Abstract
Aim:
Obesity is the most common cause of insulin resistance and metabolic syndrome (MS). These are the most important risk factors for coronary heart disease (CHD). No evidence exists regarding the prevalence of the MS in children in sSrinagar city of Kashmir India. We aimed to evaluate the prevalence of MS in 8–18-year-old school-going children of Kashmir, India.
Materials and Methods:
In this cross-sectional study, 758 respondents in 8–18 years of age were randomly selected using a simple random sampling method. The self-designed questionnaire was individually completed after receiving a written informed consent. The weight, height, waist circumference (WC), body mass index (BMI), and blood pressure were measured using standard tools. Ten milliliters of blood was taken for measuring lipid profile and fasting blood sugar (FBS) of the school children. We determined MS according to the modified Adult Treatment Panel III (ATP III) criteria.
Results:
The prevalence of the MS was 3.8% (boys: 3.9%, girls: 3.8%) and the prevalence of obesity was 9.9% (boys: 9.9%, girls: 10.6%) among the studied children. Obese subjects had the highest proportion of MS compared with those at risk for overweight and those with normal weight (30.7% vs. 2.5% and 0.5%, respectively; P = 0.000).
Conclusion:
The MS is prevalent even in young children, so we suggest screening programs for children aged 8–18 years to control obesity and MS in the developing world.
Keywords: Metabolic syndrome, obesity, prevalence, school-going children
INTRODUCTION
Individuals with metabolic syndrome (MS) are at increased risk for cardiovascular disease. MS is defined as a pattern of metabolic disturbances including central obesity, insulin resistance, hyperglycemia, dyslipidemia, and hypertension.[1] The third report of the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) recognized the MS as a secondary target of risk-reduction therapy.[2] Previous studies indicated that the process of atherosclerosis starts at an early age and is already linked to obesity and other components of the MS in childhood.[3] Although the prevalence[4,5] and associated risk factors of MS[6,7] have been extensively studied in adults, comparatively little emphasis has been laid on its prevalence in children and adolescents, such that there is no clear definition for the MS in this age group. MS in pediatric and adolescent population is not lagging far behind, as obesity has permeated into all strata of life and age groups. The studies conducted in developed countries have reported the overall prevalence of pediatric MS to be between 3.1% and 24%.[8] Based on limited reports in this field, 4.2% of American adolescents who participated in the National Health and Nutrition Examination Study (NHANES III) 1980–1992 had MS, a proportion that increased to 6.2% in NHANES 1999–2000.[9] MS is largely confined to overweight adolescents, such that 29% of overweight adolescents [body mass index (BMI)3 95th percentile for age and sex] in NHANES III 1980–1992 and 32% in NHANES 1999–2000 had this phenotype.[9] As childhood overweight increases, it is likely that the prevalence of MS in this age category will increase worldwide.[10] Recently, many studies have shown that the rate of the MS in children and adolescents has increased.[11–16] The main finding of those studies was that the prevalence of the syndrome was higher when the subjects were obese, and thus the researchers stressed that the prevalence of the MS would increase with the numbers of obese children and adolescents.[14,15] However, no standard exists for diagnosing this syndrome in children and adolescents, and it is difficult to compare the prevalence of the MS across nations and studies. The criteria used for diagnosing the MS vary. Hence, in view of high and increasing prevalence of obesity and type 2 diabetes in children and adolescents, there is an urgent need to establish internationally accepted criteria for the diagnosis of MS in children, and ethnic differences in obesity and metabolic parameters should be considered in that definition[17] The Quebec City study used skin thickness instead of waist circumference, mean blood pressure, and blood insulin,[18] while a study of Hungarian children used body fat instead of waist circumference.[19] In addition, different researchers have modified the MS criteria of the NCEP-ATP III differently for children and adolescents. Of the modified NCEP-ATP III criteria, those of Cook et al.,[11] Cruz and Goran,[12] and Ferranti et al.[13] are generally used. The three sets of criteria suggested a cut-off point for high blood pressure using the sex- and age-specific 90th percentile. Researchers worldwide preferred using NCEP-ATP III definition because it is relatively simple and much more clinically and practically applicable. In addition, NCEP-ATP III considers the proinflamatory status and prothrombotic status as components of MS.[20]
MATERIALS AND METHODS
This survey was a cross-sectional study carried out among children aged 8–18 years. Seven hundred and fifty-eight children were randomly selected using simple random sampling method. The study was approved by the Ethical Committee of Government Medical College Srinagar and its associated hospitals. The self-designed questionnaire was completed after receiving a written informed consent from all respondents and from their parents/guardians. Additionally, all children gave their verbal consent.
Weight was measured while the subjects were minimally clothed without shoes in upright position using digital scales and recorded to the nearest 0.1 kg. Height was measured in a standing position, without shoes, using a stadiometer and inch tape to the nearest 0.1 cm. BMI was calculated as weight in kilograms divided by height in meters squared. Waist circumference was measured in centimeters to the nearest of 0.1 cm using a non-stretchable measuring tape, without any pressure to body surface, at the narrowest level between the lower border of ribcage and iliac crest. To reduce subjective error, all measurements were taken by the same person. Data on family history of diabetes were collected from the subjects’ oral responses to the pretested questionnaire. The criterion for having family history of diabetes was respondents having history of diabetes in at least one first-degree relative diagnosed after 30 years of age. A qualified physician measured blood pressure two times with the subject in a seated position during physical examinations after one initial measurement for determining peak inflation level using a standard mercury sphygmomanometer. The mean of two measurements was considered to be the participant's blood pressure. The systolic blood pressure was defined as the appearance of the first sound (Korotkoff phase 1), and diastolic blood pressure was defined as the disappearance of the sound (Korotkoff phase 5) during deflation of the cuff at a 2–3 mm/s decrement rate of the mercury column. Fasting blood samples for the serum glucose and lipid profile were drawn after the subjects had fasted overnight. Fasting plasma glucose (FPG) was measured on the day of blood collection by the glucose oxidase peroxide (God Pod) method, and lipid profile was measured by enzymatic assay on a Hitchi 704 system auto-analyzer.
Obesity was determined based on BMI and the classification scheme introduced by the Centers for Disease Control and prevention (CDC). CDC charts were used for defining respondents who were at risk of overweight (BMI > 85th percentile) and obesity (BMI > 95th percentile). Abdominal obesity was defined using waist circumference percentiles by sex and age. Percentiles equal to or over 90 were defined as abdominal obesity.[4] Hypertension was detected using seventh Joint National Committee on Evaluation, Diagnosis, Treatment, and Prevention of Blood Pressure (JNC7) classification criteria. If blood pressure (systolic and diastolic) reading was at > 90th percentile based on sex and height, it was defined as pre-hypertension and readings >95 and >99 were defined as type I and II hypertension, respectively.[21]
Definition of the components of the metabolic syndrome
To allow for valid cross-study comparisons, we used the age-modified standards of the ATP III MS criteria published previously.[9,11,17] Subjects with three or more characteristics of the following components were categorized as having MS: 1) abdominal obesity (waist circumference ≥ the age- and sex-specific 90th percentile for this population); 2) elevated blood pressure (systolic and/or diastolic blood pressure ≥ the age-, sex-, and height-specific 90th percentile; 3) low high-density lipoprotein-cholesterol (HDL-C) level (≤40 mg/dL); 4) elevated serum triglycerides (TGs; ≥110 mg/dL); and 5) elevated FPG (≥110 mg/dL).
The exclusion criteria used were: 1) children who were suffering from any medical illness; 2) children who were taking hypolipidemic or hypoglycemic drugs; and 3) children who were not fasting.
Statistical methods
Entire data were described as mean ± standard deviation and percentage. All the intergroup comparisons for parametric data were done by Student's t-test, whereas non-parametric data were analyzed by Mann–Whitney U and Chi-square tests. P-value of ≤0.005 was considered significant. The software used was Statistical Package for Social Sciences (SPSS) 11.5, Minitab and MS Excel.
RESULTS
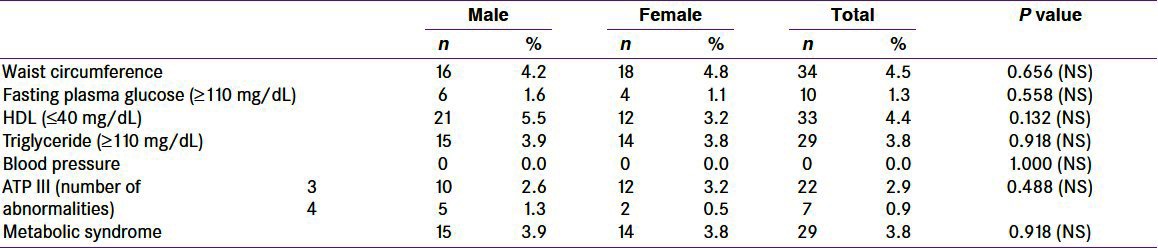
Total number of school children studied for the prevalence of MS in the age group 8–18 years was 758, among whom 385 (50.8%) were boys and 373 (49.2%) were girls; in our study, there was almost equal representation of boys and girls. Among the studied group, waist circumference was abnormal (≥90th percentile) in 34 (4.5%) children, [males = 16 (4.2%) and females = 18 (4.8%)] without any statistically significant difference (P-value = 0.656) between the two groups. Abnormal FPG (≥110 mg/dL) was present in 10 (1.3%) of the studied children; [males = 6 (1.6%) and females = 4 (1.1%)] with insignificant statistical difference (P-value = 0.558) between the two groups. HDL-C was abnormal in 33 (4.4%) children [males = 21 (5.5%) and females = 12 (3.2%)], with insignificant statistical difference (P-value = 0.132). TGs were abnormal in 29 (3.8%) among the studied children [males = 15 (3.9%) and females = 14 (3.8%)], with statistically insignificant difference (P-value = 0.918) between the two groups. No child in our study was having hypertension (P-value = 1.00) [Table 1]. In our study, prevalence of MS was seen in 29, i.e., 3.8% of the studied children [males = 15 (3.9%) and females = 14 (3.8%)], with statistically insignificant difference between the two groups (P-value = 0.918). Among the children with MS, 22 (2.9%) fulfilled three criteria and 7 (0.9%) showed four criteria [Table 1].
Table 1.
Adult treatment panel III criteria for metabolic syndrome among the studied school children (N = 758)
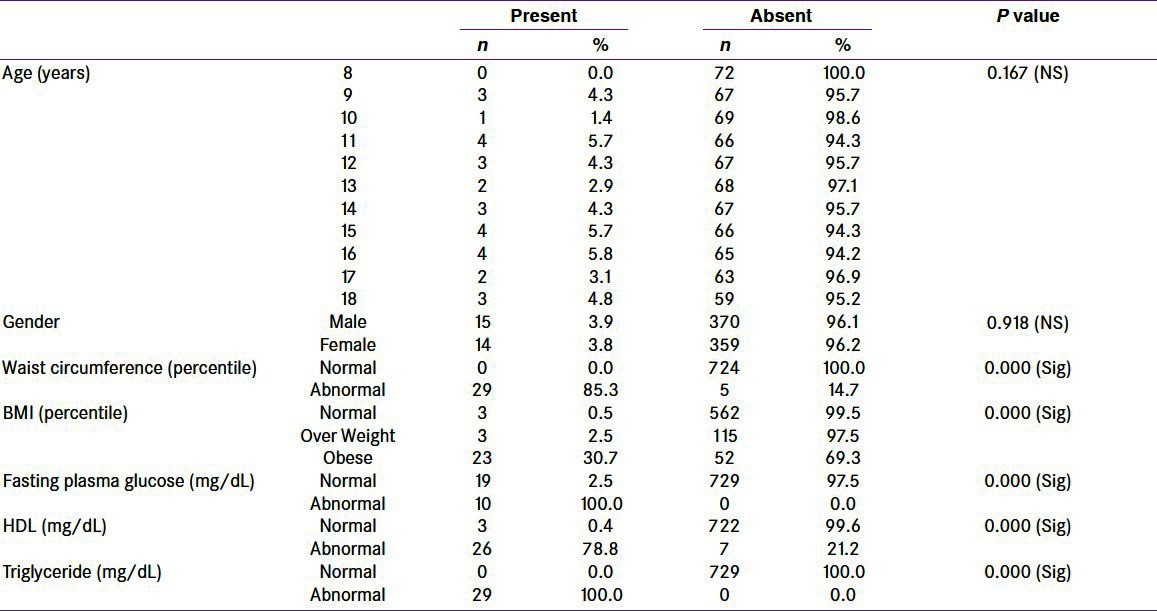
Among 34 studied children with abnormal waist circumference, MS was present in 29 (85.3%) with statistically significant difference (P-value = 0.000). Among 75 obese studied children, MS was present in 23 (30.7%) with statistically significant difference (P-value = 0.000), i.e., prevalence of MS increased from 0.5 to 30.7% with obesity. Among 33 children with abnormal HDL-C, 26 (78.8%) were having MS with statistically significant difference (P-value = 0.000). Similarly, among 10 children with abnormal FPG, MS was present in all of them with statistically significant difference (P-value = 0.000). Among 29 children with abnormal TGs, MS was present in all of them with statistically significant difference (P-value = 0.000). Overall, waist circumference and TGs were the most common parameters, whereas FPG was the least common parameter [Table 2].
Table 2.
Metabolic syndrome across the studied parameters
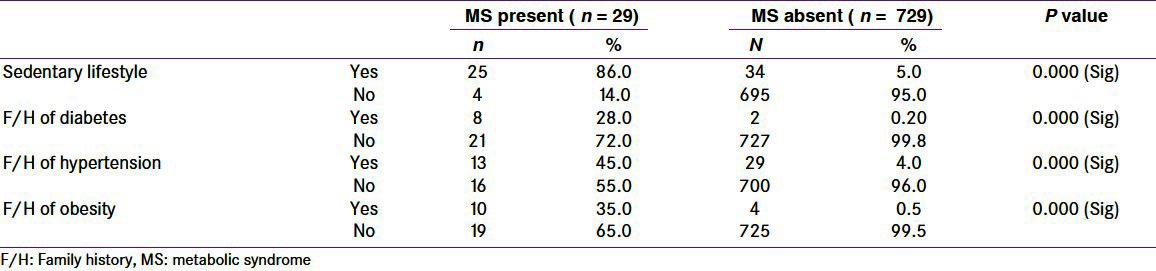
Among the studied children (N = 758), 10 (1.3%) had family history of diabetes, 42 (5.3%) had family history of hypertension, and 14 (1.8%) had family history of obesity. Among 29 children with MS, positive family history of diabetes was present in 8 (28%), positive family history of hypertension was present in 13 (45%), positive family history of obesity was present in 10 (35%), and positive history of sedentary lifestyle was present in 25 (86%). While among 729 children without MS, positive family history of diabetes was present in only 2 (0.2%), positive family history of hypertension was present in 29 (4%), positive family history of obesity was present in 4 (0.5%), and positive history of sedentary lifestyle was present in 34 (5%) [Table 3].
Table 3.
Metabolic syndrome across the studied parameters with sedentary life style and positive family risk factors
DISCUSSION
This study, conducted on an urban population of Kashmir, India, showed a prevalence of 3.8% of the MS among 8–18-year-old school children. This is the first study reporting the prevalence of the MS among adolescents in this part of world. The findings from our study provide evidence-based data on the considerable prevalence of childhood MS and its consequences in countries still grappling with the public health effects of malnutrition and micronutrient deficiency. This study, conducted in 8–18-year age group of school children of the Srinagar municipal limits showed that the MS is a common problem. The lack of previous studies about MS in Jammu and Kashmir makes it interesting to known the actual situation in one of the poorest states of the poorest country, and this would be an initial point for future studies regarding the MS. There are no clear definitions for MS in children and adolescents. The existing different criteria are used according to the place or population of the study. NCEP-ATP III definition with modified cut-off values for youth was considered for the diagnosis of the MS in the current study.
The overall prevalence of MS in the study was found to be 3.8% (males 3.9% and females 3.8%), with approximately equal sex distribution. Almost similar results were obtained with prevalence of 3.2% by Panamonta et al.[22] in a study in Thailand among the children aged between 10 and 15 years, 3.6% by Srinivasan et al.[23] in Bogalusa Heart study in 8–17 year olds, and about 2% by Kelishadi et al.[24] among the 6–18 -year-old children in Iran.
Cook et al.[11] in a study among 12–19-year-old adolescents found the prevalence of MS as per ATP III criteria was about 4.2%. Similarly, Sing et al.[25] found that the prevalence of MS among 12–17-year-old adolescents from North India was 4.2%, without any sex difference. Goodman et al.[26] found that the prevalence of MS among school children of Black, White, and Hispanic teens as per ATP III criteria was 4.2%. Similarly, Duncan et al.[9] reported the prevalence of MS in 12–19-year-old US adolescents as 4.2% in NHANES (1980–1992). These results are also nearly approximate to our finding. Our finding of almost equal sex distribution of MS (males 3.9% and females 3.8%) is consistent with the findings of Sing et al.,[25] Kelishada et al.,[24] and Lambert et al.[18]
The dramatic rise in childhood obesity in developing countries is considered a major driving force behind the increasing prevalence of MS. In our study, the prevalence of MS was 30.7% in obese children compared to only 0.5% in normal children. Almost similar results were obtained by Nasreddine et al.[27] (26.4%), Sarah et al.[8] (31.2%), Cruz et al.[28] (30%), Duncan et al.[9] (32.1%), and Cook et al.[11] (28.7%).
In a study conducted by Sing et al.[25] among North Indian adolescents, the prevalence of MS in obese children was found to be 36.6%, which was almost similar to our results. Different studies have shown that among the various risk factors, a positive family history of chronic diseases like obesity, diabetes, and/or hypertension in parents increases the risk of the MS in the children. Our study also reveals that the MS was more prevalent in those children having positive family history of diabetes (28% vs. 0.2%), hypertension (45.0% vs. 4.0%), and obesity (35% vs. 0.5%). Similar correlation has been found by Kelishadi et al.,[24] Lee et al.,[29] Juonala et al.,[30] and Wada et al.,[31] Similarly, history of sedentary lifestyle among children was associated with higher prevalence of MS. This is consistent with the finding of our study as well.
In conclusion, our study shows that MS is very well prevalent in this part of underdeveloped world and needs to be addressed on war-footed basis to prevent diabetes, hypertension, and coronary artery disease in later part of life. Therapeutic lifestyle modification, healthy food habits, maintenance of high level of physical activity and normal weight, and teachers'/parents' education about MS are the most important strategies. The high prevalence of insulin resistance in Asian Indian children who are undergoing nutritional and lifestyle transition rapidly is a worrisome feature.[17] Behavioral therapy in children needs to be included in the management plans of MS with due consideration to be given to parental involvement and reduction of inactivity, in addition to other components of behavioral therapy.[32] Recent studies established the role of probiotics and prebiotics “Pharmaco-nutritional” approach for the management of MS with possible mechanism of improving microbial balance, decreased food intake, decreased abdominal adiposity, and increased mucosal integrity with decreased inflammatory tone; however, more studies related to their role are required.[33] Thus, a multidisciplinary approach involving parents, teachers, and media is needed to address this problem.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Reaven GM. Banting lecture 1988.Role of insulin resistance in human disease. Diabetes. 1988;37:1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 2.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. Available from http://circ.ahajournals.org/content/106/25/3143.citation . [PubMed] [Google Scholar]
- 3.Berenson GS, Srinivasan SR, Bao W, Newman WP, 3rd, Tracy RE, Wattiqney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med. 1998;338:1650–6. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 4.Azizi F, Salehi P, Etemadi A, Zahedi-Asl S. Prevalence of metabolic syndrome in an urban population: Tehran Lipid and Glucose Study. Diabetes Res Clin Pract. 2003;61:29–37. doi: 10.1016/s0168-8227(03)00066-4. [DOI] [PubMed] [Google Scholar]
- 5.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: Findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–9. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 6.Esmaillzadeh A, Mirmiran P, Azizi F. Whole-grain consumption and the metabolic syndrome: A favorable association in Tehranian adults. Eur J Clin Nutr. 2005;59:353–62. doi: 10.1038/sj.ejcn.1602080. [DOI] [PubMed] [Google Scholar]
- 7.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–28. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 8.Pandit K, Goswami S, Ghosh S, Mukhopadhyag P, Chowdhury S. Metabolic syndrome in south Asian. Indian J Endocrinol Metab. 2012;16:44–55. doi: 10.4103/2230-8210.91187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duncan GE, Li SM, Zhou XH. Prevalence and trends of a metabolic syndrome phenotype among U.S. adolescents, 1999-2000. Diabetes Care. 2004;27:2438–43. doi: 10.2337/diacare.27.10.2438. [DOI] [PubMed] [Google Scholar]
- 10.Troiano RP, Flegal KM. Overweight children and adolescents: Description, epidemiology, and demographics. Pediatrics. 1998;101:497–504. [PubMed] [Google Scholar]
- 11.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: Findings from the third National Health and Nutrition Examination Survey, 1988-1994. Arch Pediatr Adolesc Med. 2003;157:821–7. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- 12.Cruz ML, Goran MI. The metabolic syndrome in children and adolescents. Curr Diab Rep. 2004;4:53–62. doi: 10.1007/s11892-004-0012-x. [DOI] [PubMed] [Google Scholar]
- 13.de Ferranti SD, Gauvreau K, Ludwig DS, Neufeld EJ, Newburger JW, Rifai N. Prevalence of the metabolic syndrome in American adolescents: Findings from the Third National Health and Nutrition Examination Survey. Circulation. 2004;110:2494–7. doi: 10.1161/01.CIR.0000145117.40114.C7. [DOI] [PubMed] [Google Scholar]
- 14.Sung EJ. A metabolic syndrome phenotype in Korean children and adolescents: Prevalence and change in characteristics over the period 1998-2001 [dissertation] Seoul: Seoul National Univ. 2005 [Google Scholar]
- 15.Chang JH, Kim DH, Kim HS, Choi IK, Cheong MY, Kim DK. Prevalence of metabolic syndrome in obese children. Korean J Pediatr. 2004;47:1149–56. [Google Scholar]
- 16.Park HS, Han JH, Choi KM, Kim SM. Relation between elevated serum alanine aminotransferase and metabolic syndrome in Korean adolescents. Am J Clin Nutr. 2005;82:1046–51. doi: 10.1093/ajcn/82.5.1046. [DOI] [PubMed] [Google Scholar]
- 17.Ganie MA. Metabolic syndrome in Indian children - An alarming rise. Indian J Endocrinol Metab. 2010;14:1–2. [PMC free article] [PubMed] [Google Scholar]
- 18.Lambert M, Paradis G, O'Loughlin J, Delvin EE, Hanley JA, Levy E. Insulin resistance syndrome in a representative sample of children and adolescents from Quebec, Canada. Int J Obes Relat Metab Disord. 2004;28:833–41. doi: 10.1038/sj.ijo.0802694. [DOI] [PubMed] [Google Scholar]
- 19.Csábi G, Török K, Jeges S, Molnár D. Presence of metabolic cardiovascular syndrome in obese children. Eur J Pediatr. 2000;159:91–4. doi: 10.1007/pl00013812. [DOI] [PubMed] [Google Scholar]
- 20.Parikh RM, Mohan V. Changing definition of metabolic syndrome. Indian J Endocrinol Metab. 2012;16:7–12. doi: 10.4103/2230-8210.91175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barkail, Paragh G. Metabolic syndrome in childhood and adolescence. Orv Hetil. 2006;1217:943–50. [PubMed] [Google Scholar]
- 22.Panamonta O, Thamsiri N, Panamonta M. Prevalance of type II diabetes and metabolic syndrome among overweight school children in Khon Kaen, Thailand. J Med Assoc Thai. 2010;93:56–60. [PubMed] [Google Scholar]
- 23.Srinivasan SR, Meyers L, Berenson GS. Predictability of childhood adiposity and insulin for developing insulin resistance syndrome (Syndrome x) in young childhood: The Bogalusa Heart study. Diabetes. 2002;51:204–9. doi: 10.2337/diabetes.51.1.204. [DOI] [PubMed] [Google Scholar]
- 24.Kelishadi R, Ardalan G, Gheiratmand R, Adeli K, Delavari A, Majdzadeh R. Pediatric metabolic syndrome and associated anthropometric indices: CASPIAN study. Acta Paediatr. 2006;95:1625–34. doi: 10.1080/08035250600750072. [DOI] [PubMed] [Google Scholar]
- 25.Singh R, Bhansali A, Sialy R, Aggarwal A. Prevalence of metabolic syndrome in adolescents from a north Indian population. Diabet Med. 2007;24:195–9. doi: 10.1111/j.1464-5491.2007.02066.x. [DOI] [PubMed] [Google Scholar]
- 26.Goodman E, Daniels SR, Morrison JA, Huang B, Dolan LM. Contrasting prevalence of and demographic disparities in the World Health Organization and National Cholesterol Education Program Adult Treatment Panel III definitions of metabolic syndrome among adolescents. J Pediatr. 2004;145:445–51. doi: 10.1016/j.jpeds.2004.04.059. [DOI] [PubMed] [Google Scholar]
- 27.Nasreddine L, Ouaijan K, Mansour M, Adra N, Sinno D, Hwalla N. Metabolic syndrome and insulin resistance in obese prepubertal children in Lebanon: A primary health concern. Ann Nutr Metab. 2010;57:135–42. doi: 10.1159/000321532. [DOI] [PubMed] [Google Scholar]
- 28.Cruz ML, Weigensberg MJ, Huang TT, Ball G, Shaibi GQ, Goran MI. The metabolic syndrome in overweight Hispanic youth and the role of insulin sensitivity. J Clin Endocrinol Metab. 2004;89:108–13. doi: 10.1210/jc.2003-031188. [DOI] [PubMed] [Google Scholar]
- 29.Lee WY, Jung CH, Park JS, Rhee EJ, Kim SW. Effects of smoking, alcohol, exercise, education, and family history on the metabolic syndrome as defined by the ATP III. Diabetes Res Clin Pract. 2005;67:70–7. doi: 10.1016/j.diabres.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Juonala M, Viikari JS, Räsänen L, Helenius H, Pietikäinen M, Raitakari OT. Young adults with family history of coronary heart disease have increased arterial vulnerability to metabolic risk factors: The Cardiovascular Risk in Young Finns Study. Arterioscler Thromb Vasc Biol. 2006;26:1376–82. doi: 10.1161/01.ATV.0000222012.56447.00. [DOI] [PubMed] [Google Scholar]
- 31.Wada K, Tamakoshi K, Yatsuya H, Otsuka R, Murata C, Zhang H, et al. Association between parental histories of hypertension, diabetes and dyslipidemia and the clustering of these disorders in offspring. Prev Med. 2006;42:358–63. doi: 10.1016/j.ypmed.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 32.Jacob JJ, Isaac R. Behavioral therapy for management of obesity. Indian J Endocrinol Metab. 2012;16:28–32. doi: 10.4103/2230-8210.91180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mallappa RH, Rokana N, Duary RK, Panwar H, Batish VK, Grover S. Management of metabolic syndrome through probiotic and prebiotic interventions. Indian J Endocrinol Metab. 2012;16:20–7. doi: 10.4103/2230-8210.91178. [DOI] [PMC free article] [PubMed] [Google Scholar]


