Abstract
Polycystic ovary syndrome affects 6 to 15% of reproductive age women worldwide. It is associated with increased risk of miscarriage, gestational diabetes mellitus, hypertensive disorders of pregnancy, preterm delivery, and birth of small for gestational age infant. Many studies on issues relating to pathophysiology and management of these complications have been published recently. These issues are being reviewed here using relevant articles retrieved from Pubmed database, especially from those published in recent past.
Keywords: Early pregnancy loss, metformin, polycystic ovary syndrome, pregnancy
INTRODUCTION
Polycystic ovary syndrome (PCOS) is a heterogeneous disorder consisting of clinical or biochemical hyperandrogenism with ovulatory dysfunction ruling out secondary causes for the same. Its diagnostic criteria has been modified along timescale[1,2] and its prevalence varies broadly from 6 to 15% in general population depending on ethnicity studied and the criteria utilized.[3,4] It is probably the most common endocrine disorder in reproductive age women.
The reproductive issues with PCOS are manifold starting with anovulatory cycles leading to subfertility. Post conception, PCOS women are at increased risk for early pregnancy loss (EPL). After having successfully passed the first trimester, they commonly encounter later pregnancy complications like gestational diabetes mellitus (GDM), pregnancy-induced hypertension (PIH), preeclampsia, preterm delivery, and birth of small for gestational age (SGA) infant. Effective tackling of metabolic and reproductive issues relating to pregnancy forms the cornerstone of management of PCOS.
Normal pregnancy milieu
Normal pregnancy is characterized by induction of insulin resistance associated with compensatory hyperinsulinemia in second and third trimesters. This insulin resistance of normal pregnancy is a physiologically advantageous adaptation designed to restrict maternal glucose uptake and to ensure shunting of nutrients to the growing fetus. It is probably mediated by increases in hormonal levels of estradiol, progesterone, prolactin, cortisol, human chorionic gonadotropin, placental growth hormone (PGH), and human placental lactogen (HPL).
HPL and PGH are the hormones mainly responsible for insulin resistance in pregnancy. HPL is responsible for adaptive increase in insulin secretion necessary for pregnancy and for diversion of maternal carbohydrate metabolism to fat metabolism in the third trimester. PGH seems to be a paracrine growth factor probably regulating the metabolic and growth needs of the fetus partially. Barbour, et al. recently demonstrated induction of hyperinsulinemic insulin resistance in transgenic mice with PGH.[5] They also demonstrated abnormalities in insulin signaling in skeletal muscle tissue of these mice, which bear a remarkable similarity to the tissue found in normal pregnant women.
There is approximately 200 to 250% increase in insulin secretion in lean women with normal glucose tolerance with advancing gestation.[6] However, there is comparatively less robust increase in insulin levels of obese women with normal glucose tolerance. In normal pregnancy, there is a decreased expression of the GLUT-4 transporter in maternal adipose tissue[7] but not in skeletal muscle. Skeletal muscle is the main site of insulin-mediated glucose disposal in vivo. Hence, the mechanisms for insulin resistance in normal pregnancy lie in the skeletal muscle either in the insulin signaling pathways or in the abnormal GLUT-4 translocation.[8]
Polycystic ovary syndrome and pregnancy
Hyperandrogenism and insulin resistance form the metabolic hallmark of PCOS women. A significant section of lean PCOS women have baseline intrinsic insulin resistance. Those with superimposed obesity have additional insulin resistance contributed by the excess adipose tissue. The baseline insulin resistance seems to be exacerbated with entry into pregnancy. There is an increased risk of pregnancy complications in PCOS women. In a population-based cohort study, women with PCOS were more often obese and more commonly used assisted reproductive technology than women without such a diagnosis.[9] PCOS was strongly associated with preeclampsia and very preterm birth and the risk of gestational diabetes was more than doubled. Infants born to mothers with PCOS were more prone to be large for gestational age and were at increased risk of meconium aspiration and having a low Apgar score (<7) at five minutes.[9] These increased risk of pregnancy complications was also confirmed by two meta-analyses which were conducted to evaluate the risk of pregnancy and neonatal complications in women with PCOS.[10,11] They are at increased risk of EPL, GDM, hypertensive disorders of pregnancy (HDP), and premature delivery.
Polycystic ovary syndrome and spontaneous miscarriage
PCOS women are at risk of EPL, defined clinically as first trimester miscarriage. EPL occurs in 30 to 50% of PCOS women compared with 10 to 15% of normal women.[12,13] The EPL rate in PCOS women has been difficult to establish due to several confounding factors. Treatment with ovulation-inducing agents is associated with a higher incidence of spontaneous EPL compared with the prevalence in the normally ovulating, naturally conceiving population.[14] Moreover, the prevalence of spontaneous EPL for women with PCOS who conceive naturally is not known. Several mechanisms underlying the increased risk of EPL in women with PCOS have been proposed and they are not exclusive [Figure 1].
Figure 1.
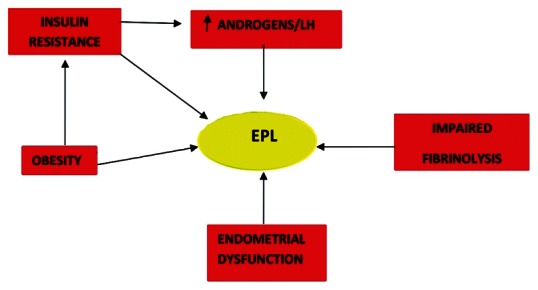
Pathogenesis of early pregnancy loss in polycystic ovary syndrome
LUTEINIZING HORMONE AND EARLY PREGNANCY LOSS
Several studies have linked elevated LH levels with EPL in women with PCOS. The likelihood of miscarriage was increased and conception rate decreased as compared to those with normal LH in PCOS women.[15] Decreased miscarriage rate in PCOS patients who underwent long-term pituitary suppression with a GnRH agonist has been documented in subsequent studies.[16,17] However, two succeeding studies with PCOS women of normal BMI have not shown the improvement in live birth rate with LH suppression using GnRH agonists.[18,19] The differing results from earlier studies may be confounded by the effects of obesity on pregnancy outcome.
ANDROGENS AND EARLY PREGNANCY LOSS
Hyperandrogenemia is hypothesized to be another probable cause of EPL in PCOS women. Hyperandrogenemia and/or clinical hyperandrogenism is currently considered as an essential prerequisite for diagnosis of PCOS.[2] Elevated free/total testosterone ratios and isolated elevated free and total testosterone levels were found to be predictive of EPL in PCOS women in two different studies.[20,21] Another study by Okon, et al. found higher testosterone concentrations among “recurrent miscarriages" patients with and without PCOS in comparison to normal fertile controls.[22] The authors postulated that high androgen levels antagonize estrogen, which may adversely affect endometrial development and implantation.
Apparao, et al. studied the poor reproductive performance observed in women with PCOS and suggested that it may be partly due to the concomitant increase in both serum androgens and elevation in endometrial androgen receptors.[23] Sex steroids regulate uterine receptivity for embryo implantation by controlling the expression of HOXA10 gene[24] which is spatially and temporally regulated during embryonic development. Elevated testosterone in PCOS downregulates the expression of HOXA10 gene, thereby decreasing the uterine receptivity and implantation.
IMPAIRED FIBRINOLYSIS AND EARLY PREGNANCY LOSS
High plasminogen activator inhibitor-1(PAI-1) activity has been found to be associated with recurrent pregnancy loss in women with unexplained recurrent miscarriages and has also been found to be significantly higher in women with PCOS independent of BMI. Glueck, et al. found PAI-1 activity to be an independent risk factor for miscarriage, possibly due to impaired fibrinolysis, which results in placental insufficiency through increased thrombosis of the placental bed.[25] Palombo, et al. documented improved pregnancy outcomes with metformin in overweight women with PCOS and correlated it to significantly reduced PAI-1 activity levels resulting from treatment.[26] The reduction in EPL in PCOS women who had reduced PAI-1 levels during treatment with metformin was confirmed in a larger cohort study.[27] Additionally, PCOS women who received metformin but did not have reductions in PAI-1 continued to have increased miscarriage rates.
INSULIN RESISTANCE AND EARLY PREGNANCY LOSS
PCOS women are believed to be strongly associated with insulin resistance and compensatory hyperinsulinemia,[28] which has been shown to be independently contributed by obesity prevalent among PCOS women.[29] This hyperinsulinemic insulin resistance is implicated in pathophysiology of EPL. However, the exact mechanism for it remains elusive currently, although different factors for its effect have been proposed. These include its effect on oocyte maturation, glucose uptake and metabolism, implantation, altered expression of HOXA10 gene, and reduction of serum glycodelin and IGF-binding protein-1 (IGFBP-1) concentrations.
Impaired glucose uptake caused by downregulation of the IGF-I receptor has been documented to result in blastocyst apoptosis.[30] Hyperglycemia in the background of hyperinsulinemic insulin resistance was shown to induce the expression of caspase, an enzyme triggering the apoptosis of blastocyst.[31] Additionally, GLUT 4 expression was revealed to be significantly lower in endometrial cells of hyperinsulinemic obese PCOS women compared with those from normoinsulinemic PCOS women or controls.[32]
ENDOMETRIAL DYSFUNCTION AND EARLY PREGNANCY LOSS
Implantation of embryo is affected by the endometrial receptivity which seems to be affected in PCOS. Initial attachment of the embryo is mediated via certain cell adhesion molecules like β3 integrin located on the luminal surface of the endometrium[33] and these molecules are decreased in PCOS women.[23] Endometrial secretory proteins like glycodelin and IGFBP-l are pivotal for implantation and maintenance of pregnancy. Glycodelin may be involved in early placental development through its modulatory effect on immune and trophoblast cells.[34] IGFBP-1 plays an important role in human female reproductive physiology regulating menstrual cycles, puberty, ovulation, decidualization, and fetal growth. Both serum glycodelin and IGFBP-l levels were shown to be significantly lower in women with EPL in first trimester by Jakubowicz, et al.[35]
OBESITY AND EARLY PREGNANCY LOSS
Increased risk of miscarriage was found in a meta-analysis investigating the association between obesity and miscarriage.[36] There is a strong inverse relationship between BMI and serum IGFBP-1 in the general population.[37] A recent meta-analysis studied the role of IGFBP-1 in PCOS pathogenesis controlling for the influence of BMI.[38] It suggested that a decreased serum level of IGFBP-1 does not have a role in the pathogenesis of PCOS but is likely to result from the high prevalence of obesity in the PCOS women. Wang, et al. conducted a study which suggested that the higher risk of spontaneous abortion observed in women with PCOS is likely to be due to their high prevalence of obesity and the type of treatment they receive.[39]
POLYCYSTIC OVARY SYNDROME AND GESTATIONAL DIABETES
GDM complicates 40 to 50% of PCOS pregnancies.[40] It intervenes in pregnancy when pancreatic β cells cannot overcome the superimposed insulin resistance of pregnancy on intrinsic insulin resistance of PCOS women. GDM has been shown to complicate PCOS pregnancy more frequently than normal pregnancy in majority of studies. Some among these had obesity as a confounding factor and hence could have increased the actual risk. Urman, et al. found PCOS women to be at increased risk of gestational diabetes independent of body mass index.[41] Li, et al. showed an increased risk of GDM and preeclampsia in non-overweight/obese PCOS women and this risk seemed to be due to PCOS itself rather than to obesity.[42] However, Haakova, et al. demonstrated that PCOS women had comparable risk of GDM or PIH as that of age-and weight-matched normal controls.[43] Two studies showed BMI >25 kg/m2 to be the greatest predictor for GDM.[44,45] A recent systematic review and meta-analysis by Toulis, et al. concluded that the higher risk of GDM in women with PCOS was a questionable finding.[46] These conflicting results may be caused by the heterogeneity of PCOS and the diversity in methodology among studies.
POLYCYSTIC OVARY SYNDROME AND HYPERTENSIVE DISEASE IN PREGNANCY
HDP occurs in 8% of PCOS pregnancies.[47] It includes PIH, defined as new-onset hypertension in pregnancy after 20 weeks of gestation, and preeclampsia, defined as PIH with proteinuria. There is inconsistent association between PCOS and HDP. Diamant, et al.[48] showed increased incidence of preeclampsia in PCOS women but the control group was not BMI matched. Haakova, et al. and Mikola, et al. documented comparable prevalence of preeclampsia between PCOS and non-PCOS women.[43,44] Radon, et al. showed a significant increase in incidence of PIH in women with PCOS women even after matching for BMI.[49] A meta-analysis by Kjeruff showed PCOS women to be at elevated risk of preeclampsia (OR-4.23) or PIH (OR-4.07).[11] De Vries, et al. demonstrated an increased prevalence of preeclampsia and a similar prevalence of PIH in PCOS group in comparison to BMI-matched non-PCOS group.[50]
POLYCYSTIC OVARY SYNDROME AND PRETERM DELIVERIES
Preterm births complicate 6 to 15% of pregnancies of PCOS women.[51] It may be associated with confounding factor of multiple pregnancies induced as a result of use of various ovulation induction regimens in PCOS women. Preeclampsia itself is a risk factor for preterm deliveries. Meta-analysis by Boomsma, et al. showed infants of PCOS women had significantly lower neonatal birth weight, although the significance was lost when only higher validity studies were analyzed.[10] Sir-Petermann, et al. demonstrated a significantly higher prevalence of SGA newborns among infants of PCOS mothers which could not be completely attributed to pregnancy complications, and seemed to be more related to the PCOS condition of the mother.[52] But other studies have failed to show any relation between preterm births and PCOS.
Treatment of complications in polycystic ovary syndrome
Insulin resistance, both intrinsically and that due to superimposed obesity, forms the most important pathogenetic mechanism for PCOS complications. It therefore seems logical to treat PCOS complications with insulin sensitizers. Metformin is the commonly used insulin sensitizer. Glueck, et al. documented the benefit of metformin for reducing EPL in an initial pilot study of PCOS women comparing with that of historic controls.[53] They later showed similar results in a larger uncontrolled cohort.[54] Their findings using metformin were confirmed in a retrospective controlled study by Jakubowicz, et al.[12] Metformin exerts its action by reducing body weight, insulin and PAI-1 levels,[26,53] androgen and LH levels,[55] and by increasing serum IGFBP-1 levels and glycodelin levels.[56] Eng, et al. demonstrated that metformin activates AMP kinase (AMPK) directly improving insulin signaling within the blastocyst, leading to improved pregnancy outcomes.[57]
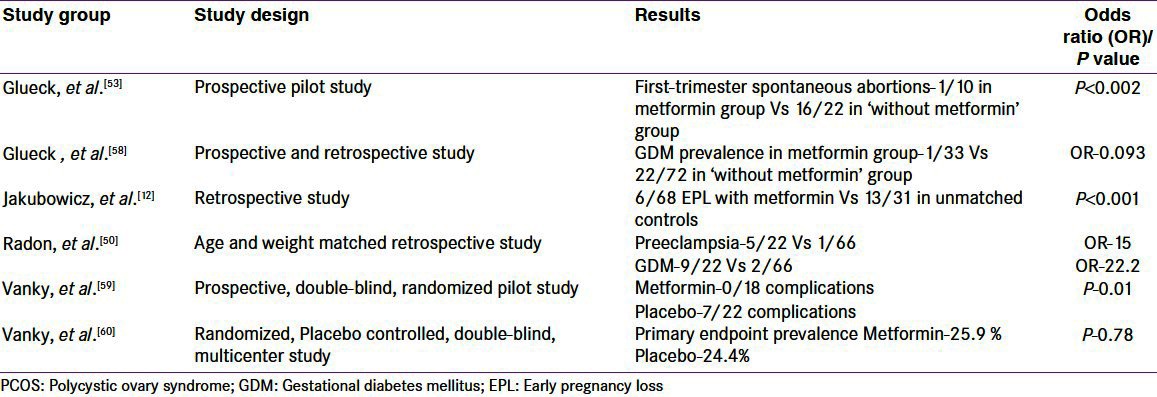
Most studies which showed the benefit of metformin in reducing EPL were either observational studies or non-randomized trials, unadjusted for major confounders and included small number of subjects [Table 1]. There is still insufficient evidence for use of metformin during pregnancy. Palomb,a et al. evaluated the effect of pregestational metformin administration on miscarriage risk in PCOS women by conducting a systematic review of randomized controlled trials till date and carrying out subsequent meta-analysis. They concluded that metformin has no effect on the miscarriage risk in PCOS women when administered before pregnancy. Although the safety of metformin for fetus in pregnancy has been documented in many studies, its use in pregnancy persists to be a contentious issue. Currently, metformin has been recognized by FDA as a class B for use in pregnancy, which means that either animal-reproduction studies have not shown a fetal risk without corresponding controlled studies in women, or animal studies have shown an adverse effect not confirmed by controlled studies in women.
Table 1.
Characteristics of important studies assessing the effect of metformin on pregnancy complications in PCOS women
The use of metformin for control of glucose intolerance in PCOS remains a controversial issue. In the recent Metformin in Gestational Diabetes (MIG) trial comparing metformin and insulin treatment in GDM, there was no significant difference in the composite fetal outcome between the metformin and insulin groups.[61] Moreover, metformin therapy during pregnancy in PCOS women was found to result in reduced incidence of GDM and shown not to have any adverse effect on infant's birth weight, height, and motor and social development at 3 and 6 months of life.[54] Khattab, et al. compared the incidences of GDM and preeclampsia in PCOS women continuing metformin throughout pregnancy with that of those who discontinued metformin use at the time of conception.[62] There was statistically significant reduction in the incidence of GDM (OR 0.17) and preeclampsia (OR 0.35) in favor of group continuous metformin throughout pregnancy.
Vanky, et al. initially did a pilot study on metformin's utility in pregnancy of PCOS women, which showed a reduced rate of severe pregnancy complications when it was taken throughout pregnancy.[59] They later followed it up with a randomized, placebo-controlled, double-blind, multicenter study to investigate the effect of metformin on pregnancy complications and pregnancy outcome in PCOS women.[60] There were no differences between the metformin and placebo groups in the primary outcome of the prevalence of preeclampsia, preterm delivery, GDM, or the composite of these three pregnancy complications. Women in the metformin group gained less weight during pregnancy compared with those in the placebo group. There was no difference in fetal birth weight between the groups. They found no evidence for use of metformin throughout all trimesters to reduce pregnancy complications in PCOS women.
The third ESHRE/ASRM-sponsored PCOS consensus workshop on women's health aspects of PCOS recommended that there is no evidence for improved live-birth rates or decreased pregnancy complications with the use of metformin either before conception or during pregnancy.[63] Currently, metformin is indicated in PCOS women with impaired glucose tolerance who does not respond adequately to calorie restriction and lifestyle changes. In the circumstance of PCOS woman becoming pregnant while being on metformin therapy, it would be advisable to stop metformin once pregnancy is confirmed. Further high-level evidences from large-scale well-powered, placebo-controlled randomized trials are required for formulation of recommendations for management of pregnancy of PCOS women.
CONCLUSIONS
Women with PCOS are at increased risk of adverse pregnancy and birth outcomes and may need increased surveillance during pregnancy and parturition. There is no evidence for benefit of metformin in management of these pregnancy complications pending further well-powered placebo-controlled randomized trials analyzing it.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. Positions statement: Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: An androgen excess society guideline. J Clin Endocrinol Metab. 2006;91:4237–45. doi: 10.1210/jc.2006-0178. [DOI] [PubMed] [Google Scholar]
- 3.Kumarapeli V, Seneviratne Rde A, Wijeyaratne CN, Yapa RM, Dodampahala SH. A simple screening approach for assessing community prevalence and phenotype of polycystic ovary syndrome in a semi-urban population in Sri Lanka. Am J Epidemiol. 2008;168:321–8. doi: 10.1093/aje/kwn137. [DOI] [PubMed] [Google Scholar]
- 4.Mehrabian F, Khani B, Kelishadi R, Ghanbari E. The prevalence of polycystic ovary syndrome in Iranian women based on different diagnostic criteria. Endokrynol Pol. 2011;62:238–42. [PubMed] [Google Scholar]
- 5.Barbour LA, Shao J, Qiao L, Pulawa LK, Jensen DR, Bartke A, et al. Human placental growth hormone causes severe insulin resistance in transgenic mice. Am J Obstet Gynecol. 2002;186:512–7. doi: 10.1067/mob.2002.121256. [DOI] [PubMed] [Google Scholar]
- 6.Catalano PM, Huston L, Amini SB, Kalhan SC. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol. 1999;180:903–16. doi: 10.1016/s0002-9378(99)70662-9. [DOI] [PubMed] [Google Scholar]
- 7.Okuno S, Akazawa S, Yasuhi I, Kawasaki E, Matsumoto K, Yamasaki H, et al. Decreased expression of the GLUT4 glucose transporter protein in adipose tissue during pregnancy. Horm Metab Res. 1995;27:231–4. doi: 10.1055/s-2007-979946. [DOI] [PubMed] [Google Scholar]
- 8.Friedman JE, Ishizuka T, Shao J, Huston L, Highman T, Catalano P. Impaired glucose transport and insulin receptor tyrosine phosphorylation in skeletal muscle from obese women with gestational diabetes. Diabetes. 1999;48:1807–14. doi: 10.2337/diabetes.48.9.1807. [DOI] [PubMed] [Google Scholar]
- 9.Roos N, Kieler H, Sahlin L, Ekman-Ordeberg G, Falconer H, Stephansson O. Risk of adverse pregnancy outcomes in women with polycystic ovary syndrome: Population based cohort study. BMJ. 2011;343:d6309. doi: 10.1136/bmj.d6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boomsma CM, Eijkemans MJ, Hughes EG, Visser GH, Fauser BC, Macklon NS. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update. 2006;12:673–83. doi: 10.1093/humupd/dml036. [DOI] [PubMed] [Google Scholar]
- 11.Kjerulff LE, Sanchez-Ramos L, Duffy D. Pregnancy outcomes in women with polycystic ovary syndrome: A metaanalysis. Am J Obstet Gynecol. 2011;204:558. doi: 10.1016/j.ajog.2011.03.021. e1-6. [DOI] [PubMed] [Google Scholar]
- 12.Jakubowicz DJ, Iuorno MJ, Jakubowicz S, Roberts KA, Nestler JE. Effects of metformin on early pregnancy loss in the polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87:524–9. doi: 10.1210/jcem.87.2.8207. [DOI] [PubMed] [Google Scholar]
- 13.Gray RH, Wu LY. Subfertility and risk of spontaneous abortion. Am J Public Health. 2000;90:1452–4. doi: 10.2105/ajph.90.9.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Homburg R. Pregnancy complications in PCOS. Best Pract Res Clin Endocrinol Metab. 2006;20:281–92. doi: 10.1016/j.beem.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Regan L, Owen EJ, Jacobs HS. Hypersecretion of luteinising hormone, infertility, and miscarriage. Lancet. 1990;336:1141–4. doi: 10.1016/0140-6736(90)92765-a. [DOI] [PubMed] [Google Scholar]
- 16.Homburg R, Levy T, Berkovitz D, Farchi J, Feldberg D, Ashkenazi J, et al. Gonadotropin-releasing hormone agonist reduces the miscarriage rate for pregnancies achieved in women with polycystic ovarian syndrome. Fertil Steril. 1993;59:527–31. doi: 10.1016/s0015-0282(16)55794-x. [DOI] [PubMed] [Google Scholar]
- 17.Balen AH, Tan SL, MacDougall J, Jacobs HS. Miscarriage rates following in vitro fertilization are increased in women with polycystic ovaries and reduced by pituitary desensitization with buserelin. Hum Reprod. 1993;8:959–64. doi: 10.1093/oxfordjournals.humrep.a138174. [DOI] [PubMed] [Google Scholar]
- 18.Clifford K, Rai R, Watson H, Franks S, Regan L. Does suppressing luteinising hormone secretion reduce the miscarriage rate. Results of a randomised controlled trial? BMJ. 1996;312:1508–11. doi: 10.1136/bmj.312.7045.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rai R, Backos M, Rushworth F, Regan L. Polycystic ovaries and recurrent miscarriage-a reappraisal. Hum Reprod. 2000;15:612–5. doi: 10.1093/humrep/15.3.612. [DOI] [PubMed] [Google Scholar]
- 20.Aksoy S, Celikkanat H, Senöz S, Gökmen O. The prognostic value of serum estradiol, progesterone, testosterone and free testosterone levels in detecting early abortions. Eur J Obstet Gynecol Reprod Biol. 1996;67:5–8. doi: 10.1016/0301-2115(96)02421-9. [DOI] [PubMed] [Google Scholar]
- 21.Tulppala M, Stenman UH, Cacciatore B, Ylikorkala O. Polycystic ovaries and levels of gonadotrophins and androgens in recurrent miscarriage: Prospective study in 50 women. Br J Obstet Gynaecol. 1993;100:348–52. doi: 10.1111/j.1471-0528.1993.tb12978.x. [DOI] [PubMed] [Google Scholar]
- 22.Okon MA, Laird SM, Tuckerman EM, Li TC. Serum androgen levels in women who have recurrent miscarriages and their correlation with markers of endometrial function. Fertil Steril. 1998;69:682–90. doi: 10.1016/s0015-0282(98)00007-7. [DOI] [PubMed] [Google Scholar]
- 23.Apparao KBC, Lovely LP, Gui Y, Lininger RA, Lessey BA. Elevated endometrial androgen receptor expression in women with polycystic ovarian syndrome. Biol Reprod. 2002;66:297–304. doi: 10.1095/biolreprod66.2.297. [DOI] [PubMed] [Google Scholar]
- 24.Cermik D, Karaca M, Taylor HS. HOXA10 expression is repressed by progesterone in the myometrium: Differential tissue-specific regulation of HOX gene expression in the reproductive tract. J Clin Endocrinol Metab. 2001;86:3387–92. doi: 10.1210/jcem.86.7.7675. [DOI] [PubMed] [Google Scholar]
- 25.Glueck CJ, Wang P, Fontaine RN, Sieve-Smith L, Tracy T, Moore SK. Plasminogen activator inhibitor activity: An independent risk factor for the high miscarriage rate during pregnancy in women with polycystic ovary syndrome. Metab Clin Exp. 1999;48:1589–95. doi: 10.1016/s0026-0495(99)90250-0. [DOI] [PubMed] [Google Scholar]
- 26.Palomba S, Orio F, Jr, Falbo A, Russo T, Tolino A, Zullo F. Plasminogen activator inhibitor 1 and miscarriage after metformin treatment and laparoscopic ovarian drilling in patients with polycystic ovary syndrome. Fertil Steril. 2005;84:761–5. doi: 10.1016/j.fertnstert.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 27.Glueck CJ, Sieve L, Zhu B, Wang P. Plasminogen activator inhibitor activity, 4G5G polymorphism of the plasminogen activator inhibitor 1 gene, and first-trimester miscarriage in women with polycystic ovary syndrome. Metab Clin Exp. 2006;55:345–52. doi: 10.1016/j.metabol.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Dunaif A. Insulin resistance and the polycystic ovary syndrome: Mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 29.Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;38:1165–74. doi: 10.2337/diab.38.9.1165. [DOI] [PubMed] [Google Scholar]
- 30.Chi MM, Schlein AL, Moley KH. High insulin-like growth factor 1 (IGF-1) and insulin concentrations trigger apoptosis in the mouse blastocyst via down-regulation of the IGF-1 receptor. Endocrinology. 2000;141:4784–92. doi: 10.1210/endo.141.12.7816. [DOI] [PubMed] [Google Scholar]
- 31.Hinck L, Thissen JP, De Hertogh R. Identification of caspase-6 in rat blastocysts and its implication in the induction of apoptosis by high glucose. Biol Reprod. 2003;68:1808–12. doi: 10.1095/biolreprod.102.010009. [DOI] [PubMed] [Google Scholar]
- 32.Mioni R, Chiarelli S, Xamin N, Zuliani L, Granzotto M, Mozzanega B, et al. Evidence for the presence of glucose transporter 4 in the endometrium and its regulation in polycystic ovary syndrome patients. J Clin Endocrinol Metab. 2004;89:4089–96. doi: 10.1210/jc.2003-032028. [DOI] [PubMed] [Google Scholar]
- 33.Lessey BA. Adhesion molecules and implantation. J Reprod Immunol. 2002;55:101–12. doi: 10.1016/s0165-0378(01)00139-5. [DOI] [PubMed] [Google Scholar]
- 34.Lee C-L, Lam KKW, Koistinen H, Seppala M, Kurpisz M, Fernandez N, et al. Glycodelin-A as a paracrine regulator in early pregnancy. J Reprod Immunol. 2011;90:29–34. doi: 10.1016/j.jri.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Jakubowicz DJ, Essah PA, Seppälä M, Jakubowicz S, Baillargeon JP, Koistinen R, et al. Reduced serum glycodelin and insulin-like growth factor-binding protein-1 in women with polycystic ovary syndrome during first trimester of pregnancy. J Clin Endocrinol Metab. 2004;89:833–9. doi: 10.1210/jc.2003-030975. [DOI] [PubMed] [Google Scholar]
- 36.Metwally M, Ong KJ, Ledger WL, Li TC. Does high body mass index increase the risk of miscarriage after spontaneous and assisted conception. A meta-analysis of the evidence? Fertil Steril. 2008;90:714–26. doi: 10.1016/j.fertnstert.2007.07.1290. [DOI] [PubMed] [Google Scholar]
- 37.Sandhu MS, Gibson JM, Heald AH, Dunger DB, Wareham NJ. Association between insulin-like growth factor-I: Insulin-like growth factor-binding protein-1 ratio and metabolic and anthropometric factors in men and women. Cancer Epidemiol Biomarkers Prev. 2004;13:166–70. doi: 10.1158/1055-9965.epi-130-3. [DOI] [PubMed] [Google Scholar]
- 38.Kelly CJ, Stenton SR, Lashen H. Insulin-like growth factor binding protein-1 in PCOS: A systematic review and meta-analysis. Hum Reprod Update. 2011;17:4–16. doi: 10.1093/humupd/dmq027. [DOI] [PubMed] [Google Scholar]
- 39.Wang JX, Davies MJ, Norman RJ. Polycystic ovarian syndrome and the risk of spontaneous abortion following assisted reproductive technology treatment. Hum Reprod. 2001;16:2606–9. doi: 10.1093/humrep/16.12.2606. [DOI] [PubMed] [Google Scholar]
- 40.Veltman-Verhulst SM, van Haeften TW, Eijkemans MJC, de Valk HW, Fauser BC, Goverde AJ. Sex hormone-binding globulin concentrations before conception as a predictor for gestational diabetes in women with polycystic ovary syndrome. Hum Reprod. 2010;25:3123–8. doi: 10.1093/humrep/deq272. [DOI] [PubMed] [Google Scholar]
- 41.Urman B, Sarac E, Dogan L, Gurgan T. Pregnancy in infertile PCOD patients. Complications and outcome. J Reprod Med. 1997;42:501–5. [PubMed] [Google Scholar]
- 42.Li G, Fan L, Zhang L, Liu XW, Sun CJ, Zhang WY, et al. Clinical characteristics and perinatal outcomes of non-overweight/obese pregnant women with polycystic ovary syndrome. Zhonghua Yi Xue Za Zhi. 2011;91:2753–6. [PubMed] [Google Scholar]
- 43.Haakova L, Cibula D, Rezabek K, Hill M, Fanta M, Zivny J. Pregnancy outcome in women with PCOS and in controls matched by age and weight. Hum Reprod. 2003;18:1438–41. doi: 10.1093/humrep/deg289. [DOI] [PubMed] [Google Scholar]
- 44.Mikola M, Hiilesmaa V, Halttunen M, Suhonen L, Tiitinen A. Obstetric outcome in women with polycystic ovarian syndrome. Hum Reprod. 2001;16:226–9. doi: 10.1093/humrep/16.2.226. [DOI] [PubMed] [Google Scholar]
- 45.Turhan NO, Seçkin NC, Aybar F, Inegöl I. Assessment of glucose tolerance and pregnancy outcome of polycystic ovary patients. Int J Gynaecol Obstet. 2003;81:163–8. doi: 10.1016/s0020-7292(03)00003-1. [DOI] [PubMed] [Google Scholar]
- 46.Toulis KA, Goulis DG, Kolibianakis EM, Venetis CA, Tarlatzis BC, Papadimas I. Risk of gestational diabetes mellitus in women with polycystic ovary syndrome: A systematic review and a meta-analysis. Fertil Steril. 2009;92:667–77. doi: 10.1016/j.fertnstert.2008.06.045. [DOI] [PubMed] [Google Scholar]
- 47.Roberts JM, Pearson G, Cutler J, Lindheimer M. Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertension. 2003;41:437–45. doi: 10.1161/01.HYP.0000054981.03589.E9. [DOI] [PubMed] [Google Scholar]
- 48.Diamant YZ, Rimon E, Evron S. High incidence of preeclamptic toxemia in patients with polycystic ovarian disease. Eur J Obstet Gynecol Reprod Biol. 1982;14:199–204. doi: 10.1016/0028-2243(82)90097-1. [DOI] [PubMed] [Google Scholar]
- 49.Radon PA, McMahon MJ, Meyer WR. Impaired glucose tolerance in pregnant women with polycystic ovary syndrome. Obstet Gynecol. 1999;94:194–7. doi: 10.1016/s0029-7844(99)00252-5. [DOI] [PubMed] [Google Scholar]
- 50.de Vries MJ, Dekker GA, Schoemaker J. Higher risk of preeclampsia in the polycystic ovary syndrome: A case control study. Eur J Obstet Gynecol Reprod Biol. 1998;76:91–5. doi: 10.1016/s0301-2115(97)00164-4. [DOI] [PubMed] [Google Scholar]
- 51.Ghazeeri GS, Nassar AH, Younes Z, Awwad JT. Pregnancy outcomes and the effect of metformin treatment in women with Polycystic Ovary Syndrome: An overview. Acta Obstet Gynecol Scand. 2012;91:658–78 [Epub ahead of print]. doi: 10.1111/j.1600-0412.2012.01385.x. [DOI] [PubMed] [Google Scholar]
- 52.Sir-Petermann T, Hitchsfeld C, Maliqueo M, Codner E, Echiburú B, Gazitúa R, et al. Birth weight in offspring of mothers with polycystic ovarian syndrome. Hum Reprod. 2005;20:2122–6. doi: 10.1093/humrep/dei009. [DOI] [PubMed] [Google Scholar]
- 53.Glueck CJ, Phillips H, Cameron D, Sieve-Smith L, Wang P. Continuing metformin throughout pregnancy in women with polycystic ovary syndrome appears to safely reduce first-trimester spontaneous abortion: A pilot study. Fertil Steril. 2001;75:46–52. doi: 10.1016/s0015-0282(00)01666-6. [DOI] [PubMed] [Google Scholar]
- 54.Glueck CJ, Wang P, Goldenberg N, Sieve-Smith L. Pregnancy outcomes among women with polycystic ovary syndrome treated with metformin. Hum Reprod. 2002;17:2858–64. doi: 10.1093/humrep/17.11.2858. [DOI] [PubMed] [Google Scholar]
- 55.Orio F, Jr, Palomba S, Cascella T, De Simone B, Manguso F, Savastano S, et al. Improvement in endothelial structure and function after metformin treatment in young normal-weight women with polycystic ovary syndrome: Results of a 6-month study. J Clin Endocrinol Metab. 2005;90:6072–6. doi: 10.1210/jc.2005-0965. [DOI] [PubMed] [Google Scholar]
- 56.Jakubowicz DJ, Seppälä M, Jakubowicz S, Rodriguez-Armas O, Rivas-Santiago A, Koistinen H, et al. Insulin reduction with metformin increases luteal phase serum glycodelin and insulin-like growth factor-binding protein 1 concentrations and enhances uterine vascularity and blood flow in the polycystic ovary syndrome. J Clin Endocrinol Metab. 2001;86:1126–33. doi: 10.1210/jcem.86.3.7295. [DOI] [PubMed] [Google Scholar]
- 57.Eng GS, Sheridan RA, Wyman A, Chi MM, Bibee KP, Jungheim ES, et al. AMP kinase activation increases glucose uptake, decreases apoptosis, and improves pregnancy outcome in embryos exposed to high IGF-I concentrations. Diabetes. 2007;56:2228–34. doi: 10.2337/db07-0074. [DOI] [PubMed] [Google Scholar]
- 58.Glueck C, Wang P, Kobayashi S, Phillips H, Sieve-Smith L. Metformin therapy throughout pregnancy reduces the development of gestational diabetes in women with polycystic ovary syndrome. Fertil Steril. 2002;77:520–5. doi: 10.1016/s0015-0282(01)03202-2. [DOI] [PubMed] [Google Scholar]
- 59.Vanky E, Salvesen KA, Heimstad R, Fougner KJ, Romundstad P, Carlsen SM, et al. Metformin reduces pregnancy complications without affecting androgen levels in pregnant polycystic ovary syndrome women: Results of a randomized study. Hum Reprod. 2004;19:1734–40. doi: 10.1093/humrep/deh347. [DOI] [PubMed] [Google Scholar]
- 60.Vanky E, Stridsklev S, Heimstad R, Romundstad P, Skogoy K, Kleggetveit O, et al. Metformin versus placebo from first trimester to delivery in polycystic ovary syndrome: A randomized, controlled multicenter study. J Clin Endocrinol Metab. 2010;95:E448–55. doi: 10.1210/jc.2010-0853. [DOI] [PubMed] [Google Scholar]
- 61.Rowan JA, Gao W, Hague WM, McIntyre HD. Glycemia and its relationship to outcomes in the metformin in gestational diabetes trial. Diabetes Care. 2010;33:9–16. doi: 10.2337/dc09-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khattab S, Mohsen IA, Aboul Foutouh I, Ashmawi HS, Mohsen MN, van Wely M, et al. Can metformin reduce the incidence of gestational diabetes mellitus in pregnant women with polycystic ovary syndrome. Prospective cohort study? Gynecol Endocrinol. 2011;27:789–93. doi: 10.3109/09513590.2010.540600. [DOI] [PubMed] [Google Scholar]
- 63.Fauser BCJM, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, et al. Consensus on women's health aspects of polycystic ovary syndrome (PCOS): The Amsterdam ESHRE/ASRM-Sponsored 3 rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97:28–38. doi: 10.1016/j.fertnstert.2011.09.024. [DOI] [PubMed] [Google Scholar]


