Abstract
Allogeneic hematopoietic stem cell transplantation recipients have an increasing risk of both hemorrhagic and thrombotic complications. However, the competing risks of two of these life-threatening complications in these complex patients have still not been well defined. We retrospectively analyzed data from 431 allogeneic transplantation recipients to identify the incidence, risk factors and mortality due to thrombosis and bleeding. Significant clinical bleeding was more frequent than symptomatic thrombosis. The cumulative incidence of a bleeding episode was 30.2% at 14 years. The cumulative incidence of a venous or arterial thrombosis at 14 years was 11.8% and 4.1%, respectively. The analysis of competing factors for venous thrombosis revealed extensive chronic graft-versus-host disease to be the only independent prognostic risk factor. By contrast, six factors were associated with an increased risk of bleeding; advanced disease, ablative conditioning regimen, umbilical cord blood transplantation, anticoagulation, acute III-IV graft-versus-host disease, and transplant-associated microangiopathy. The development of thrombosis did not significantly affect overall survival (P=0.856). However, significant clinical bleeding was associated with inferior survival (P<0.001). In allogeneic hematopoietic stem cell transplantation, significant clinical bleeding is more common than thrombotic complications and affects survival.
Introduction
Allogeneic stem cell transplantation (HSCT) is currently the standard therapy for many hematologic disorders and its use has markedly increased over the past two decades. Graft-versus-host disease (GVHD), infections and relapse are the most common complications of allogeneic HSCT. Recent studies have begun to recognize that allogeneic HSCT recipients are also prone to the complications of a higher rate of thromboembolic events (TEEs) including venous thromboembolism (VTE)1,2 and arterial events (ischemic stroke, coronary artery disease (CAD) or peripheral artery disease).3,4 In two recent studies, the 1-year incidences of allogeneic stem cell transplantation-associated VTE were 2.5% and 4.6%.5,6 The cumulative incidence of arterial events 15 years after allogeneic HSCT was 6%.4 Factors that have been suggested to increase the risk of VTE in HSCT recipients include indwelling central venous catheters, development of GVHD, and previous VTE.6 In addition, the occurrence of arterial thrombotic events was significantly higher in HSCT recipients with at least two cardiovascular risk factors.4
However, the risk of TEEs in HSCT recipients should be balanced with the risk of hemorrhagic complications. Bleeding is also a highly significant complication of allogeneic HSCT and has been associated with shorter survival.7–9 Prolonged thrombocytopenia and HSCT-specific hemorrhagic complications, such as hemorrhagic cystitis, diffuse alveolar hemorrhage, and gastrointestinal GVHD, are strong predictors of bleeding in allogeneic HSCT.10 Therefore, despite the fact that TEEs occur frequently during the post-transplant period, VTE prophylaxis is not generally used in allogeneic HSCT recipients. In addition, except for one study that simultaneously evaluated bleeding and thrombotic complications in early phases of allogeneic HSCT (<128 days),6 little is known about the competing risks of thrombosis and bleeding in this complex population. The purpose of this study was to determine the incidence, risk factors and mortality due to TEEs and bleeding in a large series of patients who underwent allogeneic HSCT in a single institution.
Design and Methods
Study subjects
Analyses were performed in accordance with the Declaration of Helsinki and the guidelines of the institutional review board of the Hospital Universitario de Salamanca. This study was approved by the Salamanca University Hospital Ethics Committee. We conducted a retrospective cohort study of 443 consecutive patients (aged over 18 years) who had undergone allogeneic HSCT between 1995 and 2011 at the Hospital Universitario de Salamanca. Twelve patients who received prophylaxis or therapeutic anticoagulation at the time of HSCT admission were excluded. Informed consent was obtained from all patients before HSCT. The main demographic and hematologic features of the 431 evaluable allogeneic HSCT recipients are shown in Table 1. Median age was 47±13 years; 61% of patients were male. Median follow up was 20 months (range 1–137 months). The conditioning regimen was myeloablative (n=193) or reduced-intensity conditioning (RIC) (n=238) (Table 1). The day of stem cell infusion was designated as Day 0. Prophylactic platelet transfusion was given when the platelet count fell below 10×109/L or 10–20×109/L in those with fever (>38°C). Post-HSCT complications, such as GVHD, vein-occlusive disease (VOD) and thrombotic microangiopathy (TMA), were also recorded. GVHD was defined according to the Seattle criteria for the diagnosis and staging of GVHD.11 VOD was diagnosed according to the Modified Seattle Criteria.12 A diagnosis of transplant-associated microangiopathy (TMA) was made in patients with microangiopathic hemolytic anemia, thrombocytopenia, renal failure and high levels of serum lactate dehydrogenase.13 As our enrollment period was very long (1995–2011), we have addressed any possible change in baseline characteristics of patients over time (1995–2004 vs. 2005–2011). The age of recipients has significantly increased in the second period (42.3 vs. 47.9, P<0.001). The number of patients who underwent an unrelated HSCT increased from 11.7% in the period 1995–2004 to 38.3% in 2005–2011, P<0.001. Also, a higher proportion of patients receiving non-myeloablative conditioning regimen was also observed in the 2005–2011 period.
Table 1.
Baseline characteristics of patients (n=431).
Indwelling central venous catheters
A central venous catheter (CVC), a triple-lumen catheter or a double-lumen Hickman was routinely implanted before infusion of stem cells. No systemic prophylaxis against deep-vein thrombosis was adopted. Each lumen of CVC was washed with 20 mL normal sterile saline and 5 mL heparinized saline (50 IU/mL) according to routine clinical practice. The CVC was removed when the patient was discharged after HSCT whilst the Hickman line was maintained until the development of complications that prompted catheter removal.
Outcomes
The primary end points of the study were the incidence, risk factors and clinical impact of post-allogeneic HSCT TEEs and hemorrhages. TEEs were subdivided into venous and arterial types. Venous TEEs were classified as deep venous thrombosis (DVT), pulmonary embolism (PE) or catheter-related venous thrombosis. The diagnosis of DVT was considered valid by venography or compression ultrasonography, according to standard methods. Pulmonary embolism was only accepted if it was demonstrated by perfusion lung scan or computerized tomography. The diagnosis of catheter-related venous thrombosis was defined as the partial or total occlusion of a vein into which a CVC had been inserted, or a DVT confirmed within 30 days of CVC removal by ultrasonography or phlebography. Arterial TEEs were classified as coronary artery disease (CAD), ischemic stroke or peripheral arterial disease, and diagnosed according to standard objective methods. Any bleeding event after allogeneic HSCT was included in the analysis, with the exception of mild petechiae. The number and location of bleeding sites were recorded. Bleeding was considered a major event if it conditioned a reduction in the hemoglobin level of at least 20 g/L, transfusion of at least two blood-pack units, or symptomatic bleeding in a critical area or organ. Transfusion events due to aplasia post chemotherapy were not included. Major bleeding was considered to be life-threatening if it resulted in: fatality, symptomatic intracranial or pulmonary bleeding, bleeding with a decrease in the hemoglobin level of at least 50 g/L, or bleeding requiring transfusion of at least four red blood-cell units or inotropic agents, or if surgery was required. All other bleeding was considered to be minor.
Statistical analysis
Data were initially included in an Excel (Microsoft) spreadsheet and a descriptive statistical analysis was performed. Results are expressed as percentages for categorical variables and as medians (and standard deviations) for continuous variables. Differences between groups were evaluated with SPSS 19.0 (SPSS, Chicago, IL, USA). Separate logistical regressions were performed using the backward conditional variable selection method to identify risk factors for the development of TEEs and bleeding. Variables associated with TEEs or bleeding in the bivariate analyses were included in the multivariate models. Cumulative incidence of vascular complications was estimated with death without a vascular event as the competing risk. Overall survival (OS) was defined as the time elapsed between HSCT Day 0 and death or last follow up, and calculated by the Kaplan-Meier method. The log rank test was used to assess differences between groups of patients with or without thrombosis, or patients with or without bleeding. Multivariate survival analysis involved developing Cox’s proportional hazards models with stepwise variable selection. All the parameters that were significant in the univariate analyses were included in the multivariate analysis. P<0.05 was considered statistically significant.
Results
Incidence and risk factors of post-allogeneic HSCT venous TEEs
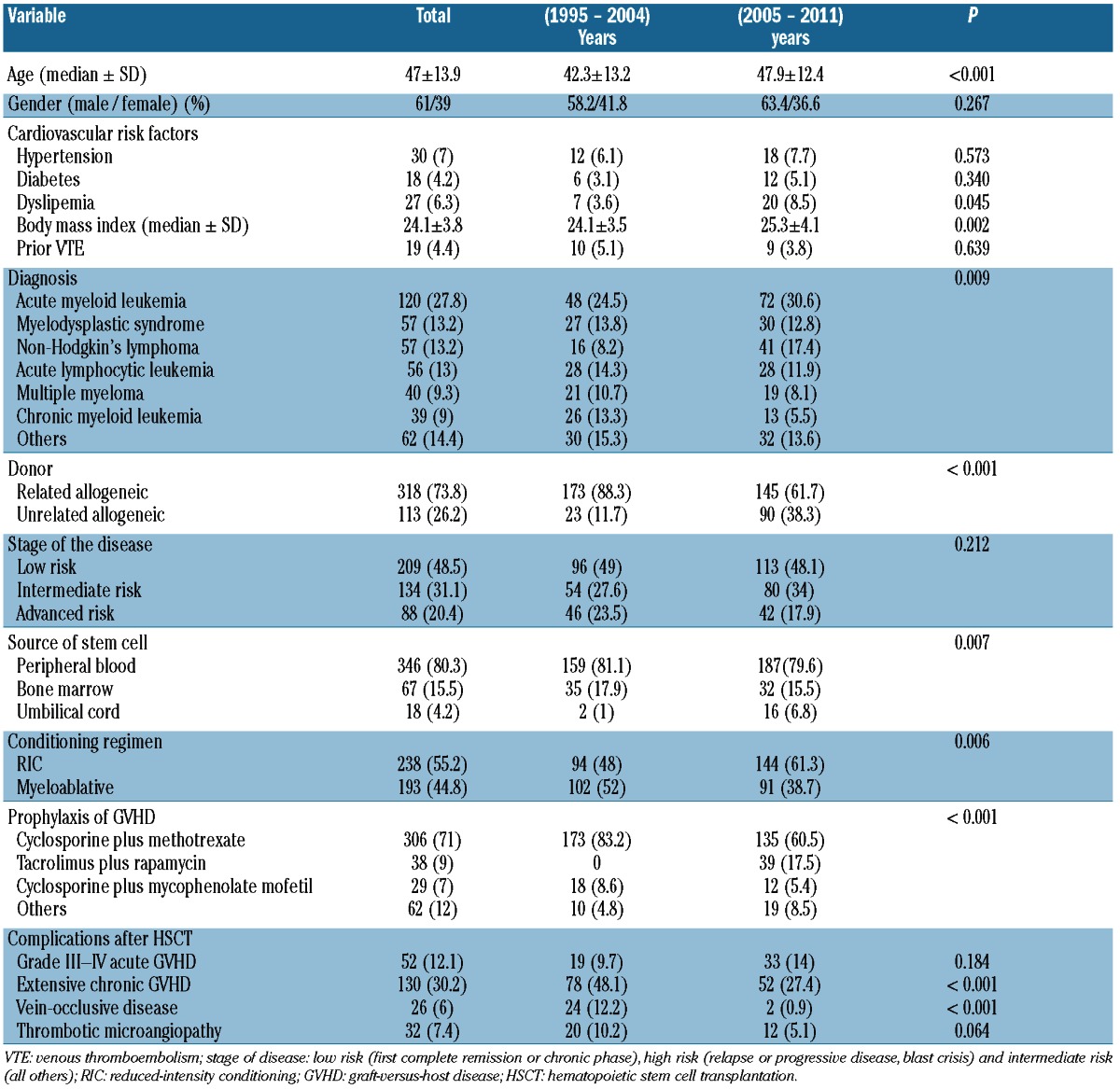
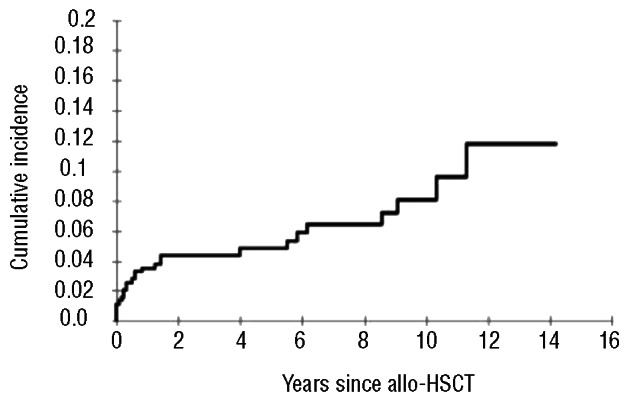
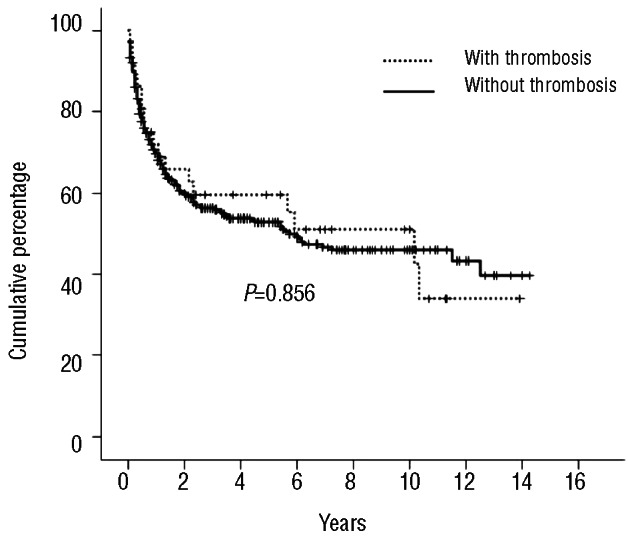
A total of 26 patients (6.03%) experienced a venous TEE after their allo-HSCT. The median time from HSC administration to diagnosis of venous TEEs was 211 days (range 9-4080 days). There were 16 venous TEEs episodes during 1995–2004 and 10 venous TEEs events during the period 2005-2011 (P=0.09). The cumulative incidence of post-allogeneic HSCT venous TEEs was 11.8% (95% CI: 7.1–19.6%) at 14 years (Figure 1). Nineteen patients (4.4%) developed a venous TEE unrelated to a CVC (11 DVTs, 5 DVTs with EP and 3 isolated PEs). Catheter-related venous thrombosis was present in 7 patients (1.6%). Anticoagulation therapy consisted of therapeutic low molecular weight heparin (LMWH) in 17 patients, and LMWH followed by acenocumarol with a target international normalized ratio of 2–3 in 7 patients. LMWH was not prescribed after CVC withdrawal in 2 patients with catheter-related venous thrombosis. The anticoagulant treatment was continued as long as it was clinically indicated. One patient with massive PE died. At the time of analysis, 5 patients (19.2%) had developed one episode of documented recurrent venous thrombosis. Table 2 shows the bivariate analysis factors for venous TEEs. The development of extensive chronic GVHD was the only risk factor for the occurrence of venous TEEs post-allogeneic HSCT (OR 2.85; 95% CI: 1.20–6.80).
Figure 1.
Cumulative incidence of a venous TEE was 3.6% (95% CI: 2.2–5.9%; 256 patients at risk) at 1 year, 4.8% (95% CI: 3.1–7.5%; 104 patients) at 5 years, 8.1% (95% CI: 5.2–12.6%; 37 patients) at 10 years, and 11.8% (95% CI: 7.1–19.6%; 3 patients) at 14 years.
Table 2.
Bivariate analysis of factors influencing venous TEEs.
Incidence and risk factors of post-allogeneic HSCT arterial TEEs
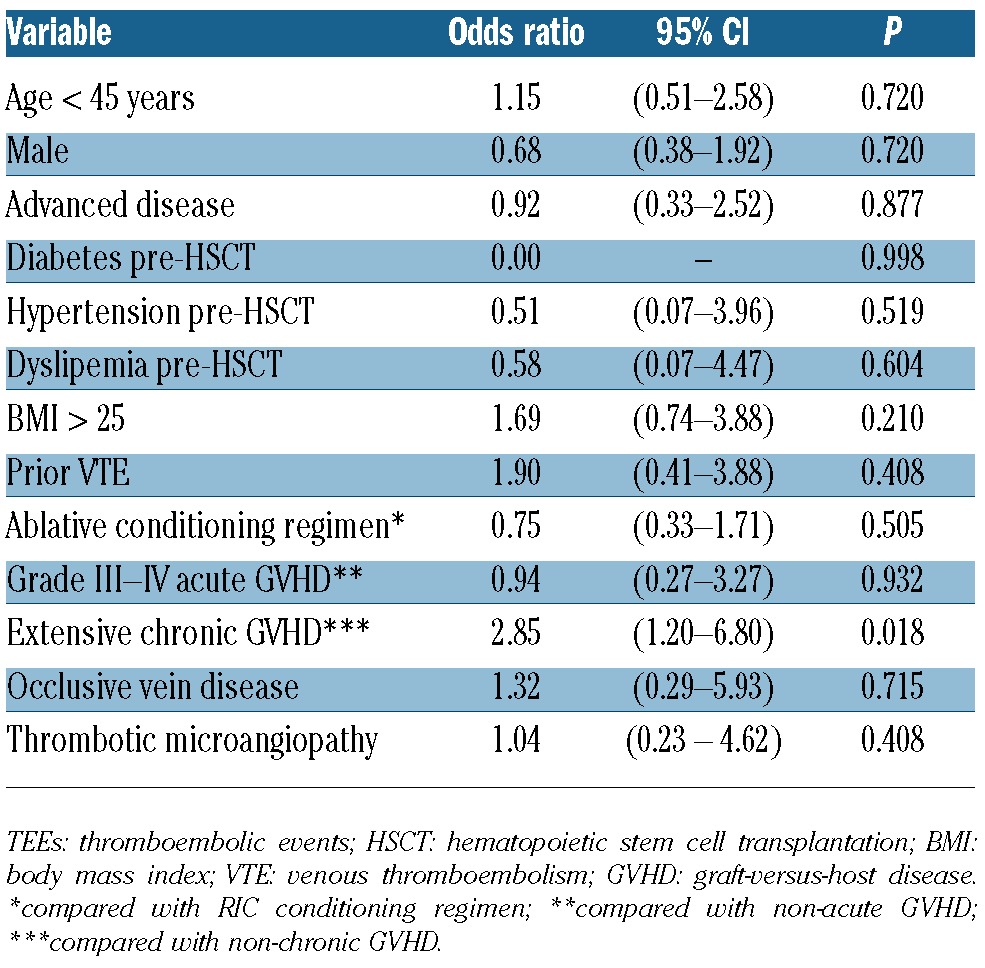
Eleven of the 431 (2.5%) patients had an arterial TEE after allogeneic HSCT (8 CAD and 3 ischemic strokes). The cumulative incidence of post-allogeneic HSCT arterial TEEs was 4.1% (95% CI: 1.8–9.3%) at 14 years (Figure 2). One patient with CAD and 2 patients with ischemic stroke were treated with acenocumarol (target INR between 2 and 3) and the 3 patients with stroke received antiplatelet agent. Two patients died because of CAD. Only a trend towards a higher rate of arterial events was observed for patients with a BMI greater than 25 (OR 2.85; 95% CI: 0.81–12.34; P=0.098).
Figure 2.
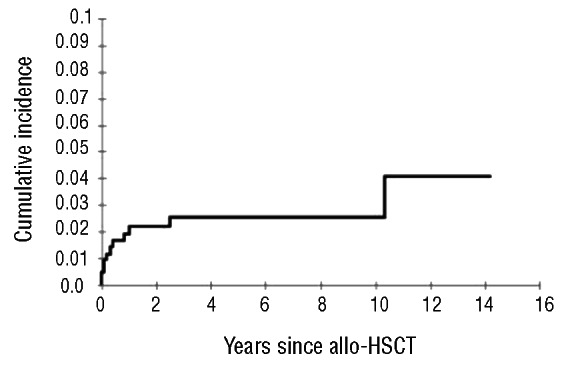
The cumulative incidence of an arterial TEEs was 2.2% (95% CI: 1.1–4.2%; 256 patients at risk) at 1 year, 2.5% (95% CI: 1.4–4.7%; 104 patients) at 5 years, and 4.1% (95% CI: 1.8–9.3%; 3 patients) at 14 years.
Incidence and risk factors of post-allogeneic HSCT bleeding
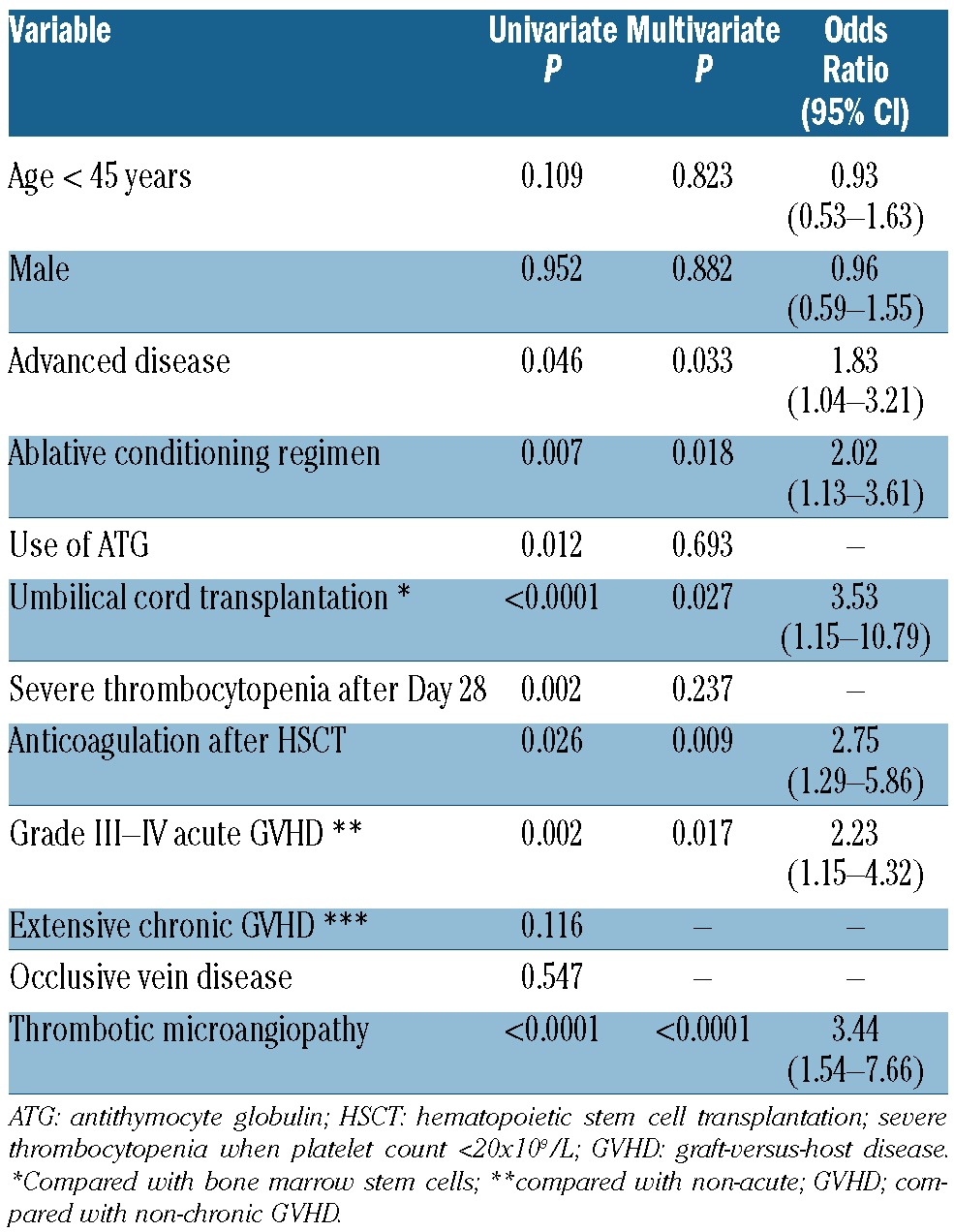
In total, 140 hemorraghic events occurred in 111 patients. The median time until the first bleeding episode was 60 days (range 2–3,660 days). The cumulative incidence of bleeding in allogeneic HSCT recipients was 30.2% (95% CI: 25.2–36.2%) at 14 years (Figure 3); 26.1% of bleeding episodes occurred during 1995–2004 and 22% in 2005–2011 (P=0.741). The majority of patients experienced bleeding at a single site with very few patients (n=25) bleeding from more than two locations. Genitourinary bleeding was the most common site for hemorrhagic complications in allogeneic HSCT recipients (32%) (Table 3), followed by gastrointestinal bleeding (22.9%). With respect to severity of hemorrhage, 73 of the bleeding events (52%) were considered major bleeding and 44 bleeding episodes (31.4%) were life-threatening events. The majority of life-threatening bleedings affected either the lungs (n=16), the gastrointestinal (GI) tract (n=10) or the central nervous system (CNS) (n=12). Twenty deaths were directly attributable to bleeding. Thirty-eight (8.8%) patients developed thrombotic and bleeding complications. Table 4 shows the univariate and multivariate analyses carried out to identify the variables capable of predicting bleeding episodes after allogeneic HSCT. Advanced disease, ablative conditioning regimen, use of ATG, umbilical cord transplantation, severe thrombocytopenia (platelet count < 20×109/L) after Day + 28, use of anticoagulation treatment after HSCT, grade III-IV acute GVHD and TMA were significantly associated with hemorrhagic complications in the univariate analysis. In multivariate analyses, six variables retained their association with bleeding events: advanced disease, ablative conditioning regimen, umbilical cord blood transplantation, anticoagulation after HSCT, grade acute III-IV GVHD and TMA. Interestingly, 10 of 111 patients (9%) had a late bleeding event (one year after HSCT). In this subgroup of patients, we analyzed the risk factors for bleeding. The use of anticoagulant therapy (P=0.003) and severe thrombocytopenia after Day 28 (P=0.004) were the risk factors associated to late bleeding.
Figure 3.
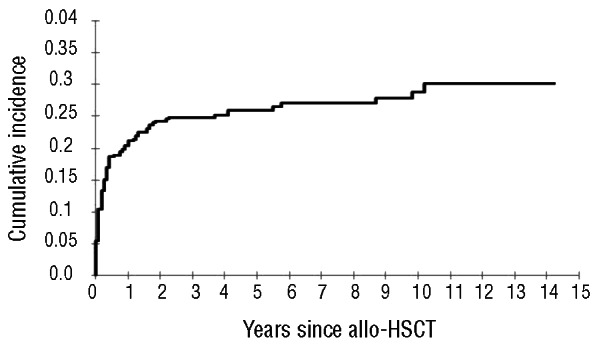
The cumulative incidence of a bleeding episode was 21.3% (95% CI: 17.7–25.5%; 237 patients at risk) at 1 year, 26.1% (95% CI: 22.1–30.8%; 100 patients) at 5 years, 28.9% (95% CI: 24.3–34.4%; 34 patients) at 10 years, and 30.2% (95% CI: 25.2–36.2%; 3 patients) at 14 years.
Table 3.
Bleeding episodes after HSCT.
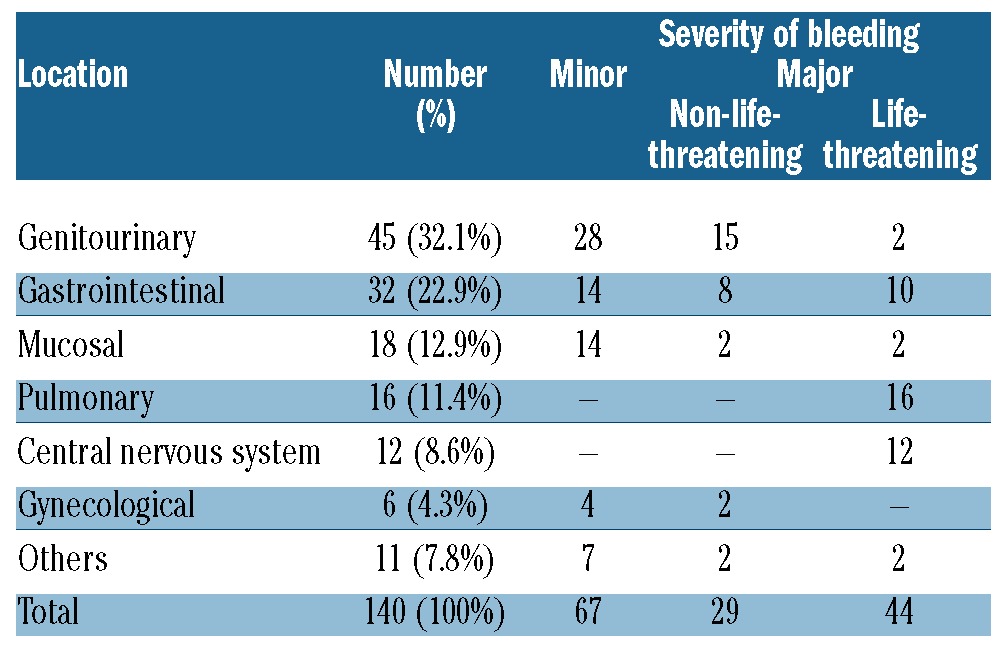
Table 4.
Univariate and multivariate analysis of factors influencing bleeding episodes.
Prognostic impact of bleeding and TEEs following allogeneic HSCT
The presence of hemorrhagic complications after HSCT was associated with an adverse outcome when compared with patients without bleeding episodes. Median OS of patients with bleeding episodes was 15 months, which was significantly lower than patients without bleeding episodes (Kaplan-Meier estimate 122 months; log rank test P<0.001) (Figure 4). In the multivariate analysis, bleeding events after allogeneic HSCT retained their independent prognostic influence (HR 3.34; 95% CI: 2.44–4.58) (Table 5). TMA was the strongest determinant of bleeding in our series and patients who developed TMA had a significantly shorter survival (log rank test P<0.001). But when TMA was included in the multivariate model, it did not remain an independent prognostic factor as it was superseded by the negative prognostic effect of grade III-IV acute GVHD. No statistical differences were identified in overall survival between patients with early and late bleeding events. Finally, there was no significant difference in OS between allogeneic HSCT recipients with or without TEEs (KaplanMeier estimate; log rank test P=0.856) (Figure 5).
Figure 4.
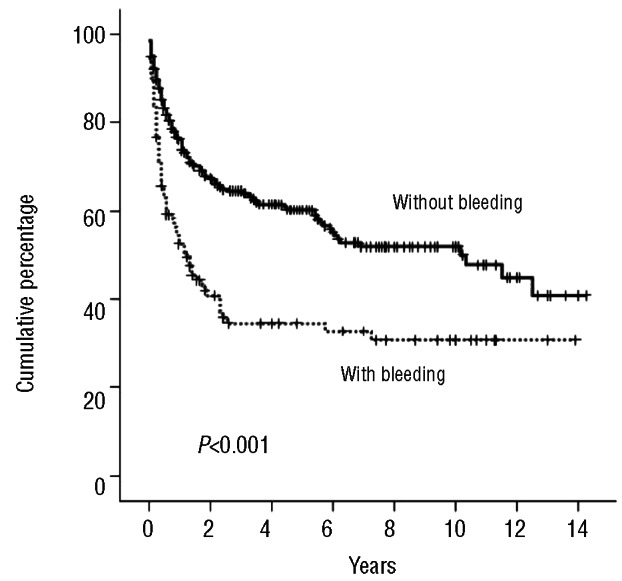
Overall survival of patients with and without bleeding after allogeneic HSCT.
Table 5.
Multivariate analysis of factors influencing overall survival.
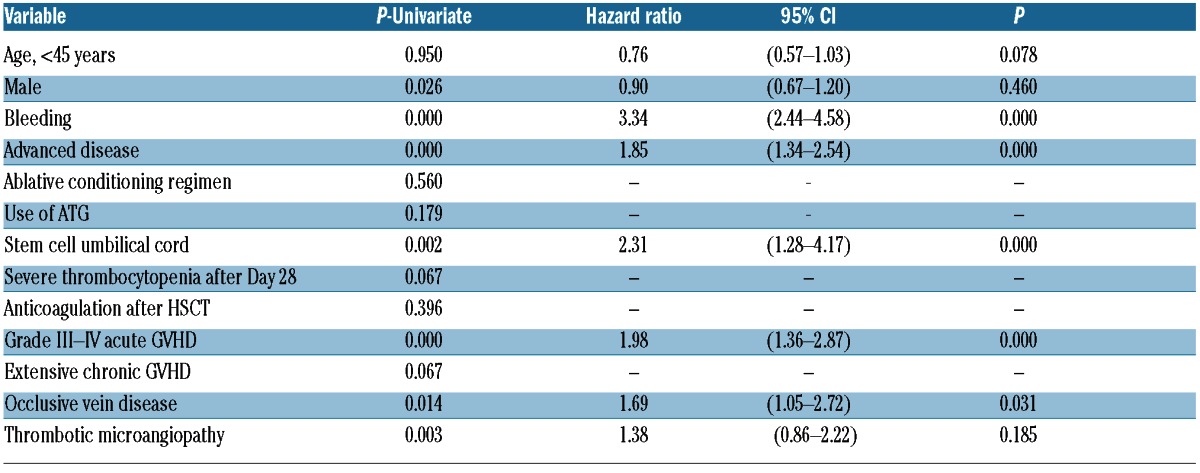
Figure 5.
Overall survival of patients with and without thrombosis after allogeneic HSCT.
Discussion
In recent years, it has begun to be recognized that the incidence of TEEs in patients who have undergone allogeneic HSCT is similar to that observed in patients with solid tumors.14 In this setting, a thromboprophylaxis strategy could be effective in preventing TEEs in selected allogeneic HSCT recipients. However, TEEs have been separately analyzed without taking into consideration the increasing risk of bleeding in allogeneic HSCT recipients. Here, we report the largest study to date of the competing risks of thrombosis and bleeding in allogeneic HSCT. Our results showed that the cumulative incidence of bleeding complications was higher than TEE. In addition, hemorrhagic complications were of significant independent prognostic value for OS on multivariate analysis, while TEE events were not.
In our series, the cumulative incidence of venous TEEs at 14 years was 11.8%. Similar incidences (3.7–14.6%) have been reported in other studies.2,5,6 Interestingly, 11 of 26 symptomatic VTE episodes occurred after one year of allogeneic HSCT. This indicates that allogeneic HSCT recipients have a similar risk of VTE in the early and late post-HSCT periods. In our series, the development of extensive chronic GVHD was the most important predictor of VTE, which is in agreement with two other studies.6,15 Pihusch et al. confirmed a higher incidence of VTE in patients with chronic GVHD2 with a limited follow up (<1 year). We have identified that the risk of VTE in GVHD was due to the development of extensive chronic GVHD. As chronic GVHD can occur for several years after HSCT, the risk of VTE would remain more than one year of allogeneic HSCT. VTE prophylaxis may be useful in extensive chronic GVHD. However, well-designed clinical trials to evaluate the risks and benefits of VTE prophylaxis in this population are needed. Over recent years, we have observed an increase in the median age of allogeneic transplant patients with the introduction of RIC regimens. An increase in unrelated and cord blood transplants has also been observed. These trends observed in our center are quite similar to those reported by the EBMT registry.16 However, our rate of bleeding/thrombotic complications has not changed over time.
The mechanism of thrombogenesis in allogeneic HSCT recipients who develop GVHD is uncertain. Release of cytokines involved in the pathophysiology of GVHD may be associated with a higher risk of VTE. Necrosis factor-alpha can modulate the endothelial hemostatic function by increasing levels of prothrombotic plasminogen activator inhibitor I or tissue factor and by decreasing those of antithrombotic factors, such as the tissue factor pathway inhibitor.2 Increasing circulating microparticles released after intensive cell activation2 could also contribute to the higher risk of VTE in GVHD.
The development of arterial thrombosis after HSCT has been of increasing concern during recent years. We found a cumulative incidence of arterial TEEs of 4.1% at 14 years. This is coincidental with two recent studies in which allogeneic HSCT recipients had a higher risk of premature arterial vascular disease.3,4 In these studies, the presence of cardiovascular risk factors was associated with higher incidences of arterial thrombosis. We found a trend towards developing arterial events in subjects with a BMI over 25.
This study illustrates that, although thrombosis is a significant problem in patients with allogeneic HSCT, it is not associated with increased mortality. In line with these results, patients on AML17 or with MM treated with lenalidomide18 who developed a VTE did not have shorter overall survival. In contrast, the development of thrombosis in solid tumors is associated with increased mortality.19 In solid tumors, the development of venous thromboembolism suggests the presence of aggressive disease. A hypercoagulable state induced by tissue factor can be crucial for metastasis.20 However, in allogeneic HSCT the hypercoagulable state would not be an important factor influencing aggressiveness.
Bleeding was more frequent than clinical thrombosis and was associated with increased mortality. However, we should take into account a possible limitation to our study since it is likely that thrombosis was not always detected (subclinical or asymptomatic), and that bleeding could be over-estimated (although we have tried to exclude transfusions due to pancytopenia).
The risk of death is higher in allogeneic HSCTs with symptomatic bleeding than in those without bleeding. The cumulative incidence of bleeding in our study is quite similar to that found by other authors.6,7,9 Recent data suggest that the excess bleeding risk after allogeneic HSCT is restricted to patients developing GVHD, and that patients without GVHD are not at increased risk of bleeding.2 We have clarified that severe acute GVHD is responsible for increased risk of bleeding. Cumulative endothelial and epithelial damage through immunological injury to the vasculature could explain the risk of bleeding in GVHD.21 We found that symptomatic bleeding can also occur in situations other than acute GVHD, such as thrombotic microangiopathy. Also, situations involving a pancytopenic period as a more intense myeloablative conditioning, and umbilical cord stem cell transplantation were predictors of bleeding.
In conclusion, in this study, which has the longest follow up to date of patients undergoing allogeneic HSCT, the incidence of VTE was relatively high during early and late phases of HSCT, but did not influence survival. Extensive chronic HSCT was the most important risk factor. In contrast, bleeding was more frequently associated with increased mortality. The principal risk factors for bleeding are myeloablative conditioning, umbilical cord blood transplantation, severe thrombocytopenia after Day 28 and development of grade III-IV acute GVHD or TMA.
Supplementary Material
Footnotes
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Tsakiris DA, Tichelli A. Thrombotic complications after haematopoietic stem cell transplantation: Early and late effects. Best Pract Res Clin Haematol. 2009;22(1):137–45 [DOI] [PubMed] [Google Scholar]
- 2.Pihusch R, Salat C, Schmidt E, Göhring P, Pihusch M, Hiller E, et al. Hemostatic complications in bone marrow transplantation: A retrospective analysis of 447 patients. Transplantation. 2002;74(9):1303–9 [DOI] [PubMed] [Google Scholar]
- 3.Tichelli A, Bucher C, Rovó A, Stussi G, Stern M, Paulussen M, et al. Premature cardiovascular disease after allogeneic stem cell transplantation. Blood. 2007;110(9):3463–71 [DOI] [PubMed] [Google Scholar]
- 4.Tichelli A, Passweg J, Wójcik D, Rovó A, Harousseau JL, Masszi T, et al. Late cardiovascular events after allogeneic hematopoietic stem cell transplantation: a retrospective multicenter study of the Late Effects Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2008;93(8):1203–10 [DOI] [PubMed] [Google Scholar]
- 5.Gonsalves A, Carrier M, Wells PS, McDiarmid SA, Huebsch LB, Allan DS. Incidence of symptomatic venous thromboembolism following hematopoietic stem cell transplantation. J Thromb Haemost. 2008;6(9):1468–73 [DOI] [PubMed] [Google Scholar]
- 6.Gerber DE, Segal JB, Levy MY, Kane J, Jones RJ, Streiff MB. The incidence of and risk factors for venous thromboembolism (VTE) and bleeding among 1514 patients undergoing hematopoietic stem cell transplantation: implications for VTE prevention. Blood. 2008;112(3):504–10 [DOI] [PubMed] [Google Scholar]
- 7.Nevo S, Vogelsang GB. Acute bleeding complications in patients after bone marrow transplantation. Curr Opin Hematol. 2001;8(5):319–25 [DOI] [PubMed] [Google Scholar]
- 8.Bacigalupo A. Haemopoietic stem cell transplants: the impact of haemorrhagic complications. Blood Rev. 2003;17(1 Suppl):S6–10 [DOI] [PubMed] [Google Scholar]
- 9.Pihusch M. Bleeding complications after hematopoietic stem cell transplantation. Semin Hematol. 2004;41(1 Suppl):93–100 [DOI] [PubMed] [Google Scholar]
- 10.Graf L, Stern M. Acute phase after haematopoietic stem cell transplantation. Bleeding and thrombotic complications. Hamostaseologie. 2012;32(1):1–7 [DOI] [PubMed] [Google Scholar]
- 11.Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man: a long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69(2):204–17 [DOI] [PubMed] [Google Scholar]
- 12.Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG, et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44(6):778–83 [DOI] [PubMed] [Google Scholar]
- 13.Ruutu T, Barosi G, Benjamin RJ, Clark RE, George JN, Gratwohl A, et al. Diagnostic criteria for hematopoietic stem cell transplant-associated microangiopathy: results of a consensus process by an International Working Group. European Group for Blood and Marrow Transplantation; European LeukemiaNet. Haematologica. 2007;92(1):95–100 [DOI] [PubMed] [Google Scholar]
- 14.Falanga A, Marchetti M. Venous thromboembolism in the hematologic malignancies. J Clin Oncol. 2009;27(29):4848–57 [DOI] [PubMed] [Google Scholar]
- 15.Stoffel N, Rysler C, Buser A, Gratwohl A, Tsakiris DA, Stern M. Leukocyte count and risk of thrombosis in patients undergoing haematopoietic stem cell transplantation or intensive chemotherapy. Thromb Haemost. 2010;103(6):1228–32 [DOI] [PubMed] [Google Scholar]
- 16.Passweg JR, Baldomero H, Gratwohl A, Bregni M, Cesaro S, Dreger P, et al. The EBMT activity survey: 1990–2010. Bone Marrow Transplant. 2012;47(7):906–23 [DOI] [PubMed] [Google Scholar]
- 17.Ku GH, White RH, Chew HK, Harvey DJ, Zhou H, Wun T. Venous thromboembolism in patients with acute leukemia: incidence, risk factors, and effect on survival. Blood. 2009;113(17):3911–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zangari M, Tricot G, Polavaram L, Zhan F, Finlayson A, Knight R, et al. Survival effect of venous thromboembolism in patients with multiple myeloma treated with lenalidomide and high-dose dexametha-sone. J Clin Oncol. 2010;28(1):132–5 [DOI] [PubMed] [Google Scholar]
- 19.Sørensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343:1846–50 [DOI] [PubMed] [Google Scholar]
- 20.Ruf W, Mueller BM. Thrombin generation and the pathogenesis of cancer. Semin Thromb Hemost. 2006;32(1 Suppl):61–8 [DOI] [PubMed] [Google Scholar]
- 21.Dumler JS, Bechorner WE, Farmer ER, Di Gennaro KA, Saral R, Santos GW. Endothelial-cell injury in cutaneous acute graft-versus-host disease. Am J Pathol. 1989;135(6):1097–103 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


