCMV) infection has been studied in germfree mice (112, 113). MCMV is a close relative of human CMV, a DNA virus that belongs to the beta-herpesvirus family. CMV infection is asymptomatic in immunocompetent host but can lead to various inflammatory diseases such as pneumonitis and gastritis in immunosuppressed patients. One early study reported that germ-free mice were more susceptible to MCMV infection than SPF mice (112). In germ-free mice infected with MCMV, the polyclonal B cell antibody response to sheep red blood cells was reduced compared to SPF mice. Later, another study used a mouse model of MCMV-associated lung disease to show that the frequency of virus-specific CD8 T cells in the lung was significantly lower in germ-free mice between 1 to 12 months after MCMV infection (113). This effect was not due to initial differences in viral loads as viral titer remained comparable between germ-free and SPF mice in the early phase of infection. Oral administration of fecal suspension containing the gut microbes from SPF mice for two weeks reconstituted the frequency of MCMV-specific CD8 T cells in germ-free mice to the level of SPF mice (113), suggesting that indigenous microbiota modulate the development of adaptive immune responses against MCMV infection. In 1964, a study by Dolowy and Muldoon demonstrated that germ-free mice were significantly more susceptible to virus-induced mortality after infection with PR8 influenza A virus (114). Twenty-one days after infection, influenza infected germ-free mice showed no detection of viral hemagglutinin-specific antibody response, suggesting that microbiota is required for adaptive immune responses against influenza virus (114). While these studies indicate a link between microbiota and antiviral immunity, the use of germ-free mice complicated the interpretation of the findings, as they have dramatically impaired lymphoid organ development and immune responses in general (71).
To more precisely understand the role of microbiota on antiviral immune defense, in a recent report, our laboratory characterized the cellular and molecular mechanism by which the gut microbiota regulate respiratory tract immune defense against influenza virus infections (115). We used adult mice under SPF conditions as they have intact lymphoid tissues and immune compartments. Mice were subjected to a four-week oral administration of antibiotic combination, vancomycin, neomycin, metronidazole, and ampicillin (V/N/M/A), which significantly altered the density and diversity of the gut microbiota in antibiotic-treated (ABT) mice. Following intranasal challenge with a sublethal dose of PR8 virus (10 PFU or 0.4 LD50), ABT mice showed critically impaired generation of virus-specific antibody, CD4 and CD8 T cell responses (115). Consequently, ABT mice exhibited delayed viral clearance in the lung compared to influenza-infected SPF mice at day 9 post infection (p.i.) (115).
To define the role of specific classes of bacteria responsible for maintaining respiratory immunity against influenza A virus, we treated mice with individual antibiotics. Treatment with neomycin alone recapitulated the effect of combination antibiotic treatment on the impaired flu-specific CD8 T cell responses. Microbiological analyses of culturable bacteria from the stool and nasal washes of mice treated with single antibiotics revealed that Lactobacillus spp. dominated the stool of ABT mice, but were almost completely eliminated from neomycin-treated mice. Instead, the stool of neomycin-treated mice was dominated by an overgrowth of Sphingomonas spp. Furthermore, we showed that treatment of mice with neomycin alone, which was not absorbed through the gastrointestinal tract into the circulation (116), significantly altered the composition and reduced the richness of the gut-resident but not nasal bacteria. Collectively, these data indicate that neomycin-sensitive bacteria in the gastrointestinal tract are required for supporting immune responses to respiratory influenza infection
We next demonstrated that the immune defect observed in ABT mice was specific to influenza virus, but not the result of global or respiratory immunodeficiency because intranasal infection with the respiratory bacterial pathogen, Legionella pneumophila, or immunization of ABT mice with adjuvant and model antigen in the footpad did not result in defective adaptive immune responses. Moreover, antibiotic treatment impaired DC homeostasis and maturation at steady state. ABT mice had reduced number of CD103+ DCs, a DC subset previously demonstrated to be required for the priming of CD8 T cells upon influenza virus infection (117). Furthermore, respiratory DCs failed to migrate from the lung to the draining lymph node shortly after influenza virus infection. As a result, DCs isolated from the draining mediastinal lymph node of ABT mice failed to prime naïve antigen-specific CD8 T cell after infection.
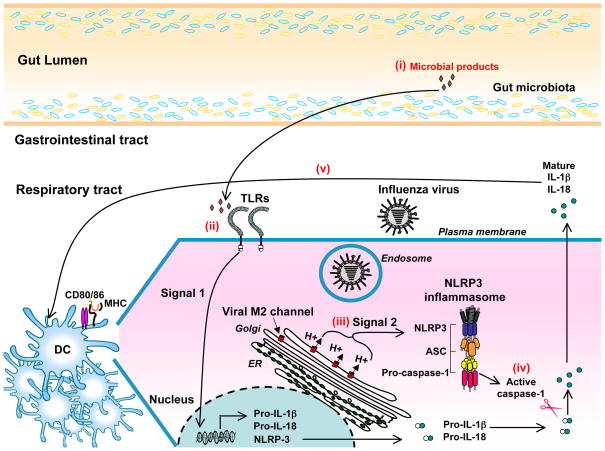
To investigate how gut microbes might specifically support immune responses against influenza virus, we focused on the requirement for inflammasome-mediated cytokine release for triggering adaptive immune responses against influenza virus (56). We found that the mRNA expression of pro-IL-1β and pro-IL-18 in the lung of ABT, but not SPF mice, was abrogated in steady state and 24 hr after influenza A infection. As a consequence, ABT mice had reduced level of IL-1β secretion in the lung upon influenza virus infection. These data indicate that intact gut-resident microbiota support the expressions of pro-IL-1β and pro-IL18 (signal 1) in steady state. Upon flu infection, virus-inflicted damage including that induced by the viral M2 ion channel (35) provides signal 2 for inflammasome-mediated release of mature IL-1β and IL-18. From our results, we speculate that the gut microbiota support respiratory immunity against influenza virus by releasing low levels of PRR ligands in circulation. In support of this idea, we found that intrarectal stimulation of ABT mice with a number of TLR agonists including LPS (TLR4), peptidoglycan (TLR2), poly(I:C) (TLR3), and CpG DNA (TLR9), could restore the impaired T- and B-cell responses in ABT mice (115). Direct PRR activation or the induction of host factors as a result of PRR signaling could provide the immediate source of signal 1 for inflammasome-mediated cytokine release in the lung at steady state and after influenza virus infection, which in turn modulate the ability of respiratory DCs to become professional antigen-presenting cells for the activation of adaptive immune defense against influenza viruses (Figure 1).
Figure 1. Proposed mechanism by which the gut microbiota support respiratory immunity against influenza virus infection.
Caspase-1-mediated inflammasome activation and IL-1β released from hematopoietic cells is required for protective adaptive immune defense against influenza virus infection (56). We speculate that the microbiota enable the host to respond optimally to influenza infection (115) through the following mechanism. (i) A selective population of gut-resident microbiota releases low levels of microbial ligands into systemic circulation. (ii) In the respiratory tract, commensal bacteria-derived microbial products induce transcriptional and translational activation of pro-IL-1β, pro-IL-18 and NLRP3 (signal 1) at steady state. (iii) In influenza-infected cells, virus-inflicted damage including ionic perturbation caused by the viral M2 proton channel expressed in the acidic trans-Golgi network provides signal 2 needed for the formation of NLRP3 inflammasome and the activation of caspase-1 (35). (iv) Active caspase-1 proteolytically cleaves the pro-IL-1β and pro-IL-18 into their mature forms for release into the extracellular space following influenza virus infection. (v) inflammasome-dependent cytokine released in the lung in turn modulate the ability of dendritic cells to upregulate the expression of costimulatory molecules and migrate to the draining lymph node where they present the processed viral antigens to activate naïve antigen-specific T cells.
Many questions remain regarding the role of the gut microbiota in antiviral response. It is unclear whether specific species of gut-resident bacteria are required to control respiratory immunity in the lung. In addition, the identity of the microbial ligands or host-derived factors induced by the gut microbiota that support stimulation of signal 1 in vivo remains unknown. Furthermore, whether other pathogens that require the inflammasomes depend on the gut microbiota for immunity is currently unknown. Interestingly, it was recently shown that MCMV infection induces the release of IL-1β mediated by the AIM2 inflammasome (34). Given the increased susceptibility of germ-free mice to MCMV infection (112), it would be interesting to test whether inflammasome-mediated cytokine secretion is impaired in germ-free or ABT mice upon MCMV infection.
Microbiome, virome and chronic virus infections
Microbiota and HIV pathogenesis
Recent evidence suggests that products from the intestinal microbiota can directly or indirectly enhance pathogenesis of disease and sustain chronic immune activation that accompanies certain viral infections. Human immunodeficiency virus 1 (HIV-1) infection is characterized by a rapid and dramatic loss of memory CD4 T cells in the gastrointestinal tract (118–120). Chronic immune activation, including systemic polyclonal B cell activation, increased frequency of activated T cells and increased levels of serum proinflammatory cytokines and chemokines, is a cardinal feature of HIV-1 infection and a strong negative prognostic factor. However, the cause of inflammation that results in chronic immune activation remains unclear. One potential mechanism is that chronic activation of the innate immune system is driven by TLR ligands directly encoded by HIV-1 (121). Another mechanism is suggested by recent studies indicating that HIV-1 infection resulted in translocation of microbiota and/or their microbial products. There is an early breach in the integrity of the mucosal immune system characterized by compromised epithelial repair and increased epithelial permeability in HIV-infected patients (122). One initial study showed translocation of a bacterial product, LPS in the serum of HIV-infected humans without overt bacterimia, which correlated with an increased number of monocytes and activated CD8 T cells (123). A more recent study showed that immunologic non-responders among HIV-1-infected highly active antiretroviral therapy (HAART)-treated patients had the highest level of circulating LPS and persistent T cell hyperactivation compared to HIV-1-infected HAART responders (124). This study also found an association between the levels of circulating LPS and bacterial DNA fragments from enterobacteria resident in the gut (124). Furthermore, HIV-1 patients have between 10–10,000 fold more opportunistic pathogens such as Pseudomonas aeruginosa and Candida albicans in the intestine and a reduced number of commensal bacteria including bifidobacteria and lactobacilli (125). The aberrant shift in the microbiome, together with a compromised mucosal barrier early on during infection, may further augment bacteria translocation and contribute to chronic immune activation in HIV-1-infected individuals, which fuels virus replication in activated CD4 T lymphocytes and progression of the disease.
Furthermore, since the gut microbiota provide important signals for the differentiation of adaptive immune cell lineages, it is possible that changes in the composition of the microbiota modulate CD4 T cell differentiation, which in turn influence the systemic immune activation observed during HIV-1 infections. Intriguingly, infection with HIV-1 in humans, or simian immunodeficiency virus (SIV) – an established model for studying human HIV disease – in rhesus-macaques, is associated with depletion of Th17 cells (126), imbalance between Th17 and Th1 (127, 128), or between Th17 and Treg cells (129). In one study, Th17 depletion was shown to result in increased systemic bacterial translocation (126), possibly allowing microbial products to enter systemic circulation and fueling the chronic immune activation. The mechanism responsible for depletion of Th17 cells during infection, however, remains unclear. Depletion of Th17 T cells due to direct HIV-1-infection, or impaired induction of Th17 cells in the intestinal mucosa due to the loss of Th17-supportive bacteria (reviewed in (130)) may account for the loss of Th17 cells. Collectively, these studies indicate that perturbation in the gut microbiota and breakdown in intestinal mucosal barrier have a profound impact on HIV-1pathogenesis.
Collective virome contributes to host immune responses
Humans are chronically infected with viruses, such as herpesviruses and polyomaviruses, which are kept in check by continuous surveillance by the immune system. In addition to exogenous viruses, eight to nine percent of the human genome contains endogenous retroviral elements (ERV), which can be transcribed into proteins in a variety of normal human tissues obtained postmortem (131). These ongoing chronic viral infections could have important impact on the innate and adaptive immune system including the cytokine environment, constitutively activation of immune cells harboring the viruses, and composition of antigen-specific T cell repertoire (reviewed in (132)). Some viruses could endow protective immune benefits in the hosts. For instance, human herpesvirus 6 (HHV6), human herpesvirus 7 (HHV) and the flavivirus GB virus C (also hepatitis G virus), all of which rarely cause any symptomatic diseases, have been shown to slow the progress of HIV-1 pathogenesis (133, 134) and increase the survival of HIV-1-infected patients (135). A seminal study by Virgin and colleagues further examined this concept in vivo. Mice latently infected with murine gammaherpesvirus 68 or MCMV, which act as a model for the human pathogens Epstein-Barr virus (EBV) and CMV, respectively displayed raised basal innate activation status. Mice chronically infected with these viruses had systemic activation of macrophages (which is not a direct consequence of viral infection) and produced elevated levels of pro-inflammatory cytokines, IFN-γ and TNF-α at steady state, rendering these mice more resistant to bacterial infections (136). These studies imply that the collective endogenous and exogenous ‘virome’ is detected by PRRs constitutively in order to prime the immune system and prepare for infections that are pathogenic to the host.
Microbiota and antiviral response in autoimmunity
Dysregulated innate stimulation by common virus infections may contribute to the pathogenesis of some autoimmune diseases. Evidence from animal experiments and human epidemiological studies has implicated a role for gastrointestinal enteroviruses, which usually cause asymptomatic or sub-clinical infections, in the development of Type 1 diabetes (T1D) (137). The most frequently involved enteroviruses in T1D patients are Coxsackievirus B. Enteroviruses are single-stranded RNA viruses belonging to the family picornaviridae, which are sensed by the RLR member, MDA5. There is a significant association between T1D and single-nucleotide polymorphisms (SNPs) in the gene encoding the RLR family member, MDA5 (also known as IFIH1) (138). Loss of function or reduced expression of MDA5 is considered protective against T1D whereas individuals with susceptible genotypes to T1D have increased expression of MDA5 in their leukocytes, suggesting a link between a robust antiviral response and T1D (reviewed in (139)). Despite these associations, the underlying mechanism by which viral sensing and antiviral response contribute to susceptibility of T1D remains unclear.
In addition to infections with common viruses such as the enteroviruses, host recognition of commensal microbiota also modulates the pathogenesis to T1D. Using a mouse model of T1D, Chervonsky and colleagues demonstrated a critical role of commensal bacteria in prevention of T1D (140). Germ-free mice of the non-obese diabetic (NOD) background strain, which develop T1D spontaneously, had higher incidence of T1D than mice kept in SPF conditions. Strikingly, Myd88−/− NOD mice were almost completely resistant to the development of T1D when housed in SPF conditions, indicating that MyD88-dependent innate immunity is critical for the development of T1D. Such protection depended on the presence of commensal bacteria because germ-free Myd88−/− mice developed robust T1D (140). This study further demonstrated that the microbiota in WT and Myd88−/− mice were different and that colonization of germ-free NOD with the microbiota of SPF Myd88−/− mice led to attenuation of T1D development, indicating that specific changes in the composition of the microbiota in Myd88−/− mice protect against T1D. Along these lines, several reports now indicate that host genomic status, particularly mutations in genes encoding innate immune genes, can dramatically alter the microbial composition, leading to inflammatory disease pathogenesis. In fact, bacteria that outgrow as a result of host genomic mutations can recapitulate the same pathology when transferred into WT hosts (141–144), cautioning us to consider the disease-causative effects of changes in the microbiota when interpreting knockout mouse studies and human genome-wide association studies (GWAS).
Since certain intestinal viruses and bacteria can trigger or modulate T1D (reviewed in (58)), could microbiota actually influence the host susceptibility to viruses that promote the development of T1D? Intriguingly, a study reported in 1963 shows that germ-free suckling mice were more susceptible to infections with Coxsackie B1 virus, with increased virus-associated mortality and rapid spread of virus to the brain, which can be rescued by monocolonization of these germ-free mice with a non-pathogenic strain of Staphylococcus aureus (145). This study raises the possibility whereby a select group of intestinal bacteria inhibit T1D through their ability to reduce host susceptibility to common viruses, particularly enteroviruses, which have been positively associated with T1D. Future studies will need to investigate the interplay, if any, between the virome and the microbiota, together with the contribution of host genetics to the development of T1D and other complex diseases.
Combating viral infections by complementary probiotic therapy
Given the role of the microbiota in shaping innate and adaptive immunity and maintaining immune homeostasis, increasing number of studies have examined the therapeutic potential of commensal bacteria in modulating the mucosal immune responses. Probiotics are live microorganisms that can confer a health benefit to the host. The most common probiotics include intestinal commensal bacteria such as Lactobacilli and Bifidobacteria. Probiotics are sometimes used in combination with prebiotics, which are non-digestible dietary supplements that selectively favor the growth of certain probiotics. The combination of probiotics and prebiotics are referred to as “synbiotics”. There are convincing data to support the use of certain probiotics in treating intestinal inflammation including inflammatory bowel disease and necrotizing enterocolitis, and the prevention of antibiotic-associated diarrhea including those caused by gastrointestinal infection with Clostridium difficile (reviewed in (146)). Human clinical studies examining the therapeutic potential of probiotics for the prevention and treatment of viral infections are still at an early stage. Below, we will examine evidence for the immunosupportive effects of probiotics during virus infections, focusing on respiratory infections and HIV-1.
Respiratory viral infections
Oral intake of probiotic or synbiotics is capable of modulating the outcome of infections in the respiratory tract. Clinical evidence from 14 human randomized controlled trials (RCT) on the use of probiotics and synbiotics for prevention and treatment of respiratory tract infections including the common cold was evaluated in a systematic review (147). Most RCTs find no difference in the incidence of respiratory tract infections between treatment groups and the placebo groups. However, a significant reduction in the severity of symptoms and the clinical course of disease is observed in five out of six RCTs and three out of nine RCTs that provided the relevant data, respectively (147). In addition, several human clinical trials have shown that certain Lactobacillus species boosted vaccine-mediated immunity. The Lactobacilli form an important part of the commensal microbiota in the intestinal tract of humans and other mammals. Recently, comparative analysis of human mucosal transcriptome responses has revealed that Lactobacillus species induced gene regulatory pathways important in immune responses, cellular proliferation and cytokine production (148). In one study, consumption of Lactobacillus GG was associated with seroconversion to a protective antibody titer against the H3N2 strain of influenza virus, but not the H1N1 or the influenza B strain (149). In another study, daily consumption of a fermented dairy drink improved antibody responses to influenza vaccination in elderly over 70 years of age (150). In addition to increasing vaccine-mediated antibody titer, one study showed that oral intake of L. fermentum CECT5716 two weeks before and after influenza vaccination was associated with an increase in the level of natural killer cells and the concentration of serum TNF-α, along with an induction of serum anti-influenza IgA level, in the probiotic-treated group after vaccination (151). In support of human clinical studies, animal studies involving the use of Lactobacillus in the prevention and treatment of respiratory viral infections have also yielded potentially promising results. In influenza-infected mice, oral intake of L. plantarum enhanced type I interferon production (152). As a result, mice that had received probiotics had lower pulmonary viral titers. A similar effect was observed in mice orally treated with L. casei strain Shirota, and here, the beneficial effect was attributed to enhanced natural- killer cell activity and increased production of IFN-γ and TNF-α (153). Collectively, these studies suggest that oral intake of probiotic bacteria can serve as an immune adjuvant in enhancing the efficacy of anti-influenza vaccines, and potentially have usefulness in therapeutic treatment of influenza infection along with antiviral drugs.
HIV-1 infections
The effect of probiotic therapy has also been investigated in human clinical trials with HIV-1-infected patients. One study conducted in Brazil involving 77 HIV-positive children showed that a two-month course of probiotic formula containing B. bifidum and S. thermophilus increased CD4 T cell counts by 17.5%, compared with a 7% decrease in the control group (154). However, another study in Nigeria involving the daily use of conventional yogurt supplemented with probiotic bacteria for two weeks in HIV-infected patients failed to induce an increase in the mean CD4 T cell counts (155). This is in contrast to a more recent observational study examining the long-term effect of probiotic yogurt consumption in HIV-1-infected patients in Tanzania, which showed that consumption of probiotic yogurt supplemented with Lactobacillus rhamnosus Fiti was significantly associated with an overall increase in CD4 T cell count, with the greatest increase occurring in the first 70 days (156). A large-scale human RCT (PRONUT study) examining the clinical and nutritional efficacy of a synbiotic, Synbiotic2000 Forte, in 795 children in Malawian included a group of 361 HIV-1-infected children (157). Although the synbiotic did not improve severe acute malnutrition outcomes, there was a reduction in overall outpatient mortality, including a tendency of reduced mortality among the subgroup of HIV-infected children. In addition to the effect of probiotics and synbiotics on modulating the progression of HIV-1 pathogenesis, other benefits have been suggested, including their impact on the prevention and cure of bacterial vaginosis and HIV-1-associated gastrointestinal infections and diarrhea (reviewed in (158)). However, very few studies have been conducted to date to assess its potential. Taken together, there are a limited number of studies to suggest that probiotic interventions in HIV-1-infected patients can directly lead to an increase CD4 T cell counts and halt the progression of HIV pathogenesis. Nevertheless, probiotic therapy appears to be safe even in individuals suffering from immunodeficiency, and has the potential to bestow a favorable outcome in HIV-1-infected patients. More studies are needed to determine whether specific groups of HIV-1-infected patients, for example, children suffering from malnutrition or those suffering from gastrointestinal infections, will benefit more from probiotic therapies. Future studies will also need to clarify which species of probiotic bacteria are best suited to reach the desired outcome, and the mechanisms by which such probiotic organisms exert beneficial effects on HIV-1-infected individuals.
Concluding Remarks
Many recent studies have highlighted the importance of PRR-mediated signaling in instructing the development of adaptive immune defense against viruses. At the same time, the use of gnotobiotic animals and genomic sequencing analysis of the microbiota has revealed a previously unknown diversity and functions of the commensal microorganisms within the mammalian immune system. Mounting evidence indicates that microbial ligands from the gut microbiota are constitutively sensed by PRRs, which modulates the setpoint of the innate and adaptive immune system in steady state. Commensal bacteria-sensing by PRRs provide tonic signals that direct intestinal and extra-intestinal immune development and prime the immune system in preparation for microbial defense against viruses and other pathogenic organisms. Crosstalk between viruses and microbiota also modulate the host immune response and disease pathogenesis during chronic virus infections. We have only begun to scratch the surface of the interface between the microbiota and antiviral responses - with the advent of high speed genome sequencing and the ability to manipulate the microbiome, the field is ripe for investigations into the mechanism by which the microbiome influences the immune system and vice versa. For example, it remains unclear why immune response to certain viruses, but not others, is more reliant on immune activation by the microbiota. This may be due to differential requirements for PRRs in establishing protective adaptive immune defense. In addition, it remains unclear whether certain species of bacteria or the overall microbial composition is important for endowing immunocompetence to the host against virus infections. Clearly, characterizing the role of microbiota in supporting antiviral defense has important implications for human health, as the interactions between host and the gut microbiota present new therapeutic targets in prevention and treatment of viral-mediated diseases. Ultimately, future advances are expected to emerge by integrating knowledge from the fields of the microbiome, virome, innate and adaptive antiviral immunity.
Acknowledgments
We thank Dr. Takeshi Ichinohe for his contributions that are discussed in this review and for his help with the figure and Dr. Ellen Foxman for editorial assistance. This work was supported by National Institutes of Health (NIH) (AI054359, AI062428, AI064705 and AI083242 to A.I.). A.I. holds an Investigators in Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund. I.K.P. was supported by the NIH National Research Service Award (T32AI07019) from the Interdisciplinary Immunology Training Program at Yale University, Department of Immunobiology. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Authors declare no conflicts of interest.
References
- 1.Janeway CA. Approaching the Asymptote? Evolution and Revolution in Immunology. Cold Spring Harbor Symposia on Quantitative Biology. 1989;54:1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Stetson DB. Connections between antiviral defense and autoimmunity. Current Opinion in Immunology. 2009;21:244–50. doi: 10.1016/j.coi.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwasaki A, Medzhitov R. Regulation of Adaptive Immunity by the Innate Immune System. Science. 327:291–5. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. Autophagy-Dependent Viral Recognition by Plasmacytoid Dendritic Cells. Science. 2007;315:1398–401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- 5.Manuse MJ, Briggs CM, Parks GD. Replication-independent activation of human plasmacytoid dendritic cells by the paramyxovirus SV5 Requires TLR7 and autophagy pathways. Virology. 405:383–9. doi: 10.1016/j.virol.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawai T, Akira S. Toll-like Receptors and Their Crosstalk with Other Innate Receptors in Infection and Immunity. Immunity. 34:637–50. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Stetson DB, Medzhitov R. Type I Interferons in Host Defense. Immunity. 2006;25:373–81. doi: 10.1016/j.immuni.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Honda K, Yanai H, Takaoka A, Taniguchi T. Regulation of the type I IFN induction: a current view. Int Immunol. 2005;17:1367–78. doi: 10.1093/intimm/dxh318. [DOI] [PubMed] [Google Scholar]
- 9.Takeuchi O, Akira S. Recognition of viruses by innate immunity. Immunol Rev. 2007;220:214–24. doi: 10.1111/j.1600-065X.2007.00562.x. [DOI] [PubMed] [Google Scholar]
- 10.Schenten D, Medzhitov R, Frederick WA. Advances in Immunology. Academic Press; The Control of Adaptive Immune Responses by the Innate Immune System; pp. 87–124. [DOI] [PubMed] [Google Scholar]
- 11.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–95. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 12.Palm NW, Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunological Reviews. 2009;227:221–33. doi: 10.1111/j.1600-065X.2008.00731.x. [DOI] [PubMed] [Google Scholar]
- 13.Barbalat R, Lau L, Locksley RM, Barton GM. Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat Immunol. 2009;10:1200–7. doi: 10.1038/ni.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reis e Sousa C. Toll-like receptors and dendritic cells: for whom the bug tolls. Semin Immunol. 2004;16:27–34. doi: 10.1016/j.smim.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Sporri R, Reis e Sousa C. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nat Immunol. 2005;6:163–70. doi: 10.1038/ni1162. [DOI] [PubMed] [Google Scholar]
- 16.Blander JM, Medzhitov R. Regulation of Phagosome Maturation by Signals from Toll-Like Receptors. Science. 2004;304:1014–8. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- 17.Burgdorf S, Scholz C, Kautz A, Tampe R, Kurts C. Spatial and mechanistic separation of cross-presentation and endogenous antigen presentation. Nat Immunol. 2008;9:558–66. doi: 10.1038/ni.1601. [DOI] [PubMed] [Google Scholar]
- 18.Luckashenak N, et al. Constitutive crosspresentation of tissue antigens by dendritic cells controls CD8+ T cell tolerance in vivo. Immunity. 2008;28:521–32. doi: 10.1016/j.immuni.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 19.Loo Y-M, Gale M., Jr Immune Signaling by RIG-I-like Receptors. Immunity. 34:680–92. doi: 10.1016/j.immuni.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato H, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–5. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 21.Schlee M, et al. Recognition of 5′ triphosphate by RIG-I helicase requires short blunt double-stranded RNA as contained in panhandle of negative-strand virus. Immunity. 2009;31:25–34. doi: 10.1016/j.immuni.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt A, et al. 5‚Ä≤-triphosphate RNA requires base-paired structures to activate antiviral signaling via RIG-I. Proceedings of the National Academy of Sciences. 2009;106:12067–72. doi: 10.1073/pnas.0900971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loo Y-M, et al. Distinct RIG-I and MDA5 Signaling by RNA Viruses in Innate Immunity. J Virol. 2008;82:335–45. doi: 10.1128/JVI.01080-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fredericksen BL, Keller BC, Fornek J, Katze MG, Gale M., Jr Establishment and Maintenance of the Innate Antiviral Response to West Nile Virus Involves both RIG-I and MDA5 Signaling through IPS-1. J Virol. 2008;82:609–16. doi: 10.1128/JVI.01305-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Venkataraman T, et al. Loss of DExD/H Box RNA Helicase LGP2 Manifests Disparate Antiviral Responses. The Journal of Immunology. 2007;178:6444–55. doi: 10.4049/jimmunol.178.10.6444. [DOI] [PubMed] [Google Scholar]
- 26.Rothenfusser S, et al. The RNA Helicase Lgp2 Inhibits TLR-Independent Sensing of Viral Replication by Retinoic Acid-Inducible Gene-I. The Journal of Immunology. 2005;175:5260–8. doi: 10.4049/jimmunol.175.8.5260. [DOI] [PubMed] [Google Scholar]
- 27.Satoh T, et al. LGP2 is a positive regulator of RIG-I‚Äì and MDA5-mediated antiviral responses. Proceedings of the National Academy of Sciences. 107:1512–7. doi: 10.1073/pnas.0912986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elinav E, Strowig T, Henao-Mejia J, Flavell RA. Regulation of the Antimicrobial Response by NLR Proteins. Immunity. 34:665–79. doi: 10.1016/j.immuni.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Schroder K, Tschopp J. The Inflammasomes. Cell. 140:821–32. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 30.Dinarello CA. Immunological and Inflammatory Functions of the Interleukin-1 Family. Annual Review of Immunology. 2009;27:519–50. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 31.Rathinam VA, Fitzgerald KA. Inflammasomes and anti-viral immunity. J Clin Immunol. 30:632–7. doi: 10.1007/s10875-010-9431-4. [DOI] [PubMed] [Google Scholar]
- 32.Kanneganti T-D. Central roles of NLRs and inflammasomes in viral infection. doi: 10.1038/nri2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poeck H, et al. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1[beta] production. Nat Immunol. 11:63–9. doi: 10.1038/ni.1824. [DOI] [PubMed] [Google Scholar]
- 34.Rathinam VAK, et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 11:395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ichinohe T, Pang IK, Iwasaki A. Influenza virus activates inflammasomes via its intracellular M2 ion channel. Nat Immunol. 11:404–10. doi: 10.1038/ni.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bauernfeind FG, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–91. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franchi L, Eigenbrod T, Nunez G. Cutting edge: TNF-alpha mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J Immunol. 2009;183:792–6. doi: 10.4049/jimmunol.0900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang K, Xie S, Sun B. Viral proteins function as ion channels. Biochim Biophys Acta. 1808:510–5. doi: 10.1016/j.bbamem.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nish S, Medzhitov R. Host Defense Pathways: Role of Redundancy and Compensation in Infectious Disease Phenotypes. Immunity. 34:629–36. doi: 10.1016/j.immuni.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jung A, et al. Lymphocytoid Choriomeningitis Virus Activates Plasmacytoid Dendritic Cells and Induces a Cytotoxic T-Cell Response via MyD88. J Virol. 2008;82:196–206. doi: 10.1128/JVI.01640-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhoj VG, et al. MAVS and MyD88 are essential for innate immunity but not cytotoxic T lymphocyte response against respiratory syncytial virus. Proceedings of the National Academy of Sciences. 2008;105:14046–51. doi: 10.1073/pnas.0804717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sabbah A, et al. Activation of innate immune antiviral responses by Nod2. Nat Immunol. 2009;10:1073–80. doi: 10.1038/ni.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lopez CB, Moltedo B, Alexopoulou L, Bonifaz L, Flavell RA, Moran TM. TLR-Independent Induction of Dendritic Cell Maturation and Adaptive Immunity by Negative-Strand RNA Viruses. J Immunol. 2004;173:6882–9. doi: 10.4049/jimmunol.173.11.6882. [DOI] [PubMed] [Google Scholar]
- 44.Heer AK, et al. TLR Signaling Fine-Tunes Anti-Influenza B Cell Responses without Regulating Effector T Cell Responses. J Immunol. 2007;178:2182–91. doi: 10.4049/jimmunol.178.4.2182. [DOI] [PubMed] [Google Scholar]
- 45.Koyama S, et al. Differential Role of TLR- and RLR-Signaling in the Immune Responses to Influenza A Virus Infection and Vaccination. J Immunol. 2007;179:4711–20. doi: 10.4049/jimmunol.179.7.4711. [DOI] [PubMed] [Google Scholar]
- 46.Seo SU, et al. MyD88 signaling is indispensable for primary influenza A virus infection but dispensable for secondary infection. J Virol. 84:12713–22. doi: 10.1128/JVI.01675-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Le Goffic R, et al. Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2006;2:e53. doi: 10.1371/journal.ppat.0020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suthar MS, et al. IPS-1 is essential for the control of West Nile virus infection and immunity. PLoS Pathog. 6:e1000757. doi: 10.1371/journal.ppat.1000757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daffis S, Samuel MA, Suthar MS, Gale M, Jr, Diamond MS. Toll-Like Receptor 3 Has a Protective Role against West Nile Virus Infection. J Virol. 2008;82:10349–58. doi: 10.1128/JVI.00935-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, Flavell RA. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med. 2004;10:1366–73. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- 51.Town T, et al. Toll-like receptor 7 mitigates lethal West Nile encephalitis via interleukin 23-dependent immune cell infiltration and homing. Immunity. 2009;30:242–53. doi: 10.1016/j.immuni.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Szretter KJ, et al. The Innate Immune Adaptor Molecule MyD88 Restricts West Nile Virus Replication and Spread in Neurons of the Central Nervous System. J Virol. 84:12125–38. doi: 10.1128/JVI.01026-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams A, Flavell RA, Eisenbarth SC. The role of NOD-like Receptors in shaping adaptive immunity. Current Opinion in Immunology. 22:34–40. doi: 10.1016/j.coi.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Allen IC, et al. The NLRP3 Inflammasome Mediates In Vivo Innate Immunity to Influenza A Virus through Recognition of Viral RNA. Immunity. 2009;30:556–65. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomas PG, et al. The Intracellular Sensor NLRP3 Mediates Key Innate and Healing Responses to Influenza A Virus via the Regulation of Caspase-1. Immunity. 2009;30:566–75. doi: 10.1016/j.immuni.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. The Journal of Experimental Medicine. 2009;206:79–87. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pang IK, Iwasaki A. Inflammasomes as mediators of immunity against influenza virus. Trends in immunology. 32:34–41. doi: 10.1016/j.it.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chervonsky AV. Influence of microbial environment on autoimmunity. Nat Immunol. 11:28–35. doi: 10.1038/ni.1801. [DOI] [PubMed] [Google Scholar]
- 59.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 140:845–58. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 60.Rescigno M. The intestinal epithelial barrier in the control of homeostasis and immunity. Trends Immunol. 32:256–64. doi: 10.1016/j.it.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 61.Round JL, O’Connell RM, Mazmanian SK. Coordination of tolerogenic immune responses by the commensal microbiota. Journal of Autoimmunity. 34:J220–J5. doi: 10.1016/j.jaut.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pfeiffer JK, Sonnenburg JL. The intestinal microbiota and viral susceptibility. Frontiers in Microbiology. 2 doi: 10.3389/fmicb.2011.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spor A, Koren O, Ley R. Unravelling the effects of the environment and host genotype on the gut microbiome. Nat Rev Microbiol. 9:279–90. doi: 10.1038/nrmicro2540. [DOI] [PubMed] [Google Scholar]
- 64.Willing BP, Russell SL, Finlay BB. Shifting the balance: antibiotic effects on host–microbiota mutualism. Nat Rev Micro. 9:233–43. doi: 10.1038/nrmicro2536. [DOI] [PubMed] [Google Scholar]
- 65.Turnbaugh PJ, Gordon JI. The core gut microbiome, energy balance and obesity. J Physiol. 2009;587:4153–8. doi: 10.1113/jphysiol.2009.174136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dethlefsen L, McFall-Ngai M, Relman DA. An ecological and evolutionary perspective on human‚Äìmicrobe mutualism and disease. Nature. 2007;449:811–8. doi: 10.1038/nature06245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu J, Gordon JI. Honor thy symbionts. Proceedings of the National Academy of Sciences. 2003;100:10452–9. doi: 10.1073/pnas.1734063100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Turnbaugh PJ, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–4. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gill SR, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–9. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qin J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith K, McCoy KD, Macpherson AJ. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Seminars in Immunology. 2007;19:59–69. doi: 10.1016/j.smim.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 72.Hill DA, et al. Metagenomic analyses reveal antibiotic-induced temporal and spatial changes in intestinal microbiota with associated alterations in immune cell homeostasis. Mucosal Immunol. 3:148–58. doi: 10.1038/mi.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6:e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brandl K, et al. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature. 2008;455:804–7. doi: 10.1038/nature07250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Endt K, et al. The Microbiota Mediates Pathogen Clearance from the Gut Lumen after Non-Typhoidal Salmonella Diarrhea. PLoS Pathog. 6:e1001097. doi: 10.1371/journal.ppat.1001097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:11070–5. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104:13780–5. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Larsen N, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 5:e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Noverr MC, Huffnagle GB. Does the microbiota regulate immune responses outside the gut? Trends Microbiol. 2004;12:562–8. doi: 10.1016/j.tim.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 80.Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med. 16:228–31. doi: 10.1038/nm.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang Z, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fau Cani Pd, Bibiloni R, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 83.Noverr MC, Falkowski NR, McDonald RA, McKenzie AN, Huffnagle GB. Development of allergic airway disease in mice following antibiotic therapy and fungal microbiota increase: role of host genetics, antigen, and interleukin-13. Infect Immun. 2005;73:30–8. doi: 10.1128/IAI.73.1.30-38.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ochoa-Repáraz J, et al. Central Nervous System Demyelina3ng Disease Protec3on by the Human Commensal Bacteroides fragilis Depends on Polysaccharide A Expression. The Journal of Immunology. 185:4101–8. doi: 10.4049/jimmunol.1001443. [DOI] [PubMed] [Google Scholar]
- 85.Hill DA, Artis D. Intestinal Bacteria and the Regulation of Immune Cell Homeostasis. Annual Review of Immunology. 28:623–67. doi: 10.1146/annurev-immunol-030409-101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bouskra D, et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456:507–10. doi: 10.1038/nature07450. [DOI] [PubMed] [Google Scholar]
- 87.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An Immunomodulatory Molecule of Symbiotic Bacteria Directs Maturation of the Host Immune System. Cell. 2005;122:107–18. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 88.Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 10:131–44. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- 89.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of Commensal Microflora by Toll-Like Receptors Is Required for Intestinal Homeostasis. Cell. 2004;118:229–41. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 90.Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology. 2004;127:224–38. doi: 10.1053/j.gastro.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 91.Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic Bacteria Direct Expression of an Intestinal Bactericidal Lectin. Science. 2006;313:1126–30. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 10:159–69. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 93.Kobayashi KS, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–4. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 94.Brandl K, Plitas G, Schnabl B, DeMatteo RP, Pamer EG. MyD88-mediated signals induce the bactericidal lectin RegIII gamma and protect mice against intestinal Listeria monocytogenes infection. J Exp Med. 2007;204:1891–900. doi: 10.1084/jem.20070563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proceedings of the National Academy of Sciences. 2008;105:20858–63. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miettinen M, Veckman V, Latvala S, Sareneva T, Matikainen S, Julkunen I. Live Lactobacillus rhamnosus and Streptococcus pyogenes differentially regulate Toll-like receptor (TLR) gene expression in human primary macrophages. Journal of Leukocyte Biology. 2008;84:1092–100. doi: 10.1189/jlb.1206737. [DOI] [PubMed] [Google Scholar]
- 97.Umenai T, et al. Eradication of the commensal intestinal microflora by oral antimicrobials interferes with the host response to lipopolysaccharide. Eur J Clin Microbiol Infect Dis. 29:633–41. doi: 10.1007/s10096-010-0905-3. [DOI] [PubMed] [Google Scholar]
- 98.Leung C-H, Lam W, Ma D-L, Gullen EA, Cheng Y-C. Butyrate mediates nucleotide-binding and oligomerisation domain (NOD) 2-dependent mucosal immune responses against peptidoglycan. European Journal of Immunology. 2009;39:3529–37. doi: 10.1002/eji.200939454. [DOI] [PubMed] [Google Scholar]
- 99.Weaver CT, Hatton RD. Interplay between the TH17 and TReg cell lineages: a (co-)evolutionary perspective. Nat Rev Immunol. 2009;9:883–9. doi: 10.1038/nri2660. [DOI] [PubMed] [Google Scholar]
- 100.Belkaid Y, Tarbell K. Regulatory T Cells in the Control of Host-Microorganism Interactions*. Annual Review of Immunology. 2009;27:551–89. doi: 10.1146/annurev.immunol.021908.132723. [DOI] [PubMed] [Google Scholar]
- 101.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–5. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 102.Round JL, et al. The Toll-Like Receptor 2 Pathway Establishes Colonization by a Commensal of the Human Microbiota. Science. 332:974–7. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proceedings of the National Academy of Sciences. 107:12204–9. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hall JA, et al. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity. 2008;29:637–49. doi: 10.1016/j.immuni.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Atarashi K, et al. Induction of Colonic Regulatory T Cells by Indigenous Clostridium Species. Science. 331:337–41. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ivanov II, et al. Specific Microbiota Direct the Differentiation of IL-17-Producing T-Helper Cells in the Mucosa of the Small Intestine. Cell Host & Microbe. 2008;4:337–49. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Atarashi K, et al. ATP drives lamina propria TH17 cell differentiation. Nature. 2008;455:808–12. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 108.Ivanov II, et al. Induction of Intestinal Th17 Cells by Segmented Filamentous Bacteria. Cell. 2009;139:485–98. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gaboriau-Routhiau V, et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31:677–89. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 110.Zaph C, et al. Commensal-dependent expression of IL-25 regulates the IL-23‚ÄìIL-17 axis in the intestine. The Journal of Experimental Medicine. 2008;205:2191–8. doi: 10.1084/jem.20080720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Geuking MB, et al. Intestinal Bacterial Colonization Induces Mutualistic Regulatory T Cell Responses. Immunity. 34:794–806. doi: 10.1016/j.immuni.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 112.Tazume S, Umehara K, Leung WC, Ando K, Yoshida T, Hashimoto K. Immunological responses in germfree mice infected with murine cytomegalovirus. Tokai J Exp Clin Med. 1990;15:13–20. [PubMed] [Google Scholar]
- 113.Tanaka K, Sawamura S, Satoh T, Kobayashi K, Noda S. Role of the indigenous microbiota in maintaining the virus-specific CD8 memory T cells in the lung of mice infected with murine cytomegalovirus. J Immunol. 2007;178:5209–16. doi: 10.4049/jimmunol.178.8.5209. [DOI] [PubMed] [Google Scholar]
- 114.Dolowy WC, Muldoon RL. STUDIES OF GERMFREE ANIMALS. I. RESPONSE OF MICE TO INFECTION WITH INFLUENZA A VIRUS. Proc Soc Exp Biol Med. 1964;116:365–71. doi: 10.3181/00379727-116-29249. [DOI] [PubMed] [Google Scholar]
- 115.Ichinohe T, et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Oudemans-van Straaten HM, van Saene HK, Zandstra DF. Selective decontamination of the digestive tract: use of the correct antibiotics is crucial. Crit Care Med. 2003;31:334–5. doi: 10.1097/00003246-200301000-00067. [DOI] [PubMed] [Google Scholar]
- 117.GeurtsvanKessel CH, et al. Clearance of influenza virus from the lung depends on migratory langerin+CD11b- but not plasmacytoid dendritic cells. J Exp Med. 2008;205:1621–34. doi: 10.1084/jem.20071365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guadalupe M, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77:11708–17. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brenchley JM, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–59. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mehandru S, et al. Primary HIV-1 Infection Is Associated with Preferential Depletion of CD4+ T Lymphocytes from Effector Sites in the Gastrointestinal Tract. The Journal of Experimental Medicine. 2004;200:761–70. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Baenziger S, et al. Triggering TLR7 in mice induces immune activation and lymphoid system disruption, resembling HIV-mediated pathology. Blood. 2009;113:377–88. doi: 10.1182/blood-2008-04-151712. [DOI] [PubMed] [Google Scholar]
- 122.Nazli A, et al. Exposure to HIV-1 Directly Impairs Mucosal Epithelial Barrier Integrity Allowing Microbial Translocation. PLoS Pathog. 6:e1000852. doi: 10.1371/journal.ppat.1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Brenchley JM, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 124.Fau Marchetti G, Bellistri GM, et al. Microbial translocation is associated with sustained failure in CD4+ T-cell reconstitution in HIV-infected patients on long-term highly active antiretroviral therapy. doi: 10.1097/QAD.0b013e3283112d29. [DOI] [PubMed] [Google Scholar]
- 125.Gori A, et al. Early Impairment of Gut Function and Gut Flora Supporting a Role for Alteration of Gastrointestinal Mucosa in Human Immunodeficiency Virus Pathogenesis. J Clin Microbiol. 2008;46:757–8. doi: 10.1128/JCM.01729-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Raffatellu M, et al. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008;14:421–8. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Brenchley JM, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112:2826–35. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cecchinato V, et al. Altered balance between Th17 and Th1 cells at mucosal sites predicts AIDS progression in simian immunodeficiency virus-infected macaques. Mucosal Immunol. 2008;1:279–88. doi: 10.1038/mi.2008.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Favre D, et al. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 2009;5:e1000295. doi: 10.1371/journal.ppat.1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ancuta P, Monteiro P, Sekaly RP. Th17 lineage commitment and HIV-1 pathogenesis. Curr Opin HIV AIDS. 5:158–65. doi: 10.1097/COH.0b013e3283364733. [DOI] [PubMed] [Google Scholar]
- 131.Seifarth W, et al. Comprehensive Analysis of Human Endogenous Retrovirus Transcriptional Activity in Human Tissues with a Retrovirus-Specific Microarray. J Virol. 2005;79:341–52. doi: 10.1128/JVI.79.1.341-352.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Virgin HW, Wherry EJ, Ahmed R. Redefining Chronic Viral Infection. Cell. 2009;138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 133.Grivel J-C, et al. Suppression of CCR5- but not CXCR4-tropic HIV-1 in lymphoid tissue by human herpesvirus 6. Nat Med. 2001;7:1232–5. doi: 10.1038/nm1101-1232. [DOI] [PubMed] [Google Scholar]
- 134.Lisco A, et al. Viral Interactions in Human Lymphoid Tissue: Human Herpesvirus 7 Suppresses the Replication of CCR5-Tropic Human Immunodeficiency Virus Type 1 via CD4 Modulation. J Virol. 2007;81:708–17. doi: 10.1128/JVI.01367-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xiang J, et al. Effect of Coinfection with GB Virus C on Survival among Patients with HIV Infection. New England Journal of Medicine. 2001;345:707–14. doi: 10.1056/NEJMoa003364. [DOI] [PubMed] [Google Scholar]
- 136.Barton ES, et al. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature. 2007;447:326–9. doi: 10.1038/nature05762. [DOI] [PubMed] [Google Scholar]
- 137.Jaidane H, Sauter P, Sane F, Goffard A, Gharbi J, Hober D. Enteroviruses and type 1 diabetes: towards a better understanding of the relationship. Rev Med Virol. 20:265–80. doi: 10.1002/rmv.647. [DOI] [PubMed] [Google Scholar]
- 138.Smyth DJ, et al. A genome-wide association study of nonsynonymous SNPs identifies a type 1 diabetes locus in the interferon-induced helicase (IFIH1) region. Nat Genet. 2006;38:617–9. doi: 10.1038/ng1800. [DOI] [PubMed] [Google Scholar]
- 139.Foxman EF, Iwasaki A. Genome‚Äìvirome interactions: examining the role of common viral infections in complex disease. Nat Rev Micro. 9:254–64. doi: 10.1038/nrmicro2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wen L, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–13. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Elinav E, et al. NLRP6 Inflammasome Regulates Colonic Microbial Ecology and Risk for Colitis. Cell. 145:745–57. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Garrett WS, et al. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell. 2007;131:33–45. doi: 10.1016/j.cell.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 144.Vijay-Kumar M, et al. Metabolic Syndrome and Altered Gut Microbiota in Mice Lacking Toll-Like Receptor 5. Science. 328:228–31. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fau Schaffer J, Beamer PR, Fau Beamer Pr, Trexler PC, Fau Trexler Pc, Breidenbach G, Fau Breidenbach G, Walcher DN, Walcher DN. Response of germ-free animals to experimental virus monocontamination. I. Observation on Coxsackie B virus. doi: 10.3181/00379727-112-28105. [DOI] [PubMed] [Google Scholar]
- 146.Gareau MG, Sherman PM, Walker WA. Probiotics and the gut microbiota in intestinal health and disease. Nat Rev Gastroenterol Hepatol. 7:503–14. doi: 10.1038/nrgastro.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Vouloumanou EK, Makris GC, Karageorgopoulos DE, Falagas ME. Probiotics for the prevention of respiratory tract infections: a systematic review. Int J Antimicrob Agents. 2009;34:197 e1–10. doi: 10.1016/j.ijantimicag.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 148.van Baarlen P, et al. Human mucosal in vivo transcriptome responses to three lactobacilli indicate how probiotics may modulate human cellular pathways. Proceedings of the National Academy of Sciences. 108:4562–9. doi: 10.1073/pnas.1000079107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Davidson LE, Fiorino AM, Snydman DR, Hibberd PL. Lactobacillus GG as an immune adjuvant for live-attenuated influenza vaccine in healthy adults: a randomized double-blind placebo-controlled trial. Eur J Clin Nutr. 65:501–7. doi: 10.1038/ejcn.2010.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Boge T, Remigy M, Vaudaine S, Tanguy J, Bourdet-Sicard R, van der Werf S. A probiotic fermented dairy drink improves antibody response to influenza vaccination in the elderly in two randomised controlled trials. Vaccine. 2009;27:5677–84. doi: 10.1016/j.vaccine.2009.06.094. [DOI] [PubMed] [Google Scholar]
- 151.Olivares M, et al. Oral intake of Lactobacillus fermentum CECT5716 enhances the effects of influenza vaccination. Nutrition. 2007;23:254–60. doi: 10.1016/j.nut.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 152.Maeda N, et al. Oral administration of heat-killed Lactobacillus plantarum L-137 enhances protection against influenza virus infection by stimulation of type I interferon production in mice. Int Immunopharmacol. 2009;9:1122–5. doi: 10.1016/j.intimp.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 153.Hori T, Kiyoshima J, Shida K, Yasui H. Augmentation of cellular immunity and reduction of influenza virus titer in aged mice fed Lactobacillus casei strain Shirota. Clin Diagn Lab Immunol. 2002;9:105–8. doi: 10.1128/CDLI.9.1.105-108.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Trois L, Cardoso EM, Miura E. Use of probiotics in HIV-infected children: a randomized double-blind controlled study. J Trop Pediatr. 2008;54:19–24. doi: 10.1093/tropej/fmm066. [DOI] [PubMed] [Google Scholar]
- 155.Anukam KC, Osazuwa EO, Osadolor HB, Bruce AW, Reid G. Yogurt containing probiotic Lactobacillus rhamnosus GR-1 and L. reuteri RC-14 helps resolve moderate diarrhea and increases CD4 count in HIV/AIDS patients. J Clin Gastroenterol. 2008;42:239–43. doi: 10.1097/MCG.0b013e31802c7465. [DOI] [PubMed] [Google Scholar]
- 156.Irvine SL, Hummelen R, Hekmat S, Looman CW, Habbema JD, Reid G. Probiotic yogurt consumption is associated with an increase of CD4 count among people living with HIV/AIDS. J Clin Gastroenterol. 44:e201–5. doi: 10.1097/MCG.0b013e3181d8fba8. [DOI] [PubMed] [Google Scholar]
- 157.Kerac M, et al. Probiotics and prebiotics for severe acute malnutrition (PRONUT study): a double-blind efficacy randomised controlled trial in Malawi. Lancet. 2009;374:136–44. doi: 10.1016/S0140-6736(09)60884-9. [DOI] [PubMed] [Google Scholar]
- 158.Hummelen R, Vos AP, van’t Land B, van Norren K, Reid G. Altered host-microbe interaction in HIV: a target for intervention with pro- and prebiotics. Int Rev Immunol. 29:485–513. doi: 10.3109/08830185.2010.505310. [DOI] [PubMed] [Google Scholar]