Abstract
The Indian Diabetes Risk Score was initially developed by the Madras Diabetes Research Foundation (MDRF-IDRS) to help detect undiagnosed Type 2 diabetes (T2DM) in the community. Soon it was found that the MDRF-IDRS could also help to predict incident diabetes, metabolic syndrome, coronary artery disease (CAD), non-alcoholic fatty liver disease as well as sleep disorders in the community. It helps to differentiate T2DM from non-T2DM. Finally, it also helps to identify those with CAD, peripheral vascular disease and neuropathy among those with T2DM. Thus, the MDRF-IDRS is a simple, virtually ‘no cost’ tool which is useful in several clinical and epidemiological settings.
Keywords: Asian Indians, coronary artery disease, diabetes risk score, metabolic syndrome, neuropathy, South Asians, type 2 diabetes
INTRODUCTION
According to the Indian Council of Medical Research-Indian Diabetes study (ICMR-INDIAB), a national diabetes study, India currently has 62.4 million people with diabetes.[1] This is set to increase to over 100 million by 2030.[2] The majority of people with diabetes (>90%) have Type 2 diabetes (T2DM). While T2DM predominantly affects older individuals in developed countries, in developing nations like India, it affects the younger population in the prime of their working lives and thus poses an even greater threat to the health of these individuals.[1,3] This epidemic of diabetes is unfortunately paralleled by a corresponding increase in the prevalence of its complications, both microvascular and macrovascular, which account for much of the premature morbidity and mortality due to diabetes in India.[4–8]
Given the rapid escalation of the diabetes epidemic, all levels of prevention (primary, secondary and tertiary diabetes prevention) need to be put into action simultaneously. Unfortunately, more than 50% of people with T2DM remain undiagnosed.[9] Thus the priority is to screen, diagnose and treat as many people with T2DM as possible. In a hugely populated country like India with over 1.2 billion people with diverse cultures, the screening and diagnosing methods for diabetes should be simple, cost-effective and less time-consuming and should also take into consideration the unique risk factors for, and increased susceptibility to, T2DM that the Asian Indians have. The latter is referred to as the “Asian Indian Phenotype”.[9,10]
The Indian Diabetes Risk Score (IDRS) was initially developed by us at the Madras Diabetes Research Foundation (MDRF, Chennai) as a simple tool to help detect undiagnosed T2DM in the community.[11] Others in India have also developed similar risk scores.[12] Hence for the purpose of this article we have called our risk score as the MDRF-IDRS to distinguish our score from other scores in India. We later found that the MDRF-IDRS can be used in many other clinical and epidemiological settings. This paper will review the development of MDRF-IDRS and its expanding role and application in the field of diabetes and related metabolic disorders.
EVOLUTION AND DEVELOPMENT OF MADRAS DIABETES RESEARCH FOUNDATION - INDIAN DIABETES RISK SCORE
The Chennai Urban Rural Epidemiology Study (CURES) is a large cross-sectional study carried out in Chennai (formerly Madras), the largest city in Southern India and the fourth largest in India, with a population of about 7 million people. The sampling for CURES was based on the model of systematic random sampling, wherein from the 155 wards, 46 were selected from which 26,001 individuals were selected to represent all the 10 corporation zones of Chennai. The detailed methodology is described elsewhere and hence will not be detailed further here.[13] The data from CURES was used to develop IDRS.
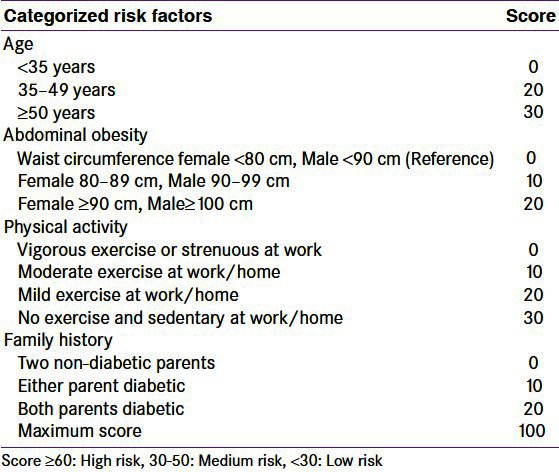
The IDRS was developed using four simple parameters, namely age, family history of diabetes, waist circumference and physical activity based on a multiple logistic regression model used to help identify undiagnosed diabetes in the community as described elsewhere.[11] Briefly, the information for these risk factors was obtained by four questions and a simple waist circumference which were given scores derived from the logistic regression as shown in Table 1. Subjects with an IDRS value of <30 were categorized as low risk, those between 30 and 50 as medium risk and those with ≥60 as high risk for diabetes. Receiver Operating Curves (ROC) were constructed to identify the optimum value (≥60%) of IDRS for determining diabetes as diagnosed using WHO Consulting Group Criteria.[14] We found that an IDRS value ≥60 had the optimum sensitivity (72.5%) and specificity (60.1%) for determining undiagnosed diabetes in the community with a positive predictive value of 17.0%, negative predictive value of 95.1%, and accuracy of 61.3%.[11] Thus, a simple tool, the MDRF-Indian Diabetes Risk Score (MDRF–IDRS) was developed to identify undiagnosed diabetic subjects in the population.
Table 1.
VALIDATION OF MDRF-IDRS BY OTHER STUDIES
The MDRF-IDRS was later validated in the Boolor Diabetes study in Karnataka state[15] where using IDRS, screening of nearly one-third of the population of Boloor locality in Mangalore was done. In that study using an IDRS score ≥60, 62.2% of people living with undiagnosed diabetes in that population could be detected, with a specificity of 73.7% which is almost identical to our study.[11]
Further validation of MDRF-IDRS was done by Gupta, et al., by estimating the prevalence of diabetes in rural and urban Tamil Nadu.[16,17] The prevalence of diabetes was 8.3% in urban areas and 5.9% in rural areas. The subjects with diabetes had higher IDRS scores (76% in urban and 56% in rural) than the general population (31%) and the differences were significant.[16,17] This indicates that IDRS has excellent predictive value for detecting undiagnosed diabetes in the community and IDRS was also a much stronger risk indicator than examining individual risk factors like age, family history, obesity or physical activity.
INCIDENCE OF DIABETES
The prevalence of diabetes in India has been reported by several cross-sectional studies. However, there are virtually no longitudinal, population-based studies on the incidence of diabetes from India. The Chennai Urban Population Study [CUPS] is one of the few longitudinal epidemiological studies on diabetes conducted in India till date. The baseline study was completed in 1996-97 and the follow-up was conducted after a mean period of eight years.[18] The CUPS[17] showed that subjects with IDRS score ≥60 at baseline also had the highest proportion of conversion to diabetes (27.8%) followed by those with medium risk score of IDRS (16.9%) and was lowest in those with low IDRS (<30), (5.6%, P < 0.001). Moreover, 38.4% of ‘converters’ to either diabetes or pre-diabetes had high IDRS scores at baseline. IDRS had the highest relative risk (RR) for predicting incident diabetes. Even after adjusting for age and gender, the RR for incident diabetes remained significant (IDRS ≥ 60: RR 3.1, P = 0.035, IDRS 30-50: RR 2.7, P = 0.032).[18] Thus a high IDRS can be useful to identify those who are likely to develop diabetes or pre-diabetes in the future, even if they have normal glucose tolerance now.
INDIAN DIABETES RISK SCORE AND CLASSIFICATION OF TYPES OF DIABETES
The optimal treatment of diabetes starts with accurate classification of diabetes mellitus. Currently, a clinician must analyze a variety of risk factors such as family history, age, weight, and symptoms plus expensive biochemical studies to accurately classify a patient with diabetes. This then helps the clinician to advise on the treatment options like lifestyle and pharmacological interventions for T2DM or for immediately starting insulin, if the patient has Type 1 diabetes (T1DM). In clinic-based settings, we often end up with limited time to classify diabetes patients, counsel them, and initiate treatment. Moreover, not all patients can afford costly investigations to classify diabetes, like C-Peptide assay or Glutamic Acid Decarboxylase GAD–antibodies. This highlights the need for a simple and inexpensive tool which can be used as an initial step to screen for and classify diabetes.
In a study done by Sharma, et al.,[19]8747 diabetic patients attending our centre were independently classified by a diabetologist (who was not aware of the IDRS score) as “T2DM" or “non-T2DM" which included T1DM and other types of diabetes. The IDRS was then calculated for each patient at first visit by an independent observer who was unaware of the clinical status of the patient. Receiver Operating Characteristic (ROC) curves were constructed and the optimal IDRS cutoff points for predicting T2DM and non-T2DM were calculated. Of the 8747 patients analyzed, 204 (2.3%) were classified as non-T2DM and 8543 (97.7%) as T2DM. In the ROC analysis, an IDRS ≥ 60 had an area under the curve (AUC) of 0.894 with a sensitivity of 83.8% and specificity of 81.0% to predict T2DM. Conversely, an IDRS <60 had an AUC of 0.882 with sensitivity of 79.9% and specificity of 83.8% to predict non-T2DM.[19]
This was confirmed in another study[20] conducted at Manipal in Karnataka to see whether a low IDRS score could pick up patients with secondary diabetes, more specifically steroid-induced diabetes.[20] It was found that all patients with steroid-induced diabetes had IDRS <60. Thus, a low IDRS could be used as a simple tool to alert the clinician that the patient may be having other types of diabetes (other than T2DM) while a score of ≥60 can be used as a pointer to T2DM.[19,20] This is not surprising as the risk factors included in the IDRS, namely age, family history of diabetes, physical inactivity and waist circumference are all risk factors of T2DM and not for T1DM, secondary diabetes or other forms of diabetes.
IDRS AND DETECTION OF COMPLICATIONS OF DIABETES
With the prevalence of diabetes on the rise, the complications of T2DM, such as retinopathy, nephropathy, neuropathy, peripheral vascular disease (PVD) and coronary artery disease (CAD) are also rising in India and this could have devastating results.[3–8] Asian Indian T2DM subjects may be at greater lifetime risk for these complications due to the earlier onset of their disease.[3,21] We took up a study to see whether the diabetic individuals identified by IDRS might also possibly have a higher prevalence of diabetes-related complications. This study showed that the prevalence of several diabetic complications was higher among subjects in the high-risk category of IDRS ≥60.[22] Thus, CAD [9.2% vs. 5.4%, P = 0.043], diabetic peripheral neuropathy [29.2% vs. 8.8%, P < 0.001] and PVD [4.8% vs. 1.9%, P = 0.038] were all significantly higher among subjects in the high-risk category [IDRS ≥ 60] compared to those with IDRS score <60. In the regression analysis, the odds ratio (OR) for neuropathy was 4.27 (95% CI: 2.74-6.67, P < 0.001), for PVD it was 2.57 (95% CI: 1.02-6.46, P = 0.045) and for CAD it was 1.79 (95% CI: 1.01-3.18, P = 0.046) in subjects with IDRS ≥ 60. Even after adjusting for the duration of diabetes, neuropathy [OR: 4.03, 95% CI: 2.55-6.37, P < 0.001); and PVD [OR: 2.54, 95% CI: 1.01–6.41, P = 0.049] were associated with IDRS ≥60[22]. However, IDRS did not show any significant association with diabetic retinopathy or macroalbuminuria.
Thus, use of IDRS for targeted screening in T2DM patients could help pick up those who are likely to have CAD, PVD and neuropathy.
IDRS AND CARDIO-METABOLIC DISEASE
Diabetes shares many risk factors with other non-communicable diseases like age, physical inactivity, waist circumference, insulin resistance, dyslipidemia and high blood pressure. Diabetes is also a cardiovascular risk equivalent.[23] Hence, we did studies to determine the association of IDRS with individual cardiovascular risk factors and with metabolic syndrome as well as with CAD.
With increasing IDRS scores, <30, 30-50, and ≥60, the prevalence of hypertension: 9.4, 22.1 and 38.2% (P for trend: <0.001), hypertriglyceridemia: 8.8, 19.9 and 25.3% (P for trend: <0.001) and hypercholesterolemia: 7.2, 20.3 and 34.9% (P for trend: <0.001) also increased. Moreover, the mean IDRS increased significantly with worsening glucose intolerance (NGT, 48 ± 17; IGT, 57 ± 16; NDD, 61 ± 15; KD, 68 ± 12; and P for trend <0.001). The prevalence of metabolic syndrome in normal glucose-tolerant subjects also increased with IDRS scores—IDRS <30:1.8%, 30-50:14.6% and ≥60: 30.3 % (P for trend <0.001)[24] [Figure 1]. Thus the prevalence of metabolic syndrome markedly increases in those with high IDRS Scores. This is not surprising as one of the factors included in the IDRS is waist circumference which is one of the components of the metabolic syndrome.
Figure 1.
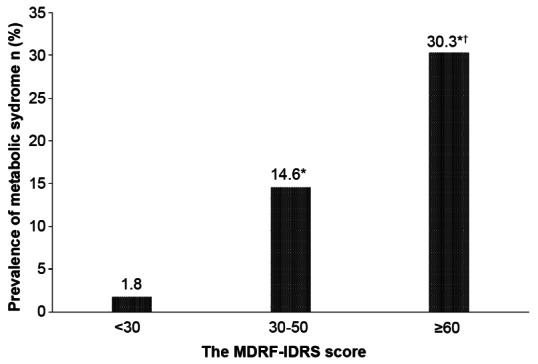
Prevalence of metabolic syndrome with increasing MDRF-Indian Diabetes Risk Score (IDRS) in subjects with normal glucose tolerance (*P value <0.001 compared with low risk. †P value <0.001 compared with medium risk)
The prevalence of CAD in the high-risk IDRS group was significantly higher (2.2%) compared with the medium-risk IDRS group (0.8% P = 0.05) and the low-risk IDRS group (0.06% P = 0.03).[24]
IDRS AND NON-ALCOHOLIC FATTY LIVER DISEASE
We recently showed that among non-diabetic subjects, the prevalence of NAFLD increased with an increase in IDRS scores. Subjects with IDRS ≥ 60 had significantly higher prevalence of NAFLD (30.4%) than subjects with low IDRS score (15%) (P = 0.022). In stepwise logistic regression analysis, along with glycated hemoglobin and Alanine aminotransferase/Aspartate aminotransferase (ALT/AST) ratio; a score of IDRS ≥ 60 had the odds of 1.78 for NAFLD (CI 1.04-3.06, P = 0.035).[25] Thus, IDRS not only helps to identify T2DM but also to identify metabolic syndrome, CAD and NAFLD in the community.
IDRS AND ARTERIAL STIFFNESS
Coronary atherosclerosis has been shown to be initiated early in life, many years before clinical manifestations of CAD occur.[26] Changes in the arterial wall can lead to increased arterial stiffness, which has been shown to influence cardiovascular prognosis adversely.[27] Augmentation index (AI) is a non-invasive index of arterial stiffness and has been shown to be associated with the presence of coronary atherosclerosis and increased cardiovascular risk.[28,29] A study done by us[30] on non-diabetic subjects showed that arterial stiffness values increased with an increase in IDRS. Subjects with IDRS ≥ 60 had significantly higher AI (24.6 ± 7.2) compared to subjects with IDRS 30-60 (16.4 ± 5.5) and with IDRS <30 (13.3 ± 4.5), and the trend was statistically significant (P < 0.001). Moreover, IDRS was independently associated with arterial stiffness with an OR of 6.4, (P < 0.001), even after adjusting for smoking, blood pressure, insulin resistance and lipid profile.[30]
IDRS AND SLEEP ABNORMALITIES
It is well known that sleep abnormalities are common in those with obesity, diabetes and cardiovascular disease.[31,32,33] We therefore investigated the prevalence and risk factors of sleep abnormalities and their relationship to cardio-metabolic factors in urban South India. It was found that the prevalence of both ‘snoring’ and ‘daytime sleepiness’ increased dramatically with an increase in IDRS scores (prevalence of snoring: 22.2% in IDRS <30, 31.4% IDRS 30-50, and 48.2% IDRS ≥ 60, trend χ2: 11.14, P = 0.001; Daytime sleepiness: 33.3% in IDRS < 30, 54.5% in IDRS 30-50, and 63.7% in IDRS ≥ 60, trend χ2:5.12, P = 0.024).[34] This forms one more extension for the use of the MDRF-IDRS.
COMPARISON OF IDRS AND TCF7L2 GENOTYPING FOR DETECTING OF DIABETES
With increasing number of people with diabetes worldwide and particularly in India there is a necessity for low-cost screening tests which can apply on a large scale. Recently, the TCF7L2 gene had emerged as the strongest genetic marker for T2DM.[35,36] Some companies have started marketing TCF7L2 genotyping to predict diabetes in the community.[37] We wanted to compare the IDRS vs. TCF7L2 genotype to screen for diabetes in the community. We studied subjects without known diabetes, (n = 961) and compared the effectiveness and costs of screening tests for undiagnosed Type 2 diabetes mellitus using: 1. Oral glucose tolerance testing (OGTT) in all individuals, 2. OGTT only in those with a high IDRS, 3. OGTT after genotyping of the TCF7L2 gene (rs 12255372 and rs 7903146 polymorphisms).[38] In this study, doing OGTT on all individuals identified 72 subjects with newly diagnosed diabetes (NDD). IDRS screening (cutoff ≥ 60) yielded 413 positive subjects which included 54 (75%) of the 72 NDD identified by OGTT. Genotyping of TCF7L2 yielded 493 positive subjects which only included 36 (50%) of the 72 NDD subjects showing less discriminatory power of genotyping. Screening with genotyping missed 27 (37.5%) of NDD subjects identified by IDRS. In contrast, IDRS missed only 9 (12.5%) of the NDD subjects identified by genotyping.[38]
Thus it is clear that for predicting the presence of undiagnosed diabetes in India, the MDRF-IDRS is more efficient and much less expensive than genotyping or even doing OGTT on the whole population.
IDRS AND POST-MENOPAUSAL WOMEN
The Santiniketan Women Study[39] was done in West Bengal to evaluate the use MDRF-IDRS with respect to menopausal status in Asian Indian women. This study revealed that of the 102 pre-menopausal women, 9% had high IDRS, whereas, of the 34 post-menopausal women, 38% had high IDRS (χ2= 16.13; P < 0.001). Furthermore, there was a trend for decreased IDRS with increasing education level suggesting that with better education, exercise levels were higher and central obesity rates were lower.
CONCLUSION
Figure 2 summarizes the multiple applications and the uses of the MDRF-IDRS. It helps to identify undiagnosed diabetic subjects in the population[11,15] and in both urban and rural settings.[16,17] IDRS helps to discriminate T2DM from secondary causes of diabetes like steroid-induced diabetes[20] and also helps to distinguish Type 2 from all forms of ‘non-Type 2’ diabetes mellitus.[19] It helps to identify metabolic syndrome, and cardio-metabolic risks in normoglycemic subjects.[24] Also, among non-diabetic individuals, IDRS is associated with NAFLD, and arterial stiffness;[25,30] IDRS is also associated with complications of diabetes, like neuropathy and peripheral vascular disease.[22] For large-scale screening for diabetes, using IDRS is much less expensive than genotyping and it also makes it more cost-effective than doing OGTT on the whole population to detect undiagnosed T2DM in India.[38]
Figure 2.

The multiple applications of the MDRF-IDRS
One of the limitations of the MDRF-IDRS is that, as it was derived from an Asian Indian population, its use is probably restricted to South Asians and for other populations similar scores might have to be developed.
The major advantage of the MDRF-IDRS is that it can even be done online and thus can reach millions of people in India (to calculate IDRS through a website, http://www.drmohansdiabetes.com/). Thus in short, the MDRF-IDRS is a simple, very low-cost screening tool which has multiple potential applications in clinical and in epidemiological settings in India.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Anjana RM, Pradeepa R, Deepa M, Datta M, Sudha V, Unnikrishnan R, et al. ICMR-INDIAB Collaborative Study Group: Prevalence of diabetes and prediabetes (impaired fasting glucose or/and impaired glucose tolerance) in rural and urban India: Phase 1 results of the Indian Council of Medical Research-INdiaDIABetes (INDIAB) study. Diabetologia. 2011;54:3022–7. doi: 10.1007/s00125-011-2291-5. [DOI] [PubMed] [Google Scholar]
- 2.Unwin N, Whiting D, Guariguata L, Ghyoot G, Gan D, editors. 5th ed. Belgium: International Diabetes Federation; 2011. In Diabetes Atlas. [Google Scholar]
- 3.Mohan V, Alberti KG. Diabetes in the tropics. In: International Text Book of Diabetes Mellitus (Second Edition) In: Alberti KGMM, Zimmet P, Defronzo RA, Keen H, editors. Chichester. U.K: John Wiley and Sons Ltd; 1997. pp. 171–87. [Google Scholar]
- 4.Rema M, Premkumar S, Anitha B, Deepa R, Pradeepa R, Mohan V. Prevalence of diabetic retinopathy in urban India: The Chennai Urban Rural Epidemiology Study (CURES) Eye Study I. Invest Ophthalmol Vis Sci. 2005;46:2328–33. doi: 10.1167/iovs.05-0019. [DOI] [PubMed] [Google Scholar]
- 5.Unnikrishnan RI, Rema M, Pradeep R, Deepa M, Shanthirani CS, Deepa R, et al. Prevalence and risk factor of diabetic nephropathy in an urban south Indian population; The Chennai Urban Rural Epidemiology study (CURES-45) Diabetes Care. 2007;30:2019–24. doi: 10.2337/dc06-2554. [DOI] [PubMed] [Google Scholar]
- 6.Pradeepa R, Rema M, Vignesh J, Deepa M, Deepa R, Mohan V. Prevalence and risk factors for diabetic neuropathy in an urban south Indian population: The Chennai Urban Rural Epidemiology Study (CURES-55) Diabet Med. 2008;25:407–12. doi: 10.1111/j.1464-5491.2008.02397.x. [DOI] [PubMed] [Google Scholar]
- 7.Premalatha G, Shanthi Rani CS, Deepa R, Markovitz J, Mohan V. Prevalence and risk factors of peripheral vascular disease in a selected south Indian population - The Chennai Urban Population Study (CUPS) Diabetes Care. 2000;23:295–1300. doi: 10.2337/diacare.23.9.1295. [DOI] [PubMed] [Google Scholar]
- 8.Mohan V, Deepa R, Shanthi Rani S, Premalatha G. Prevalence of coronary artery disease and its relationship to lipids in a selected population in south India. J Am Coll Cardiol. 2001;38:682–7. doi: 10.1016/s0735-1097(01)01415-2. [DOI] [PubMed] [Google Scholar]
- 9.Joshi SR, Das AK, Vijay VJ, Mohan V. Challenges in Diabetes Care in India: Sheer Numbers, Lack of Awareness and Inadequate Control. J Assoc Physicians India. 2008;56:443–50. [PubMed] [Google Scholar]
- 10.Deepa R, Sandeep S, Mohan V. Abdominal obesity, visceral fat and Type 2 diabetes – “Asian Indian Phenotype”. Under the Aegis of SASAT. In: Mohan V, Rao HR, Gundu, editors. Type 2 Diabetes in South Asians: Epidemiology, Risk Factors and Prevention. Under the Aegis of SASAT. Delhi: Jaypee Brothers Medical Publishers; 2006. pp. 138–52. [Google Scholar]
- 11.Mohan V, Deepa R, Deepa M, Somannavar S, Datta M. A simplified Indian Diabetes Score for screening for undiagnosed diabetic subjects. J Assoc Physicians India. 2005;53:759–63. [PubMed] [Google Scholar]
- 12.Ramachandran A, Snehalatha C, Vijay V, Wareham NJ, Colagiuri S. Derivation and validation of diabetes risk score for urban Asian Indians. Diabetes Res Clin Pract. 2005;70:63–70. doi: 10.1016/j.diabres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 13.Deepa M, Pradeepa R, Rema M, Mohan A, Deepa R, Shanthirani S, et al. The Chennai Urban Rural Epidemiology Study (CURES)—study design and methodology (urban component) (CURES-I) J Assoc Physicians India. 2003;51:863–70. [PubMed] [Google Scholar]
- 14.Alberti KG, Zimmet PZ. Definition diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus, provisional report of a WHO Consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 15.Adhikari P, Pathak R, Kotian S. Validation of the MDRF-Indian Diabetes Risk Score (IDRS) in another south Indian population through the Boloor Diabetes Study (BDS) J Assoc Physicians India. 2010;58:434–6. [PubMed] [Google Scholar]
- 16.Gupta SK, Singh Z, Purty AJ, Kar M, Vedapriya D, Mahajan P, et al. Diabetes prevalence and its risk factors in rural area of Tamil Nadu. Indian J Community Med. 2010;35:396–9. doi: 10.4103/0970-0218.69262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta SK, Singh Z, Purty AJ, Mohan V. Diabetes prevalence and its risk factors in urban Pondicherry. Int J Diabetes Dev Ctries. 2009;29:166–9. doi: 10.4103/0973-3930.57348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohan V, Deepa M, Anjana RM, Lanthorn H, Deepa R. Incidence of diabetes and pre-diabetes in a selected urban south Indian population (CUPS-19) J Assoc Physicians India. 2008;56:152–7. [PubMed] [Google Scholar]
- 19.Sharma KM, Ranjani H, Nguyen H, Shetty S, Datta M, Narayan KM, et al. Indian Diabetes Risk Score Helps to Distinguish Type 2 from Non-Type 2 Diabetes Mellitus (GDRC-3) J Diabetes Sci Technol. 2011;5:419–25. doi: 10.1177/193229681100500232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shanbhogue VV, Vidyasagar S, Madken M, Varma M, Prashant CK, Seth P, et al. Indian Diabetic Risk Score and its utility in steroid induced diabetes. J Assoc Physicians India. 2010;58:202. [PubMed] [Google Scholar]
- 21.Bjork S, Kapur A, King H, Nair J, Ramachandran A. Global policy: Aspects of diabetes in India. Health Policy. 2003;66:61–72. doi: 10.1016/s0168-8510(03)00044-7. [DOI] [PubMed] [Google Scholar]
- 22.Mohan V, Vassy JL, Pradeepa R, Deepa M, Subashini S. The Indian type 2 diabetes risk score also helps identify those at risk of macrovasvular disease and neuropathy (CURES-77) J Assoc Physicians India. 2010;58:430–3. [PubMed] [Google Scholar]
- 23.DECODE Study Group, the European Diabetes Epidemiology Group. Glucose tolerance and cardiovascular mortality: Comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med. 2001;161:397–405. doi: 10.1001/archinte.161.3.397. [DOI] [PubMed] [Google Scholar]
- 24.Mohan V, Sandeep S, Deepa M, Gokulakrishnan K, Datta M, Deepa R. A diabetes risk score helps identify metabolic syndrome and cardiovascular risk in Indians-the Chennai Urban Rural Epidemiology Study (CURES-38) Diabetes Obes Metab. 2007;9:337–43. doi: 10.1111/j.1463-1326.2006.00612.x. [DOI] [PubMed] [Google Scholar]
- 25.Anbalagan VP, Vijayachandrika V, Vamsi M, Deepa M, Mohan V. A simple Indian Diabetes Risk Score could help identify nondiabetic individuals at high risk of Non Alcoholic Fatty Liver Disease (CURES-117) J Diabetes Sci Technol. 2012 Nov 1; doi: 10.1177/193229681200600624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stary HC. Evolution and progression of atherosclerotic lesions in coronary arteries of children and young adults. Arteriosclerosis. 1989;9(1 Suppl):119–32. [PubMed] [Google Scholar]
- 27.O'Rourke MF, Mancia G. Arterial stiffness. J Hypertens. 1999;17:1–4. doi: 10.1097/00004872-199917010-00001. [DOI] [PubMed] [Google Scholar]
- 28.Nürnberger J, Keflioglu-Scheiber A, Opazo Saez AM, Wenzel RR, Philipp T, Schäfers RF. Augmentation index is associated with cardiovascular risk. J Hypertens. 2002;20:2407–14. doi: 10.1097/00004872-200212000-00020. [DOI] [PubMed] [Google Scholar]
- 29.Weber T, Auer J, O'Rourke MF, Kvas E, Lassnig E, Berent R, et al. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation. 2004;109:184–9. doi: 10.1161/01.CIR.0000105767.94169.E3. [DOI] [PubMed] [Google Scholar]
- 30.Mohan V, Gokulakrishnan K, Ganesan A, Kumar SB. Association of Indian diabetes Risk Score with arterial stiffness in Asian Indian nondiabetic subjects: The Chennai Urban Rural Epidemiology Study (CURES-84) J Diabetes Sci Technol. 2010;4:337–43. doi: 10.1177/193229681000400214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Punjabi NM, Polotsky VY. Disorders of glucose metabolism in sleep apnea. J Appl Physiol. 2005;99:1998–2007. doi: 10.1152/japplphysiol.00695.2005. [DOI] [PubMed] [Google Scholar]
- 32.Resnick HE, Redline S, Shahar E, Gilpin A, Newman A, Walter R, et al. Sleep Heart Health Study. Diabetes and sleep disturbances: Findings from the Sleep Heart Health Study. Diabetes Care. 2003;26:702–9. doi: 10.2337/diacare.26.3.702. [DOI] [PubMed] [Google Scholar]
- 33.Seicean S, Kirchner HL, Gottlieb DJ, Punjabi NM, Resnick H, Sanders M, et al. Sleep-disordered breathing and impaired glucose metabolism in normal-weight and overweight/obese individuals: the Sleep Heart Health Study. Diabetes Care. 2008;31:100–6. doi: 10.2337/dc07-2003. [DOI] [PubMed] [Google Scholar]
- 34.Roopa M, Deepa M, Indulekha K, Mohan V. Prevalence of sleep abnormalities and their association with metabolic syndrome among Asian Indians: Chennai Urban Rural Epidemiology Study (CURES-67) J Diabetes Sci Technol. 2010;4:1524–31. doi: 10.1177/193229681000400630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bodhini D, Radha V, Dhar M, Narayani N, Mohan V. The rs12255372 (G/T) and rs7903146(C/t) polymorphisms of the TCF7L2 gene are associated with type 2 diabetes mellitus in Asian Indians.(CURES-42) Metabolism. 2007;56:1174–8. doi: 10.1016/j.metabol.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 36.Grant SF, Thorleifsson G, Reynisdottir I, Benediktsson R, Manolescu A, Sainz J, et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006;38:320–3. doi: 10.1038/ng1732. [DOI] [PubMed] [Google Scholar]
- 37. [Last accessed on 2012 Aug 06]. Available from: http://www/decodehealth.com/type--diabetes .
- 38.Mohan V, Goldhaber-Fiebert JD, Radha V, Gokulakrishnan K. Screening with OGTT alone or in combination with the Indian diabetes risk score or genotyping of TCF7L2 to detect undiagnosed type 2 diabetes in Asian Indians. Indian J Med Res. 2011;133:294–9. [PMC free article] [PubMed] [Google Scholar]
- 39.Ghosh A, Bhagat M. Indian diabetes risk score according to menopausal status in Asian Indian women: The Santiniketan Women study. J Diabetes. 2009;1:140–1. doi: 10.1111/j.1753-0407.2009.00035.x. [DOI] [PubMed] [Google Scholar]


