Abstract
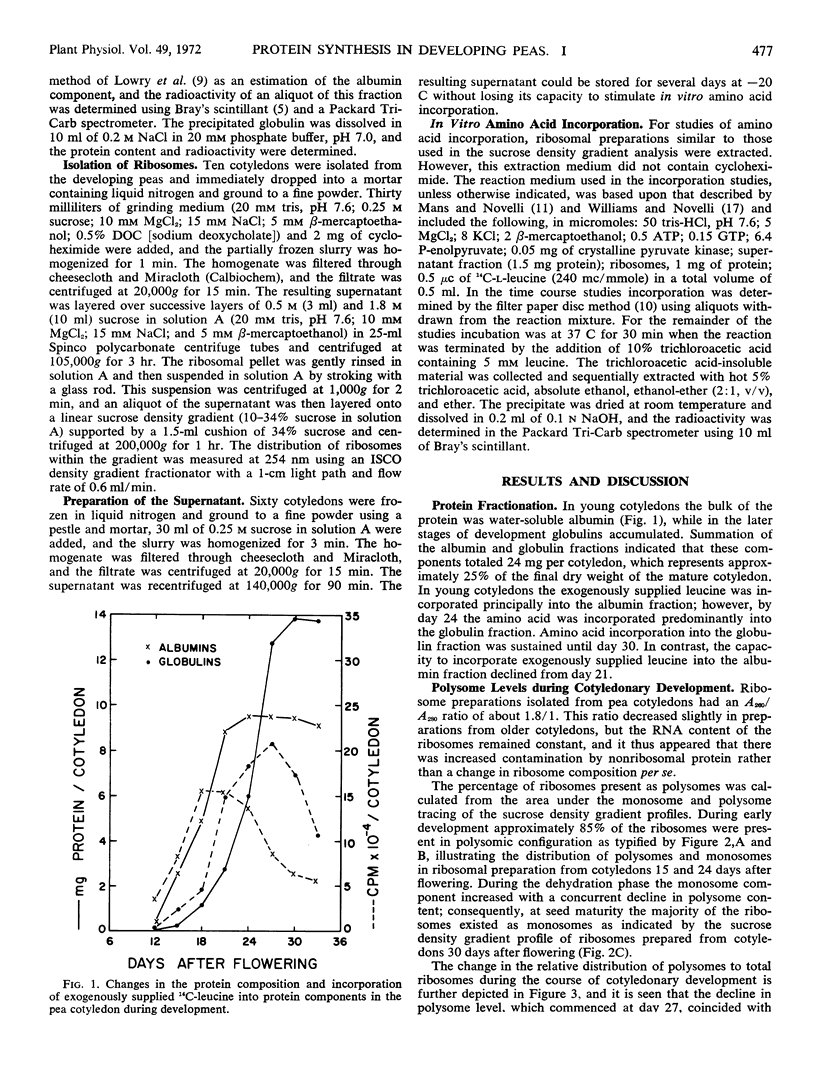
The changes in protein content of pea cotyledons have been followed during the period from 9 to 33 days after flowering. Initially protein content increased gradually with a rapid period of deposition occurring between days 21 and 27 after flowering. After the 28th day the rate of accumulation of protein declined as the seed dehydrated and matured. At maturity the pea cotyledon contained approximately 25% protein which was divided into albumins and globulins in the ratio of 1:1.4.
Analytical data and the incorporation of exogenously supplied 14C-leucine indicated that albumins were synthesized early in cotyledon development whereas globulin synthesis predominated with increasing maturity.
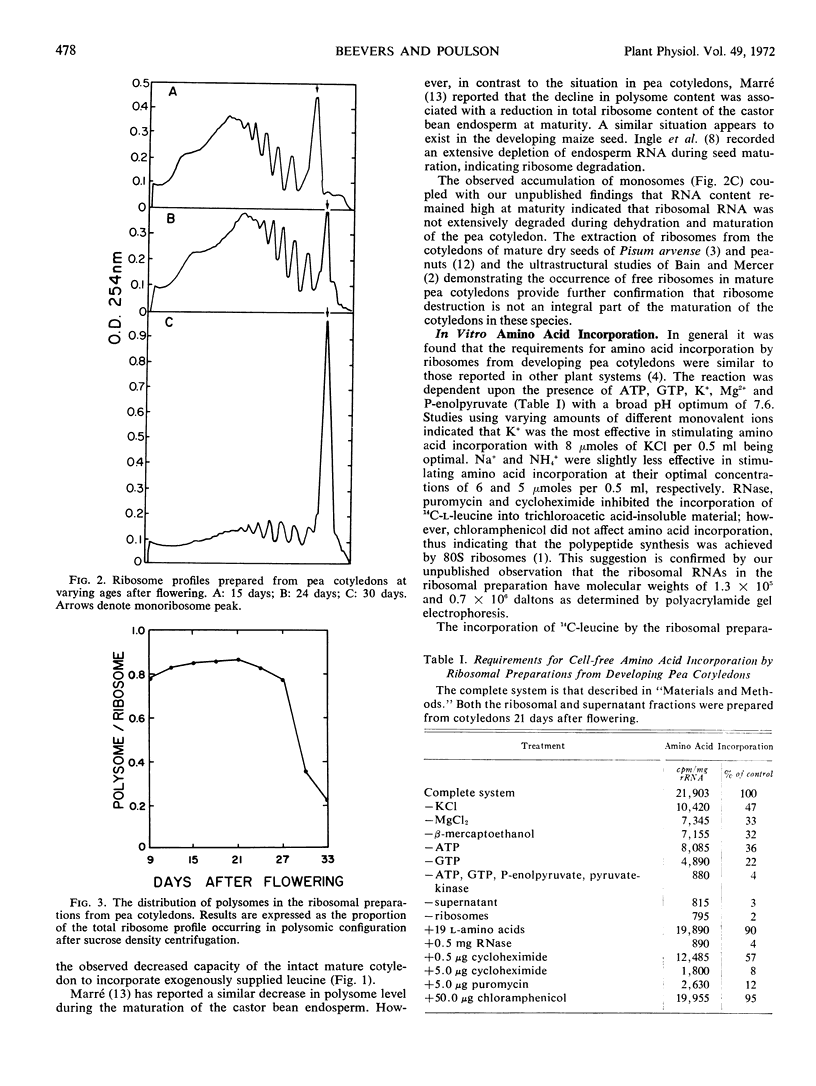
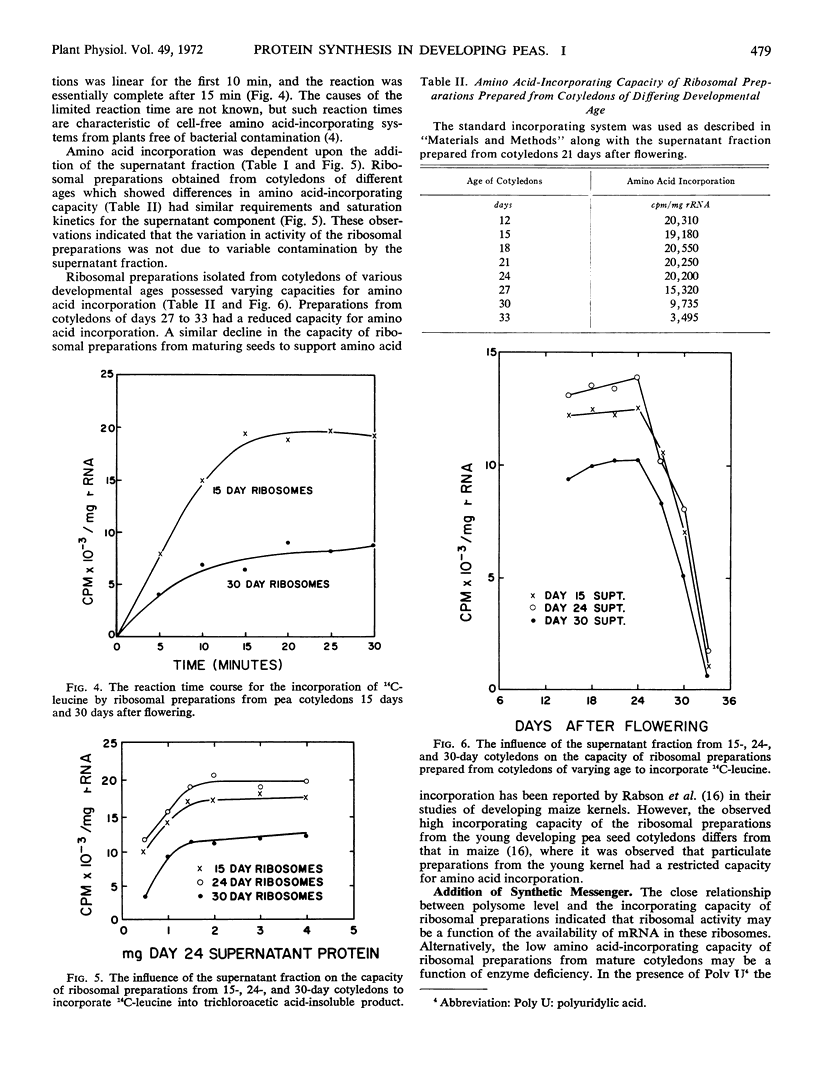
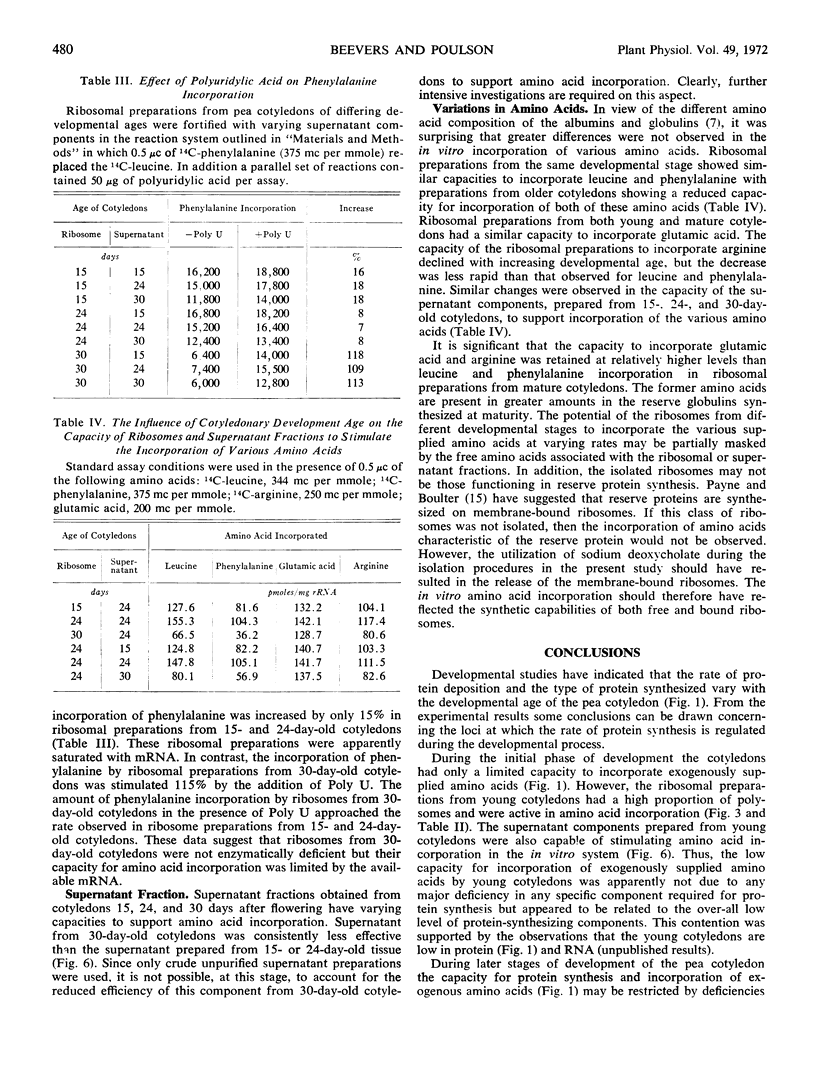
Ribosomal preparations extracted from seeds during the period of rapid protein synthesis contained a high percentage of polysomes. Preparations from older cotyledons with a declining capacity for protein synthesis had few polysomes and an abundance of monosomes. The amino acid-incorporating capacity of ribosomal preparations from cotyledons of varying age was related to the polysomic content. The phenylalanine-incorporating capacity of ribosomal preparations from mature pea seed could be stimulated by the addition of polyuridylic acid. The distribution of polysomes and the in vitro incorporation data suggested that protein synthesis could be partially restricted by the availability of messenger RNA at maturity.
However, reciprocal mixing experiments of supernatant and ribosomal fractions from cotyledons of different developmental age indicated that the supernatant fractions have varying capacities to stimulate in vitro amino acid incorporation. Thus the possibility of the regulation of protein synthesis at the translational level was not precluded.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. A., Smillie R. M. Binding of chloramphenicol by ribosomes from chloroplasts. Biochem Biophys Res Commun. 1966 May 25;23(4):535–539. doi: 10.1016/0006-291x(66)90762-5. [DOI] [PubMed] [Google Scholar]
- Barker G. R., Rieber M. The development of polysomes in the seed of Pisum arvense. Biochem J. 1967 Dec;105(3):1195–1202. doi: 10.1042/bj1051195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsson C. E. Seed globulins of the Gramineae and Leguminosae. Biochem J. 1949;44(4):387–400. doi: 10.1042/bj0440387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingle J., Beitz D., Hageman R. H. Changes in Composition during Development and Maturation of Maize Seeds. Plant Physiol. 1965 Sep;40(5):835–839. doi: 10.1104/pp.40.5.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES R. W., BABCOCK G. E., TAYLOR N. W., SENTI F. R. Molecular weights of wheat gluten fractions. Arch Biochem Biophys. 1961 Sep;94:483–488. doi: 10.1016/0003-9861(61)90076-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARCUS A., FEELEY J. PROTEIN SYNTHESIS IN IMBIBED SEEDS. II. POLYSOME FORMATION DURING IMBIBITION. J Biol Chem. 1965 Apr;240:1675–1680. [PubMed] [Google Scholar]
- RABSON R., MANS R. J., NOVELLI G. D. Changes in cell-free amino acid incorporating activity during maturation of maize kernels. Arch Biochem Biophys. 1961 Jun;93:555–562. doi: 10.1016/s0003-9861(61)80052-0. [DOI] [PubMed] [Google Scholar]
- Williams G. R., Novelli G. D. Ribosome changes following illumination of dark-grown plants. Biochim Biophys Acta. 1968 Jan 29;155(1):183–192. doi: 10.1016/0005-2787(68)90348-1. [DOI] [PubMed] [Google Scholar]


