Abstract
McCune- Albright Syndrome (MAS) is a rare fibrosseous lesion, characterized by a classic triad of polyostotic fibrous dysplasia (PFD), café –au-lait macules (CALM) and underlying endocrinopathies. We present the oral findings of an interesting case of MAS with relevant review of literature. A 30-year-old male presented to us with swelling of both jaws over a period of two years. Cutaneous examination revealed café - au – lait macule over the back, crossing the midline. Skeletal survey showed expansile, osteolytic, mixed radiolucent- radiopaque lesions in skull and jaw bones. Serum alkaline phosphatase was elevated (388 IU/L), with normal calcium, phosphorus, parathyroid hormone and 25 hydroxy vitamin D levels. Diagnosis of McCune- Albright syndrome was made and he was treated with parenteral bisphosphonates (intravenous Zoledronate 4 mg) and is under follow up for surgical recontouring of the jaws. Early recognition facilitates better treatment and improves prognosis by reducing the morbidity.
Keywords: Albright's syndrome, fibrous dysplasia of the jaws, fibroosseous lesion, polyostotic fibrous dysplasia
INTRODUCTION
Fibroosseous lesions of the jaws are characterized by replacement of medullary bone by fibrous stroma containing newly formed mineralized tissue like woven bone or cementum. Fibroosseous lesions comprise a diverse group of lesions like fibrous dysplasia, ossifying fibromas and osseous dysplasias.[1] Fibrous dysplasia (FD) is a benign, non inheritable fibro osseous lesion, characterized by bone pain, recurrent pathologic fractures and skeletal deformities. It may affect a single bone (monostotic form), multiple bones (polyostotic form) or as a component of McCune -Albright syndrome (MAS).[2]
MAS is characterized by a classic triad of polyostotic fibrous dysplasia (PFD), café –au-lait macules (CALM) and underlying endocrinopathies.[1] We recently encountered an interesting case of MAS presenting as maxillofacial fibrous dysplasia, hence, being reported with the relevant review of literature.
CASE REPORT
A-30-year old man presented with a slow growing painless swelling involving both jaws, over a period of 2 years. He stated that the swelling was initially small, but gradually enlarged to cause spacing between teeth and noticeable facial asymmetry. This swelling was not associated with pain, paresthesia or pus discharge. History taking from his parents revealed recurrent fractures since the age of 4 years, brown pigmented patches over his trunk since birth, attainment of puberty at the age of 12 years and no similar features in other family members. There was no history of visual impairment, nasal stuffiness or hearing disability. He was a product of non consanguineous marriage, born full term with normal birth weight and milestones. Physical examination showed short stature in comparison to parents (height-142 cm), multiple deformities due to recurrent fractures, shortened right leg, limping and a large café au lait macule over the back crossing the midline. Facial examination revealed frontal bossing, asymmetry on the right side, with an enlargement of the right malar and maxillary bone and chin button deviation towards the left side [Figures 1 and 2]. He had a prognathic mandible, with lengthening of the body on the right side [Figure 3].
Figure 1.
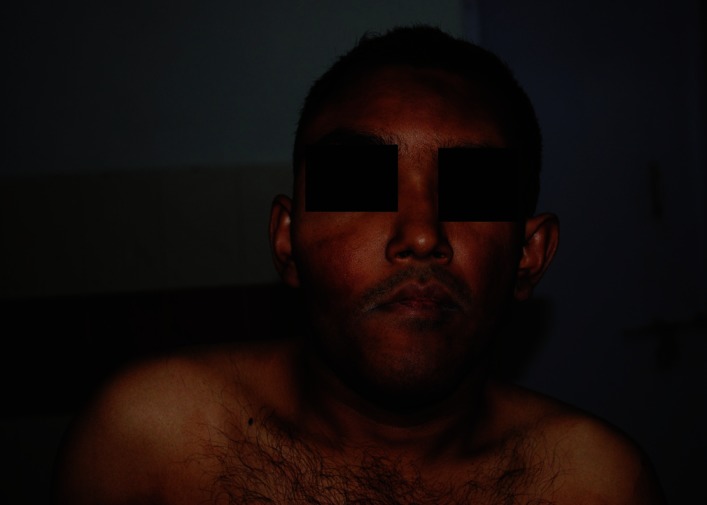
Extraoral view showing facial asymmetry
Figure 2.
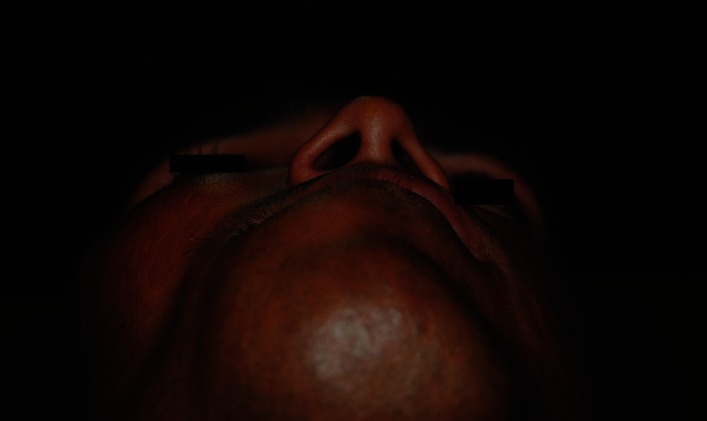
Birds eye view showing deviated chin button toward right side.
Figure 3.
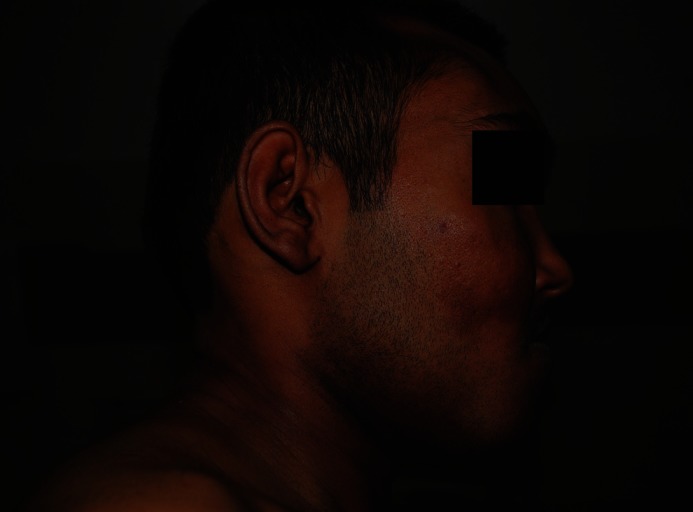
Profile view showing lengthening of the body of the mandible
Intraoral examination revealed well defined uniform expansion of the mandible up to the inferior border, extending from the third molar on the right side to the premolars on the left side. The labial and right buccal vestibules of the mandible were shallow due to the swelling. The mucosa over the swelling showed black patchy pigmentation and dilated superficial veins. The swelling was non tender and bony hard on palpation. The right maxilla was enlarged diffusely from the lateral incisor to the second molar with normal mucosa and was nontender with bony hard consistency [Figure 4].
Figure 4.
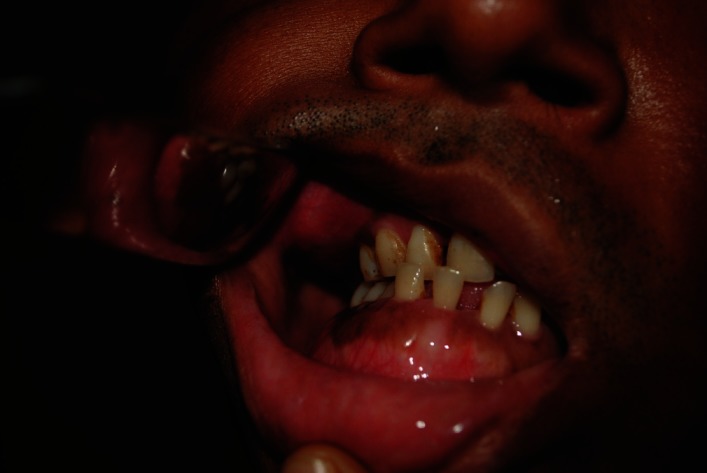
Intraoral view showing enlargement of the maxilla and the mandible
The midline of both maxillary and mandibular teeth had shifted to the left, with an anterior cross bite and spacing between maxillary and mandibular anterior teeth. Grade I mobility and labial tipping were seen in 41, 42. The overjet between the right maxillary and mandibular posterior teeth was reduced. He was diagnosed clinically as a case of MAS. Hormonal profile revealed normal thyroid, adrenal and gonadal axes evaluation. The serum alkaline phosphatase was elevated (388 IU/L), with normal calcium, phosphorus, parathyroid hormone and 25 hydroxy vitamin D levels.
Skeletal survey by paranasal sinus view of the skull revealed expansile, ill defined mixed radiopaque- radiolucent lesions in the frontal bone, ground glass radiopaque lesion in the right maxilla causing obliteration of the maxillary sinus and an expansion of the right body of the mandible [Figure 5]. The OPG showed an extensive multilocular lesion, extending 1 cm below the sigmoid notch of the ascending ramus of the right side to mesial aspect of the mandibular left first molar [Figure 6]. The lesion caused an increase in width of the right body and symphysis with a loss of normal trabecular pattern. The mandibular canal was displaced inferiorly towards an intact lower border. The right maxillary sinus was occluded and the right maxillary alveolar process in the posterior region showed orange peel pattern. Loss of lamina dura was noted for teeth located within the lesion. Expansive, osteolytic lesions were also seen in right hip and shoulder. He was treated with parenteral bisphosphonates (intravenous Zoledronate 4 mg) and is under follow up for surgical recontouring of the jaws.
Figure 5.
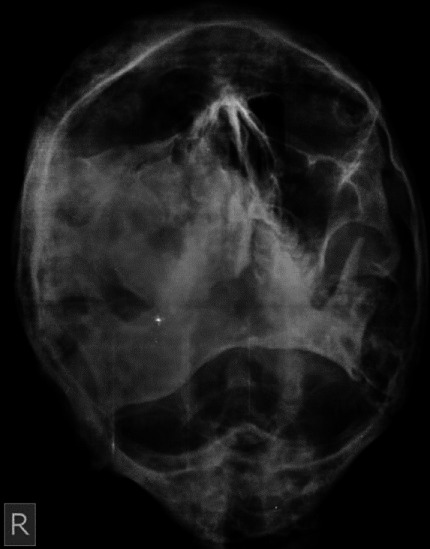
PNS view showing expansile, ill defined mixed radiopaque-radiolucent lesions in the frontal bone, ground glass radiopaque lesion in the right maxilla
Figure 6.
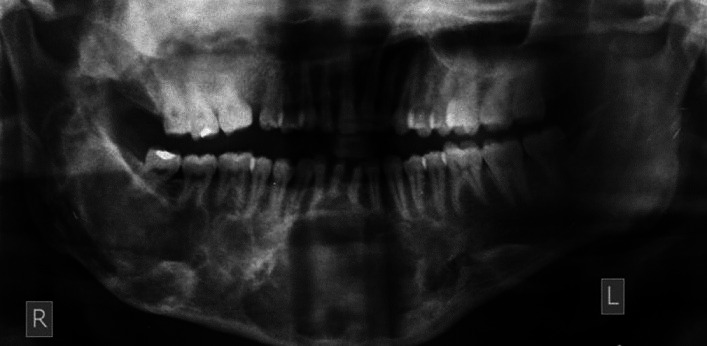
OPG showing extensive multilocular lesion of the right ascending ramus extending upto the mandibular left first molar
DISCUSSION
MAS consists of a triad of polyostotic fibrous dysplasia, CALM and hormone excess syndromes. Our patient had all components of the triad with unusual features like craniofacial dysplasia, CALM crossing midline and a possible precocity. Polyostotic fibrous dysplasia may affect 75% of the skeleton, exhibiting slow, progressive bone growth, that may result in pathological fractures and deformities of multiple bones.[3] Café – au – lait skin pigmentation is commonly seen as large hypermelanotic macules of irregular and serpiginous borders on buttocks, thorax, back, shoulder and posterior area of the neck.[3] The cutaneous and bone lesions tend to respect the midline, occurring on one side of the body.[4] The endocrinopathies include precocious puberty, hyperthyroidism, acromegaly, Cushing's syndrome and renal phosphate wasting.[4] The clinical diagnosis of MAS can be made when any two of the three above mentioned classic features are present.[5] Our patient presented with a history of recurrent pathological fractures, frontal bossing, asymmetrical painless expansion of the maxilla and mandible along with an involvement of the right shoulder, hip and right limb bones. The bone lesions were predominantly seen on the right side, but the CALM on the back had crossed the midline. Though definite history of precocity was not available in our patient, the features like short stature and attainment of puberty by 12 years of age suggests the possibility of precocious puberty.
MAS is reported with a prevalence of 1 in 10 lakh people.[4] MAS is seen in children of 1st and 2nd decades without specific gender predilection, but infants as young as 18 months also have been affected.[5] The condition is due to an embryonic postzygomatic somatic mutation in the Gsa gene, located on chromosome 20q13.2-13.3.[4,6,7] This mutation causes proliferation of the osteoprogenitor cells, leading to formation of fibrous matrix with woven bone.[7] Craniofacial fibrous dysplasia may cause ocular effects like globe displacement and loss of vision due to the involvement of sphenoid and ethmoid bones.[6] Dental findings observed in a series of fibrous dysplasia (n = 32) included malocclusion, delayed eruption, tooth displacement and tooth anomalies like tooth rotation, oligodontia and taurodontism. They occur due to direct activation in tooth mutations or indirect effect due to proximity to abnormal bone.[8] Our patient did not have ocular effects and the dental findings observed were anterior cross bite, spacing between teeth and labial tipping of 41,42.
Radiographically, the lesions might progress from a totally radiolucent multilocularity, mixed radiolucent – radiopaque stage to a totally radiopaque stage, depending upon the degree of maturation.[9] Some of the common radiologic presentations include ground glass appearance, salt and pepper, orange peel or thumb print appearance.[9,10] The lesion has ill defined borders, with an absence of a capsule or cortical border and gradually merge into the surrounding bone. The mandibular canal may be displaced in any direction depending on the epicenter of the lesion, with an upward displacement, being the most common.[9,10] Fibrous dysplasia of the maxilla may cause obliteration of the maxillary sinus and displacement of the orbit. In our case, multilocular radiolucency was seen in the mandible with loss of lamina dura. The mandibular canal was displaced inferiorly which could be explained by a superior location of the epicenter.[9] The maxillary alveolar process shows a typical orange peel and ground glass appearance, with complete obliteration of the maxillary sinus. The frontal bone shows a mixed radiolucent radiopaque lesion. Thus, all the three stages of maturation were seen in different facial bones in our patient. Computed tomography was not done in our patient, to avoid a high dose exposure to radiation which predisposes to malignancy in these patients.[11]
Bisphosphonates are used to prevent recurrent fractures and they act as antiresorptive agents. Surgical treatment of FD consists of either excision followed by reconstruction or surgical recontouring of the affected bone to improve esthetics and function.[6]
To conclude, we present a case of MAS, with unusual features like craniofacial fibrous dysplasia, CALM extending beyond the midline and possible precocity. The radiological findings showed the three stages of maturation in different facial bones of our patient. The patient was treated with bisphosphonates and is under follow up for surgical recontouring.
ACKNOWLEDGEMENT
The author would like to thank the patient for providing consent to use his photograph in this article
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Worawongvasu R, Songkampol K. Fibroosseous lesions of the jaws: An analysis of 122 cases in Thailand. J Oral Pathol Med. 2010;39:703–8. doi: 10.1111/j.1600-0714.2010.00932.x. [DOI] [PubMed] [Google Scholar]
- 2.Bhadada SK, Bhansali A, Das S, Singh R, Sen R, Agarwal A, et al. Fibrous dysplasia & McCune-Albright syndrome: An experience from a tertiary care centre in north India. Indian J Med Res. 2011;133:504–9. [PMC free article] [PubMed] [Google Scholar]
- 3.Xavier SP, Ribeiro MC, Sicchieri LG, Brentegani LG, Lacerda SA. Clinical, microscopic, and imaging findings associated to McCune-Albright syndrome: A report of two cases. Braz Dent J. 2008;19:165–70. doi: 10.1590/s0103-64402008000200014. [DOI] [PubMed] [Google Scholar]
- 4.Dumitrescu CE, Collins MT. McCune-Albright syndrome. Orphanet J Rare Dis. 2008;3:12. doi: 10.1186/1750-1172-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ravi Kumar P. Unusually early presentation of McCune- Albright syndrome in a male child with precocious puberty. IJCCI. 2011;2:4–7. [Google Scholar]
- 6.Chen YR, Chang CN, Tan YC. Craniofacial fibrous dysplasia: An update. Chang Gung Med J. 2006;29:543–9. Review. [PubMed] [Google Scholar]
- 7.Sepideh S, Ensiyeh S. McCune-Albright syndrome: A case report. Arch Iran Med. 2010;13:245–7. [PubMed] [Google Scholar]
- 8.Akintoye SO, Lee JS, Feimster T, Booher S, Brahim J, Kingman A, et al. Dental characteristics of fibrous dysplasia and McCune-Albright syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:275–82. doi: 10.1016/s1079-2104(03)00225-7. [DOI] [PubMed] [Google Scholar]
- 9.Sontakke SA, Karjodkar FR, Umarji HR. Computed tomographic features of fibrous dysplasia of maxillofacial region. Imaging Sci Dent. 2011;41:23–8. doi: 10.5624/isd.2011.41.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steven RS, Muralidhar M, Joseph R. Clinical and radiographic features of chronic monostotic fibrous dysplasia of the mandible. J Can Dent Assoc. 2004;70:548–52. [PubMed] [Google Scholar]
- 11.Ruggieri P, Sim FH, Bond JR, Unni KK. Malignancies in fibrous dysplasia. Cancer. 1994;73:1411–24. doi: 10.1002/1097-0142(19940301)73:5<1411::aid-cncr2820730516>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]


