Imatinib mesylate (IM, Glivec®) and the 2nd generation tyrosine kinase inhibitors (TKIs) (dasatinib and nilotinib) are the standard treatment for children and adults with chronic myeloid leukemia (CML). IM inhibits all ABL tyrosine kinases and selectively suppresses the ATP-binding site of platelet-derived growth factor receptor (PDGF-R) and c-KIT that are expressed in various types of neoplasms. Indeed, the efficacy of IM has been proven in other malignancies expressing at least one IM-sensitive TK receptor, including those occurring in children.1 An altered bone and mineral metabolism, a reduction in testosterone and gynecomastia referred to PDGF-R and c-KIT inhibition in normal cells have been reported in adults treated with IM.2–4 So far, most of the data on the long-term side-effects of IM in children who have received this treatment concern its impact on growth.5–7
We report 4 CML pre-pubertal patients (3 males and 1 female; median age at start of IM treatment 10.6 years) in complete cytogenetic response (CCyR) with IM who were regularly monitored between May 2004 and June 2011 to evaluate the bone and mineral metabolism, as well as growth and pubertal development. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Ethics Committee as a part of a retrospective study (CML-Petit-01). Patients received IM at a dosage ranging from 275 to 324 mg/m2/day (median 314 mg). One patient stopped IM after 3.4 years of treatment because of a cytogenetic relapse. Starting from January 2008, 3 patients in major molecular response (MMolR, BCR-ABL/ABL <0.05%) were scheduled to receive intermittent therapy consisting of the same dosage of IM taken three weeks on and one week off every month.
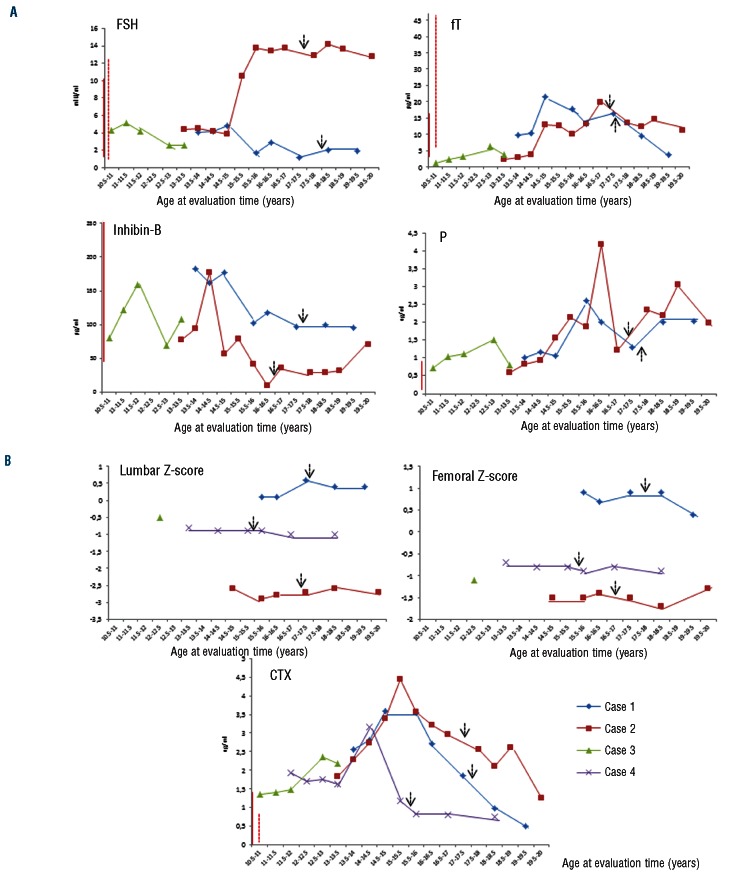
Follicle stimulating hormone (FSH), luteinizing hormone (LH), testosterone (T), free-testosterone (fT), inhibin-B, progesterone (P), 17β-estradiol (E2), growth hormone (GH), insulin-like growth factor-1 (IGF-1), thyroid stimulating hormone (TSH), prolactin (PRL) and Tanner stage were assessed to define pubertal and sexual development (Online Supplementary Table S1). T, fT and testis volume for male patients, and E2, breast development and the menstrual cycle for females, rose according to the appearance of signs of puberty. For both genders, the Tanner stage reached the adult target and a normal sexual phenotype was observed. As shown in Figure 1A, P levels rose above the normal range in a background of normal values of T, E2 and PRL in all males. The high P levels could result from the IM inhibition of PDGFR and c-kit tyrosine kinases that in turn inhibit ∆5–3 β-hydroxysteroid dehydrogenase and 17,20 lyase, both responsible for P formation. A similar effect of IM on P levels has been also found in adult males.3 Whether this finding may have clinical relevance is not known. One boy (case n. 2) continued to show a reduced inhibin-B/FSH ratio, as previously reported,8 combined with severe oligozoospermia even during intermittent therapy with IM, suggesting a durable risk of fertility reduction.
Figure 1.
(A) Hormonal markers of puberty in 3 complete cytogenetic responder (CCyR) boys during imatinib mesylate (IM) treatment. Continuous line and dotted line near the y axis of the graphic indicate the normal range for subjects aged under 16 years and for adults, respectively, where applicable. FSH: follicle stimulating hormone; fT: free-testosterone; P: progesterone. The arrows indicate intermintent IM treatment. (B) Bone mineral density and the bone resorption marker (CTX) in 4 CCyR patients (3 boys and 1 girl) during IM treatment. Continuous line and the dotted line near the y axis of the graphic indicate the normal range for subjects aged 13–16 (years) and for adults, respectively.
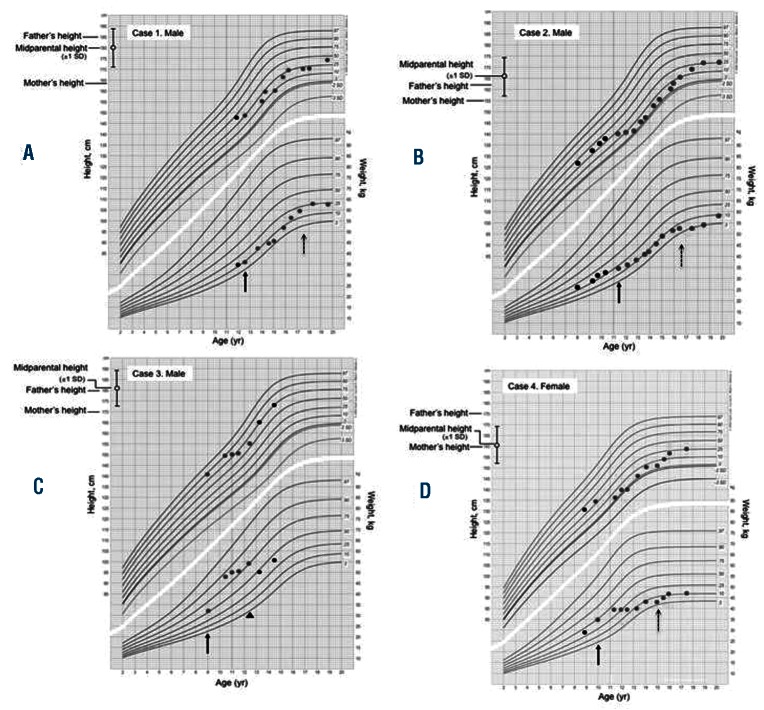
C-terminal telopeptides of type I collagen (CTX), parathyroid hormone (PTH), serum calcium (Ca), serum phosphate (PO4-), serum magnesium (Mg), bone-specific alkaline phosphatase (BAP), 25-hydroxyvitamin-D (D3) and bone mineral density (BMD) were assessed to evaluate bone growth and mineralization (Online Supplementary Table S2). We observed a deceleration of longitudinal growth in all patients since the administration of IM (Figure 2), in the presence of normal values of TSH, GH and IGF-1. All patients reached the mid-parental height during intermittent IM schedule, suggesting a relationship between the continuous and prolonged exposure to the TKI and the growth deceleration in pre-pubertal children. Our data are similar to those previously reported in CML children treated with IM.5–7 The mechanism by which TKI treatment causes a growth delay in children remains unknown. Recently, Hobernicht et al. suggested that the BCR-ABL targeted TKIs resulted in a decreased GH secretion at the pituitary level rather than an impaired action on downstream targets.9 All patients showed the bone resorption marker CTX above the highest reference value for age-matched controls (Figure 1B). Furthermore, a reduction in the chronological age BMD was recorded in 2 patients (case ns. 2 and 3), resistant, severe and associated with serum Ca in the upper normal limit and urinary Ca higher than normal (896 mg/day, n.v. 100–300 mg/day) in one of them (case n. 2). PTH, serum and urinary Ca, PO4-, BAP and Mg serum levels were normal in all other patients. Interestingly, case n. 3 recovered normal BMD values within 12 months after IM discontinuation. Our data suggest the biggest effect of IM is on bone resorption rather than on osteogenic promotion in children treated with IM. In contrast, available data in adults suggest that IM stimulates bone formation and retrains bone resorption, resulting in a sequestration of calcium and phosphate in the bone with concomitant lower serum levels of these minerals.4,10 The dysregulated bone remodeling probably results from both the inhibition of osteoclasts, by blocking c-FMS, c-KIT, carbonic anhydrase II and PDGFR signaling, and the activation of osteoblast activity through inhibition of PDGFR.4 The discrepancy between the osteogenic promotion in adults and the resorptive effect observed in our pediatric population could be explained by a different age-dependent impact of IM on bone metabolism. This hypothesis has been recently supported by demonstrations in rats that inhibition of TK receptors during longitudinal bone growth leads to long-term adverse effects on bone growth and remodeling.11
Figure 2.
Growth chart for boys and girls according to ethnic origin, showing height and weight of patients and their parental target range. Arrow indicates start of IM treatment. Red dots indicate height and weight during IM treatment, while black dots refer to the period before and after IM. The arrowhead in panel C indicates interruption of IM treatment. The longitudinal growth, between the 50th and the 10th percentile before IM, slowed below the 1st percentile in 2 patients (B and D).
In conclusion, the side-effects observed in our patients raise some concerns that children having long exposure to IM prior to puberty may have different characteristics to adults. Since the number of children on treatment with TKIs is constantly increasing, the possible side-effects of these long-term targeted therapies may play an important role in the treatment strategy. In particular, bone metabolism and growth velocity should be closely monitored in all children treated with TKIs. Furthermore, bosutinib, a dual Scr/Abl TKI with minimal inhibitory activity against c-KIT and PDFR, that has shown efficacy in patients previously treated with 2 or more TKIs12 could offer a new effective therapeutic option also for children with CML.
Supplementary Material
Footnotes
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org
References
- 1.Geoerger B, Morland B, Ndiaye A, Doz F, Kalifa G, Geoffray A, et al. Target-driven exploratory study of imatinib mesylate in children with solid malignancies by the Innovative Therapies for Children with Cancer (ITCC) European Consortium. Eur J Cancer. 2009; 45(13):2342–51 [DOI] [PubMed] [Google Scholar]
- 2.Berman E, Nicolaides M, Maki RG, Fleisher M, Chanel S, Scheu K, et al. Altered bone and mineral metabolism in patients receiving imatinib mesylate. N Engl J Med. 2006;354(19):2006–13 [DOI] [PubMed] [Google Scholar]
- 3.Gambacorti-Passerini C, Tornaghi L, Cavagnini F, Rossi P, Pecori-Giraldi F, Mariani L, et al. Gynaecomastia in men with chronic myeloid leukaemia after imatinib. Lancet. 2003;361(9397):1954–6 [DOI] [PubMed] [Google Scholar]
- 4.Jönsson S, Olsson B, Ohlsson C, Lorentzon M, Mellström D, Wadenvik H. Increased cortical bone mineralization in imatinib treated patients with chronic myelogenous leukemia. Haematologica. 2008;93(7):1101–3 [DOI] [PubMed] [Google Scholar]
- 5.Millot F, Baruchel A, Guilhot J, Petit A, Leblanc T, Bertrand Y, et al. Imatinib is efficient but has a negative impact in children with previously untreated chronic myelogenous leukemia (CML) in early chronic phase (CP): results of the French national phase IV trial. 51st ASH Meeting, Blood. 2009;114 (22):(Abstract 863). [Google Scholar]
- 6.Shima H, Tokuyama M, Tanizawa A, Tono C, Hamamoto K, Muramatsu H, et al. Distinct impact of imatinib on growth at pre-pubertal and pubertal ages of children with chronic myeloid leukemia. J Pediatr. 2011;159(4):676–81 [DOI] [PubMed] [Google Scholar]
- 7.Bansal D, Shava U, Varma N, Trehan A, Marwaha RK. Imatinib has adverse effect on growth in children with chronic myeloid leukemia. Pediatr Blood Cancer. 2012;59(3):481–4 [DOI] [PubMed] [Google Scholar]
- 8.Mariani S, Giona F, Basciani S, Brama M, Gnessi L. Low bone density and decreased inhibin-B/FSH ratio in a boy treated with imatinib during puberty. Lancet. 2008;372(9633):111–2 [DOI] [PubMed] [Google Scholar]
- 9.Hobernicht SL, Schweiger B, Zeitler P, Wang M, Hunger SP. Acquired growth hormone deficiency in a girl with chronic myelogenous leukemia treated with tyrosine kinase inhibitor therapy. Pediatr Blood Cancer. 2011;56(4): 671–3 [DOI] [PubMed] [Google Scholar]
- 10.Fitter S, Dewar AL, Kostakis P, Hughes TP, Roberts MM, Lynch K, et al. Long-term imatinib therapy promotes bone formation in CML patients. Blood. 2008;111(5):2538–47 [DOI] [PubMed] [Google Scholar]
- 11.Nurmio M, Joki H, Kallio J, Määttä JA, Väänänen HK, Toppari J, et al. Receptor tyrosine kinase inhibition causes simultaneous bone loss and excess bone formation within growing bone in rats. Toxicol Appl Pharmacol. 2011;254(3):267–79 [DOI] [PubMed] [Google Scholar]
- 12.Khoury HJ, Cortes JE, Kantarjian HM, Gambacorti-Passerini C, Baccarani M, Kim DW, et al. Bosutinib is active in chronic phase chronic myeloid leukemia after imatinib and dasatinib and/or nilotinib therapy failure. Blood. 2012;119(15):3403–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.