Abstract
Hepcidin, an important regulator of iron homeostasis, is suggested to be causally related to anemia of inflammation. The aim of this study was to explore the role of plasma hepcidin in anemia among older persons from the general population. The Leiden 85-Plus Study is a population-based study of 85-year olds in Leiden, the Netherlands. Eighty-five-year old inhabitants of Leiden were enrolled between September 1997 and September 1999. At the age of 86, plasma hepcidin was determined with time of flight mass spectrometry in 490 participants [160 (32.7%) male, 114 (23.3%) with anemia]. Anemia was defined according to criteria of the World Health Organization (hemoglobin level <13 g/dL for men and hemoglobin <12 g/dL for women). The median plasma hepcidin level was 3.0 nM [interquartile range (IQR) 1.8–4.9]. We found strong correlations between plasma hepcidin and body iron status, C-reactive protein and erythropoietin levels. Significantly higher hepcidin levels were found in participants with anemia of inflammation (P<0.01), in participants with anemia of kidney disease (P=0.01), and in participants with unexplained anemia (P=0.01) than in participants without anemia. Participants with iron-deficiency anemia had significantly lower plasma hepcidin levels than participants without anemia (P<0.01). In conclusion, older persons with anemia of inflammation have higher hepcidin levels than their counterparts without anemia. The potential clinical value of hepcidin in future diagnostic algorithms for anemia has to be explored.
Introduction
Anemia is a common disorder in older individuals. The prevalence of anemia increases with age to more than 20% in people aged 85 years and over.1,2 Approximately one fifth of older persons with anemia are considered to have anemia of inflammation,2 previously known as anemia of chronic disease, which is commonly found in patients with infections or inflammatory disorders, such as rheumatoid arthritis, inflammatory bowel disease, cancer and chronic kidney disease.3,4 Although the exact pathophysiological mechanism of this condition is unclear, it is likely to be mediated by immune-driven processes.3,4
Hepcidin, a 25 amino-acid peptide hormone produced by the liver, has been hypothesized to play a crucial role in anemia of inflammation.5,6 Hepcidin is the main regulator of iron homeostasis.7 When more iron is needed for erythropoiesis, hepcidin production is down-regulated, leading to an increased availability of iron for red blood cell production. In contrast, pro-inflammatory cytokines, particularly interleukin-6, induce the production and secretion of hepcidin.8 Hepcidin binds to the membrane protein ferroportin, an iron efflux channel on the surface of absorptive enterocytes, macrophages and hepatocytes, and induces its internalization and degradation in lysosomes, thereby sequestering iron in the cytoplasm of these cells.9 As a result, plasma iron levels drop rapidly.5,6
Transgenic mice over-expressing hepcidin and mice receiving synthetic hepcidin develop mild-to-moderate microcytic, hypochromic anemia.10–13 In humans, increased hepcidin levels have also been found in diseases characterized by overt inflammation, such as rheumatoid arthritis and sepsis.14–16 In addition, higher hepcidin levels have been found in anemic patients with chronic inflammation,15 chronic kidney disease 17,18 and cancer.19 Hepcidin is, therefore, considered to be the main mediator of anemia of inflammation.3–6
Aging is often associated with a low-grade pro-inflammatory state.20,21 This mild pro-inflammatory state is thought to elicit a chronic elevation of circulating hepcidin leading to impaired availability of plasma iron, limiting hemoglobin synthesis and eventually causing anemia of inflammation.5,6 Whether hepcidin also plays a role in anemia in older people from the general population is debated. In the InChianti study, a population-based study of older subjects in Tuscany (Italy), urinary hepcidin levels were not higher in older individuals with anemia of inflammation.22 However, hepcidin concentrations in urine may not always accurately reflect plasma hepcidin concentrations, since urinary hepcidin concentrations may also depend on glomerular filtration, tubular re-absorption, and local production by tubular epithelial cells.5,23 Moreover, the (pre)-analytical variation for measurements of hepcidin in urine has been found to be relatively high.24 So far, no data are available on levels of plasma hepcidin in older subjects with anemia in the general population. Recently, more reliable plasma hepcidin assays have become available,5,25 allowing us to investigate the relation between plasma hepcidin levels and anemia of inflammation in older persons in the general population.
We used data from the Leiden 85-Plus Study, a population-based prospective study of older individuals in Leiden, the Netherlands, to: (a) explore correlates of plasma hepcidin in older people in the general population, and (b) to investigate the role of plasma hepcidin in anemia in old age by testing two hypotheses; (i) older people with anemia of inflammation have higher levels of plasma hepcidin and (ii) older people with iron-deficiency anemia have lower levels of plasma hepcidin.
Design and Methods
Design, setting and participants
The Leiden 85-Plus Study is a population-based prospective follow-up study of 85-year-old inhabitants of Leiden, the Netherlands. Between September 1997 and September 1999, 705 inhabitants of Leiden reached the age of 85 and were eligible and invited to participate in the study.26 Fourteen subjects died before enrollment in the study and 92 subjects refused to participate (response 87%). Of the 599 participants aged 85, 47 died before the age of 86, 39 refused further participation and 16 refused blood sampling. For seven participants, plasma hepcidin level could not be measured because of small sample volume. Plasma hepcidin levels were available for 490 participants aged 86.
Participants were visited at their place of residence for face-to-face interviews, the assessment of functional tests and the collection of a venous blood sample. All participants gave their informed consent. The Medical Ethical Committee of the Leiden University Medical Center approved the study.
Laborator y measurements
Non-fasting blood samples were drawn before 11 a.m. Plasma samples were stored at −80°C.
In 2010, plasma hepcidin-25 levels at age 86 were measured in one batch by a combination of weak cation exchange chromatography and time-of-flight mass spectrometry (TOF-MS), using a Microflex LT matrix-enhanced laser desorption/ionisation (MALDI) TOF-MS platform (Bruker Daltonics, Bremen, Germany).25 An internal standard (synthetic hepcidin-24; Peptide International Inc., Louisville, KT, USA) was used for quantification.27 The lower limit of detection was 0.5 nM.
Ferritin was determined in one batch using the Dual Count Solid Phase No Boil Assay (Diagnostic Products Corp, Los Angeles, CA, USA). Iron [colorimetric method, sensitivity 0.97 μmol/L (5.4 μg/dL) and coefficient of variation ≤4.7%] and transferrin [immunoturbidimetric method, sensitivity 0.09 g/L (9 mg/dL), and coefficient of variation ≤5.0%] were measured on an Architect clinical chemistry analyzer from Abbott (Chicago, IL, USA). Total iron binding capacity (TIBC) was calculated as follows: TIBC (mmol/L) = transferrin (g/L) × 25.14.28 Transferrin iron saturation (%) was defined as plasma iron divided by TIBC multiplied by 100. Low iron status was considered present when the ferritin level was <20 μg/L for men and <15 μg/L for women, iron level was <10 μmol/L, transferrin level was >3.7 g/L, or transferrin iron saturation was <20%.28 When a participant fulfilled one or more of the criteria, he or she was considered to have a low iron status.
Hemoglobin concentration and mean corpuscular volume (MCV) were determined on the day the blood sample was drawn by a fully automated system (Sysmex XE-2100, TOA Medical Electronics, Kobe, Japan). Anemia was defined according to the World Health Organization criteria (hemoglobin level <13 g/dL for men and hemoglobin <12 g/dL for women).29
Plasma levels of vitamin B12 and folate at age 86 were determined in one batch using the Dual Count Solid Phase No-Boil Assay (Diagnostic Products Corp, Los Angeles, CA, USA). Vitamin B12 deficiency was defined as plasma vitamin B12 levels lower than 150 pmol/L; folate deficiency was defined as plasma folate levels lower than 7.0 nmol/L.30
Plasma creatinine concentration was measured according to the Jaffe method on the day the blood sample was drawn. A fully automated Hitachi 747 system (Hitachi, Tokyo, Japan) was used. We estimated creatinine clearance using the Cockcroft–Gault formula.31 A creatinine clearance <30 mL/min was considered to be low.
C-reactive protein (CRP) and interleukin-6 levels were determined as markers of inflammation. CRP was measured with the Hitachi 747 automated analyzer on the day the sample was drawn (Hitachi, Tokyo, Japan). CRP levels >5 mg/L were considered to be high. Standard enzyme-linked immunosorbent assays (ELISA) were performed according to the manufacturer’s guidelines (Central Laboratory of the Blood Transfusion Service, Amsterdam, the Netherlands) to measure interleukin-6 levels. Levels of inter-leukin-6 >10 ng/L were considered to be high.
In 2008, plasma erythropoietin levels were measured in one batch with the use of an immunoassay (Immulite 2500, Siemens Medical Diagnostics, Tarrytown, USA; sensitivity 1.2 IU/L, coefficient of variation <6%).
Subtypes of anemia
Participants with anemia were subdivided according to the specific subtype of anemia, using the following criteria: (i) participants with low iron status were classified as having iron-deficiency anemia; (ii) participants with either vitamin B12 deficiency or folate deficiency were considered to have vitamin B12/folate deficiency anemia; (iii) participants with low creatinine clearance were classified as having anemia of kidney disease; and (iv) participants with either (a) elevated CRP levels only, or (b) elevated CRP levels and ferritin level >300 μg/L, and iron level <10 μmol/L or transferrin iron saturation <20%, were considered to have anemia of inflammation. Participants in whom more than one of the above-mentioned laboratory abnormalities were identified were considered to have anemia of multiple causes. As a low iron status was hypothesized to be associated with lower plasma hepcidin levels, and the other causes of anemia with higher hepcidin levels, separate groups were constructed for those with multiple causes including iron deficiency and for those with multiple causes but normal iron status. Participants with anemia and normal iron status, normal vitamin B12, normal folate, normal renal function, and normal CRP were classified as having unexplained anemia.
Other clinical parameters
Information on the presence of disease (stroke, myocardial infarction, severe cognitive impairment, diabetes mellitus, Parkinson disease, hip fracture, arthritis, obstructive lung disease and malignant disease) was obtained from general practitioners, nursing home physicians, pharmacy records and laboratory findings.32 Diabetes mellitus was considered present when diagnosed by the primary care physician, when non-fasting glucose levels were >11.0 mmol/L, or when a participant was taking anti-diabetic medication. The presence of severe cognitive impairment was based on either a diagnosis by the general practitioner or a MiniMental State Examination (MMSE) score <19 points.33
Statistical analysis
Participants with hepcidin values below the detection limit of 0.5 nM were analyzed as having a hepcidin level of 0.5 nM. Participants were further grouped according to quartiles of plasma hepcidin levels. Differences in characteristics between participants in the four quartiles were tested with the χ2 test (linear-linear, one degree of freedom) for categorical variables and the Jonckheere–Terpstra test for continuous variables. To investigate which factors were independently associated with plasma hepcidin, we performed a multiple linear regression analysis, with ferritin, iron, CRP and erythropoietin as independent variables and hepcidin as the dependent variable.
Differences in hematologic parameters between different subtypes of anemia were tested with Kruskal-Wallis tests. Plasma hepcidin levels for participants without anemia and participants with different subtypes of anemia are displayed as medians with corresponding interquartile ranges (IQR) and 95% confidence intervals (CI). Differences in hepcidin levels between subjects with the different subtypes of anemia and those without anemia were analyzed with Mann-Whitney U tests.
We analyzed the data with PASW 17.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
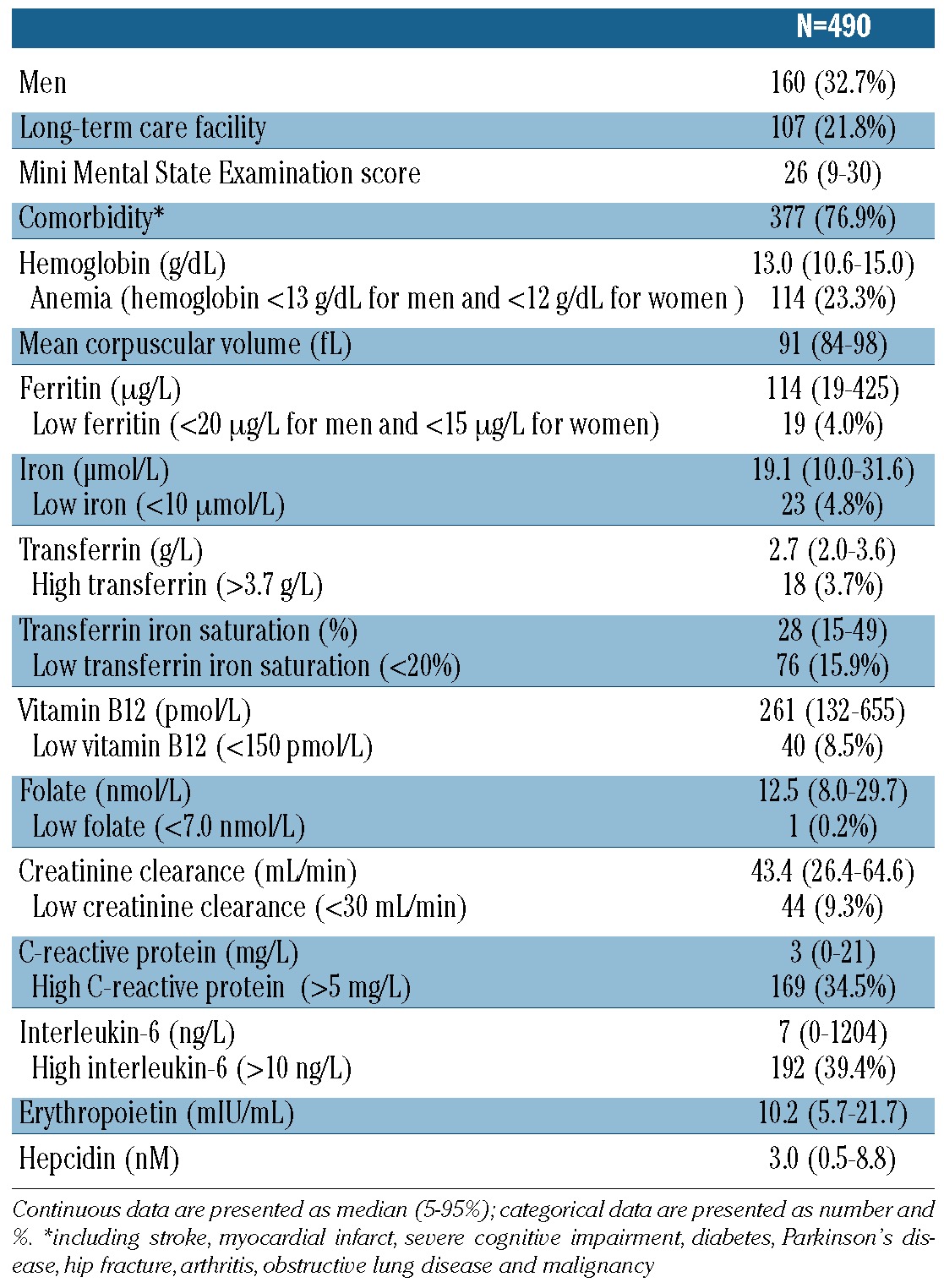
Table 1 presents the characteristics of the study participants, all aged 86. In total, 160/490 (32.7%) of the study population were male and 107/490 (21.8%) lived in a long-term care facility. The median MMSE score was 26 (IQR 22–28). Comorbidity was present in 377/490 (76.9%) participants. The prevalence of anemia was 23.3% (114/490). High CRP levels (>5 mg/L) were found in 169 participants (34.5%).
Table 1.
Characteristics of the study population at the age of 86 years.
Plasma hepcidin levels in the total study population ranged from <0.5 nM through 18.7 nM with a median of 3.0 nM (IQR 1.8–4.9). No differences in plasma hepcidin levels were found between men and women [median hepcidin 3.1 nM (IQR 1.8–5.1) versus 3.0 nM (IQR 1.8–4.8), P=0.58].
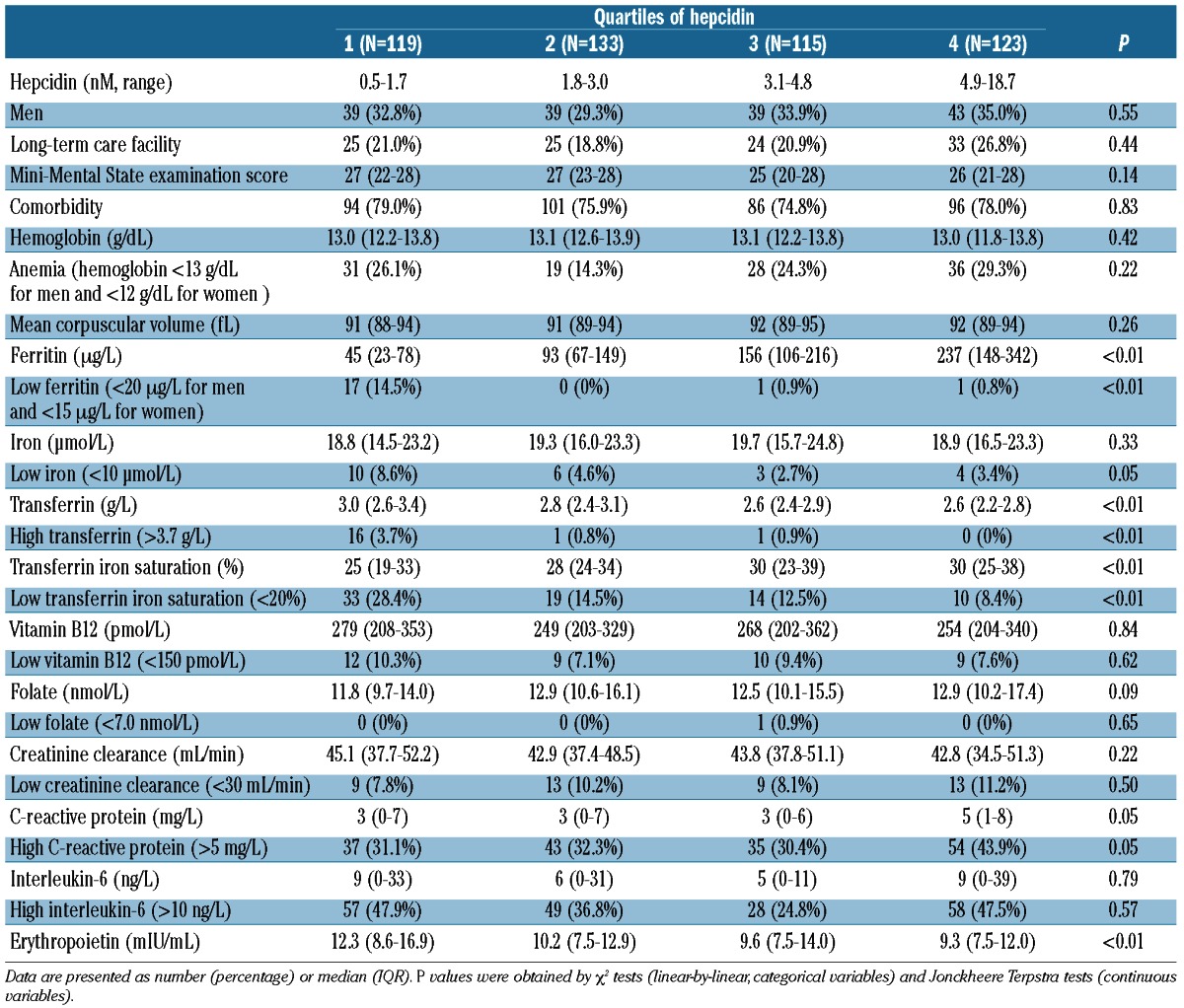
Table 2 shows the characteristics of the study population at the age of 86, divided according to quartiles of hepcidin concentration. Median hemoglobin levels were similar in the four hepcidin quartiles (P=0.42). Plasma hepcidin levels correlated with body iron status, CRP levels and levels of erythropoietin. In a multiple linear regression analysis, ferritin (β=0.01, P<0.01), CRP (β=0.03, P<0.01) and erythropoietin (β=−0.04, P<0.01) were independently associated with hepcidin. Iron was not associated with hepcidin in this multiple linear regression model (β=−0.02, P=0.20).
Table 2.
Characteristics of the study population at the age of 86 years, divided according to quartiles of hepcidin.
As hypothesized, hepcidin levels were significantly lower in participants with low iron status (n=89) than in participants with normal iron status (n=401) [median 2.0 nM (IQR 0.5–3.5) versus 3.2 nM (IQR 2.0–5.2), P<0.01]. Participants with high CRP levels (n=169) had higher hepcidin levels than those with normal CRP levels (n=321): median hepcidin 3.3 nM (IQR 1.9–5.9) versus 2.9 (IQR 1.7–4.6) (P=0.01). No difference was observed in hepcidin levels between participants with high interleukin-6 levels [(>10 ng/L) n=192, 2.9 nM (IQR 1.5–5.5)] and participants with low interleukin-6 levels [n=295, 3.1 nM (IQR 2.0–4.7), P=0.69].
No difference was observed in hepcidin levels between participants with anemia in general (n=114) and their counterparts without anemia (n=376), [median hepcidin 3.7 nM (IQR 1.4–6.0) versus 3.0 nM (IQR 1.8–4.7), P=0.19].
Table 3 shows the hematologic characteristics of the participants according to their subtype of anemia. The lowest hemoglobin levels were observed in those with iron deficiency anemia [10.9 g/dL (IQR 10.1–11.8)]; participants with unexplained anemia had a hemoglobin level of 11.7 g/dL (IQR 10.8–12.0). As expected, lower levels of ferritin and iron, higher levels of transferrin and lower transferrin iron saturation were observed in those with iron deficiency anemia. The lowest vitamin B12 levels were found in those with vitamin B12/folate deficiency. Creatinine clearance was lowest among individuals with anemia of kidney disease. The highest CRP levels were observed in subjects with anemia of inflammation and in those with anemia with multiple causes. In addition, the highest serum iron levels were found in subjects with unexplained anemia. Participants with anemia of kidney disease had the lowest erythropoietin levels. The highest levels of erythropoietin were observed in those with iron-deficiency anemia. The erythropoietin level in the participants with unexplained anemia was 11.1 mIU/mL (IQR 7.5–13.9).
Table 3.
Characteristics of the study population at the age of 86 years, divided according to subtype of anemia.
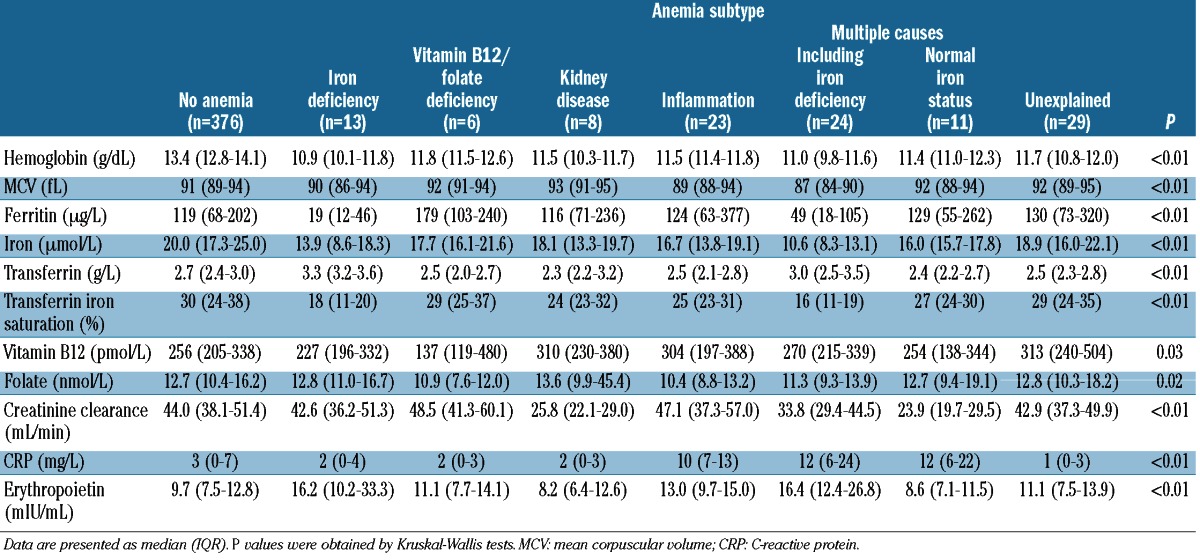
Figure 1 presents hepcidin levels according to anemia subtype. Higher hepcidin levels were found in participants with anemia of inflammation [n=23, 4.7 nM (IQR 2.8–9.1), P<0.01], in participants with anemia of kidney disease [n=8, 5.4 nM (IQR 3.8–7.2), P=0.01], and in participants with unexplained anemia [n=29, 4.2 nM (IQR 2.7–7.2), P=0.01]. Participants with iron-deficiency anemia [based on ferritin, iron, transferrin and transferrin iron saturation (n=13)] had significantly lower plasma hepcidin levels [1.4 nM (IQR 0.5–2.7)] than participants without anemia [n=376, 3.0 nM (IQR 1.8–4.7), P<0.01]. There were six subjects with anemia with iron deficiency defined by the presence of low ferritin only, irrespective of iron, transferrin and transferrin iron saturation. As expected, compared to subjects defined as having iron-deficiency anemia on the basis of ferritin, iron, transferrin or transferrin iron saturation, the median hepcidin level in these six subjects dropped to 0.5nM (IQR 0.5–4.0; P=0.05 versus those without anemia). Lower hepcidin levels were found in participants with anemia of multiple causes including iron deficiency [n=24, 1.8 nM (0.5–3.8), P=0.03]. Hepcidin levels did not differ between participants with anemia of multiple causes but normal iron status (n=11) and participants without anemia (P=0.63). Plasma hepcidin levels did not differ between participants with vitamin B12 deficiency anemia or folate deficiency anemia (n=6) and participants without anemia (P=0.83).
Figure 1.
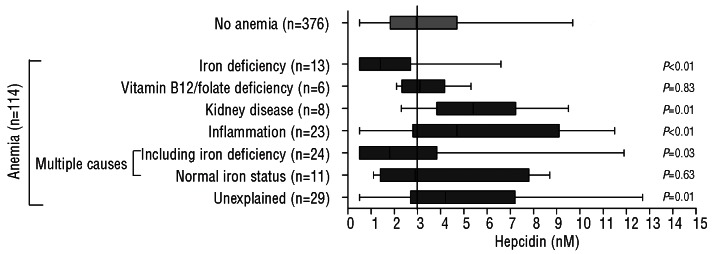
Hepcidin levels according to subtype of anemia. P values were obtained by the Mann-Whitney U test with the “No anemia” group as the reference. The reference line represents the hepcidin value of the “No anemia” group (3.0 nM).
Discussion
In the present study conducted among older members of the general population, we found strong correlations between plasma hepcidin levels, and body iron status, CRP and erythropoietin. Lower hepcidin levels were found in the older subjects with iron-deficiency anemia than in their peers without anemia. Higher hepcidin levels were found in subjects with anemia of inflammation, anemia of kidney disease, and unexplained anemia.
These findings concur with our hypotheses that elderly people with iron-deficiency anemia have lower levels of hepcidin and that older subjects with anemia of inflammation have higher levels of hepcidin. Our findings are in line with those of studies in animals and other selected human populations that showed strong associations between hepcidin levels and ferritin levels and inflammatory mark-ers.15,16,22,25,34,35 Earlier studies have also reported lower or even undetectable hepcidin levels in iron-deficiency anemia.15,22,25,36
In contrast to the findings of the InChianti study on hepcidin measured in urine,22 we are the first to report higher hepcidin levels in older members of the general population with anemia of inflammation, anemia of kidney disease, and unexplained anemia. These conflicting findings between the two studies may perhaps be explained by differences in the definition of subtypes of anemia, and iron status in particular, or by differences in hepcidin measurements between the InChianti study and our study, e.g. urine versus plasma, or total hepcidin versus hepcidin-25, respectively.5
In our study, the highest hepcidin levels were found in those participants with anemia of chronic kidney disease, perhaps because excretion and degradation of hepcidin by the kidney is hampered.5,17 Although in previous studies patients with chronic kidney disease were mostly found to have increased plasma hepcidin levels, the association between the glomerular filtration rate and hepcidin levels in such patients has not yet been precisely examined.5,17,18,37 As we found erythropoietin to be highest in participants within the lowest hepcidin quartile, and vice versa, erythropoiesis or erythropoietin itself may inhibit hepcidin production. Detailed studies are necessary to shed further light on the underlying pathophysiological mechanisms.
A combination of several age-related physiological changes are thought to contribute to the development of unexplained anemia, such as (subclinical) renal insufficiency, stem cell aging, androgen insufficiency, low-grade chronic inflammation, and myelodysplasia or other types of bone marrow failure.2,38,39 Interestingly, we found higher levels of plasma hepcidin in those participants with unexplained anemia. Further studies should establish the underlying pathophysiological mechanism, as iron status, inflammatory status and markers of erythropoiesis were normal in these participants. It is possible that in unexplained anemia, bone marrow failure is associated with lower iron utilization by the developing erythrocytes, resulting in higher plasma iron concentrations and higher iron stores and, consequently, with increased hepcidin production by hepatocytes.5,40
Iron is vital for micro-organisms.41 Humans have developed several mechanisms to deprive microbes of iron during infections.41–43 These mechanisms include an increased production of iron-binding proteins such as lactoferrin,43 but also a systemic response by rapidly lowering plasma iron concentrations, mediated by a cytokine-driven increase in hepcidin.41–43 As such, hepcidin functions as the main regulator of iron homeostasis.5–7 At supraphysiological concentrations, hepcidin exhibits antifungal and antibacterial activities44 which resemble cysteine-rich antimicrobial peptides defensins and protegrins.45 Hepcidin binds to the membrane protein ferroportin, after which duodenal enterocytes deliver less dietary iron to extracellular fluid, macrophages fail to release iron that is recycled from senescent erythrocytes and hepatocytes retain stored iron, all causing a drop in iron levels.5,6 We found strong correlations between plasma hepcidin levels and body iron status, CRP levels and erythropoietin indicating that the regulatory mechanisms that drive hepcidin production are intact until very old age.
The strengths of the present study are its large population-based sample of older individuals and the state-of-the-art measurement of plasma hepcidin levels instead of urinary hepcidin levels.5,6,24
Hepcidin levels were measured more than 13 years after blood collection in non-fasting blood samples. It is not yet known whether plasma hepcidin concentrations are directly affected by the iron content of the food that was eaten prior to the measurement. More studies need to be done on the influence of dietary intake and the diurnal rhythm of hepcidin. However, we found circadian differences in serum hepcidin levels24 and, in a recent study among healthy human volunteers, hepcidin-25 concentrations displayed a diurnal variation that was not influenced by food intake.46 The stability of plasma hepcidin over longer periods is also unknown and hepcidin tends to aggregate and adhere to surfaces, and may even be oxidized or degraded.5,6 This may have led to measurement errors. However, all comparisons were within the same study population, and all samples were collected before 11 a.m., irrespective of the subtype of anemia that the subjects had. In addition, there is no reason to assume that iron content of the food prior to the measurement or degradation of hepcidin was different in subjects with different types of anemia. We, therefore, consider it unlikely that the relative differences presented here are due to eating habits, diurnal rhythm of hepcidin levels or storage conditions. However, these factors may impair direct comparison with findings from other studies using the same methodology with respect to absolute hepcidin levels.
The median hepcidin level in our study population (n=490), all of whom were 86 years old, was 3.0 nM (IQR 1.8–4.9). No differences were observed in hepcidin levels between men and women. In the Nijmegen Biomedical Study, a population-based survey in Nijmegen (the Netherlands), serum hepcidin was measured in 2998 adults aged 18 years and over, including 16 people aged 85 years and over. The median reference level of serum hep-cidin-25 was 4.5 nM for men, 2.0 nM for premenopausal women, and 4.9 nM for postmenopausal women.25,34,47 The median serum hepcidin level was 6.8 nM (95% reference range 1.6–12.8) in men aged 85 years and over (n=7) and 3.7 nM (95% reference range <0.5–15.4) in women aged 85 years and over (n=9).34,47 The median hepcidin levels observed in our population differ from those in subjects aged 85 years and over in the Nijmegen Biomedical Study, which may be explained by the small number of persons aged 85 years and over in the Nijmegen Biomedical Study. In order to be able to establish reference ranges for serum hepcidin in older people in the general population, our findings need to be confirmed in other large studies in the same age group.
Another limitation of this study is that the etiological classification of anemia was not based on clinical gold standards. Because of the epidemiological nature of this study, the underlying causes of the anemia could not be definitively confirmed by clinical and laboratory assessments and follow-up. Our study can be considered a first exploration of the role of plasma hepcidin in anemia in older people in the general population. Further clinical studies on this topic using comprehensive clinical and laboratory assessments and clinical follow-up are needed. In addition, a limitation of our study is its relatively small sample size, especially with regards to each of the different subtypes of anemia.
The potential clinical value of hepcidin in future diagnostic algorithms for anemia should also be explored, as hepcidin levels may help to discriminate between classic iron-deficiency anemia (low hepcidin levels) and iron deficiency in the context of anemia of inflammation (high hepcidin levels).5,6,14,48 Hepcidin levels could then be used to predict the therapeutic response to iron administration.5,6 Future studies should also establish whether hepcidin antagonists, e.g. anti-interleukin-6 receptor antibodies and hepcidin-neutralizing agents,49 or targets against the hepcidin binding site of ferroportin, have potential value in the treatment of anemia of inflammation in older people.6
Supplementary Material
Acknowledgements
The Leiden 85-Plus Study was partly funded by an unrestricted grant from the Dutch Ministry of Health, Welfare and Sports (1997–2001).
The sponsor had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.den Elzen WP, Willems JM, Westendorp RG, de Craen AJ, Assendelft WJ, Gussekloo J. Effect of anemia and comorbidity on functional status and mortality in old age: results from the Leiden 85-plus Study. CMAJ. 2009;181(3–4):151–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104(8):2263–8 [DOI] [PubMed] [Google Scholar]
- 3.Zarychanski R, Houston DS. Anemia of chronic disease: a harmful disorder or an adaptive, beneficial response? CMAJ. 2008;179(4):333–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352(10):1011–23 [DOI] [PubMed] [Google Scholar]
- 5.Kroot JJ, Tjalsma H, Fleming RE, Swinkels DW. Hepcidin in human iron disorders: diagnostic implications. Clin Chem. 2011;57(12):1650–69 [DOI] [PubMed] [Google Scholar]
- 6.Ganz T, Nemeth E. Hepcidin and disorders of iron metabolism. Annu Rev Med. 2011;62347–60 [DOI] [PubMed] [Google Scholar]
- 7.Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003;102(3):783–8 [DOI] [PubMed] [Google Scholar]
- 8.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113(9):1271–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090–3 [DOI] [PubMed] [Google Scholar]
- 10.Nicolas G, Bennoun M, Porteu A, Mativet S, Beaumont C, Grandchamp B, et al. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc Natl Acad Sci USA. 2002;99(7):4596–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roy CN, Mak HH, Akpan I, Losyev G, Zurakowski D, Andrews NC. Hepcidin antimicrobial peptide transgenic mice exhibit features of the anemia of inflammation. Blood. 2007;109(9):4038–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rivera S, Liu L, Nemeth E, Gabayan V, Sorensen OE, Ganz T. Hepcidin excess induces the sequestration of iron and exacerbates tumor-associated anemia. Blood. 2005;105(4):1797–802 [DOI] [PubMed] [Google Scholar]
- 13.Rivera S, Nemeth E, Gabayan V, Lopez MA, Farshidi D, Ganz T. Synthetic hepcidin causes rapid dose-dependent hypoferremia and is concentrated in ferroportin-containing organs. Blood. 2005;106(6):2196–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Santen S, van Dongen-Lases EC, de Vegt F, Laarakkers CM, van Riel PL, van Ede AE, Swinkels DW. Hepcidin and hemoglobin content parameters in the diagnosis of iron deficiency in rheumatoid arthritis patients with anemia. Arthritis Rheum. 2011;63(12):3672–80 [DOI] [PubMed] [Google Scholar]
- 15.Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101(7):2461–3 [DOI] [PubMed] [Google Scholar]
- 16.van Eijk LT, Kroot JJ, Tromp M, van der Hoeven JG, Swinkels DW, Pickkers P. Inflammation-induced hepcidin-25 is associated with the development of anemia in septic patients: an observational study. Crit Care. 2011;15(1):R9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashby DR, Gale DP, Busbridge M, Murphy KG, Duncan ND, Cairns TD, et al. Plasma hepcidin levels are elevated but responsive to erythropoietin therapy in renal disease. Kidney Int. 2009;75(9):976–81 [DOI] [PubMed] [Google Scholar]
- 18.Peters HP, Laarakkers CM, Swinkels DW, Wetzels JF. Serum hepcidin-25 levels in patients with chronic kidney disease are independent of glomerular filtration rate. Nephrol Dial Transplant. 2010;25(3):848–53 [DOI] [PubMed] [Google Scholar]
- 19.Ukarma L, Johannes H, Beyer U, Zaug M, Osterwalder B, Scherhag A. Hepcidin as a predictor of response to epoetin therapy in anemic cancer patients. Clin Chem. 2009;55(7):1354–60 [DOI] [PubMed] [Google Scholar]
- 20.Bruunsgaard H, Pedersen BK. Age-related inflammatory cytokines and disease. Immunol Allergy Clin North Am. 2003;23(1):15–39 [DOI] [PubMed] [Google Scholar]
- 21.Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, Guralnik JM, Longo DL. The origins of age-related proinflammatory state. Blood. 2005;105(6):2294–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrucci L, Semba RD, Guralnik JM, Ershler WB, Bandinelli S, Patel KV, et al. Proinflammatory state, hepcidin and anemia in older persons. Blood. 2010;115(18):3810–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kulaksiz H, Theilig F, Bachmann S, Gehrke SG, Rost D, Janetzko A, Cetin Y, Stremmel W. The iron-regulatory peptide hormone hepcidin: expression and cellular localization in the mammalian kidney. J Endocrinol. 2005;184(2):361–70 [DOI] [PubMed] [Google Scholar]
- 24.Kroot JJ, Hendriks JC, Laarakkers CM, Klaver SM, Kemna EH, Tjalsma H, Swinkels DW. (Pre)analytical imprecision, between-subject variability, and daily variations in serum and urine hepcidin: implications for clinical studies. Anal Biochem. 2009;389(2):124–9 [DOI] [PubMed] [Google Scholar]
- 25.Kroot JJ, Laarakkers CM, Geurts-Moespot AJ, Grebenchtchikov N, Pickkers P, van Ede AE, et al. Immunochemical and mass-spectrometry-based serum hepcidin assays for iron metabolism disorders. Clin Chem. 2010;56(10):1570–9 [DOI] [PubMed] [Google Scholar]
- 26.der Wiel AB, van Exel E, de Craen AJ, Gussekloo J, Lagaay AM, Knook DL, Westendorp RG. A high response is not essential to prevent selection bias: results from the Leiden 85-plus study. J Clin Epidemiol. 2002;55(11):1119–25 [DOI] [PubMed] [Google Scholar]
- 27.Swinkels DW, Girelli D, Laarakkers C, Kroot J, Campostrini N, Kemna EH, Tjalsma H. Advances in quantitative hepcidin measurements by time-of-flight mass spectrometry. PLoS ONE. 2008;3(7):e2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samenwerkende Artsenlaboratoria en diagnostische centra in Nederland. Online SAN Memoboek voor diagnostiek in de eerstelijn, http://memoboek.dynapaper.nl Accessed September 2011
- 29.Nutritional anaemias. Report of a WHO scientific group. World Health Organ Tech Rep Ser. 1968;4055–37 [PubMed] [Google Scholar]
- 30.den Elzen WP, Westendorp RG, Frolich M, de Ruijter W, Assendelft WJ, Gussekloo J. Vitamin B12 and folate and the risk of anemia in old age. The Leiden 85-Plus Study. Arch Intern Med. 2008;168(20):2238–44 [DOI] [PubMed] [Google Scholar]
- 31.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41 [DOI] [PubMed] [Google Scholar]
- 32.Bootsma-van der Wiel A, de Craen AJ, van Exel E, MacFarlane PW, Gussekloo J, Westendorp RG. Association between chronic diseases and disability in elderly subjects with low and high income: the Leiden 85-plus Study. Eur J Public Health. 2005;15(5):494–7 [DOI] [PubMed] [Google Scholar]
- 33.Heeren TJ, Lagaay AM, von Beek WC, Rooymans HG, Hijmans W. Reference values for the Mini-Mental State Examination (MMSE) in octo- and nonagenarians. J Am Geriatr Soc. 1990;38(10):1093–6 [DOI] [PubMed] [Google Scholar]
- 34.Galesloot TE, Vermeulen SH, Geurts-Moespot AJ, Klaver SM, Kroot JJ, van TD, et al. Serum hepcidin: reference ranges and biochemical correlates in the general population. Blood. 2011;117 (25):e218–e225 [DOI] [PubMed] [Google Scholar]
- 35.Lee P, Gelbart T, Waalen J, Beutler E. The anemia of ageing is not associated with increased plasma hepcidin levels. Blood Cells Mol Dis. 2008;41(3):252–4 [DOI] [PubMed] [Google Scholar]
- 36.Kemna EH, Tjalsma H, Podust VN, Swinkels DW. Mass spectrometry-based hepcidin measurements in serum and urine: analytical aspects and clinical implications. Clin Chem. 2007;53(4):620–8 [DOI] [PubMed] [Google Scholar]
- 37.Tomosugi N, Kawabata H, Wakatabe R, Higuchi M, Yamaya H, Umehara H, Ishikawa I. Detection of serum hepcidin in renal failure and inflammation by using ProteinChip System. Blood. 2006;108(4):1381–7 [DOI] [PubMed] [Google Scholar]
- 38.Makipour S, Kanapuru B, Ershler WB. Unexplained anemia in the elderly. Semin Hematol. 2008;45(4):250–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patel KV. Epidemiology of anemia in older adults. Semin Hematol. 2008;45(4):210–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pak M, Lopez MA, Gabayan V, Ganz T, Rivera S. Suppression of hepcidin during anemia requires erythropoietic activity. Blood. 2006;108(12):3730–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jurado RL. Iron, infections, and anemia of inflammation. Clin Infect Dis. 1997;25(4):888–95 [DOI] [PubMed] [Google Scholar]
- 42.Nemeth E, Ganz T. Regulation of iron metabolism by hepcidin. Annu Rev Nutr. 2006;26323–42 [DOI] [PubMed] [Google Scholar]
- 43.Ashrafian H. Hepcidin: the missing link between hemochromatosis and infections. Infect Immun. 2003;71(12):6693–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276(11):7806–10 [DOI] [PubMed] [Google Scholar]
- 45.Vyoral D, Petrak J. Hepcidin: a direct link between iron metabolism and immunity. Int J Biochem Cell Biol. 2005;37(9):1768–73 [DOI] [PubMed] [Google Scholar]
- 46.Troutt JS, Rudling M, Persson L, Stahle L, Angelin B, Butterfield AM, Schade AE, Cao G, Konrad RJ. Circulating human hepcidin-25 concentrations display a diurnal rhythm, increase with prolonged fasting, and are reduced by growth hormone administration. Clin Chem. 2012;58(8):1225–32 [DOI] [PubMed] [Google Scholar]
- 47.www.hepcidinanalysis.com, accessed June 1, 2012
- 48.Theurl I, Aigner E, Theurl M, Nairz M, Seifert M, Schroll A, et al. Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: diagnostic and therapeutic implications. Blood. 2009;113(21):5277–86 [DOI] [PubMed] [Google Scholar]
- 49.Sasu BJ, Cooke KS, Arvedson TL, Plewa C, Ellison AR, Sheng J, et al. Antihepcidin antibody treatment modulates iron metabolism and is effective in a mouse model of inflammation-induced anemia. Blood. 2010;115(17):3616–24 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


