Abstract
Regenerative therapies in the musculoskeletal system are based on the suitable application of cells, biomaterials, and/or factors. For an effective approach, numerous aspects have to be taken into consideration, including age, disease, target tissue, and several environmental factors. Significant research efforts have been undertaken in the last decade to develop specific cell-based therapies, and in particular adult multipotent mesenchymal stem cells hold great promise for such regenerative strategies. Clinical translation of such therapies, however, remains a work in progress. In the clinical arena, autologous cells have been harvested, processed, and readministered according to protocols distinct for the target application. As outlined in this review, such applications range from simple single-step approaches, such as direct injection of unprocessed or concentrated blood or bone marrow aspirates, to fabrication of engineered constructs by seeding of natural or synthetic scaffolds with cells, which were released from autologous tissues and propagated under good manufacturing practice conditions (for example, autologous chondrocyte implantation). However, only relatively few of these cell-based approaches have entered the clinic, and none of these treatments has become a “standard of care” treatment for an orthopaedic disease to date. The multifaceted reasons for the current status from the medical, research, and regulatory perspectives are discussed here. In summary, this review presents the scientific background, current state, and implications of clinical mesenchymal stem cell application in the musculoskeletal system and provides perspectives for future developments.
Keywords: Tissue regeneration, Adult stem cells, Bone marrow stromal cells, Cellular therapy, Chondrogenesis, Cell transplantation, Marrow stromal stem cells, Mesenchymal stem cells
Introduction
Mesenchymal stem cells (MSCs) hold great promise for regenerative therapies in the musculoskeletal system. The limited resources of differentiated cells and tissues are thought to be overcome by MSC populations that can be readily harvested from bone marrow, blood, or mesenchymal tissues, including bone, cartilage, meniscus, ligaments, and tendons, among others [1]. Techniques of MSC isolation, expansion, and differentiation have been added to the therapeutic repertoire for musculoskeletal regeneration [1]. In spite of sporadic reports of adverse events that were reported in association with adult MSC delivery in experimental settings, such as heterotopic bone or tumor formation [2], controlled MSCs delivery is generally perceived as a safe procedure, and the number of clinical trials using ex vivo expanded stromal cell populations for therapeutic purposes are rapidly increasing (see http://www.clinicaltrials.gov).
To date, MSCs derived from adult donors are usually applied in the clinical setting, and have limited differentiation capacities. However, human embryonic stem cells (ESCs) derived from the inner cell mass of blastocysts, which exhibit unlimited in vitro self-renewal capacity and pluripotency, reflect another potential cell source for the repair of diseased human musculoskeletal tissues [3, 4]. The predisposition of ESCs to form teratomas, even after predifferentiation ex vivo because of residual undifferentiated cell populations, complicates their possible clinical application [3, 4]. Furthermore, the remaining unsolved political and ethical concerns and barriers concerning the use of ESCs currently prevent this cell type from entering the clinical and also research arena in numerous countries. To circumvent the use of ESCs or allogeneic cells in general, researchers of several groups have achieved reprogramming of adult fibroblasts to ESC-like, pluripotent stages, by retroviral transduction with variable different transcription factors, including c-MYC, Klf4, Nanog, Lin28, Oct-4, and SOX-2 [5–8]. Although these inducible pluripotent cells elicit some promising features that might allow their application in tissue regeneration [9], more refined protocols for such applications have to be developed along with techniques to circumvent the numerous problems associated with gene transfer in the clinical setting. Therefore, MSCs from adult donors are thought to be the most promising candidate cell type to date to augment cell-based orthopaedic tissue regeneration in the clinical setting.
Despite the large amount of research findings on the use of MSCs for various regenerative approaches in the musculoskeletal system in experimental settings in vitro and in vivo, as reviewed elsewhere [1], there are only limited amounts of published clinical studies using MSC-based approaches for such purposes to date, due to medical, research, and regulatory reasons. Therefore, in this review we have chosen to highlight some unique therapeutic features of adult MSCs, summarize their current state of clinical application in the musculoskeletal system, and provide prospects for future developments.
Principles of Adult MSC Biology and Delivery
Adult MSCs represent usually a heterogeneous population of cells, with a positive immunophenotype for STRO-1, CD73, CD146, and CD106 and a negative one for CD11b, CD45, CD34, CD31, and CD117, based on a recommendation of the International Society for Cellular Therapy [10]. MSCs act via multifaceted pathways that are not completely understood to date to augment regeneration, including mechanisms that mediate homing of administered MSCs to sites of injury. However, two main inherent functions of MSCs can be distinguished. The first is the secretory or “trophic” function of MSCs [11], which includes the secretion of a wide spectrum of factors with immunomodulatory, anti-inflammatory, antiapoptotic, proangiogenic, proliferative, or chemoattractive capacities, among others [12–14]. Second, administered MSCs are thought to orchestrate the differentiation process together with differentiated or undifferentiated resident cells for functional tissue restoration [12, 14]. For regeneration, these MSC functions can be augmented by the coadministration of soluble growth and differentiation factors, nucleic acids, natural or synthetic biomaterials, or mechanical stimuli. Given the complexity of each of the therapeutic components involved, along with the different modes of delivery of each alone and in combination, there is currently no fully developed treatment for any orthopaedic application [1]. Given the different modes of MSC delivery as well as the time and efforts necessary for performing meaningful randomized controlled clinical trials, the search for such an optimized procedure has remained elusive. However, several studies have been conducted that use mesenchymal stromal cell-based approaches in the clinical setting (Table 1), and some of the basic principles of stromal cell delivery are now outlined.
Table 1.
Present status of clinical cell delivery therapies for musculoskeletal regeneration
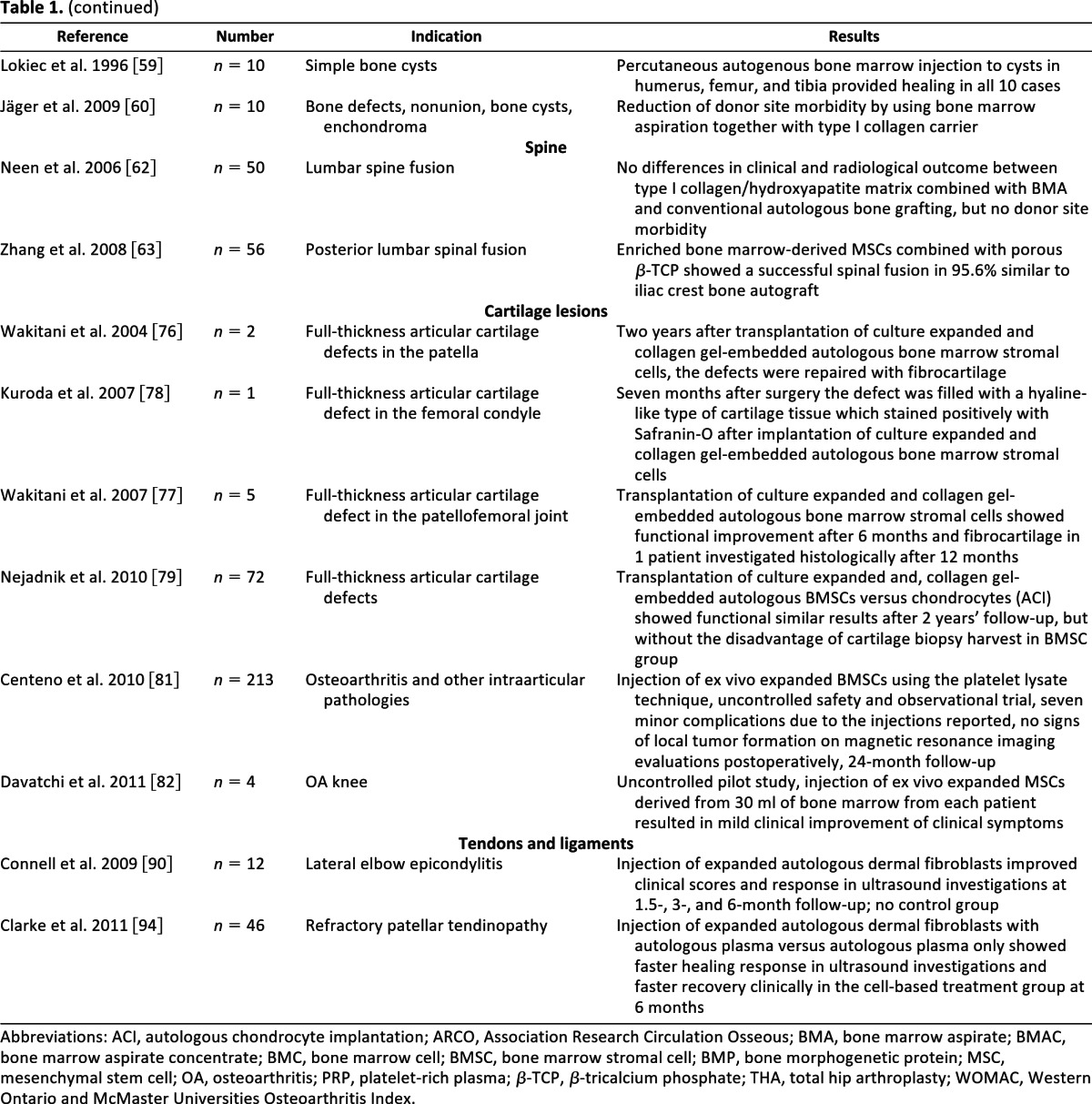
Abbreviations: ACI, autologous chondrocyte implantation; ARCO, Association Research Circulation Osseous; BMA, bone marrow aspirate; BMAC, bone marrow aspirate concentrate; BMC, bone marrow cell; BMSC, bone marrow stromal cell; BMP, bone morphogenetic protein; MSC, mesenchymal stem cell; OA, osteoarthritis; PRP, platelet-rich plasma; β-TCP, β-tricalcium phosphate; THA, total hip arthroplasty; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
A successful regenerative approach based on the use of autologous material usually comprises the harvest of cells either derived from blood by venous puncture or derived from bone marrow by, for example, aspiration from iliac crest or by surgical removal from a donor site, with increasing levels of invasiveness (Fig. 1, red box). Further processing of such autologous materials can be conducted via several pathways (Fig. 1, yellow box). The simplest means of cell delivery to the musculoskeletal system is direct injection of cells. Although injection of whole blood or marrow, or concentrates thereof, usually can be performed in the operating room without major regulatory obstacles, the administration of ex vivo processed cell preparations requires strict compliance with good medical practice (GMP) and also nation-specific requirements. Depending on the application, the suspension can be injected directly into the diseased tissue, where the cells eventually populate the target site and stimulate repair via autocrine or paracrine pathways (Fig. 1, yellow box). Examples of such direct approaches are MSC injection therapies for the treatment of arthritis, tendinitis, or tendon ruptures [15, 16]. In cases where large tissue defects need to be restored, cells are usually delivered via a scaffold to the target tissue. Such constructs are used to regenerate structural defects in bone or cartilage [17–19]. For this purpose, large numbers of natural or synthetic biomaterials have been tested specifically for almost every target cell and tissue, as reviewed extensively elsewhere [1, 20–28]. The physicochemical characteristics of such materials should ideally facilitate appropriate cell adherence, proliferation, differentiation, and matrix synthesis, while providing biodegradability, as well as suitable mechanical strength [26, 27]. Given the heterogeneity of the different orthopaedic tissues, these requirements vary greatly, making refined strategies necessary.
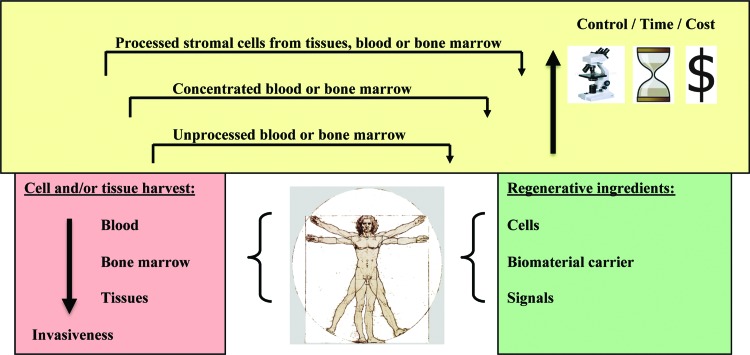
Figure 1.
Schematic illustration of cell delivery for musculoskeletal regeneration. Cell delivery can be facilitated in various fashions for the purpose of musculoskeletal regeneration. First, the cells can be retrieved from the human body and readministered without further modification, such as injection of autologous blood or bone marrow. Although marrow suspensions are thought to contain a higher percentage of stromal cells than peripheral blood, both represent crude mixtures with overall low abundance of mesenchymal stem cells (MSCs). MSC density can be increased by centrifugation of whole blood or marrow or recovery from cell filters, clotting, or other concentration methods. These methods are relatively simple, making them appealing for single-step usage procedures in the operating room, and, therefore, are not associated with high regulative hurdles. However, blood and marrow concentrates or coagulates still have to be considered very crude and undefined mixtures. Alternatively, cells and in particular MSCs can be harvested from blood, bone marrow, and tissues by digestion and adherent culture. Such cell populations are usually maintained in tissue culture for amplification under controlled good medical practice conditions with usually autologous serum. This method offers the opportunity for close monitoring of the cells under safety and quality aspects, as well as further cell selection. However, this approach presents the highest regulatory levels, in the context of specific requirements of each country and continent (European Medicines Agency and U.S. Food and Drug Administration). The use of unprocessed as well as ex vivo processed cells might be enhanced and supplemented by the use of a biomaterial scaffold, soluble factor, nucleic acid, or mechanical stimulation. Additional specific regulatory requirements must be met for the use of each of these supplements, as well as for their combined usage, indicating the complexity for such approaches from a biological, medical, and regulatory perspective.
An important issue for the success of any stem cell therapy is the quality of the cells administered, with regard to cellular integrity, age, senescence, proliferative and differentiative capacity, and intact paracrine function [29, 30]. In this regard, the use of cells that are harvested intraoperatively, such as whole blood, marrow, or concentrates thereof, has the advantage that cells have not been grossly manipulated and subjected to stress factors during cell culture that may impair their regenerative functions such as induced replicative senescence (Fig. 1, yellow box). However, the disadvantage is that such suspensions are heterogeneous and contain unidentified components that may be disadvantageous for therapy, such as high ratios of red blood cells or fibrin within neocartilage regenerates. Ex vivo cell preparations are subjected to additional external influences and stress factors but will allow for enhancements, such as predifferentiation, selection, and control of the desired subpopulation of cells (Fig. 1, yellow box).
All of the mentioned modes of cell delivery might be augmented by the use of other regenerative ingredients such as biomaterials, soluble factors, nucleic acids, or physical stimuli (Fig. 1, green box). However, the best possible combinations of these regenerative elements need to be determined. Many of these complex approaches have been reviewed extensively elsewhere for tissue engineering of cartilage, bone, muscle, tendons and ligaments, disc, and meniscus [1]. Another approach is to build on currently approved clinical practices, which are discussed below.
Clinical Mesenchymal Stromal Cell Applications for Musculoskeletal Regeneration
Mesenchymal stromal cells have been used clinically for several regenerative approaches in the musculoskeletal system, as outlined in Table 1. Some of the specifics, including the type of cell preparation used, the nature and extent of the disease treated, and other study parameters, are detailed below.
Bone Regeneration
Bone has a significant natural tendency towards spontaneous healing and functional restoration without significant scarring. However, there are many conditions where this feature is impaired, such as in large defects of bone due to trauma, tumor, infection, aseptic loosening, or nonunions. Therefore, substantial research efforts in the field of orthopaedics and regenerative medicine have been devoted towards the development of improved treatments for such critical bone defects [31–33]. These investigations have led to the identification of a number of factors with strong osteogenic potency, including several of the bone morphogenetic proteins (BMPs), of which the human recombinant BMP-2 and BMP-7 (OP-1) have been approved by the U.S. Food and Drug Administration (FDA) in 2004 for restricted clinical use. Such factors might be applied together with a suitable biomaterial and/or cell preparation to devise a potentially effective approach to reconstructive surgery [34]. In fact, in the last 10–20 years, a number of clinical studies have shown that cell preparations, including bone marrow, bone marrow concentrates, and ex vivo cell preparations, can be efficiently used to facilitate bone healing in nonunions, critical-sized segmental defects, or osteonecrosis, as summarized in Table 1.
Large Bone Defects and Nonunions
Successes in several small- and large-animal models of disease have set the stage for the use of MSCs to restore large bone defects in the clinical setting [35]. In 1998, Connolly initially reported successful treatment of tibial nonunions in 18 of 20 patients examined at 6–8-month follow-up [36]. Using a similar approach, Hernigou and colleagues correlated the quantity of mineralized bone formation with the number of colony-forming units (CFUs) within the grafted marrow following percutaneous injection to the tibial nonunions and reported good results in 60 patients [37]. The percutaneous injection technique was not performed in strong deformities, which could be considered to be a limitation of this procedure [37]. However, because of its biological value and low associated risks, bone marrow aspirates have been used in several other studies investigating open surgery procedures for bone healing in high-tibial osteotomies combined with lyophilized bone chips [38], healing of bone defects (n = 125) of variable sizes combined with devitalized bone allografts [39], and ectopic bone formation together with titanium carriers and recombinant BMP-7 [40]. No additional effects were seen when nonirradiated frozen bone-bank allografts were compared with freeze-dried irradiated bone allografts vitalized with autologous bone marrow for the treatment of acetabular defects in revision total hip arthroplasties [41].
To increase the numbers of CFU fibroblasts within the cell preparation, centrifugation methods for the generation of bone marrow concentrates have been applied in the context of bone regeneration that still avoids any ex vivo cell culture. The safety of the application of such bone marrow aspirate concentrates (BMACs) has been shown in 101 bone defects of variable ethiology, including pseudarthrosis, avascular necrosis, and others [42]. A comparative follow-up study by the same group showed superior results when BMAC was applied together with a hydroxyapatite (HA) matrix, compared with a collagen matrix, in a total of 39 defects [43]. However, biomaterial-only controls were not included in this study [43].
In contrast to the single-step procedures, several studies have also investigated the use of ex vivo expanded mesenchymal stromal cells for the regeneration of bone. In an initial phase I clinical trial, segmental bone defects of three patients were successfully treated with MSCs that were used together with HA carriers [44]. When bones were treated with culture expanded bone marrow cells together with platelet-rich plasma (PRP) during distraction osteogenesis approaches that included 20 [45] or 46 [46] cases, significantly higher healing rates were observed than in the control groups without cell-based therapies [45, 46].
Osteonecrosis of the Hip
Osteonecrosis is caused by bone death in the femoral head that occurs due to poor blood supply, and there are four commonly distinguished grades to describe the severity of the disease (Association Research Circulation Osseous [ARCO]/Steinberg) [19]. Cell-based procedures have been thought to have therapeutic potential in osteonecrosis [34, 37, 47, 48], especially to augment the core decompression procedure, which was originally described by Ficat and Arlet more than 30 years ago [49], to lower the elevated levels of intraosseous pressure and enhance neovascularization and osteogenesis at the defect site [50, 51].
The first clinical trials for cell-based treatment of hip osteonecrosis used percutaneous application of different marrow suspensions via small drill holes and without any supporting matrix material [48, 52, 53]. A more recent publication reported the use of ex vivo expanded MSCs combined with β-tricalcium phosphate (β-TCP) matrix and vascularized fibula grafts in combination with core decompression for femoral head necrosis treatment in three patients [54]. In our own study, using an almost similar approach, we dispersed an ex vivo expanded autologous bone marrow cell preparation (tissue repair cells, Aastrom, Ann Arbor, MI, http://www.aastrom.com) on β-TCP carrier granules to fill stage II (ARCO) osteonecrosis areas after debridement in three patients [19]. Follow-up examinations showed intact femoral heads and entirely filled bone canals at 6 weeks upon radiographic and magnetic resonance imaging (MRI) examination (Fig. 2). It remains to be proven, in large-scale randomized controlled clinical trials compared with no-cell controls, whether such procedures can improve long-term clinical outcome after core decompression.
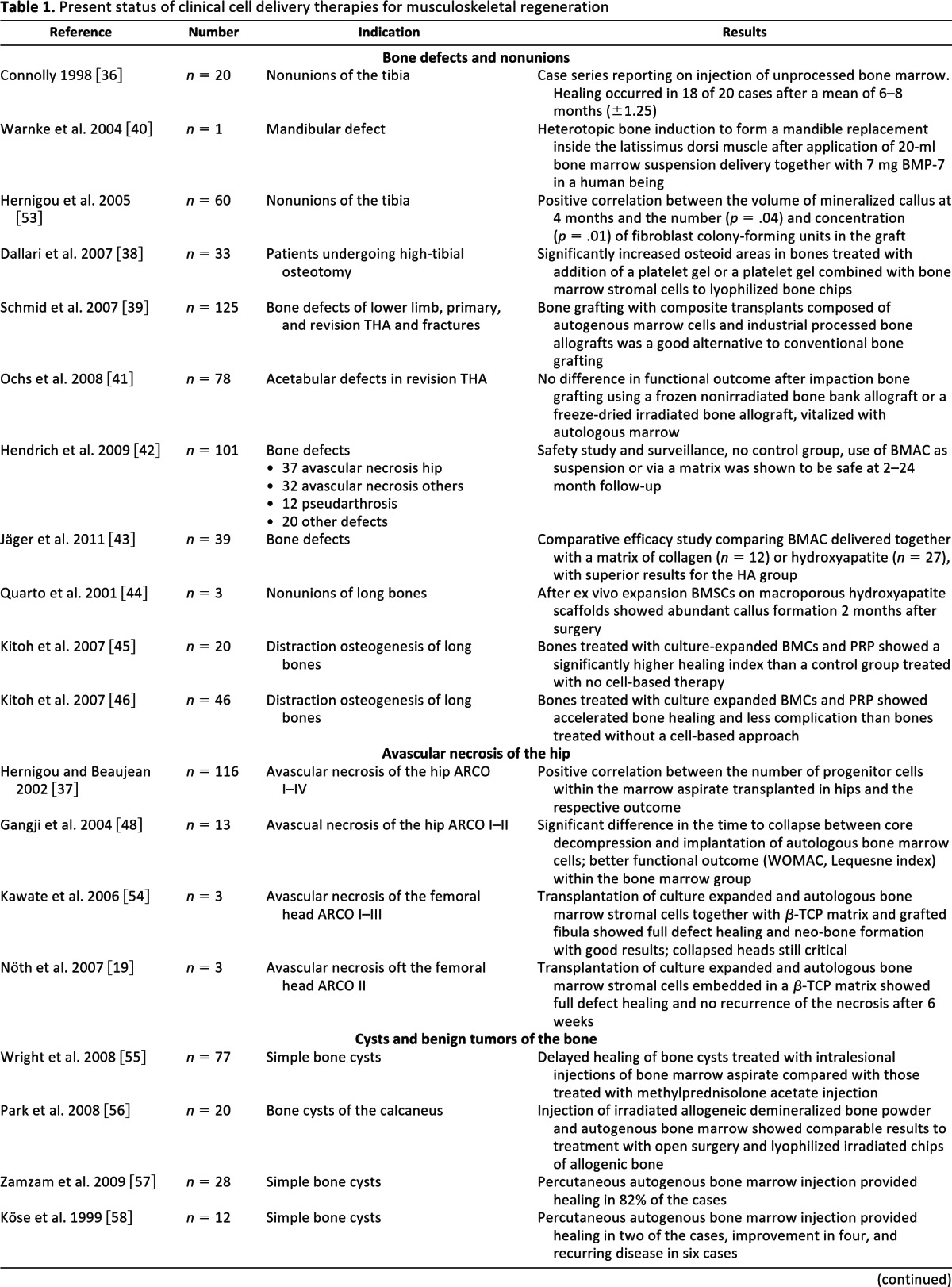
Figure 2.
Example of clinical mesenchymal stromal cell delivery following ex vivo expansion for the treatment of avascular necrosis of the femoral head. (A): Autologous bone marrow aspiration from the posterior iliac crest. (B): For delivery, β-tricalcium phosphate (β-TCP) granules (black arrow) were soaked with an autologous stromal cell suspension and immersed in autologous fibrin (white arrow) to facilitate subsequent handling. (C): Adhesive stromal cell/β-TCP constructs were delivered to the bony defects via tubings that were placed into the canals of the core decompression. (D): Postoperative radiography displays a 10-mm drill hole 6 weeks after core decompression completely filled with the stromal cell/β-TCP construct in a 34-year-old male patient with avascular necrosis (stage ARCO II) of the femoral head.
Bone Cysts and Tumors
For the treatment of bone cysts, marrow injections have proven useful in several clinical studies, with some variability in the healing outcome after simple marrow injections ranging from 18%–100% [55–60]. However, intralesional marrow injections have shown to be not as efficient as intralesional injection with methylprednisolone in a comparative study [55]. When marrow aspirates were injected together with a bone powder, similarly satisfactory results were obtained compared with open surgery and grafting of allogenic lyophilized bone chips [56]. In addition, collagen type I has been shown to be an appropriate carrier for the use of marrow aspirates in this setting [60].
Spinal Fusion
For spinal fusion, MSCs have been successfully tested in several small and large animal disease models as reviewed in [61]. These preclinical data set the stage for two initial phase I clinical trials for this indication [62, 63]. The first involved the use of bone marrow aspirated together with hydroxyapatite biomaterial carriers to augment lumbar spine fusion, compared with conventional bone grafting from the iliac crest in 55 patients, and yielded similar results in both groups, whereasthe donor site morbidity in the latter one was acknowledged [62]. Similar successful spinal fusion rates (95%) were seen when ex vivo enriched bone marrow stromal cells were combined with porous β-TCP for posterior lumbar spine fusion in 56 patients compared with iliac crest bone autograft [63].
From numerous in vitro and in vivo experimental studies of bone healing, it is conceivable that other osteogenic or angiogenic agents, such as growth factors (e.g., bone morphogenetic proteins: BMP-2, BMP-7), may also be incorporated in the procedure to elicit a greater healing response at the osseous defect site [64–68]. Overall, the experimental outcomes have been remarkable, suggesting the feasibility of future clinical application of this technology. However, it remains to be seen whether such approaches will be sufficient to induce an efficient osteogenic response in settings where patients are older, diabetic, traumatized, or smokers, and more rigorous evaluations in preclinical studies are needed.
Cartilage
Localized Articular Cartilage Defects
Substantial experimental research in the field of cell-based treatment of cartilage pathologies, recently reviewed in [15], has set the stage for the use of cell-based treatments in the clinical setting. For focal articular cartilage defects, the autologous chondrocyte implantation (ACI) procedure, first described by Brittberg and colleagues [69], became an established treatment modality for defects larger than 4 cm2 or as a second-line treatment, where initial treatments such as microfracture failed to induce satisfactory healing [70]. For this procedure, a cartilage biopsy is harvested arthroscopically from a non-load-bearing area, followed by enzymatic digestion to release chondrocytes, which are subsequently expanded before reimplantation. The first generation of ACI used a periosteal flap sutured onto the defect, followed by injection of the cell suspension underneath the periosteum within the defect cavity, and then sealing with fibrin glue. This technique elicited predominantly good to very good long-term clinical results in the majority of the patients [71, 72]. Because of several problems that were noted using this technology, including transplant hypertrophy, calcification, delamination, and cell leakage [73, 74], the ACI was subsequently developed further using biocompatible scaffolds in which the chondrocytes were seeded in three-dimensional (3D) constructs that are implanted in the second and third generation of ACI procedures [19, 75].
To circumvent autologous tissue harvest from healthy cartilage, which is very limited in quantity, initial clinical attempts were directed towards the use of MSCs seeded in 3D collagen carriers for the repair of focal full-thickness cartilage defects [76]. In this study, full-size patella defects in two patients were treated with collagen gel constructs that were seeded with ex vivo expanded MSCs and covered with a periosteal flap, and good defect filling, as well as markedly improved patient outcome, was observed at 1-year follow-up [76]. The same group also reported on the treatment of nine defects, in five knees of three patients, with similar good results at 12 months [77]. A biopsy of the regenerate tissue from one patient after 12 months revealed formation of a fibrocartilage repair tissue that was rich in proteoglycans [77]. Using a similar approach, this technology was also used to treat a full-thickness cartilage defect in the medial femoral condyle of one individual, with similar good clinical and histological outcomes [78]. A recent randomized controlled cohort study compared the use of autologous bone-marrow-derived MSCs (BMSCs) with the transplantation of autologous chondrocytes by means of the ACI procedure, and similar functional outcomes were observed in both groups in up to 2 years' follow-up, with less cost and donor site morbidity in the BMSC group [79].
Although promising, the clinical data on MSC delivery to cartilage defects still have to be considered as very preliminary. Several randomized controlled clinical studies that investigate the use of bone marrow concentrates and MSCs via delivery as suspension or three-dimensional constructs to cartilage defects are under way and officially registered (http://www.clinicaltrials.gov). However, to our knowledge these data are not published yet, and the data emerging from these controlled in vivo studies in the next few years need to be analyzed to determine whether MSC-based treatments can compete with current treatment modalities.
Arthritides
As opposed to focal articular cartilage defects, approaches to treat arthritides such as rheumatoid arthritis (RA) or osteoarthritis (OA) with stem cell-based approaches have to take into consideration the larger sizes of the defects, as well as the underlying disease process, as vulnerable neocartilage constructs may face rapid degradation when delivered to inflamed joints. Therefore, the underlying pathology has to be effectively under control, or any cell-based treatment of OA or RA is unlikely to be successful long-term.
For the treatment of OA, the intraarticular injection of MSC suspensions represents conceptually the simplest approach. Using such an approach, cells are distributed throughout the joint space to provide their therapeutic effects, as seen in an vivo study in goats, where delivery of autologous stromal cell suspensions were able to mediate regeneration of inflamed menisci after resection, along with reduction of arthritic changes to the cartilage, including osteophytic remodeling, and subchondral sclerosis [80]. Initial casuistic pilot studies are in the literature that evaluated injection of ex vivo expanded marrow stromal cell populations in patients with OA of the knee [81, 82], and they reported safety and feasibility of the procedure; MRI evaluations of one patient revealed increased cartilage and meniscus thickness after 24 months. However, these data still have to be considered as very preliminary, and long-term evaluations in controlled randomized clinical trials are necessary to determine efficacy.
Because of their immunosuppressive effects, MSCs from autologous and allogeneic origin have been considered for several autoimmune diseases, including RA [83, 84]. Allogeneic MSC transplantation in four patients with refractory RA was considered safe but revealed only short-term clinical improvement in two patients, and none of the patients reached the stage of remission [85]. However, in RA this treatment option has not been pursued further, because of disease relapses and the good success with standard therapies that were complemented with the use of the antagonist in recent years [84].
As opposed to systemic transplantation, fibroblastic cells were used to deliver anti-inflammatory cytokines to RA diseased joints intraarticularly following genetic modification experimentally and clinically [86]. In the first clinical trial, the IL-1 receptor antagonist (IL-1Ra) was chosen as the transgene and delivered to the metacarpophalangeal joints of nine individuals with severe RA via synovial fibroblasts, which were genetically modified using a retrovirus [87]. This phase I study revealed the safety of the procedure and also transgene expression in all treated joints [87]. Comparable studies were performed at other centers with different vector- and cell preparations and showed comparable outcomes, which are reviewed elsewhere [86]. However, it remains to be seen whether such costly and laborious genetic therapies will find the way to broad clinical application, and the safety issue remains of concern in diseases that are not life-threatening.
Tendon
Because of the beneficial experiences of cell-based treatments of tendinopathy in experimental studies [88], autologous blood injections were used clinically with some success for the treatment of medial [89] or lateral epicondylitis with [90, 91] or without dry needling, as well as refractory patellar tendinopathy [92]. A recent systematic review of four injection therapies for lateral epicondylitis, namely, prolotherapy, polidocanol, whole blood, and PRP, revealed that all were comparatively effective without any major differences that could be extracted from the literature [93]. In the quest for the development of improved treatments for the tendinopathies, the injection of ex vivo expanded autologous dermal fibroblasts has been evaluated as an effective and safe treatment for refractory lateral elbow epicondylitis in an initial pilot study [90]. The same group of investigators compared the injection of dermal fibroblasts together with autologous serum versus autologous serum only for the treatment of refractory patellar tendinopathy, and they reported a faster healing response as observed by ultrasound and faster clinical recovery in the cell-based treatment group at 6 months [94].
Other Orthopaedic Applications
In the field of regenerative orthopaedics, there are solid research data that support the beneficial use of MSCs for treatment of meniscus [95], intervertebral disc [96], ligaments and tendon [97, 98], or muscle injuries [99], which are extensively reviewed elsewhere [1]. For all of these applications, clinical trials are currently under way that use MSCs from autologous or allogeneic origin (http://www.clinicaltrials.gov). Taken together, these promising features of cell-based therapy approaches support the feasibility of the development of these treatments to clinical use, and the initial clinical trials will clarify whether such approaches will find the way to broad clinical application.
Apart from applications in musculoskeletal regeneration, there is a rapidly growing body of data from clinical trials that have used MSCs for the treatment of myocardial infarction, nerve regeneration after stroke or spinal cord injuries, graft versus host disease, certain Mendelian hereditary disorders, and others, which have been recently extensively reviewed elsewhere [100–103]. Of particular interest to the orthopaedic field are the studies that used allogeneic bone marrow-derived MSC transplantation to replace defective collagen type I synthesis by oseoblasts of patients suffering from osteogenesis imperfecta (OI), a genetic disorder that leads to a variety of skeletal pathologies, including deformities and incidental fractures [104, 105]. In this context, transplantation of allogeneic MSCs from foreign donors [105], or human leukocyte antigen (HLA)-compatible sibling donors [104], led to successful cell engraftment, significant increase of bone mineral content, and reduced bone fracture frequencies in children with OI. Therefore it remains to be seen whether such therapies can be further developed to broad successful clinical application.
Perspectives for Stem Cell Therapies for Musculoskeletal Regeneration
In the orthopaedic and sports medicine community, regenerative biological therapies are rapidly emerging and have received increasing attention over the last years. For example, the clinical application of PRP has been added to the repertoire of treatment options to enhance healing for almost all musculoskeletal tissues, including tendon, bone, cartilage, muscle, and others, and almost outpaced the research in the field [106]. However, as more and more clinical data surface, the limitations of PRP therapies have become evident [106]. It is perceivable that stem cells might prove ideal to complement or enhance such readily available treatment modalities, where desired results are often not achieved. It remains to be seen for every specific application whether the addition of cellular entities derived from whole blood or marrow, concentrates thereof, or ex vivo processed cell preparations (Fig. 1) is required and effective to augment optimized healing results.
Another great potential for healing enhancement is the facilitation of endogenous tissue stem cells. In view of the mounting evidence demonstrating the paracrine and trophic functions of MSCs, it is not known to date whether the stem cell therapies outlined above in this review act by stimulating regenerative cell populations in situ, and to what extent this mechanism contributed to the therapeutic effects seen. Also, does PRP attract MSCs, and what are the relevant mechanisms of action? As information on tissue stem cells becomes more evident, local resident cell populations might be a more effective cell therapy target in the future [1]. Once the relevant regenerative mechanisms of stem cells are more precisely understood, it is conceivable that these functions might be augmented with the use of appropriate growth factors and biomaterials. For bone regeneration, human recombinant BMP-2 and BMP-7 (OP-1) have been brought to broad clinical application and found their place in the therapeutic regimen of bone diseases, where standard therapies failed [107]. For such cases, the addition of MSCs might further improve the approach, as the preliminary clinical data suggest [40]. These may represent the steps of the “therapeutic escalator” that lead to the regeneration of other musculoskeletal tissues, such as cartilage, where no satisfying treatment options are available in the clinic. These approaches will require that the most appropriate material(s), factor(s), and cell preparation(s) for the target tissue are identified.
Furthermore, what researchers and clinicians have to clarify before they pursue therapeutic efforts is what they can and cannot realistically expect from their stem cell or regenerative therapy. In other words, what are the aspects of healing they would like to enhance, and which tools are therefore needed? An MSC-based therapy per se might not be the solution for every problematic defect in the musculoskeletal system, and chances and risks have to be well considered to avoid failures that might be disadvantageous for the patient and the field. Such information needs to be communicated to patients and also to the sponsors and regulators of the health system, in order to gain public support for the development of novel therapies for more effective treatments in the future that will prove to be cost-effective in the long term.
Lastly, several ethical and regulatory aspects have to be considered before such cell-based treatments can be conducted. Any application of cells that involves ex vivo expanded or processed cells is considered advanced therapy medicinal products (ATMPs) by the competent international and national regulatory agencies, for example, the FDA or the European Medicines Agency. Approval of such ATMPs usually requires several specific quality-control and safety criteria that are based on the use of standardized GMP conditions for cell processing, the conduct of appropriate preclinical animal models, and meaningful controlled phase I/II clinical trials [108, 109]. Meeting these stringent criteria will help guarantee that the efficacy and safety of innovative stem cell interventions will be rigorously established, while also protecting study participants. However, the laborious and time-consuming nature of these regulative processes must be taken into consideration in developing clinical applications of such innovative stem cell-based approaches.
Conclusion
There is an increasing demand for MSC-based regenerative approaches in the musculoskeletal system, the current clinical status of which is listed in Table 1. MSCs have been delivered as whole blood or marrow, concentrates, or ex vivo expanded cell populations, either in suspension or seeded in carrier matrices with or without stimulating factors, to injured tissues. However, there is no orthopaedic application where MSC-based therapies have been advanced to broad clinical application. Delivery of MSCs for the repair of bone, cartilage, and tendon cells has shown safety and initial efficacy in several phase I clinical trials, but large comparative prospective randomized clinical trials are required for adequate comparison of the MSC-based therapies to standard treatment modalities. In other arenas, such as meniscus, intervertebral disc, ligament, or muscle regeneration, MSCs have only been used experimentally with some success. Such MSC-based therapies are thought to be improved by the use of certain bioactive materials and stimuli that aid distinct aspects of repair. However, the ideal combinations of cell preparation, bioactive factor(s), and material(s) for each application have to be identified that also meet the desired safety and cost requirements in a satisfactory manner. Only when such therapies for non-life-threatening diseases can show improved outcomes compared with standard treatments will they be able to prove long-term cost-effectiveness and find their place in the therapeutic regimen of the discipline.
Acknowledgments
This work is supported in part by the Deutsche Forschungsgemeinschaft (DFG STE1051/2-1, A.F.S. and U.N.), Bayerische Forschungsstiftung (FORZEBRA TP2-WP5, A.F.S. and U.N.), the Interdisziplinäres Zentrum für Klinische Forschung Würzburg (IZKF D-101 and D-219, A.F.S.), the European Union (seventh Framework Program-HEALTH VascuBone and ADIPOA, U.N. and L.R.), and the Commonwealth of Pennsylvania Department of Health (R.S.T.).
Author Contributions
A.F.S., L.R., U.N., R.S.T.: conception and design, manuscript writing, final approval of manuscript, financial support; F.G.: conception and design, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interests.
References
- 1.Nöth U, Rackwitz L, Steinert AF, et al. Cell delivery therapeutics for musculoskeletal regeneration. Adv Drug Deliv Rev. 2010;62:765–783. doi: 10.1016/j.addr.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Kuhn NZ, Tuan RS. Regulation of stemness and stem cell niche of mesenchymal stem cells: Implications in tumorigenesis and metastasis. J Cell Physiol. 2010;222:268–277. doi: 10.1002/jcp.21940. [DOI] [PubMed] [Google Scholar]
- 3.Roobrouck VD, Vanuytsel K, Verfaillie CM. Concise review: Culture mediated changes in fate and/or potency of stem cells. Stem Cells. 2011;29:583–589. doi: 10.1002/stem.603. [DOI] [PubMed] [Google Scholar]
- 4.Jukes JM, van Blitterswijk CA, de Boer J. Skeletal tissue engineering using embryonic stem cells. J Tissue Eng Regen Med. 2010;4:165–180. doi: 10.1002/term.234. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Nakagawa M, Koyanagi M, Tanabe K, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 7.Meissner A, Wernig M, Jaenisch R. Direct reprogramming of genetically unmodified fibroblasts into pluripotent stem cells. Nat Biotechnol. 2007;25:1177–1181. doi: 10.1038/nbt1335. [DOI] [PubMed] [Google Scholar]
- 8.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 9.Illich DJ, Demir N, Stojkovic M, et al. Concise review: Induced pluripotent stem cells and lineage reprogramming: Prospects for bone regeneration. Stem Cells. 2011;29:555–563. doi: 10.1002/stem.611. [DOI] [PubMed] [Google Scholar]
- 10.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 11.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 12.Caplan AI. Review: Mesenchymal stem cells: Cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005;11:1198–1211. doi: 10.1089/ten.2005.11.1198. [DOI] [PubMed] [Google Scholar]
- 13.Petrie Aronin CE, Tuan RS. Therapeutic potential of the immunomodulatory activities of adult mesenchymal stem cells. Birth Defects Res C Embryo Today. 2010;90:67–74. doi: 10.1002/bdrc.20174. [DOI] [PubMed] [Google Scholar]
- 14.Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: Regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9:204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nöth U, Steinert AF, Tuan RS. Technology insight: Adult mesenchymal stem cells for osteoarthritis therapy. Nat Clin Pract Rheumatol. 2008;4:371–380. doi: 10.1038/ncprheum0816. [DOI] [PubMed] [Google Scholar]
- 16.Hogan MV, Bagayoko N, James R, et al. Tissue engineering solutions for tendon repair. J Am Acad Orthop Surg. 2011;19:134–142. doi: 10.5435/00124635-201103000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Marcacci M, Kon E, Moukhachev V, et al. Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng. 2007;13:947–955. doi: 10.1089/ten.2006.0271. [DOI] [PubMed] [Google Scholar]
- 18.Brittberg M. Autologous chondrocyte implantation—technique and long-term follow-up. Injury. 2008;39(suppl 1):S40–S49. doi: 10.1016/j.injury.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 19.Nöth U, Reichert J, Reppenhagen S, et al. Cell based therapy for the treatment of femoral head necrosis. Orthopade. 2007;36:466–471. doi: 10.1007/s00132-007-1087-2. [DOI] [PubMed] [Google Scholar]
- 20.Christenson EM, Anseth KS, van den Beucken JJ, et al. Nanobiomaterial applications in orthopedics. J Orthop Res. 2007;25:11–22. doi: 10.1002/jor.20305. [DOI] [PubMed] [Google Scholar]
- 21.Ma PX. Biomimetic materials for tissue engineering. Adv Drug Deliv Rev. 2008;60:184–198. doi: 10.1016/j.addr.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dawson JI, Oreffo RO. Bridging the regeneration gap: Stem cells, biomaterials and clinical translation in bone tissue engineering. Arch Biochem Biophys. 2008;473:124–131. doi: 10.1016/j.abb.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 23.Khan Y, Yaszemski MJ, Mikos AG, et al. Tissue engineering of bone: Material and matrix considerations. J Bone Joint Surg Am. 2008;90(suppl 1):36–42. doi: 10.2106/JBJS.G.01260. [DOI] [PubMed] [Google Scholar]
- 24.Mano JF, Silva GA, Azevedo HS, et al. Natural origin biodegradable systems in tissue engineering and regenerative medicine: Present status and some moving trends. J R Soc Interface. 2007;4:999–1030. doi: 10.1098/rsif.2007.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rezwan K, Chen QZ, Blaker JJ, et al. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27:3413–3431. doi: 10.1016/j.biomaterials.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 26.Kuo CK, Li WJ, Mauck RL, et al. Cartilage tissue engineering: Its potential and uses. Curr Opin Rheumatol. 2006;18:64–73. doi: 10.1097/01.bor.0000198005.88568.df. [DOI] [PubMed] [Google Scholar]
- 27.Chen FH, Rousche KT, Tuan RS. Technology insight: Adult stem cells in cartilage regeneration and tissue engineering. Nat Clin Pract Rheumatol. 2006;2:373–382. doi: 10.1038/ncprheum0216. [DOI] [PubMed] [Google Scholar]
- 28.Li WJ, Tuan RS. Fabrication and application of nanofibrous scaffolds in tissue engineering. Curr Protoc Cell Biol. 2009 doi: 10.1002/0471143030.cb2502s42. Chapter 25:Unit 25.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinert AF, Ghivizzani SC, Rethwilm A, et al. Major biological obstacles for persistent cell-based regeneration of articular cartilage. Arthritis Res Ther. 2007;9:213. doi: 10.1186/ar2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lepperdinger G. Inflammation and mesenchymal stem cell aging. Curr Opin Immunol. 2011;23:518–524. doi: 10.1016/j.coi.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tuan RS. Role of adult stem/progenitor cells in osseointegration and implant loosening. Int J Oral Maxillofac Implants. 2011;26(suppl):50–62. discussion 63–69. [PubMed] [Google Scholar]
- 32.Rauh J, Milan F, Gunther KP, et al. Bioreactor systems for bone tissue engineering. Tissue Eng Part B Rev. 2011;17:263–280. doi: 10.1089/ten.TEB.2010.0612. [DOI] [PubMed] [Google Scholar]
- 33.Jones E, Yang X. Mesenchymal stem cells and bone regeneration: Current status. Injury. 2011;42:562–568. doi: 10.1016/j.injury.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 34.Cancedda R, Bianchi G, Derubeis A, et al. Cell therapy for bone disease: A review of current status. Stem Cells. 2003;21:610–619. doi: 10.1634/stemcells.21-5-610. [DOI] [PubMed] [Google Scholar]
- 35.Beyth S, Schroeder J, Liebergall M. Stem cells in bone diseases: Current clinical practice. Br Med Bull. 2011;99:199–210. doi: 10.1093/bmb/ldr035. [DOI] [PubMed] [Google Scholar]
- 36.Connolly JF. Clinical use of marrow osteoprogenitor cells to stimulate osteogenesis. Clin Orthop Relat Res. 1998;355(suppl):S257–S266. doi: 10.1097/00003086-199810001-00026. [DOI] [PubMed] [Google Scholar]
- 37.Hernigou P, Beaujean F. Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res. 2002;405:14–23. doi: 10.1097/00003086-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Dallari D, Savarino L, Stagni C, et al. Enhanced tibial osteotomy healing with use of bone grafts supplemented with platelet gel or platelet gel and bone marrow stromal cells. J Bone Joint Surg Am. 2007;89:2413–2420. doi: 10.2106/JBJS.F.01026. [DOI] [PubMed] [Google Scholar]
- 39.Schmid U, Thielemann F, Weise K, et al. A novel therapeutic approach to bone replacement: Vitalisation of industrial processed allogenic bone graft with autologous bone marrow. Z Orthop Unfall. 2007;145:221–229. doi: 10.1055/s-2007-965204. [DOI] [PubMed] [Google Scholar]
- 40.Warnke PH, Springer IN, Wiltfang J, et al. Growth and transplantation of a custom vascularised bone graft in a man. Lancet. 2004;364:766–770. doi: 10.1016/S0140-6736(04)16935-3. [DOI] [PubMed] [Google Scholar]
- 41.Ochs BG, Schmid U, Rieth J, et al. Acetabular bone reconstruction in revision arthroplasty: A comparison of freeze-dried, irradiated and chemically-treated allograft vitalised with autologous marrow versus frozen non-irradiated allograft. J Bone Joint Surg Br. 2008;90:1164–1171. doi: 10.1302/0301-620X.90B9.20425. [DOI] [PubMed] [Google Scholar]
- 42.Hendrich C, Franz E, Waertel G, et al. Safety of autologous bone marrow aspiration concentrate transplantation: Initial experiences in 101 patients. Orthop Rev (Pavia) 2009;1:e32. doi: 10.4081/or.2009.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jäger M, Herten M, Fochtmann U, et al. Bridging the gap: Bone marrow aspiration concentrate reduces autologous bone grafting in osseous defects. J Orthop Res. 2011;29:173–180. doi: 10.1002/jor.21230. [DOI] [PubMed] [Google Scholar]
- 44.Quarto R, Mastrogiacomo M, Cancedda R, et al. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344:385–386. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- 45.Kitoh H, Kitakoji T, Tsuchiya H, et al. Distraction osteogenesis of the lower extremity in patients with achondroplasia/hypochondroplasia treated with transplantation of culture-expanded bone marrow cells and platelet-rich plasma. J Pediatr Orthop. 2007;27:629–634. doi: 10.1097/BPO.0b013e318093f523. [DOI] [PubMed] [Google Scholar]
- 46.Kitoh H, Kitakoji T, Tsuchiya H, et al. Transplantation of culture expanded bone marrow cells and platelet rich plasma in distraction osteogenesis of the long bones. Bone. 2007;40:522–528. doi: 10.1016/j.bone.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 47.Petrigliano FA, Lieberman JR. Osteonecrosis of the hip: Novel approaches to evaluation and treatment. Clin Orthop Relat Res. 2007;465:53–62. doi: 10.1097/BLO.0b013e3181591c92. [DOI] [PubMed] [Google Scholar]
- 48.Gangji V, Hauzeur JP, Matos C, et al. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. A pilot study. J Bone Joint Surg Am. 2004;86-A:1153–1160. doi: 10.2106/00004623-200406000-00006. [DOI] [PubMed] [Google Scholar]
- 49.Ficat F, Arlet J. Biopsy-forage in the treatment of primary femoro-capital osteonecrosis. Presse Med. 1971;79:581. [PubMed] [Google Scholar]
- 50.Hungerford DS. Pathogenetic considerations in ischemic necrosis of bone. Can J Surg. 1981;24:583–587. 590. [PubMed] [Google Scholar]
- 51.Kiaer T, Pedersen NW, Kristensen KD, et al. Intra-osseous pressure and oxygen tension in avascular necrosis and osteoarthritis of the hip. J Bone Joint Surg Br. 1990;72:1023–1030. doi: 10.1302/0301-620X.72B6.2246284. [DOI] [PubMed] [Google Scholar]
- 52.Gangji V, Hauzeur JP. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. Surgical technique. J Bone Joint Surg Am. 2005;87(suppl 1):106–112. doi: 10.2106/JBJS.D.02662. [DOI] [PubMed] [Google Scholar]
- 53.Hernigou P, Poignard A, Manicom O, et al. The use of percutaneous autologous bone marrow transplantation in nonunion and avascular necrosis of bone. J Bone Joint Surg Br. 2005;87:896–902. doi: 10.1302/0301-620X.87B7.16289. [DOI] [PubMed] [Google Scholar]
- 54.Kawate K, Yajima H, Ohgushi H, et al. Tissue-engineered approach for the treatment of steroid-induced osteonecrosis of the femoral head: Transplantation of autologous mesenchymal stem cells cultured with beta-tricalcium phosphate ceramics and free vascularized fibula. Artif Organs. 2006;30:960–962. doi: 10.1111/j.1525-1594.2006.00333.x. [DOI] [PubMed] [Google Scholar]
- 55.Wright JG, Yandow S, Donaldson S, et al. A randomized clinical trial comparing intralesional bone marrow and steroid injections for simple bone cysts. J Bone Joint Surg Am. 2008;90:722–730. doi: 10.2106/JBJS.G.00620. [DOI] [PubMed] [Google Scholar]
- 56.Park IH, Micic ID, Jeon IH. A study of 23 unicameral bone cysts of the calcaneus: Open chip allogeneic bone graft versus percutaneous injection of bone powder with autogenous bone marrow. Foot Ankle Int. 2008;29:164–170. doi: 10.3113/FAI.2008.0164. [DOI] [PubMed] [Google Scholar]
- 57.Zamzam MM, Abak AA, Bakarman KA, et al. Efficacy of aspiration and autogenous bone marrow injection in the treatment of simple bone cysts. Int Orthop. 2009;33:1353–1358. doi: 10.1007/s00264-008-0619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Köse N, Gokturk E, Turgut A, et al. Percutaneous autologous bone marrow grafting for simple bone cysts. Bull Hosp Jt Dis. 1999;58:105–110. [PubMed] [Google Scholar]
- 59.Lokiec F, Ezra E, Khermosh O, et al. Simple bone cysts treated by percutaneous autologous marrow grafting. A preliminary report. J Bone Joint Surg Br. 1996;78:934–937. doi: 10.1302/0301-620x78b6.6840. [DOI] [PubMed] [Google Scholar]
- 60.Jäger M, Jelinek EM, Wess KM, et al. Bone marrow concentrate: A novel strategy for bone defect treatment. Curr Stem Cell Res Ther. 2009;4:34–43. doi: 10.2174/157488809787169039. [DOI] [PubMed] [Google Scholar]
- 61.Pneumaticos SG, Triantafyllopoulos GK, Chatziioannou S, et al. Biomolecular strategies of bone augmentation in spinal surgery. Trends Mol Med. 2011;17:215–222. doi: 10.1016/j.molmed.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 62.Neen D, Noyes D, Shaw M, et al. Healos and bone marrow aspirate used for lumbar spine fusion: A case controlled study comparing healos with autograft. Spine (Phila Pa 1976) 2006;31:E636–E640. doi: 10.1097/01.brs.0000232028.97590.12. [DOI] [PubMed] [Google Scholar]
- 63.Zhang P, Gan YK, Tang J, et al. Clinical study of lumbar fusion by hybrid construct of stem cells technique and biodegradable material. Zhonghua Wai Ke Za Zhi. 2008;46:493–496. [PubMed] [Google Scholar]
- 64.Oakes DA, Lieberman JR. Osteoinductive applications of regional gene therapy: Ex vivo gene transfer. Clin Orthop Relat Res. 2000;379(suppl):S101–S112. doi: 10.1097/00003086-200010001-00014. [DOI] [PubMed] [Google Scholar]
- 65.Lieberman JR, Ghivizzani SC, Evans CH. Gene transfer approaches to the healing of bone and cartilage. Mol Ther. 2002;6:141–147. doi: 10.1006/mthe.2000.0663. [DOI] [PubMed] [Google Scholar]
- 66.Lieberman JR, Daluiski A, Stevenson S, et al. The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats. J Bone Joint Surg Am. 1999;81:905–917. doi: 10.2106/00004623-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 67.Baltzer AW, Lieberman JR. Regional gene therapy to enhance bone repair. Gene Ther. 2004;11:344–350. doi: 10.1038/sj.gt.3302195. [DOI] [PubMed] [Google Scholar]
- 68.Tang TT, Lu B, Yue B, et al. Treatment of osteonecrosis of the femoral head with hBMP-2-gene-modified tissue-engineered bone in goats. J Bone Joint Surg Br. 2007;89:127–129. doi: 10.1302/0301-620X.89B1.18350. [DOI] [PubMed] [Google Scholar]
- 69.Brittberg M, Lindahl A, Nilsson A, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 70.Behrens P, Bosch U, Bruns J, et al. Indications and implementation of recommendations of the working group “Tissue Regeneration and Tissue Substitutes” for autologous chondrocyte transplantation (ACT) Z Orthop Ihre Grenzgeb. 2004;142:529–539. doi: 10.1055/s-2004-832353. [DOI] [PubMed] [Google Scholar]
- 71.Peterson L, Minas T, Brittberg M, et al. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: Results at two to ten years. J Bone Joint Surg Am. 2003;85-A(suppl 2):17–24. doi: 10.2106/00004623-200300002-00003. [DOI] [PubMed] [Google Scholar]
- 72.Horas U, Pelinkovic D, Herr G, et al. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am. 2003;85-A:185–192. doi: 10.2106/00004623-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 73.Harris JD, Siston RA, Brophy RH, et al. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19:779–791. doi: 10.1016/j.joca.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 74.Niemeyer P, Salzmann GM, Hirschmüller A, et al. Factors that influence clinical outcome following autologous chondrocyte implantation for cartilage defects of the knee [in German] Z Orthop Unfall. 2012;150:83–88. doi: 10.1055/s-0030-1270894. [DOI] [PubMed] [Google Scholar]
- 75.Iwasa J, Engebretsen L, Shima Y, et al. Clinical application of scaffolds for cartilage tissue engineering. Knee Surg Sports Traumatol Arthrosc. 2009;17:561–577. doi: 10.1007/s00167-008-0663-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wakitani S, Mitsuoka T, Nakamura N, et al. Autologous bone marrow stromal cell transplantation for repair of full-thickness articular cartilage defects in human patellae: Two case reports. Cell Transplant. 2004;13:595–600. doi: 10.3727/000000004783983747. [DOI] [PubMed] [Google Scholar]
- 77.Wakitani S, Nawata M, Tensho K, et al. Repair of articular cartilage defects in the patello-femoral joint with autologous bone marrow mesenchymal cell transplantation: Three case reports involving nine defects in five knees. J Tissue Eng Regen Med. 2007;1:74–79. doi: 10.1002/term.8. [DOI] [PubMed] [Google Scholar]
- 78.Kuroda R, Ishida K, Matsumoto T, et al. Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthritis Cartilage. 2007;15:226–231. doi: 10.1016/j.joca.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 79.Nejadnik H, Hui JH, Feng Choong EP, et al. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: An observational cohort study. Am J Sports Med. 2010;38:1110–1116. doi: 10.1177/0363546509359067. [DOI] [PubMed] [Google Scholar]
- 80.Murphy JM, Fink DJ, Hunziker EB, et al. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48:3464–3474. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- 81.Centeno CJ, Schultz JR, Cheever M, et al. Safety and complications reporting on the re-implantation of culture-expanded mesenchymal stem cells using autologous platelet lysate technique. Curr Stem Cell Res Ther. 2010;5:81–93. doi: 10.2174/157488810790442796. [DOI] [PubMed] [Google Scholar]
- 82.Davatchi F, Abdollahi BS, Mohyeddin M, et al. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int J Rheum Dis. 2011;14:211–215. doi: 10.1111/j.1756-185X.2011.01599.x. [DOI] [PubMed] [Google Scholar]
- 83.Larghero J, Vija L, Lecourt S, et al. Mesenchymal stem cells and immunomodulation: Toward new immunosuppressive strategies for the treatment of autoimmune diseases? Rev Med Interne. 2009;30:287–299. doi: 10.1016/j.revmed.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 84.Kötter I, Schmalzing M, Henes J, et al. Current value of stem-cell transplantation in autoimmune diseases. Z Rheumatol. 2008;67:716–722. doi: 10.1007/s00393-008-0386-2. [DOI] [PubMed] [Google Scholar]
- 85.Liang J, Li X, Zhang H, et al. Allogeneic mesenchymal stem cells transplantation in patients with refractory RA. Clin Rheumatol. 2012;31:157–161. doi: 10.1007/s10067-011-1816-0. [DOI] [PubMed] [Google Scholar]
- 86.Evans CH, Ghivizzani SC, Robbins PD. Gene therapy of the rheumatic diseases: 1998 to 2008. Arthritis Res Ther. 2009;11:209. doi: 10.1186/ar2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Evans CH, Robbins PD, Ghivizzani SC, et al. Gene transfer to human joints: Progress toward a gene therapy of arthritis. Proc Natl Acad Sci U S A. 2005;102:8698–8703. doi: 10.1073/pnas.0502854102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kuo CK, Marturano JE, Tuan RS. Novel strategies in tendon and ligament tissue engineering: Advanced biomaterials and regeneration motifs. Sports Med Arthrosc Rehabil Ther Technol. 2010;2:20. doi: 10.1186/1758-2555-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Suresh SP, Ali KE, Jones H, et al. Medial epicondylitis: Is ultrasound guided autologous blood injection an effective treatment? Br J Sports Med. 2006;40:935–939. doi: 10.1136/bjsm.2006.029983. discussion 939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Connell D, Datir A, Alyas F, et al. Treatment of lateral epicondylitis using skin-derived tenocyte-like cells. Br J Sports Med. 2009;43:293–298. doi: 10.1136/bjsm.2008.056457. [DOI] [PubMed] [Google Scholar]
- 91.Connell DA, Ali KE, Ahmad M, et al. Ultrasound-guided autologous blood injection for tennis elbow. Skeletal Radiol. 2006;35:371–377. doi: 10.1007/s00256-006-0081-9. [DOI] [PubMed] [Google Scholar]
- 92.James SL, Ali K, Pocock C, et al. Ultrasound guided dry needling and autologous blood injection for patellar tendinosis. Br J Sports Med. 2007;41:518–521. doi: 10.1136/bjsm.2006.034686. discussion 522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rabago D, Best TM, Zgierska AE, et al. A systematic review of four injection therapies for lateral epicondylosis: Prolotherapy, polidocanol, whole blood and platelet-rich plasma. Br J Sports Med. 2009;43:471–481. doi: 10.1136/bjsm.2008.052761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Clarke AW, Alyas F, Morris T, et al. Skin-derived tenocyte-like cells for the treatment of patellar tendinopathy. Am J Sports Med. 2011;39:614–623. doi: 10.1177/0363546510387095. [DOI] [PubMed] [Google Scholar]
- 95.Makris EA, Hadidi P, Athanasiou KA. The knee meniscus: Structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials. 2011;32:7411–7431. doi: 10.1016/j.biomaterials.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Meyerrose T, Olson S, Pontow S, et al. Mesenchymal stem cells for the sustained in vivo delivery of bioactive factors. Adv Drug Deliv Rev. 2010;62:1167–1174. doi: 10.1016/j.addr.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lui PP, Rui YF, Ni M, et al. Tenogenic differentiation of stem cells for tendon repair-what is the current evidence? J Tissue Eng Regen Med. 2011;5:e144–e163. doi: 10.1002/term.424. [DOI] [PubMed] [Google Scholar]
- 98.Hsu SL, Liang R, Woo SL. Functional tissue engineering of ligament healing. Sports Med Arthrosc Rehabil Ther Technol. 2010;2:12. doi: 10.1186/1758-2555-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Quintero AJ, Wright VJ, Fu FH, et al. Stem cells for the treatment of skeletal muscle injury. Clin Sports Med. 2009;28:1–11. doi: 10.1016/j.csm.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Giordano A, Galderisi U, Marino IR. From the laboratory bench to the patient's bedside: An update on clinical trials with mesenchymal stem cells. J Cell Physiol. 2007;211:27–35. doi: 10.1002/jcp.20959. [DOI] [PubMed] [Google Scholar]
- 101.Tolar J, Le Blanc K, Keating A, et al. Concise review: Hitting the right spot with mesenchymal stromal cells. Stem Cells. 2010;28:1446–1455. doi: 10.1002/stem.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Trounson A, Thakar RG, Lomax G, et al. Clinical trials for stem cell therapies. BMC Med. 2011;9:52. doi: 10.1186/1741-7015-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Malgieri A, Kantzari E, Patrizi MP, et al. Bone marrow and umbilical cord blood human mesenchymal stem cells: State of the art. Int J Clin Exp Med. 2010;3:248–269. [PMC free article] [PubMed] [Google Scholar]
- 104.Marino R, Martinez C, Boyd K, et al. Transplantable marrow osteoprogenitors engraft in discrete saturable sites in the marrow microenvironment. Exp Hematol. 2008;36:360–368. doi: 10.1016/j.exphem.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Horwitz EM, Gordon PL, Koo WK, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci U S A. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kon E, Filardo G, Di Martino A, et al. Platelet-rich plasma (PRP) to treat sports injuries: evidence to support its use. Knee Surg Sports Traumatol Arthrosc. 2011;19:516–527. doi: 10.1007/s00167-010-1306-y. [DOI] [PubMed] [Google Scholar]
- 107.Argintar E, Edwards S, Delahay J. Bone morphogenetic proteins in orthopaedic trauma surgery. Injury. 2011;42:730–734. doi: 10.1016/j.injury.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 108.Schneider CK, Salmikangas P, Jilma B, et al. Challenges with advanced therapy medicinal products and how to meet them. Nat Rev Drug Discov. 2010;9:195–201. doi: 10.1038/nrd3052. [DOI] [PubMed] [Google Scholar]
- 109.Salmikangas P, Flory E, Reinhardt J, et al. Regulatory requirements for clinical trial and marketing authorisation application for cell-based medicinal products. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2010;53:24–29. doi: 10.1007/s00103-009-0991-5. [DOI] [PubMed] [Google Scholar]