Abstract
Background:
Dental caries remains the most common dental disease facing mankind. Prevention of initiation and interruption in progression of early lesions are the desirable modes of caries management. There is a scope for agents, which may be used to enhance anti - caries activity. This need has redirected research to develop novel preventive agents that can act as an adjunct to fluoride or independent of it. Casein Phosphopeptide – Amorphous Calcium Phosphate (CPP-ACP) is one such agent that has been proposed to have anti cariogenic properties.
Aim:
The purpose of this in vitro study was to evaluate the effect of paste containing CPP-ACP, MI Paste, on enamel remineralization.
Materials and Methods:
This study consisted of 30 samples embedded in orthodontic resin with either the buccal or lingual surface exposed. The samples were assigned to either a CPP-ACP containing paste; Fluoridated toothpaste; or a control group. The groups were then subjected to cycling in a demineralizing solution and a remineralizing solution. Groups II and III received prior application of MI paste and Fluoridated toothpaste respectively followed by cycling in a demineralizing solution and a remineralizing solution. Following 14 days of cycling, the samples were sectioned and examined using confocal microscopy. The lesion depth, were evaluated.
Statistical Analysis:
Image Proplus software was used to analyze the images. The values were statistically evaluated using one – way ANOVA and Scheffe's Test.
Results and Conclusion:
Within the limitations of the study it was concluded that enamel surfaces treated with the CPP-ACP paste exhibited the least lesion depths followed by the enamel surfaces treated with the fluoridated tooth paste and control group respectively.
Keywords: Caries, casein phosphopeptide-amorphous calcium phosphate, fluoride
INTRODUCTION
Dental caries is an infectious microbiologic disease of the teeth that results in localized dissolution and destruction of the calcified tissues. The largest increase in the prevalence of caries has been associated with dietary changes. Today, dental caries remains the most common dental disease facing mankind.[1] Prevention of initiation and interruption in progression of early lesions are the desirable modes of caries management. Fluoride is a preventive agent that has almost mesmerized the dental research. It is one of the elements categorized as strongly cariostatic. Although fluoride has had a profound effect on the level of caries progression, it is far from being a complete cure. It is unlikely that there is any concentration of fluoride that will eliminate caries totally.[2] High fluoride strategy cannot be followed in most instances to avoid potential for adverse effects due to overexposure to fluoride.
Thus, there is a scope for agents that may be used with fluoride to enhance anti-caries activity.[2] This need has redirected research to develop novel preventive agents that can act as an adjunct to fluoride or independent of it. Casein phosphopeptides (CPP)-amorphous calcium phosphate (ACP) is one such agent that has been proposed to have anti-cariogenic properties. Phosphopeptides of casein are produced from the tryptic digest of milk protein “casein” by aggregation with calcium and phosphate.
Casein phosphopeptide is a sticky protein that binds and stabilizes calcium and phosphate ions in an amorphous state. It readily binds to saliva pellicle, plaque, soft tissues and even to the hydroxyapatite component of enamel. CPP-ACP maintains saturation of levels of minerals, especially calcium and phosphate, at the tooth surface thereby depressing demineralization and enhancing remineralization.
AIMS AND OBJECTIVE
To determine whether enamel samples treated with CPP-ACP containing paste can resist acidic challenge in vitro when measured with confocal microscopy
To determine whether there is a significant difference in lesion depth between untreated enamel surfaces, enamel surfaces treated with CPP-ACP containing paste and enamel surfaces treated with fluoride containing paste.
MATERIALS AND METHODS
Thirty extracted human premolars without enamel defects or decalcification were used for this study. Roots were sectioned at the cementoenamel junction and the crowns were sectioned into buccal and lingual halves using a high-speed water-cooled hand piece and a carborundum disc. The crowns were placed in a glass container with deionized water.
Tooth crown samples were embedded in orthodontic acrylic with the buccal or lingual surface exposed. A 2 × 2 mm window of exposed enamel was created in the middle of the sample surface by applying a uniform coat of the nail varnish around it. Each mounted specimen was assigned to one of three different experimental groups and it was stored in deionized water until further use.
The demineralizing solution used was:
mM CaCl2
2.2 mM NaH2PO4
50 mM Acetic acid.
The remineralizing solution used was:
20 mM HEPES
1.5 mM Ca2+ as CaCl2
0.9 mM phosphate as KH2PO4
1 ppm Fluoride as NaF.
Treatment groups
Teeth in the group I were placed in a demineralizing solution with pH 4.46 for a period of 8 h and then removed and placed in artificial saliva for 1 h. After 1 h, the teeth were placed in a remineralizing solution with pH 7.00 for the balance of 24 h (15 h). This cycling continued for 14 days
Fluoridated tooth paste was applied to the exposed enamel surface of teeth in group III with a rubber glove and allowed to sit for 5 min. The specimens in this group were further treated similar to group I
CPP-ACP containing paste (MI paste RECALDENT™) was applied to the exposed enamel surface of teeth in group II with a rubber glove, according to the manufacturer's directions, and allowed to sit for a period of 5 min. The specimens in this group were further treated similar to group I.
Carborundum disc was used to section the samples in a buccolingual direction to obtain samples of ~200 μm thickness.
0.1 mM of rhodamine B solution was prepared by adding 23.95 mg of rhodamine B dye to 500 ml of deionized water.
The sectioned specimens were stored in 0.1 mM rhodamine B for 24 h and then placed in deionized water until further use. Rhodamine B from the solution incorporates in demineralized tooth structure and does not penetrate sound tooth structure or orthodontic resin.
Use of confocal microscopy
A confocal laser scanning microscope was used at ×10 magnification to view the images. HeNe 543 nm wavelength laser source was used with a rhodamine filter to excite the rhodamine B dye. The images were analyzed using Image proplus software [Figure 1].
Figure 1.
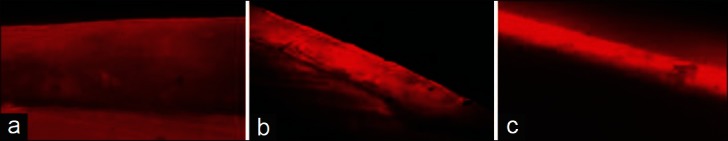
Depth of rhodamine B dye penetration (a) control group, (b) fluoride group and (c) CPP-ACP group
One-way ANOVA and Scheffe's test were used to analyze the data.
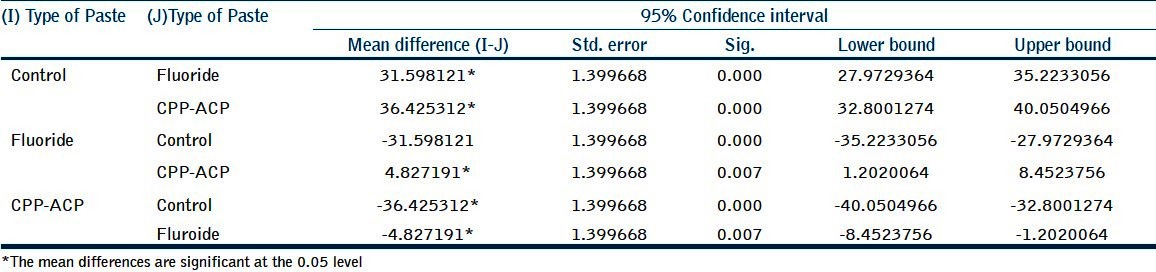
Significance was established at P < 0.05 [Tables 1 and 2].
Table 1.
Mean lesion depth (μm) for the three groups
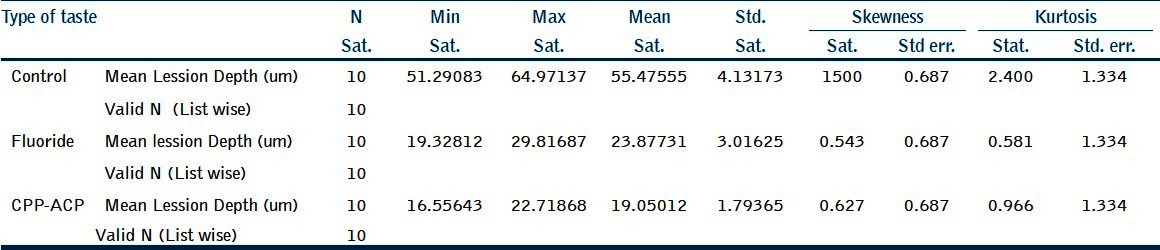
Table 2.
Statistical analysis
RESULTS
The mean lesion depth observed in the control group was 55.47 μm. The mean lesion depth observed in the fluoridated tooth paste-treated group was 23.88 μm. The mean lesion depth observed in the CPP-ACP tooth paste-treated group was 19.05 μm [Figure 2].
Figure 2.
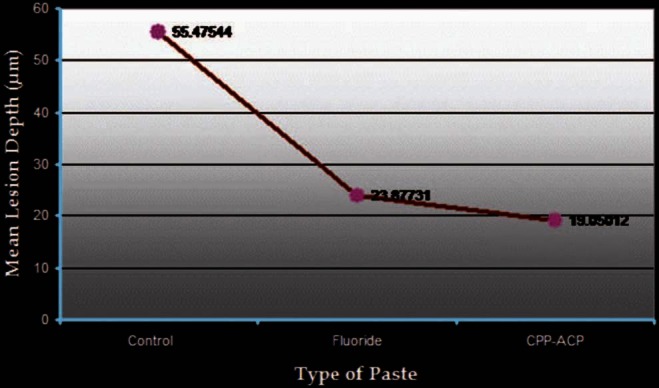
Mean lesion depth for the three groups
The percentage reduction in mean lesion depth between the control group and the fluoridated tooth paste-treated group was 56.96%. The percentage reduction in mean lesion depth between the control group and the CPP-ACP tooth paste-treated group was 65.66%. The percentage reduction in mean lesion depth between the fluoridated tooth paste-treated group and the CPP-ACP tooth paste-treated group was 20.22%.
DISCUSSION
The administration of fluoride has been proposed, and used, as a method of reducing enamel susceptibility to decalcification. Sub-ppm levels of fluoride in saliva are effective in shifting the balance from demineralization to remineralization. This is attributed to the fluoride-enhanced precipitation of calcium and phosphates and the formation of high-quality fluorapatite in the dental tissue that aids remineralization and inhibits glycolysis of plaque microorganisms. Low fluoride levels are found in the saliva after tooth brushing with fluoride-containing dentrifices. Salivary fluoride concentrations decrease exponentially in a biphasic manner to very low concentrations within a few hours. Similar concentrations are ineffective in interfering with processes of growth and metabolism of bacteria, and also do not result in a significantly reduced dissolution of tooth mineral. Fluoride slow release devices, in the form of fluoride-releasing restorative materials, may serve to increase the fluoride levels in saliva and plaque to levels at which caries can be prevented.
In this study, enamel surfaces treated with fluoridated tooth paste exhibited a 56.96% reduction in lesion depth when compared with the untreated enamel surfaces. This is in accordance with the study done by Hicks et al.,[3] where a 27% reduction in lesion depth was observed. These results suggest that treatment with fluoridated toothpaste does have a protective influence on the enamel surfaces.
Casein phosphopeptide – Amorphous calcium phosphate
CPP are peptides that are derived from the milk protein casein that are complexed with calcium and phosphate. CPP contain a cluster of phosphoseryl residues that stabilize nanoclusters of ACP in metastable solution. The CPP binds to spontaneously forming ACP nanoclusters and prevents their growth to the critical size required for nucleation and precipitation. It has been proposed that the mechanism of anticariogenicity for CPP-ACP is that it substantially increases the level of calcium phosphate in plaque, which decreases enamel demineralization and enhances remineralization. CPP will bind to surfaces such as plaque, bacteria, soft tissue and dentin (owing to its sticky nature), providing a reservoir of bioavailable calcium and phosphate in the saliva and on the surface of the tooth. The ACP is released from the CPP complex during oral acidic challenges. The acid could be from plaque bacteria. Under these conditions, the CPP-bound ACP would buffer plaque pH, and, in so doing, would dissociate to calcium and phosphate ions. This would counteract a drop in pH thus preventing enamel demineralization. The increased calcium phosphate in the plaque buffers free calcium and phosphate ion activities and maintains a state of super-saturation of the ions in close approximation with the tooth.
MI paste is the first product for professional use that contains RECALDENT™ (CPP-ACP). MI paste is water-based sugar-free flavored crème that is applied to the tooth surface or oral cavity. The flavoring helps to stimulate salivary flow to enhance the effectiveness of the RECALDENT™ CPP-ACP. The longer the paste is maintained in the mouth, the more effective is the result. MI paste has substantial buffering capabilities, giving a sustained release of calcium phosphate ions for over 3 h. Initial clinical trials in patients with severe xerostomia have also revealed positive results in terms of caries prevention and mouth moistening as reported by Hay et al.[4]
Rahiotis et al.[5] conducted an in vitro study on sound human dentine and observed that the presence of CPP-ACP on dentin surfaces provoked lower demineralization in comparison with the dentin surfaces without agent. Similar results were observed by Yamaguchi et al.[6] Mazzaoui et al.[7] incorporated CPP-ACP into glass ionomer cement (GIC) and showed a significant increase in microtensile bond strength (33%), compressive strength (23%) and release of calcium, phosphate and fluoride ions at neutral pH. Cai et al.[8] demonstrated that CPP-ACP containing rinsing solutions produced higher remineralization than the controls. Walsh et al.[9] reported that the combination of CPP-ACP and PAD proved to be very effective for stabilizing root surface caries in the clinical practice. Tantbirojn et al.[10] conducted an in vitro study in which cola-softened enamel surfaces were used. Treatment with CPP-ACP rehardened the surfaces significantly as compared with fluoride-treated surfaces.
This study evaluated the effects of MI paste containing CPP-ACP and fluoridated toothpaste in reducing decalcification and promoting remineralization in vitro. The design of the current study combined cycling of demineralization and remineralization to simulate clinical conditions.
In this study, the confocal laser scanning microscope has been used to view the lesion depth of the various samples. The lesion depth corresponds to the depth of demineralization. This type of light microscope can be considered as being midway between optical and electron microscopy. Surface images of samples can be produced that are similar in character to those of the scanning electron microscope (SEM).[11] The improved resolution and removal of out-of-focus blur allows much more information to be gained from fluorescence microscopy techniques, with the images capable of 3-D reconstruction of the sample.
In this study, the enamel surfaces treated with CPP-ACP paste showed a significant reduction in lesion depth as compared with the untreated enamel surfaces and enamel surfaces treated with fluoridated tooth paste. In comparison with the untreated enamel surfaces, the reduction in lesion depth of the CPP-ACP-treated enamel surfaces was 65.66%; and in comparison with the enamel surfaces treated with fluoridated toothpaste, the lesion depth reduction in the CPP-ACP-treated group was 20.22%. These results are in accordance with other studies that have shown that CPP-ACP-containing products prevent demineralization and promote remineralization. In an in vitro study by Reynolds et al.,[12] CPP-stabilized calcium phosphate solutions were shown to remineralize subsurface lesions in human 3rd molar enamel. Iijima et al.[13] conducted a study in which enamel pieces worn in the mouth were subjected to treatment with CPP-ACP-containing chewing gum and then exposed to the effects of acid for 8 h and 16 h. It was found that treatment with CPP-ACP chewing gum resulted in significant remineralization compared with the control. Similar results were observed by Shen et al.[14] and Itthagarun et al.[15] Hicks et al. evaluated the effects of sodium fluoride and CPP-ACP on enamel caries formation and observed that a thrice-daily application of sodium fluoride resulted in a 40% reduction in lesion depth and CPP-ACP paste resulted in a 59% reduction in lesion depth.
Fluoride requires a good source of calcium and phosphate for remineralization of tooth enamel with the more acid-resistant fluorapatite; RECALDENT™ (CPP-ACP) provides this in an amorphous soluble form.
In a study conducted by Jayarajan et al.,[16] the remineralizing ability of CPP-ACP and CPP-ACPF were analyzed using DIAGNOdent® (KaVo) and a SEM. Groups treated with CPP-ACP and CPP-ACPF showed significantly higher amounts of remineralization than the untreated group. However, because of the added benefit of fluoride (NaF 0.2%), CPP-ACPF showed marginally more amount of remineralization than CPP-ACP.
The results of this study, in conjunction with the results of the above-mentioned studies, suggest that CPP stabilizes and localizes ACP at the tooth surface. It maintains a supersaturation level of the calcium and phosphate ions in close proximity to the tooth surface. It thereby buffers plaque pH, depresses enamel demineralization and enhances remineralization.
CONCLUSION
Within the limitations of the study, it can be concluded that enamel surfaces treated with the CPP-ACP paste exhibited the least lesion depths, followed by the enamel surfaces treated with the fluoridated tooth paste and control group, respectively. Thus, it can be said that although the fluoridated toothpaste offered some protective potential, samples treated with CPP-ACP paste were best able to resist the acidic challenge. CPP-ACP is a valuable new product to complement the usual toothpaste. It is a highly effective means of practising active caries prevention in patients susceptible to dental caries.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Zero DT. Dentifrices, mouthwashes, and remineralization/caries arrestment strategies. BMC Oral Health. 2006;6:S9. doi: 10.1186/1472-6831-6-S1-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts AJ. Role of models in assessing new agents for caries prevention – Non-fluoride systems. Adv Dent Res. 1995;9:304–11. doi: 10.1177/08959374950090031601. [DOI] [PubMed] [Google Scholar]
- 3.Hicks MJ, Flaitz CM. Enamel caries formation and lesion progression with a fluoride dentifrice and a calcium-phosphate containing fluoride dentifrice: A polarized light microscopic study. ASDC J Dent Child. 2000;67:21–8. [PubMed] [Google Scholar]
- 4.Hay KD, Thomson WM. A clinical trial of the anticaries efficacy of casein derivatives complexed with calcium phosphate in patients with salivary gland dysfunction. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:271–5. doi: 10.1067/moe.2002.120521. [DOI] [PubMed] [Google Scholar]
- 5.Rahiotis C, Vougiouklakis G. Effect of a CPP-ACP agent on the demineralization and remineralization of dentine in vitro. J Dent. 2007;35:695–8. doi: 10.1016/j.jdent.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Yamaguchi K, Miyazaki M, Takamizawa T, Inage H, Kurokawa H. Ultrasonic determination of the effect of casein phosphopeptide-amorphous calcium phosphate paste on the demineralization of bovine dentin. Caries Res. 2007;41:204–7. doi: 10.1159/000099319. [DOI] [PubMed] [Google Scholar]
- 7.Mazzaoui SA, Burrow MF, Tyas MJ, Dashper SG, Eakins D, Reynolds EC. Incorporation of casein phosphopeptide-amorphous calcium phosphate into a glass-ionomer cement. J Dent Res. 2003;82:914–8. doi: 10.1177/154405910308201113. [DOI] [PubMed] [Google Scholar]
- 8.Cai F, Shen P, Morgan MV, Reynolds EC. Remineralization of enamel subsurface lesions in situ by sugar-free lozenges containing casein phosphopeptide-amorphous calcium phosphate. Aust Dent J. 2003;48:240–3. doi: 10.1111/j.1834-7819.2003.tb00037.x. [DOI] [PubMed] [Google Scholar]
- 9.Vlacic J, Meyers IA, Walsh LJ. Combined CPP-ACP and photoactivated disinfection (PAD) therapy in arresting root surface caries: A case report. Br Dent J. 2007;203:457–9. doi: 10.1038/bdj.2007.947. [DOI] [PubMed] [Google Scholar]
- 10.Tantbirojn D, Huang A, Ericson MD, Poolthong S. Change in surface hardness of enamel by a cola drink and a CPP-ACP paste. J Dent. 2008;36:74–9. doi: 10.1016/j.jdent.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Watson TF. Applications of confocal scanning optical microscopy to dentistry. Br Dent J. 1991;171:287–91. doi: 10.1038/sj.bdj.4807695. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds EC. Remineralization of enamel subsurface lesions by casein phosphopeptide-stabilized calcium phosphate solutions. J Dent Res. 1997;76:1587–95. doi: 10.1177/00220345970760091101. [DOI] [PubMed] [Google Scholar]
- 13.Iijima Y, Cai F, Shen P, Walker G, Reynolds C, Reynolds EC. Acid resistance of enamel subsurface lesions remineralized by a sugar-free chewing gum containing casein phosphopeptide-amorphous calcium phosphate. Caries Res. 2004;38:551–6. doi: 10.1159/000080585. [DOI] [PubMed] [Google Scholar]
- 14.Shen P, Cai F, Nowicki A, Vincent J, Reynolds EC. Remineralization of enamel subsurface lesions by sugar-free chewing gum containing casein phosphopeptide-amorphous calcium phosphate. J Dent Res. 2001;80:2066–70. doi: 10.1177/00220345010800120801. [DOI] [PubMed] [Google Scholar]
- 15.Itthagarun A, King NM, Yiu C, Dawes C. The effect of chewing gums containing calcium phosphates on the remineralization of artificial caries-like lesions in situ. Caries Res. 2005;39:251–4. doi: 10.1159/000084806. [DOI] [PubMed] [Google Scholar]
- 16.Jayarajan J, Janardhanam P, Jayakumar P, Deepika Efficacy of CPP-ACP and CPP-ACPF on enamel remineralization-An in vitro study using scanning electron microscope and DIAGNOdent. Indian J Dent Res. 2011;22:77–82. doi: 10.4103/0970-9290.80001. [DOI] [PubMed] [Google Scholar]


