Abstract
Out of 153 newly referred human T-lymphotropic virus type I infected patients, 42 (27%) had 5% or more abnormal lymphocytes, consistent with the diagnosis of smoldering adult T-cell leukemia/lymphoma. The abnormal lymphocyte percentage was higher in patients with human T-lymphotropic virus type I associated inflammatory disease compared with asymptomatic carriers (P=0.006). Over 4.5 years median follow up, 4 patients, all with 10 or more human T-lymphotropic virus type I DNA copies/100 peripheral blood mononuclear cells at presentation, but only one with 5% or more abnormal lymphocytes at presentation, developed adult T-cell leukemia/lymphoma. Thus, high pre-morbid human T-lymphotropic virus type I proviral load, rather than fulfilment of the classification criteria for smoldering adult T-cell leukemia/lymphoma, was associated with an increased risk of developing aggressive adult T-cell leukemia/lymphoma.
Introduction
Human T-lymphotropic virus type I (HTLV-I) is the etiological agent of HTLV-I-associated myelopathy (HAM), and adult T-cell leukemia/lymphoma (ATLL).1,2 In the Shimoyama classification, four subtypes of ATLL are recognized: smoldering, chronic, lymphoma and acute.3 Essential diagnostic criteria are histological/cytological evidence of T-cell malignancy, abnormal peripheral blood T lymphocytes (except in lymphoma) and anti-HTLV-I antibodies in serum. Sub-classification is based on lymphocyte count, abnormal lymphocyte percentage, lactate dehydrogenase (LDH), corrected calcium and the pattern of tissue involvement. Smoldering ATLL is diagnosed when 5% or more abnormal lymphocytes of a T-cell nature are present in the peripheral blood but total lymphocyte count is less than 4× 109/L, corrected calcium is normal and LDH is less than 1.5 times the upper limit of normal (ULN), or in the presence of histologically proven skin or lung involvement. In the classification study, cutaneous lesions were present in 48.9% and lung involvement in 15.6%; thus abnormal lymphocytes of 5% or more would have been the only abnormality in the remainder. Abnormal lymphocytes in this context include flower cells, blast-like cells, giant cells with cerebriform nuclei, and small atypical lymphocytes with nuclear pleomorphism.4,5 A further category of lymphocytes with ‘unusual morphology’, including lymphoblasts and lymphocytes with features including cytoplasmic granulation and vacuolation, has been described in ATLL.6 The classification study reported 62.8% 4-year survival for smoldering ATLL. In another retrospective study, 11 of 26 (42%) smoldering ATLL transformed to aggressive ATLL after a median of 81 months.7
Atypical lymphocytes are commonly observed in peripheral blood films of non-ATLL HTLV-I infected patients attending the HTLV service at St Mary’s Hospital, London, UK. Therefore, a prospective analysis of all such patients (1991-2008) was conducted to identify whether the presence of 5% or more abnormal lymphocytes in peripheral blood or other risk factors were associated with risk of incident aggressive ATLL.
Design and Methods
Essential inclusion criteria were serological confirmation of HTLV-I infection (gag and env antibodies on Western blot) and a peripheral blood film at presentation. Patients were characterized as either asymptomatic HTLV-I carriers (ACs) or patients with any HTLV-I-associated inflammatory disease (HAID). Patients with a prior diagnosis of cutaneous, chronic, acute or lymphomatous ATLL were excluded.
Demographic data included gender, ethnic origin, age, disease status at last follow up or cause of death. Laboratory data included full blood and differential white cell counts, CD3+CD4+ and CD3+CD8+ subsets, CD4 and CD25 expression, corrected calcium, globulin, creatine kinase (CK), LDH and β2 microglobulin (β2M). HTLV-I proviral load (PVL) was quantified by real-time PCR using the Roche LightCycler (Mannheim, Germany).8 Briefly, genomic DNA extracted from 1×103 peripheral blood mononuclear cells (PBMCs) was used as a template for amplification. Two separate PCRs were carried out for quantification of HTLV-I Tax and β-globin DNA. Standard curves were generated for both PCRs using genomic DNA from MT-2 cells (7 Tax copies per cell) and the copy numbers in individual samples were estimated by interpolation from the standard curves. The HTLV-I PVL was then calculated as:
Peripheral blood films were stained according to the method of May-Grünwald-Giemsa and 100 PBMCs were examined microscopically by 2 of the authors (BJB and AH).
Statistical analyses were performed using R version 2.12.1 (The R Foundation for Statistical Computing). Potential risk factors were examined for correlation with 5% or more abnormal lymphocytes using the Mann-Whitney Test for continuous variables and Pearson’s χ2 test with Yates’s continuity correction for binary variables, with a 5% level of significance. β2M, CK, LDH and globulin were converted to binary variables (within or above normal limits), and HTLV-I PVL was analyzed on a logarithmic scale. Bonferroni’s correction for multiple comparisons was applied to all P values obtained. Variables significantly associated (before Bonferroni’s correction for multiple comparisons) with 5% or more abnormal lymphocytes were further analyzed through logistical regression. For continuous variables, likelihood ratio tests were performed to test for any departures from a linear trend. Multivariate logistical regression models were built through both backward stepwise exclusion and forward stepwise selection to ensure a consistent model was produced.
Person-years follow up for each participant were calculated from initial presentation to last follow up or development of ATLL.
This study was approved by the Joint Research Office of the Imperial College Healthcare National Health Service Trust.
Results and Discussion
Of the 153 eligible patients, 117 were female, 76% African/Afro-Caribbean, 19% Caucasian and 5% of other ethnic origin. Ninety-two (60%) were ACs and 57 of 61 with HAID had HAM. Demographic and laboratory data are summarized in Table 1. The median values of globulin, CPK and β2M, surrogate markers associated with inflammation, approach the ULN. Median follow up was 4.5 years, being longer than five years in 38% AC and 51% in patients with HAID. All patients under regular follow up were seen at least every six months.
Table 1.
Demographic and laboratory data of the NCHR cohort.
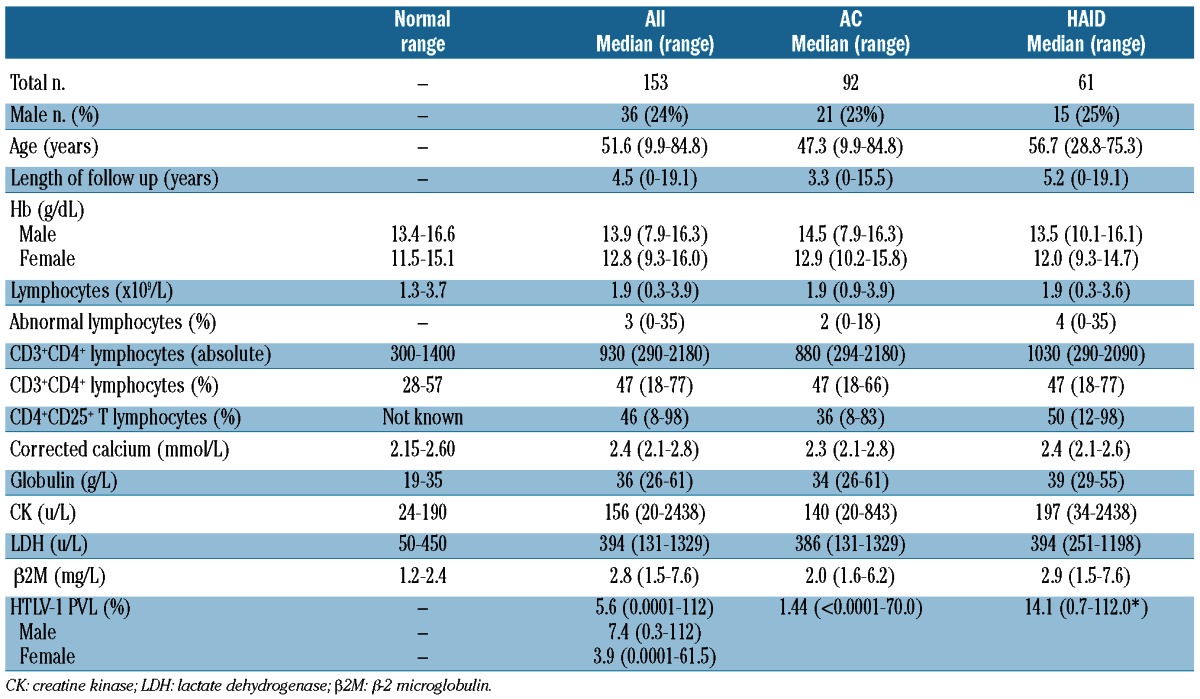
Absolute CD4+ lymphocyte counts and atypical lymphocyte percentages but not total lymphocyte counts, were higher in patients with HAID (P<0.05). Globulin, CK and β2M were higher in the HAID group while the neutrophil count was lower (all P<0.05). HTLV-I PVL was higher in males than females and in patients with HAID than ACs (both P<0.05).
There was no difference in lymphocyte count, atypical lymphocyte percentage or β2M between African/Afro-Caribbean and Caucasian subjects. Lower hemoglobin concentration and neutrophil counts, and higher LDH and CK were observed in African/Afro-Caribbean subjects (all P<0.05).
Abnormal peripheral blood lymphocytes were observed in 75% of patients. An irregular or convoluted nucleus (Figure 1A) was the most frequent feature, but other morphological abnormalities such as cleft or bi-lobed nuclei (Figure 1B and C), atypical large granular lymphocytes (LGLs) (Figure 1D), flower cells (Figure 1E) and abnormally large lymphocytes (Figure 1F) were frequently observed, often in the same patient. Flower cells were identified in 16% of cases and their presence was associated with both a higher abnormal lymphocyte percentage (4% vs. 2%, P=0.007) and higher HTLV-I PVL (9.3% vs. 5.3%, P=0.049). The frequency of flower cells did not differ significantly between patients with HAID (12 of 61, 20%) and ACs (13 of 92, 14%) (P=0.38).
Figure 1.
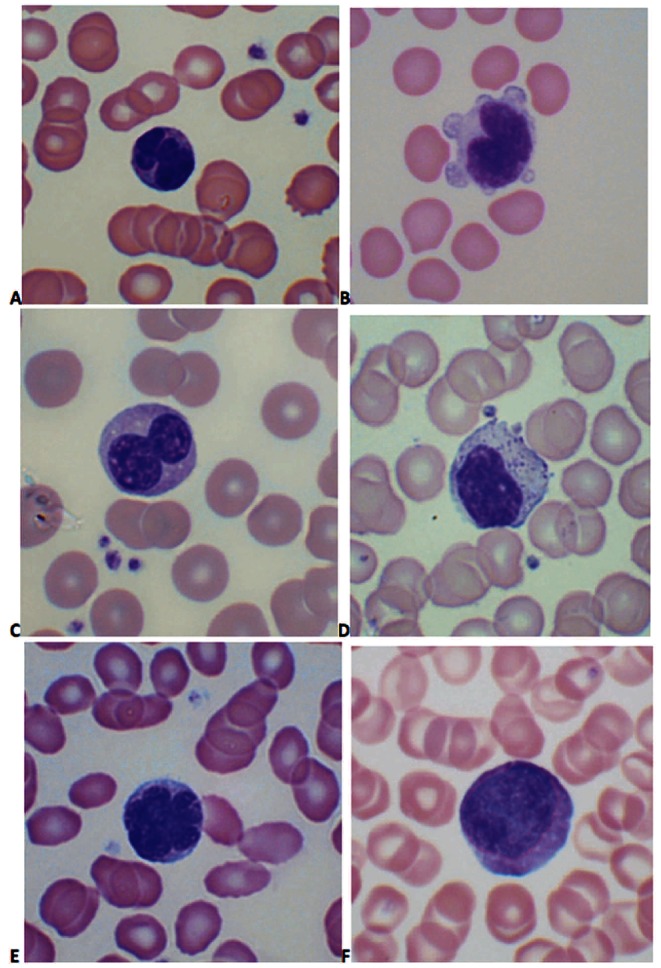
Lymphocyte morphology in asymptomatic carriers of HTLV-1: (A) convoluted nucleus; (B) cleft nucleus with cytoplasmic blebbing; (C) bi-lobed nucleus; (D) large granular lymphocyte; (E) flower cell; (F) abnormally large cell. (Images were obtained using a Nikon Eclipse E600 microscope, 100x objective, Olympus DP-12 camera; Adobe Photoshop used to adjust contrast and brightness only).
Forty-two patients had 5% or more circulating abnormal lymphocytes (median 7%, range 5–35%), and 111 patients had less than 5% abnormal lymphocytes (median 1%, range 0–4%). Presence of 5% or more abnormal lymphocytes showed a strong association with HAID (P<0.001, P=0.005 after Bonferroni’s correction) being found in 44% patients with HAID (27 of 61) and 16% AC (15 of 92).
Platelet counts (P=0.025), CPK (P=0.027), β2M (P<0.001), CD4+CD25+ (P=0.040) and HTLV-I PVL (P=0.017) were associated with 5% or more abnormal lymphocytes, although of these only β2M maintained a significant association after Bonferroni’s correction for multiple comparisons (P=0.007).
Upon univariate logistical regression, presence of HAID (OR 4.08, range 1.93–8.62, P<0.001) and above normal β2M (OR 5.55, range 1.98-15.52, P=0.001) were strongly associated with 5% or more abnormal lymphocytes. Additionally, PVL (OR 1.55, range 1.06–2.27) per log increase, P=0.024), CD4+CD25+ lymphocyte percentage (OR 1.030, range 1.00–1.06, per unit increase, P=0.03) and above normal CK (OR 2.50, range 1.18–5.29, P=0.02) were associated with 5% or more abnormal lymphocytes. Likelihood ratio tests gave no evidence against a linear trend for all continuous variables used in logistical regression.
In the multivariate logistical model, above normal β2M was the only independent predictor of 5% or more abnormal lymphocytes. Probably due to the strong association between HAID and β2M (&x003C7;2=19.99, df=1, P<0.001) the association between HAID and 5% or more abnormal lymphocytes was no longer present. Patients with raised β2M are over three times more likely to have 5% or more abnormal lymphocytes after controlling for HAID than patients with normal β2M (OR 3.45, range 1.098–10.827, P=0.034).
None of the 97 patients with an HTLV-I PVL of less than 10% developed aggressive ATLL after a median 3.1-year follow up. Median follow up of the 56 patients with an HTLV-I PVL 10% or more (range 10-61.5) was 4.2 years. Four (7%) developed aggressive ATLL, (3 lymphoma and one acute ATLL) of whom 2 were male. Of the 56, 41 had HAID and 15 were ACs; thus 3 of 41 (7.3%) with HAID and 1 of 15 (6.7%) ACs with PVL 10% or more developed ATLL (P=not significant). At initial presentation, only one had 5% or more abnormal lymphocytes, including flower cells. All 4 patients maintained an HTLV-I PVL 10% or more throughout the mean 5.8 (range 2–10) years preceding the emergence of ATLL.
The overall incidence of aggressive ATLL in our cohort (4 of 153) equates to 5.4 cases per 1,000 person-years (95% CI: 0.83–11.9). Among patients with an HTLV-I PVL 10% or more, the incidence of aggressive ATLL was 13.3 cases per 1,000 person years.
Long-term observational cohort studies are essential to accurately predict risk of aggressive ATLL in HTLV-I carriers, but few such studies exist. In the Miyazaki cohort study 27% of the 1,790 cohort members enrolled were HTLV-I positive while five incident cases of ATLL were observed between 1984 and 1995.9 Although the low incidence of ATLL also prevents us from developing a robust risk prediction model, several important observations can be made.
1) The presence of 5% or more abnormal lymphocytes at presentation was positively associated with the presence of HAID, higher serum levels of LDH, CK, β2M, globulin and increased percentage of CD4+CD25+ suggesting an inflammatory stimulus to abnormal lymphocyte production.
2) CD4+CD25+ co-expression was significantly higher in subjects with HAID (median 50%, range 8-98%, excluding the patients who subsequently developed ATLL). Distinction of these activated T cells from a malignant population with the characteristic CD3+CD4+CD25+ ATLL immunophenotype requires further investigation and the presence of a high percentage of CD4+CD25+ percentile on flow cytometry alone should not be used to diagnose ATLL. We conclude that these atypical lymphocytes are activated lymphocytes and are not pre-malignant.
3) The observation that 7% of patients with HTLV-I PVL of 10% or more developed aggressive ATLL during a median 4.5-year follow up suggests that this subgroup has a much higher lifetime risk of ATLL than the general HTLV-I-positive population. In subjects with HTLV-I PVL of 10% or more, although numbers were small, there was no apparent difference in development of ATLL in subjects with or without HAID. Of those with HAID who developed ATLL, none had been previously treated with steroids or immunosuppression. Higher HTLV-I PVL has been reported to predict the development of ATLL in asymptomatic HTLV-I carriers.10,11 In a 2010 study, 14 of 1,218 AC developed ATLL (10 smoldering, 2 lymphoma and 2 acute ATLL) after a median follow up of one year, all of whom had an over 4% HTLV-I proviral load while asymptomatic.11 The incidence of aggressive ATLL in this Japanese AC cohort was 2 per 1000 person-years; this was less than but not dissimilar to that seen in our non-Japanese cohort that also included patients with HAID. Exclusion of these patients would underestimate the overall risk of ATLL since this group has higher HTLV-I PVL.
Supplementary Material
Footnotes
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Hinuma Y, Nagata K, Hanaoka M, Nakai M, Matsumoto T, Kinoshita KI, et al. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci USA. 1981;78(10):6476–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gessain A, Barin F, Vernant JC, Gout O, Maurs L, Calender A, et al. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;2(8452):407–10 [DOI] [PubMed] [Google Scholar]
- 3.Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984–87). Br J Haematol. 1991;79(3):428–37 [DOI] [PubMed] [Google Scholar]
- 4.Ohshima K, Jaffe E, Kikuchi M. Adult T-cell leukaemia/lymphoma. In: Swerdlow S, Campo E, Harris N, Jaffe E, Pileri S, Stein H, et al. (eds.) WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC; 2008 [Google Scholar]
- 5.The world health organization classification of malignant lymphomas in japan: incidence of recently recognized entities Lymphoma Study Group of Japanese Pathologists. Pathol Int. 2000;50(9):696–702 [DOI] [PubMed] [Google Scholar]
- 6.Tsukasaki K, Imaizumi Y, Tawara M, Fujimoto T, Fukushima T, Hata T, et al. Diversity of leukaemic cell morphology in ATL correlates with prognostic factors, aberrant immunophenotype and defective HTLV-1 genotype. Br J Haematol. 1999;105(2):369–75 [DOI] [PubMed] [Google Scholar]
- 7.Ishitsuka K, Ikeda S, Utsunomiya A, Saburi Y, Uozumi K, Tsukasaki K, et al. Smouldering adult T-cell leukaemia/lymphoma: a follow-up study in Kyushu. Br J Haematol. 2008;143(3):442–4 [DOI] [PubMed] [Google Scholar]
- 8.Goon PK, Igakura T, Hanon E, Mosley AJ, Barfield A, Barnard AL, et al. Human T cell lymphotropic virus type I (HTLV-I)-specific CD4+ T cells: immunodominance hierarchy and preferential infection with HTLV-I. J Immunol. 2004;172(3):1735–43 [DOI] [PubMed] [Google Scholar]
- 9.Hisada M, Okayama A, Shioiri S, Spiegelman DL, Stuver SO, Mueller NE. Risk factors for adult T-cell leukemia among carriers of human T-lymphotropic virus type I. Blood. 1998;92(10):3557–61 [PubMed] [Google Scholar]
- 10.Okayama A, Stuver S, Matsuoka M, Ishizaki J, Tanaka G, Kubuki Y, et al. Role of HTLV-1 proviral DNA load and clonality in the development of adult T-cell leukemia/lymphoma in asymptomatic carriers. Int J Cancer. 2004;110(4):621–5 [DOI] [PubMed] [Google Scholar]
- 11.Iwanaga M, Watanabe T, Utsunomiya A, Okayama A, Uchimaru K, Koh KR, et al. Human T-cell leukemia virus type I (HTLV-1) proviral load and disease progression in asymptomatic HTLV-1 carriers: a nationwide prospective study in Japan. Blood. 2010;116(8):1211–9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


