Abstract
Background:
Polycystic ovary syndrome (PCOS) is common diagnosis in women presenting with infertility. All the dimensions of PCOS have not been completely explored. Many studies have tried to characterize the exact presentation of the disease. In this study we studied clinical features of PCOS in Indian women to characterize different phenotypes of this syndrome. Prevalence of acanthosis nigricans (AN) as surrogate marker of insulin resistance, obesity, hirsutism and hypothyroidism in PCOS women have been simultaneously studied.
Materials and Methods:
Present work is a non comparative cross-sectional open label study carried out over a period of 18 months in an endocrinology hospital in western Maharashtra, India.
Results and Conclusion:
Authors conclude that PCOS occurs both in obese and non-obese women; AN and hirsutism occur in equal proportion of patients. AN is correlated with obesity. Hormonal dysfunctions in PCOS manifested together or independently. PCOS women can be sub grouped based on clinical features suggestive of endocrinological malfunctions and can be investigated accordingly for selection of appropriate treatment modalities.
Keywords: Acanthosis nigricans, hirsutism, Indian women, polycystic ovary syndrome
INTRODUCTION
Polycystic Ovary Syndrome is one of the most common endocrinopathy affecting women. In 1935 Irving F. Stein and Michael L. Leventhal described a symptom complex due to anovulation.[1] Oligomenorrhea, hirsutism and obesity together with enlarged polycystic ovary (PCO) were the diagnostic criteria of PCOS. It is now accepted that this problem is arising from persistent anovulation with a spectrum of etiologies and clinical manifestations.
There is no universally accepted definition of PCOS. It is a complex clinical presentation and is traditionally thought of as a triad of oligomenorrhea, hirsutism and obesity, and is now recognized as a heterogeneous disorder that results in overproduction of androgens, primarily from the ovary, and is associated with insulin resistance. The first recognition of an association between glucose intolerance and hyperandrogenism (HA) was the famous report of the bearded diabetic woman by Archard and Thiers in 1921.[2]
PCOS may present with amenorrhea, infertility, features of hyperandrogenemia (HA), signs of metabolic disturbances like insulin resistance, and dyslipidemia. The apparent underlying reason is persistent anovulation over a prolonged period. Different endocrinopathies can lead to anovulation and the subsequent emergence of polycystic ovaries. Thus PCO can be considered as a functional derangement. The clinical picture and ovarian status seen in PCOS can reflect any of the dysfunctional states. As these women are vulnerable to type II diabetes, dyslipidemia, premature arteriosclerosis, and endometrial carcinoma, treatment of PCOS should also aim to search these abnormalities. Treatment of these concurrent abnormalities in individual PCOS woman will result in a better outcome. It may correct the signs and symptoms and also prevent anticipated and or unanticipated future adverse outcomes. Infertility as a result of anovulation is a complication of PCOS. PCOS women may present with a complaint of failure to conceive. PCOS can result in primary or secondary infertility.
The exact prevalence of PCOS is not known as the syndrome is not defined precisely. The estimated prevalence in women of reproductive age is 5-10%. Under the new criteria (Rotterdam-2003), the prevalence among the general female population will raise up to 10%.[3] Nidhi, et al. prospectively studied 460 girls aged 15-18 years from a residential college in Andhra Pradesh, South India. The authors have reported a prevalence of PCOS in 9.13% of the Indian adolescents.[4] Consideration of a one definitive endocrine or clinical criterion for the diagnosis of the PCOS may result in biased selection of patients focusing on an isolated segment of a wide clinical spectrum. This can influence the incidence and prevalence of PCOS, thereby masking the gravity of the problem.
Studies highlighting the impact of ethnicity in PCOS have considered the metabolic aspects of the syndrome, including insulin resistance, glucose intolerance, lipid abnormalities, and coronary artery diseases. Williamson, et al. reported that PCOS women of different ethnicity presented with different clinical manifestation of PCOS.[5] Studies conducted on Indian PCOS women suggested that abnormalities of the insulin receptor are more common in Indian women with PCOS compared to white women with PCOS.[6]
In the present study, we are reporting clinical presentation in Indian PCOS women of Western Maharashtra.
MATERIALS AND METHODS
Study design
This was a non-comparative cross-sectional open label study carried out over the period of 18 months. A total of 120 newly diagnosed post-pubertal PCOS patients consulting endocrinology hospital were studied. Patients complaining of irregular menses and /or infertility were enrolled as per inclusion and exclusion criteria after taking written informed consent. Presence of at least two criteria from clinical, hormonal, and abdominal USG category were considered diagnostic of PCOS. Women with complain of irregular menses or oligomenorrhea (absence of menses for 35-182 days) or amenorrhea (absence of menses for > 182 days), signs or symptoms of HA, abdominal USG showing at least 12 follicles (2-9 mm in diameter) arranged peripherally around a dense core of ovarian stroma or scattered throughout an increased amount of stroma were enrolled in the study. Patients having any major systemic illness, congenital adrenal hyperplasia, hyperprolactinemia, acromegaly, functional hypothalamic amenorrhea, and patients receiving drugs for any other systemic illness (except hypothyroidism) were excluded. The study was approved by Institutional Ethic committee. All the guidelines of Helsinki were followed.
Detailed menstrual history, marital status, and parity were recorded. In patients complaining of amenorrhea, pregnancy was ruled out whenever necessary.
Cut-off body mass index (BMI) with body fat as Standard Consensus Statement for Indian population was considered, i.e., Normal BMI: 18.0-22.9 kg/m2 , Overweight: 23.0-24.9 kg/m2 Obesity: >25 kg/m2 BMI ≥ 25 was considered as obese.[7]
Hirsutism was scored according to modified Ferriman Gallaway score.[8] Grading of severity based on the score was assessed as <4 - mild, 4-7 - moderate, ≥8 - severe. Serum dehydroepiandrosterone (DHEA) and testosterone levels were measured in patients showing any sign of HA.
Data were analyzed using Fisher's exact test. P value < 0.05 was considered significant.
RESULTS
Out of 120 patients studied, 47 were married and 73 were unmarried.
Seventy-five women were obese, 15 women were overweight, and 25 women had normal BMI and 5 women had less than normal BMI.
Two women had subclinical hypothyroidism (one obese, one overweight). Sixteen women had hypothyroidism and were receiving treatment for the same.
Serum DHEA level was tested in 70 subjects and its level was found to be elevated in 22 subjects (maximum level 995 μg/dl), whereas testosterone levels were normal or low (tested in 50 subjects).
DISCUSSION
The precise triggering factor(s) and the chronology of events which lead to PCOS remain less well-known. Researchers think that PCOS is a maladaptation of the evolutionary phenomenon that is adrearche. During pubertal development, adolescents typically have relative androgenemia, insulin resistance, cystic ovaries and anovulatory cycles, which transits to an estrogenic state later in puberty. Failure to make this transition may result in PCOS secondary to abnormal pubertal development.[9]
In 1935, Stein and Leventhal described seven women presenting with oligo/amenorrhea combined with the presence of bilateral polycystic ovaries established during surgery. Three of these seven patients also presented with obesity, whereas five showed signs of hirsutism. Only one woman was both obese and showed hirsutism.[1] These findings imply that in case polycystic ovary (PCO) is diagnosed by morphology in women with oligo/anovulation, not all the features which are believed to be associated with PCOS need to be present. Likewise, with the use of transvaginal ultrasonography, it has become evident that women with oligo/amenorrhea, obesity and hirsutism do not all have the typical PCO morphology. The occurrence of considerable heterogeneity in clinical symptoms and endocrine features associated with PCOS implies that some women with PCO on ultrasound scan may even exhibit none of the other features of PCOS. In the present study, a diverse presentation of PCOS was observed.
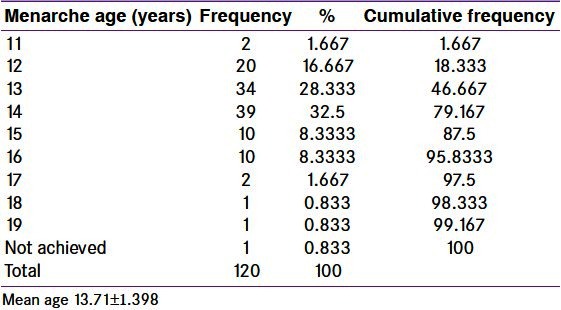
In the present study, young PCOS patients were studied. The mean age was 22.05 ± 4.649 [Table 1]. The mean age of menarche was 13.71 ± 1.398 [Table 2].
Table 1.
Frequency distribution of age in polycystic ovary syndrome women
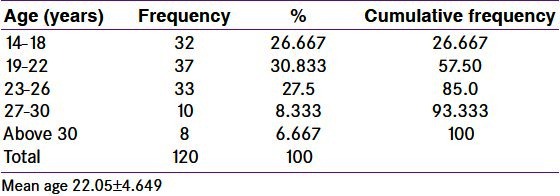
Table 2.
Age of menarche in polycystic ovary syndrome women
Anovulation and infertility
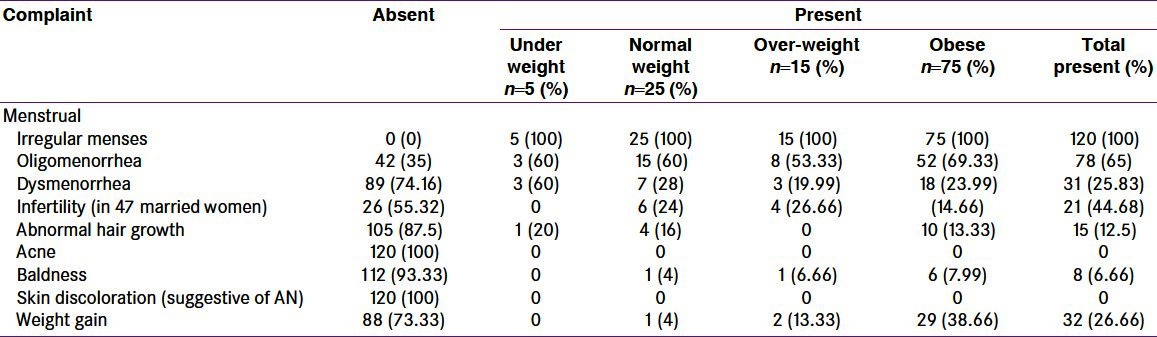
The complexity and diversity of both underlying pathology as well as clinical manifestations renders difficulty in defining better predictors of PCOS. Normal ovulation results in regular menstrual cycles (28-35 days cycle). Anovulation is the pathognomic feature of PCOS and results in irregular menstrual cycles. Therefore, persistent menstrual irregularities (resulting from anovulation) seem to be better predictors compared to biochemical parameters. Oligomenorrhea is one of the diagnostic criteria of PCOS. Documentation of anovulation is usually not necessary in view of menstrual irregularities with periods of amenorrhea. In the present study all patients complained of irregular menses. Oligomenorrheoa was present in 65% patients [Table 3]. Though more number of obese patients had oligomenorrheoa, the difference between obese and non-obese was not significant. Oligomenorrheoa is considered as a highly predictive surrogate marker of PCOS. Nurses’ Health study II reported that over an 8-year period, the conversion rate to type 2 diabetes among oligomenorrheic women was approximately two-fold greater than that for eumenorrheic women, regardless of whether the oligomenorrheic women were obese or lean, indicating that oligomenorrhea was an independent predictor of type 2 diabetes.[10] On the background of this association the higher percentage of oligomenorrhea in the present study warrants a serious thought toward this menstrual problem. Majumdar and Singh have compared the clinical features of PCOS in Indian women. The authors have reported prevalence of menstrual irregularities as 79.2% vs. 44% in obese vs. non-obese women.[11] In the present study, we are reporting the prevalence of oligomenorrhea as 66.67% in obese and 60% in non-obese women. In the present study, 44.68% married PCOS women complained of infertility [Table 3]. Out of these 21 women, 6 women were hypothyroid. In the remaining euthyroid women, 6 were obese, 3 were overweight, and 6 non-obese. There is no significant difference between obese PCOS and non-obese PCOS women as far as infertility is concerned. Though the number of study subjects is low, we suggest that PCOS patients, irrespective of weight, are at risk of infertility. Pfeifer SM have also mentioned infertility as one of the long term sequellae of PCOS.[12]
Table 3.
Presenting complaints in polycystic ovary syndrome women
Obesity
Obesity is common in women who have PCOS. Presence of obesity is a risk factor to amplify the consequences of PCOS. It increases the risk for metabolic dysfunction. Insulin resistance is worsened by presence of obesity. The prevalence of obesity in these women varies; many patients of PCOS have a normal BMI. Obesity is not essential to make the diagnosis of PCOS.[13] The results of present study show that PCOS was present in both obese and non-obese subjects. Mean BMI was 27.32 ± 6 [Table 4].
Table 4.
Body mass index in polycystic ovary syndrome women
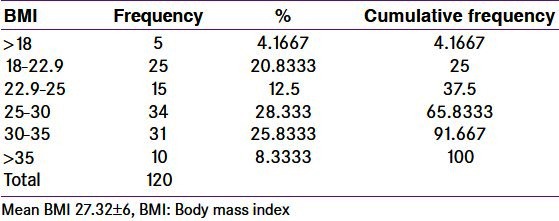
The number of obese and overweight women was more (75%) [Table 4], the ratio of obese to non-obese was 3:1. 16 women also had hypothyroidism (14 obese and 2 non-obese). Subclinical hypothyroidism was present in one obese and one overweight (BMI 24.39) patient. On excluding women with thyroid dysfunction, the percentage of obese women with PCOS dropped to 72.5 and the ratio of euthyroid obese PCOS women to euthyroid non-obese PCOS decreased to 2.26:1. Our result is different from that of the previous study conducted by Kalra, et al. in which the percentage of obese, overweight and normal BMI in Indian POCS women (n = 65) based on ACOG criteria was 15.38%, 44.61%, and 40%, respectively.[13] The discrepancy may be because of the cut-off BMI. Considering obese and overweight women together in their study percentage of obese women is 60. We have used BMI cut-off as suggested by Consensus statement for Asian Indian.[7] Asian Indians have higher percentage body fat, abdominal adiposity at lower or similar BMI levels as compared to white Caucasians. Asian Indians are more predisposed to develop insulin resistance and cardiovascular risk factors at lower levels of BMI as compared to other ethnic groups.[14] Excess clustering of cardiovascular risk factors could be attributed to a large extent by differences in body composition of Asian Indians vis-a-vis white Caucasians. Asian Indians have higher percentage body fat, abdominal adiposity at lower or similar BMI levels as compared to white Caucasians. Studies indicate that the cut-off BMI corresponding to the cut-off of percentage body fat is lower for Asian Indians from various parts of India. As obesity in growing children is a major health problem, more PCOS adolescent girls are put at risk of increase in the severity of the symptoms and health hazards. In fact, with the rising prevalence of childhood obesity, adolescent girls who may have never displayed symptoms of PCOS, with the exception of irregular cycles may now experience symptoms of anovulation and androgen excess.
It is also a possibility that obese women, because of obesity become more health conscious, and/or have co-morbidities and seek medical advice as compared to non-obese women. The present study was conducted in an endocrinology hospital. This can influence the selection of patients; more number of patients having other endocrinological diseases like hypothyroidism or presence of obesity may be selected as compared to community-based studies. In spite of this the percentage of non-obese women in the present study is considerable. Therefore a large community-based study is required to assess the prevalence of PCOS in non-obese women. We suggest that though obese women are at a higher risk of PCOS, body weight is not a limiting factor for the occurrence of PCOS. Though not significant, the average age in obese (23 year) and non-obese (20 year) was different. Increase in weight as the age advances is a natural phenomenon, nonetheless young PCOS should be categorically advised to have or maintain a normal BMI. Obesity exacerbates the PCOS phenotype in previously asymptomatic individuals.[15] Weight reduction in adult women has been shown to improve free androgen levels, insulin sensitivity and ovulatory function.[16] Differentiating subgroups based on BMI in PCOS will help in selecting the modalities of treatment including weight reduction as mentioned above.
Acanthosis nigricans
In the present study, 44.16% patients showed presence of AN, a surrogate marker of insulin resistance [Table 5]. Over last two and half decades attention has been focused on the association of insulin resistance with PCOS. AN is a velvety, mossy, hyper pigmented skin disorder. Presence of AN appears to be more a sign of insulin resistance or medication reaction than distinct disease itself. Other pathological conditions rarely associated with AN are insulinoma and malignant disease, especially adenocarcinoma of the stomach. Usually dorsal surface of the neck and intertriginous areas, such as the upper thigh and axilla are involved. Acanthosis is more common in obese PCOS patients. Hyperkeratosis and papillomatosis are the histological characteristics of AN. AN is a frequent occurrence in HA and diabetes mellitus.[17] In 1976, Kahn and colleagues found out association of HA, insulin resistance and AN-a distinct disorder in adolescent girls called as TYPE A syndrome-(mutation of insulin receptor) with the features of virilization, increased muscle bulk, clitomegaly, temporal balding, deepening of voice and insulin resistance with striking AN and Type B syndrome - extreme insulin resistance, AN in post menopausal women with autoimmune diseases thought to be caused by endogenous anti-insulin antibodies.[18] The presence of AN in hyper androgenic women depends on the presence and severity of hyperinsulinemia. It correlates with the magnitude of peripheral insulin resistance and less well with the hyperinsulinemia measured by a glucose tolerance test. The mechanism responsible for the development of AN is uncertain. Conflicting studies suggest mediation through various growth factor receptors, not just insulin or insulin-like growth factor.
Table 5.
Clinical findings in polycystic ovary syndrome women
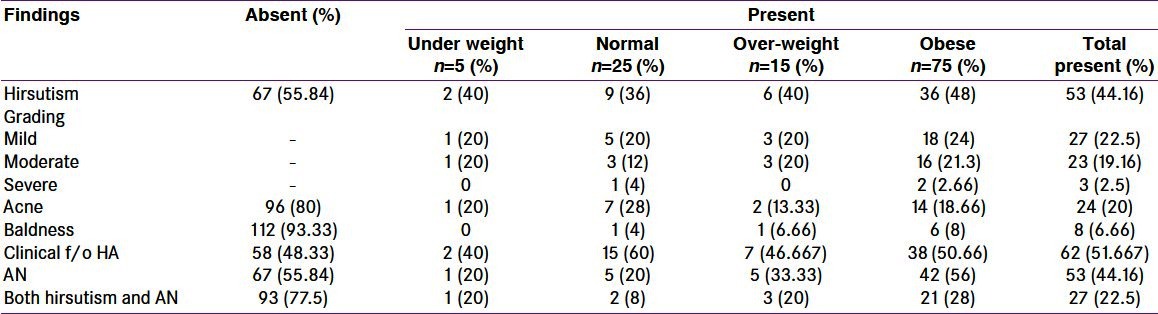
In the present study, 52.22% obese women vs. 20% non-obese had AN. AN positively correlated with obesity [Table 6]. Not all obese patients had AN. This suggests that either insulin resistance is not present in all obese PCOS patients or development of acanthosis in insulin resistant obese PCOS patients requires presence of some co factor. It is also possible that some patients are genetically predisposed to this dermal manifestation whereas a preventing factor is present in others. The consequences of metabolic syndrome are accentuated in PCOS. We have defined obesity on the basis of a comparatively low cut-off BMI. A positive correlation between AN and obesity considering the cut-off is suggestive of higher risk of insulin resistance at a comparatively lower range of BMI in Indian PCOS women. In the previous studies, it has been reported that insulin resistance is more common in Indian women.[14] The higher percentage of PCOS women having AN, a surrogate marker of insulin resistance in our study as compared to percentage of PCOS women with impaired glucose tolerance may be because of less number of study subjects. Dunaif, et al. found that women with PCOS are profoundly insulin resistant and are at an increased risk of development of NIDDM. Insulin resistance in these women occurs at an early age (3rd-4th decade) than it does in the general population.[19]
Table 6.
Acanthosis nigricans in non-obese vs. obese polycystic ovary syndrome women
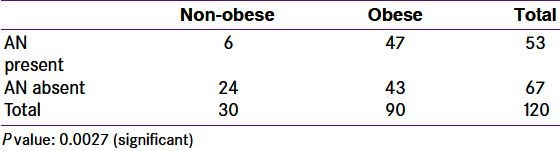
Consideration for AN as a measure of insulin resistance in the present study was attempted because though it is appropriate to investigate fasting glucose and insulin levels, this is not always practical in India. An easier and acceptable marker of insulin resistance could be welcome. Given the fact that none of the study subject noticed or complained of skin discoloration herself, there is a scope to educate women about the importance of AN, its association with insulin resistance and its presence in PCOS. Many studies in Indian PCOS women have discussed association of insulin resistance but not of AN.
Hirsutism, acne and baldness
PCOS women may present with a complaint of male pattern hair growth. Hirsutism is a common disorder resulting from androgen activity specified in women as excessive growth of terminal hair in androgen-dependent regions of the body. The cause of hirsutism in most women is PCOS. Androgens have been shown to be at least partly responsible for promoting the anagen phase (growth phase) of hair cycle, leading to larger hair follicles (and bringing about a change from vellus to terminal hair status. The anagen phase is influenced by insulin-like growth factor (IGF-I). IGF-I is carried in the circulation by IGFBPs (IGF binding protein). The activity of this growth factor depends on a number of factors including binding proteins, which in turn, are also influenced by the actions of insulin. Thus women with PCOS may demonstrate abnormalities in the metabolism of both of the major factors responsible for hirsutism: Androgens and insulin/growth factors. The method of visually assessing the amount of facial and body terminal hairs (0.5 mm in length) most readily used today is a modification of the system originally proposed by FG (mFG). In most populations, with the exception of Mongoloid races, an mFG score of ≥ 6-8 signifies hirsutism. However, a ‘normal’ amount of hair may be an mFG of <2-3, and a significant proportion of women with mFG scores of 2-6 have androgen excess, primarily PCOS, regardless of ethnicity. Although there are a number of objective methods available for the measurement of hair growth, density, and diameter, these methods are not suitable for routine clinical practice mainly due to their complexity and cost.[20] HA commonly manifests itself as hirsutism (60-83%), acne (11-43%).[21] Hirsutism is not only associated with increased androgen levels but also with sensitivity of the hair follicles to it. Ethnicity, sensitivity of the hair follicles, and levels of androgens can all cause variations in the extent of the hirsutism seen in PCOS women. We have followed the lower range of score (2 to 6, as per mFG score) to diagnose hirsutismand this scoring can be justified because women from the study area are overall less hairy. Hirsutism in the study population was diagnosed by an experienced endocrinologist from the local area.
In the present study, 12.5% patients reported abnormal hair growth [Table 3]; clinically 44.16% women had hirsutism [Table 5]. Few patients had severe hirsutism (two obese and one normal weight). Though DHEA levels were elevated in most subjects, but they were not high enough to suggest adrenal pathology. Moreover, serum testosterone levels were either normal or low in most patients (except one with serum levels 13.37 ng/ml), ruling out ovarian neoplasms.
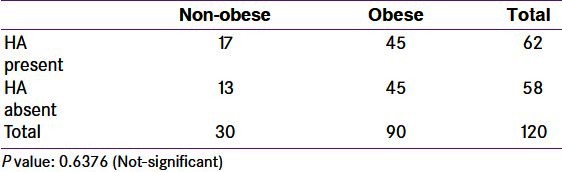
Though more obese women had hirsutism, there was no correlation between hirsutism and obesity [Table 7]. Acne (20%) and baldness (6.66%) were not common. In some women, acne and hirsutism were present independently. The percentage of HA is 51.67, considering hirsutism and acne together [Table 5]. The prevalence of HA in Indian PCOS women is reported to be 74.2% vs. 50.6% in obese and non-obese subjects, respectively.[11] In the present study we found the prevalence of HA as 66.67% vs. 60% in obese and non-obese subjects, respectively.
Table 7.
Clinical features of hyper-androgenism in non-obese vs. obese polycystic ovary syndrome women
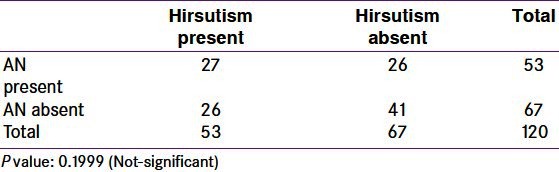
Pathophysiology of PCOS involves abnormalities in insulin function (insulin resistance) as well as abnormal androgen function (HA). Both dysfunctions may be interlinked. Insulin resistance and hyperinsulinemia may increase androgen levels by decreasing sex hormone binding globulin (SHGB). Based on this, AN and hirsutism can be expected to be present together in majority of the PCOS women. In the present study, prevalence of AN and hirsutism is similar (44.16%). Correlation between these two was not significant [Table 8]. 27 patients (22.5%) had both acanthosis and hirsutism. Out of these 21 patients were obese. This indicates that obesity accentuates interlink between hyperinsulinemia and HA. It is also possible that, in addition to hyperinsulinemia, IGF-1 is involved in these patients. Acne, another feature of increased androgenic activity, increases the proportion of hyper androgenic women over women with AN. This is either because insulin resistance may be a lesser problem than hyperandrogenicity, or development of marker of insulin resistance is a delayed process as compared with those of hyperandrogenicity and/or may need involvement of other factors. The former explanation has some reservations as we have not labeled insulin resistance based on glucose tolerance test or HOMA index. We suggest that these patients should be treated as a subgroup of PCOS and treatment modalities should be tailored accordingly.
Table 8.
Correlation of acanthosis nigricans and hirsutism in polycystic ovary syndrome women
Thyroid dysfunction
In the present study, thyroid dysfunction was present in 15% of the patients. Our result is in accordance with that of Ozdemir, et al. The authors have studied prevalence of thyroid pathologies in patients with PCOS and reported that in 107 PCOS women, 15.9% had hypothyroidism.[22] Women with thyroid dysfunction are another subgroup of PCOS. Though measurement of thyroid hormones is usually a part of investigations of PCOS, it is not followed as a rule. In the present study, patients with clinical manifestation of thyroid dysfunction were evaluated for thyroid function test. Further studies are needed to reveal the association of thyroid dysfunction and PCOS.
PCOS can present as conglomerate of various clinical features like multiple follicles on USG, oligomenorrhea, obesity, AN and hirsutism. These findings may be present alone or in various combinations with one another. Some of the common combinations and percentage of patients under these have been depicted in Table 9. Combination of multiple follicles with oligomenorrhea was the commonest presentation. More number of obese patients had co-presence of AN and/or hirsutism. Three or more features together were a common occurrence. Multiple follicles without any other clinical presentation was also observed and the prevalence was comparable with its combination with hirsutism.
Table 9.
Prevalence of main clinical features (alone or in combination) in polycystic ovary syndrome
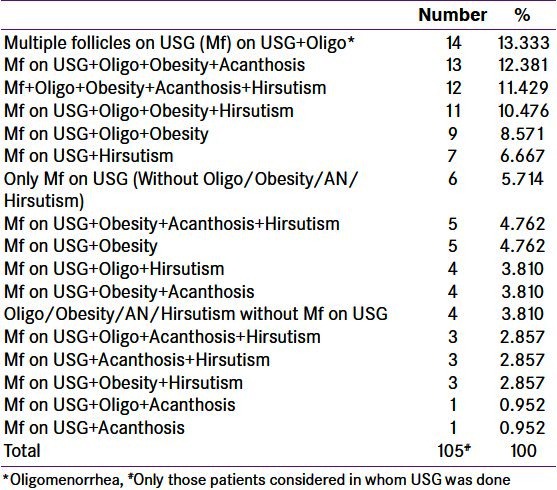
For many years, PCOS was treated as a single abnormality. As a consequence, managing this entity became less flexible. Recommendations to consider this symptom complex as a syndrome and to avoid disease-like term have been made. The results of the present study are confirmative of existence of diverse subgroups of PCOS.
CONCLUSION
PCOS, an ill defined symptom complex needs its due attention. As ethnicity plays an important role in this symptom complex, there is a greater need to know the characteristics of the same in different populations. Imbalance of different hormonal functions can affect ovarian homeostasis resulting in anovulation, which will manifest as PCOS. Women presenting with oligomenorrhea should be further investigated for PCOS and treated accordingly. Overall occurrence of a single or couple of features is less common as compared to that of multiple characters. This is suggestive of a chain of pathological and hormonal reactions. Timely therapeutic intervention can halt this ongoing process. Nevertheless, complaint related to a single feature should not be neglected. Though obesity is common in PCOS, non-obese women are also at risk of PCOS. Prevalence of AN and hirsutism in PCOS is comparable. There is a need to increase awareness regarding obesity as well as AN, as very few patients were aware of their abnormal BMI and none was aware of skin discoloration. PCOS women can be sub-grouped based on clinical features suggestive of endocrinological malfunctions and can be investigated accordingly for selection of appropriate treatment modalities.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Stein IF, Leventhal ML. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935;29:181. [Google Scholar]
- 2.Dunaif A. Insulin resistance and the polycystic ovary syndrome: Mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 3.Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Nidhi R, Padmalata V, Nagarathna R, Amritanshu R. Prevalence of polycystic ovarian syndrome in Indian adolescents. J Pediatr Adolesc Gynecol. 2011;24:223–7. doi: 10.1016/j.jpag.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Williamson K, Gunn AJ, Johnson N, Milsom SR. The impact of ethnicity on the presentation of polycystic ovarian syndrome. Aust N Z J Obstet Gynaecol. 2001;41:202–6. doi: 10.1111/j.1479-828x.2001.tb01210.x. [DOI] [PubMed] [Google Scholar]
- 6.Norman RJ, Mahabeer S, Masters S. Ethnic differences in insulin and glucose response to glucose between white and Indian women with polycystic ovary syndrome. Fertil Steril. 1995;63:58–62. doi: 10.1016/s0015-0282(16)57297-5. [DOI] [PubMed] [Google Scholar]
- 7.Misra A, Chowbey P, Makkar BM, Vikram NK, Wasir JS, Chadha D, et al. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Physicians India. 2009;57:163–70. [PubMed] [Google Scholar]
- 8.Hatch R, Rosenfield RL, Kim MH, Tredway D. Hirsutism: Implications, etiology, and management. Am J Obstet Gynecol. 1981;140:815–30. doi: 10.1016/0002-9378(81)90746-8. [DOI] [PubMed] [Google Scholar]
- 9.Nader S. Adrenarche and polycystic ovary syndrome: A tale of two hypotheses. J Pediatr Adolesc Gynecol. 2007;20:353–60. doi: 10.1016/j.jpag.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Solomon CG, Hu FB, Dunaif A, Rich-Edwards J, Willett WC, Hunter DJ, et al. Long or highly irregular menstrual cycles as a marker for risk of type 2 diabetes mellitus. JAMA. 2001;286:2421–6. doi: 10.1001/jama.286.19.2421. [DOI] [PubMed] [Google Scholar]
- 11.Majumdar A, Singh TA. Comparison of clinical features and health manifestations in lean vs. obese Indian women with polycystic ovarian syndrome. J Hum Reprod Sci. 2009;2:12–7. doi: 10.4103/0974-1208.51336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfeifer SM, Kives S. Polycystic ovary syndrome in the adolescent. Obstet Gynecol Clin North Am. 2009;36:129–52. doi: 10.1016/j.ogc.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Kalra A, Nair S, Rai L. Association of obesity and insulin resistance with dyslipidemia in Indian women with polycystic ovarian syndrome. Indian J Med Sci. 2006;60:447–53. [PubMed] [Google Scholar]
- 14.McKeigue PM, Shah B, Marmot MG. Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet. 1991;337:382–6. doi: 10.1016/0140-6736(91)91164-p. [DOI] [PubMed] [Google Scholar]
- 15.Blank SK, Helm KD, McCartney CR, Marshall JC. Polycystic ovary syndrome in adolescence. Ann N Y Acad Sci. 2008;1135:76–84. doi: 10.1196/annals.1429.005. [DOI] [PubMed] [Google Scholar]
- 16.Kiddy DS, Hamilton-Fairley D, Bush A, Short F, Anyaoku V, Reed MJ, et al. Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clin Endocrinol (OXf) 1992;36:105–11. doi: 10.1111/j.1365-2265.1992.tb02909.x. [DOI] [PubMed] [Google Scholar]
- 17.Kierland RR. Acanthosis nigricans; an analysis of data in 22 cases and a study of its frequency in necropsy material. J Invest Dermatol. 1947;9:299–305. doi: 10.1038/jid.1947.102. [DOI] [PubMed] [Google Scholar]
- 18.Kahn CR, Flier JS, Bar RS, Archer JA, Gorden P, Martin MM, et al. The syndromes of insulin resistance and acanthosis nigricans. Insulin-receptor disorders in man. N Engl J Med. 1976;294:739–45. doi: 10.1056/NEJM197604012941401. [DOI] [PubMed] [Google Scholar]
- 19.Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;38:1165–74. doi: 10.2337/diab.38.9.1165. [DOI] [PubMed] [Google Scholar]
- 20.Yildiz BO, Bolour S, Woods K, Moore A, Azziz R. Visually scoring hirsutism. Hum Reprod Update. 2010;16:51–64. doi: 10.1093/humupd/dmp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falsetti L, Gambera A, Andrico S, Sartori E. Acne and hirsutism in polycystic ovary syndrome: Clinical, endocrine-metabolic and ultrasonographic differences. Gynecol Endocrinol. 2002;16:275–84. doi: 10.1080/gye.16.4.275.284. [DOI] [PubMed] [Google Scholar]
- 22.Ozdemir D. Prevalence of thyroid pathologies in patients with polycystic ovary syndrome Presented at European Congress of Endocrinology 2011, Rotterdam, The Netherlands. Endocrine Abstracts 26 P92. [Last accessed 2012 Jul 01]. Available from: http://www.endocrine-abstractsorg/ea/0026/ea0026p92.htm .


