Abstract
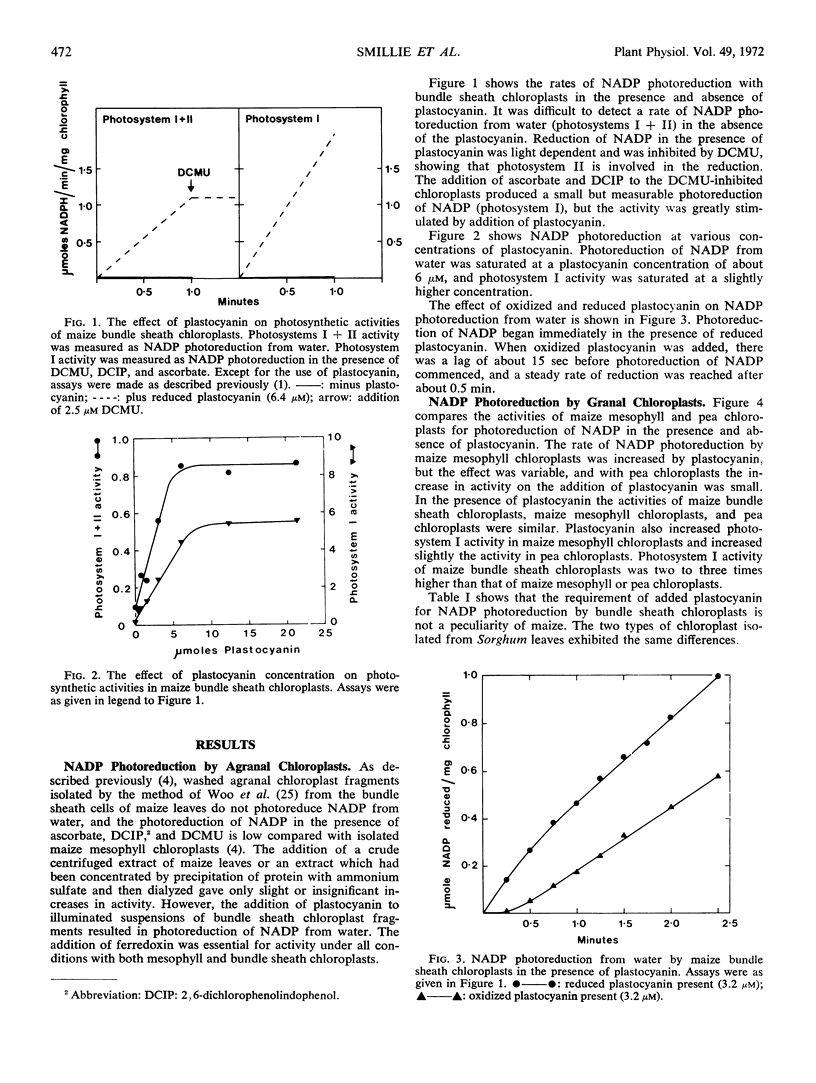
Photoreduction of NADP from water in agranal chloroplasts isolated from the leaf bundle sheath cells of Zea mays (var. DS 606A) or Sorghum bicolor (var. Texas 610) was dependent upon addition of plastocyanin as well as ferredoxin. Activity was further increased by the addition of ferredoxin NADP-reductase. Saturation for plastocyanin was reached at about 6 micromolar. In contrast, grana-containing chloroplasts isolated from leaf mesophyll cells of these plants or from pea (Pisum sativum L.) leaves did not require either plastocyanin or ferredoxin NADP-reductase for NADP photoreduction from water, although with some preparations plastocyanin stimulated the activity.
Photosystem I activity, which was low in washed preparations of bundle sheath chloroplasts, was also stimulated by plastocyanin. The effect of plastocyanin on photosystem I activity in the grana-containing chloroplasts was similar to that on NADP photoreduction from water.
In the presence of plastocyanin, the rates of NADP photoreduction from water were about the same in the agranal and granal chloroplasts, but photosystem I activity was considerably higher in bundle sheath chloroplasts. In these chloroplasts photosystem II appeared to limit the rate of NADP photoreduction.
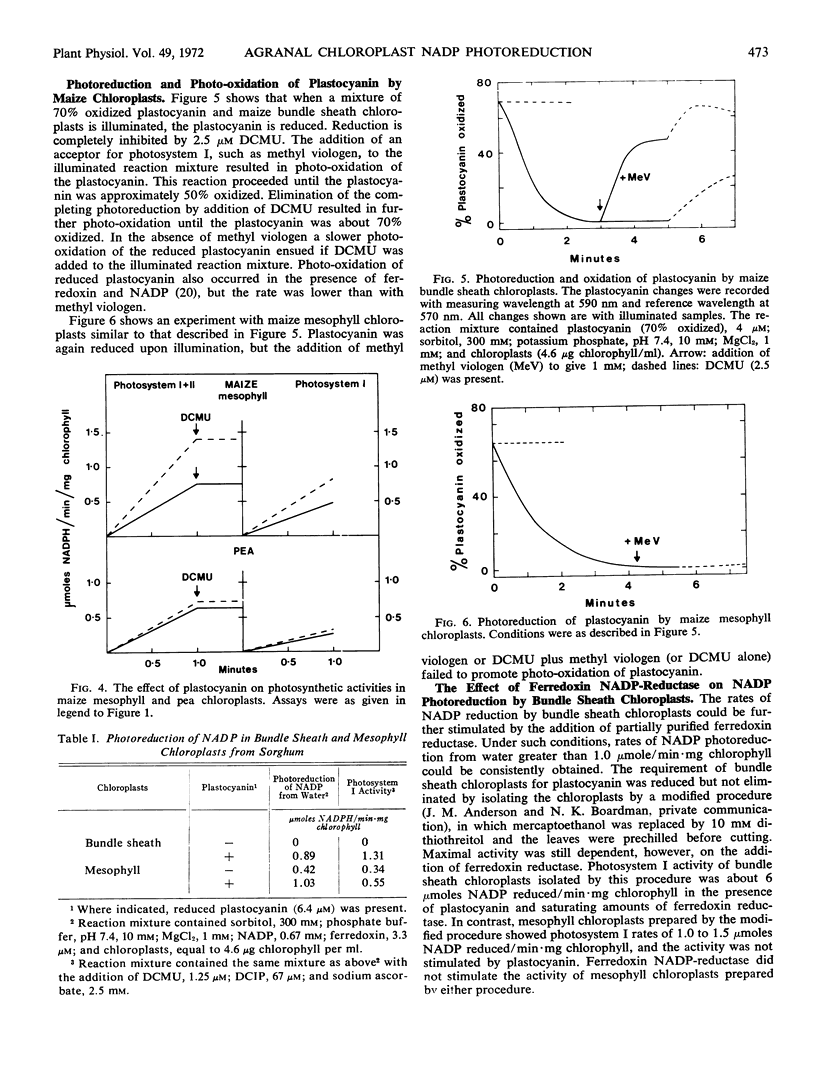
The results indicated that the agranal bundle sheath chloroplasts reduced plastocyanin via photosystem II and oxidized it via photosystem I. Both types of maize chloroplast photoreduced oxidized plastocyanin, but in the presence of methyl viologen, reduced plastocyanin was photo-oxidized only by the bundle sheath chloroplasts.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen K. S., Bain J. M., Bishop D. G., Smillie R. M. Photosystem II Activity in Agranal Bundle Sheath Chloroplasts from Zea mays. Plant Physiol. 1972 Apr;49(4):461–466. doi: 10.1104/pp.49.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I., Chain R. K., McSwain B. D., Tsujimoto H. Y., Knaff D. B. Evidence from chloroplast fragments for three photosynthetic light reactions. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1404–1409. doi: 10.1073/pnas.67.3.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. G., Andersen K. S., Smillie R. M. Incomplete membrane-bound photosynthetic electron transfer pathway in agranal chloroplasts. Biochem Biophys Res Commun. 1971 Jan 8;42(1):74–81. doi: 10.1016/0006-291x(71)90364-0. [DOI] [PubMed] [Google Scholar]
- Bishop D. G., Andersen K. S., Smillie R. M. Photoreduction and Oxidation of Cytochrome f in Bundle Sheath Cells of Maize. Plant Physiol. 1972 Apr;49(4):467–470. doi: 10.1104/pp.49.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black C. C., Mayne B. C. P(700) activity and chlorophyll content of plants with different photosynthetic carbon dioxide fixation cycles. Plant Physiol. 1970 Jun;45(6):738–741. doi: 10.1104/pp.45.6.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman N. K. The photochemical systems of photosynthesis. Adv Enzymol Relat Areas Mol Biol. 1968;30:1–79. doi: 10.1002/9780470122754.ch1. [DOI] [PubMed] [Google Scholar]
- Edwards G. E., Lee S. S., Chen T. M., Black C. C. Carboxylation reactions and photosynthesis of carbon compounds in isolated mesophyll and bundle sheath cells of Digitaria sanguinalis (L.) Scop. Biochem Biophys Res Commun. 1970 May 11;39(3):389–395. doi: 10.1016/0006-291x(70)90589-9. [DOI] [PubMed] [Google Scholar]
- Gorman D. S., Levine R. P. Photosynthetic Electron Transport Chain of Chlamydomonas reinhardi VI. Electron Transport in Mutant Strains Lacking Either Cytochrome 553 or Plastocyanin. Plant Physiol. 1966 Dec;41(10):1648–1656. doi: 10.1104/pp.41.10.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hind G. The site of action of plastocyanin in chloroplasts treated with detergent. Biochim Biophys Acta. 1968 Jan 15;153(1):235–240. doi: 10.1016/0005-2728(68)90165-5. [DOI] [PubMed] [Google Scholar]
- KATOH S., SHIRATORI I., TAKAMIYA A. Purification and some properties of spinach plastocyanin. J Biochem. 1962 Jan;51:32–40. doi: 10.1093/oxfordjournals.jbchem.a127497. [DOI] [PubMed] [Google Scholar]
- Katoh S., San Pietro A. Activities of chloroplast fragments. I. Hill reaction and ascorbate-indophenol photoreductions. J Biol Chem. 1966 Aug 10;241(15):3575–3581. [PubMed] [Google Scholar]
- Katoh S., San Pietro A. The role of C-type cytochrome in the Hill reaction with Euglena chloroplasts. Arch Biochem Biophys. 1967 Feb;118(2):488–496. doi: 10.1016/0003-9861(67)90377-3. [DOI] [PubMed] [Google Scholar]
- Katoh S., Takamiya A. Restoration of NADP photoreducing activity of sonicated chloroplasts by plastocyanin. Biochim Biophys Acta. 1965 Apr 26;99(1):156–160. doi: 10.1016/s0926-6593(65)80014-5. [DOI] [PubMed] [Google Scholar]
- Kok B., Rurainski H. J., Harmon E. A. Photooxidation of Cytochromes c, f, and Plastocyanin by Detergent Treated Chloroplasts. Plant Physiol. 1964 Jul;39(4):513–520. doi: 10.1104/pp.39.4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smillie R. M., Andersen K. S., Bishop D. G. Plastocyanin-dependent photoreduction of NADP by agranal chloroplasts from maize. FEBS Lett. 1971 Apr 2;13(6):318–320. doi: 10.1016/0014-5793(71)80250-8. [DOI] [PubMed] [Google Scholar]
- Vernon L. P., Shaw E. R., Ke B. A photochemically active particle derived from chloroplasts by the action of the detergent Triton X-100. J Biol Chem. 1966 Sep 10;241(17):4101–4109. [PubMed] [Google Scholar]
- Wessels J. S. Isolation of a chloroplast fragment fraction with NADP+-photoreducing activity dependent on plastocyanin and independent of cytochrome f. Biochim Biophys Acta. 1966 Nov 8;126(3):581–583. doi: 10.1016/0926-6585(66)90016-1. [DOI] [PubMed] [Google Scholar]
- Woo K. C., Anderson J. M., Boardman N. K., Downton W. J., Osmond C. B., Thorne S. W. Deficient Photosystem II in Agranal Bundle Sheath Chloroplasts of C(4) Plants. Proc Natl Acad Sci U S A. 1970 Sep;67(1):18–25. doi: 10.1073/pnas.67.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]


