Abstract
JAK2 fusion genes are rare but recurrent abnormalities associated with diverse, clinically heterogeneous hematologic malignancies. Here we assess the JAK1/2 inhibitor ruxolitinib as therapy for patients with JAK2-rearrangement associated myeloproliferative neoplasms (MPN). Ruxolitinib-treated Ba/F3 cells transformed to IL3 independence by ETV6-JAK2 showed reduced proliferation and survival (IC50 = 370 nM) compared with KG1A or Ba/F3 cells transformed by BCR-ABL1, SPBN1-FLT3 and ZMYM2-FGFR1 (IC50 > 10 μM for all). Inhibition was associated with reduced phosphorylation of ETV6-JAK2, ERK, STAT5 and AKT. Primary cell growth from 2 patients with JAK2 rearrangement and one patient with JAK2 amplification was assessed in methylcellulose assays. Reduced colony growth was seen for all patients in ruxolitinib-treated cultures compared with healthy controls (n=7). Fluorescence in situ hybridization showed reduced growth of JAK2-rearrangement positive colonies compared to JAK2-rearrangement negative colonies. Our data, therefore, provide evidence that ruxolitinib is a promising therapy for treatment of patients with JAK2 fusion genes.
Introduction
Chromosomal translocations targeting JAK2 are rare but recurrent abnormalities are seen in myeloproliferative neoplasms (MPN), acute myeloid leukemia, acute lymphoblastic leukemia and lymphoma.1–7 Three fusion variants in MPN, namely PCM1-JAK2, BCR-JAK2 and ETV6-JAK2, are thought to be constitutively activated drivers of the disease process analogous to BCR-ABL1 in chronic myeloid leukemia. JAK2-rearranged malignancies are typically aggressive and, apart from stem cell transplantation, current therapies have limited efficacy.
Ruxolitinib (INC424) (Novartis/Incyte) is an orally available, selective JAK1/2 inhibitor recently approved by the United States Food and Drug Administration for the treatment of intermediate or high-risk myelofibrosis. JAK2 inhibitors are obvious candidates for treatment of patients with JAK2 fusion genes8 and, therefore, we have examined the activity of ruxolitinib in primary patient material and cell line models. We present data showing that ruxolitinib is a promising candidate for treatment of patients with MPN and chromosomal abnormalities involving JAK2.
Design and Methods
To assess response to ruxolitinib in cell line models, Ba/F3 cells were transfected with pcDNA3.1-ETV6-JAK2 (ETV6 amino acid 154 fused to JAK2 amino acid 534; kindly provided by Dr Dwayne Barber, Ontario Cancer Institute, Toronto, Canada). Clones were isolated and transformed to IL3 independence. Ba/F3 cells transformed to IL3 independence by BCR-ABL1 (kindly provided by Dr Junia Melo, University of Adelaide, Australia), ZMYM2-FGFR19 or SPTBN1-FLT310 and the KG1A cell line were used as controls. All cell lines were grown in RPMI-1640 plus 10% serum.
To assess proliferative response to ruxolitinib (provided by Novartis, Basel, Switzerland), cell lines were grown in 96-well plates (Ba/F3 lines at 1×105/mL, KG1A at 2–3×105/mL) with concentrations of 10 nM to 10 μM at half-log intervals in triplicate in 100 μl volumes. CellTiter 96 Aqueous One Solution Cell Proliferation Assay (MTS) (Promega, Madison, WI, USA) was used to measure live cells at 0 and 48 h. Absorbance was measured using an MRX Microplate Reader (Thermo Labsystems, Waltham, MA, USA). Each experiment was performed three times. Cellular IC50 values were calculated using GraphPad Prism 4 software (GraphPad Software, San Diego, CA, USA).
The effect of ruxolitinib on phosphorylation was assessed by Western blot. Cells were washed of serum then incubated with inhibitor for 4 h. At each inhibitor concentration equal numbers of cells were lysed in SDS-lysis buffer (62.5 mM Tris pH 6.8, 2% SDS, 10% glycerol, 0.1% bromphenol blue) with addition of protease inhibitors. Proteins were separated by sodium dodecyl sulphatepolyacrylamide gel electrophoresis (SDS-PAGE). Antibodies were: anti-STAT5 (#9363, Cell Signalling Technology, Beverley, MA, USA), anti-phospho-STAT5 (#9359, Cell Signalling Technology), anti-ERK (sc-94, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-phos-pho-ERK (sc-7383, Santa Cruz Biotechnology, CA, USA), anti-AKT (ab8805, Abcam, Cambridge, UK), anti-phospho-AKT (ab66138, Abcam), anti-JAK2 (#2863-1, Epitomics, Burlingame, CA, USA) and anti-phospho-JAK2 (#TA303559, Origene, Rockville, MD, USA). Signals were detected using enhanced chemiluminescence (ECL) plus Western blotting detection reagent (Amersham Biosciences, Little Chalfont, UK).
Primary peripheral blood mononuclear cells from 2 patients with JAK2 rearrangements and one patient with a JAK2 amplification were used in in vitro response assays. Peripheral blood samples from 7 healthy individuals and bone marrow samples from 3 chronic myeloid leukemia (CML) patients were used as controls. The study was approved by the relevant internal review boards and/or ethics committees, and informed consent was provided according to the Declaration of Helsinki.
Case #1
A 51-year old male presented with a history of night sweats. Peripheral blood showed left-shifted leukocytosis of 12×109/L with a hemoglobin level of 13.7g/dL and a platelet count of 138×109/L. Eosinophils and basophils were not elevated. Spleen size was increased (20×10 cm). Bone marrow was hypercellular with pronounced granulopoiesis, prominent eosinophilia, and reduced numbers of megakaryocytes and dysplastic erythropoiesis. Marrow fibrosis was grade 2. Cytogenetic analysis revealed a 46,XY,t(8;9)(p22;p24) in 17 of 20 metaphases and realtime polymerase chain reaction (RT-PCR) amplified a chimeric mRNA with JAK2 exon 11 fused to PCM1 exon 36.
Case #2
The peripheral blood of a 68-year old male patient showed left-shifted leukocytosis (39×109/L) with 9% eosinophils (absolute numbers 3.5×109/L), thrombocytosis (780×109/L) and normal hemoglobin (13 g/dL). Liver and spleen were slightly enlarged (13×8 cm). Trephine biopsy revealed a hypercellular marrow with pronounced granulopoiesis predominantly caused by eosinophils and reduced numbers of erythropoietic precursors and megakary ocytes. Blasts were slightly elevated.
Marrow fibrosis was grade 1. Cytogenetic analysis revealed 46,XY,t(9;18)(p24;q12),t(14;18)(q21;q23)[24]. A rearrangement of JAK2 was confirmed by fluorescence in situ hybridization (FISH) with probes flanking the JAK2 gene (Kreatech, Amsterdam, The Netherlands). The putative JAK2 fusion partner remains to be identified.
Case #3
A 71-year old male patient presented with moderate leukocytopenia (3.4×109/L), transfusion-dependent anemia (Hb 7.6 g/dl) and thrombocytopenia (53×109/L). The spleen was slightly enlarged. Bone marrow biopsy showed approximately 50% ringed sideroblasts with dysplastic granulopoiesis and megakaryopoesis leading to refractory cytopenia with multilineage dysplasia. No marrow fibrosis could be detected.
Cytogenetics revealed a complex karyotype (46,XY,der(5)t(3;5)(q26;q14)[1]/46,XY,der(5)t(3;5)(q26;q14), der(7)t(7;9)(q21;p24),r(9)(p13q34),der(12)t(12;18)(p12;p11),r(18)(p1 1q21)[19). FISH and comparative genomic hybridization (CGH) analysis revealed overamplification of JAK2 and genetic analysis showed the patient to be negative for JAK2 V617F.
Primary cells were set up at 2×105/mL in methylcellulose with cytokines without erythropoietin (Stem Cell Technologies, Vancouver, BC, Canada) in triplicate for each inhibitor dose. Colonies greater than 50 cells were counted on Day 7 and colonies greater than 100 cells were counted on Day 14. An index of growth response was calculated as described previously.11 FISH was used to assess any differential effect of ruxolitinib upon colonies with or without rearranged or amplified JAK2. Colonies were plucked into 3:1 methanol/acetic acid, stored at –20°C until required, and then pipetted onto slides. Split-apart FISH with differentially labeled bacmid probes RP11-3H3 (5′ JAK2) and RP11–28A9 (3′ JAK2) was performed according to established techniques12 with the addition of a 70% acetic acid wash immediately after slide making and a refix in 1% paraformaldehyde in PBS for 10 min before hybridization.
Results and Discussion
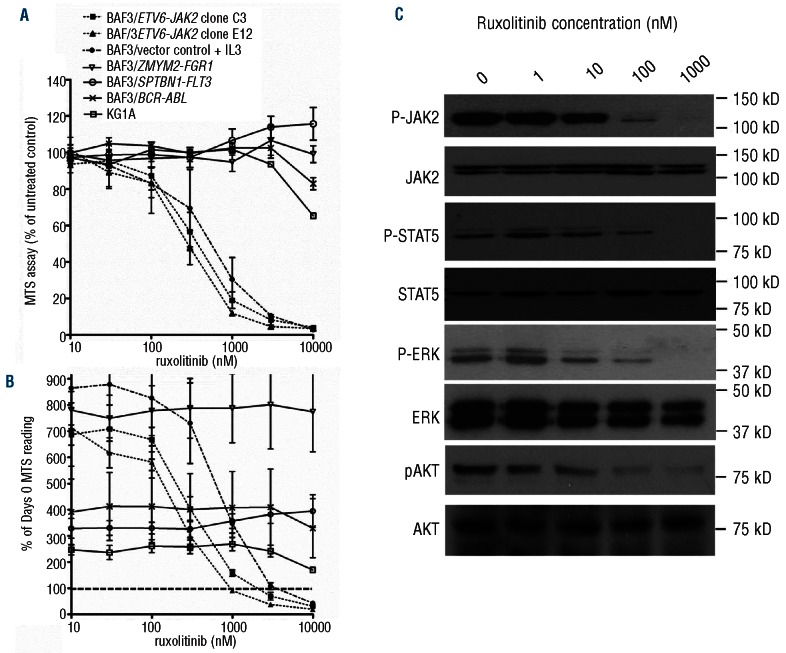
The IL3-dependent Ba/F3 cell line can be transformed to IL3 independence by expression of activated oncogenes such as ETV6-JAK2 and thereby provides a model system for assessing tyrosine kinase inhibitors.1 In initial experiments, Ba/F3-ETV6-JAK2 clones and untransformed Ba/F3 cells (grown with IL3) showed similar responses to ruxolitinib consistent with the requirement of JAK2 for IL3 signaling.13 We therefore used the FGFR1OP2-FGFR1 positive KG1A cell line14 plus Ba/F3 cells transformed to IL3 independence by BCR-ABL1, SPTBN1-FLT3 and ZMYM2-FGFR1 as negative controls since FGFR1, ABL1 and FLT3 are ruxolitinib insensitive. The mean cellular IC50 value for the two Ba/F3-ETV6-JAK2 clones was 370 nM whilst for all other cell lines the IC50 was over 10,000 nM (Figure 1A). A net loss of cells over the 48-h experimental period was seen with Ba/F3-ETV6-JAK2 at higher ruxolitinib concentrations, i.e. where the MTS reading at 48 h falls below 100% of the Day 0 MTS reading (Figure 1B). In contrast, almost no reduction was seen in survival of control cell lines. Increasing inhibitor concentration was accompanied by reduced phosphorylation of the JAK2 fusion protein, ERK, STAT5 and AKT (Figure 1C). Together with previous findings on ruxolitinib pharmacology (25 mg dose gives a Cmax of 934 nM),15 our data therefore, suggest that effective inhibition of JAK2 fusion proteins would be readily obtainable in vivo.
Figure 1.
Ruxolitinib is active against Ba/F3 cells transformed by ETV6-JAK2. (A) KG1A cells (which contain FGFR1OP2-FGFR1) and Ba/F3 cells transformed to IL3-independence by the fusion genes ETV6-JAK2, SPTBN1-FLT3, ZMYM2-FGFR1 or BCR-ABL1 were exposed to ruxolitinib for 48 h. Values are means +/− SE of 3 independent experiments. (B) Expression as a percentage of Day 0 MTS reading (where line crosses y-axis at 100%) indicates a net reduction in numbers of Ba/F3-ETV6-JAK2 cells over 48 h. Values are means +/− SE of 2 independent experiments. (C) Treatment of cells with ruxolitinib for 4 h resulted in a dose-dependent reduction in phosphorylation of ETV6-JAK2, ERK, STAT5 and AKT. Translation from an internal methionine results in two isoforms of ETV6-JAK2;2 we found only the larger to be phosphorylated.
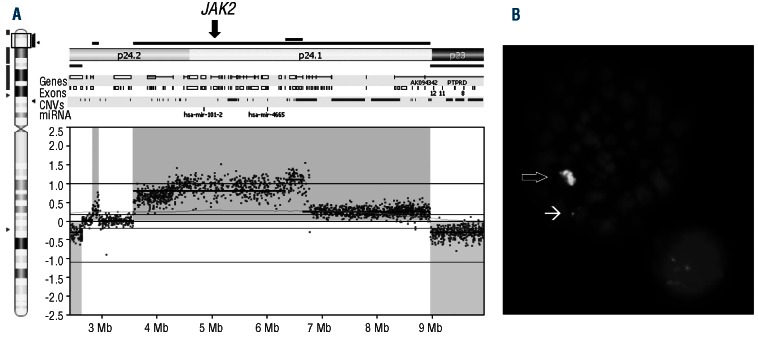
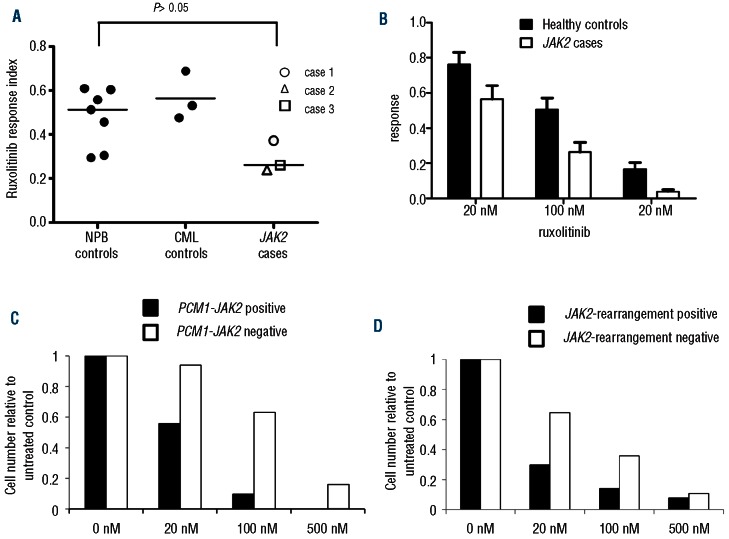
Ruxolitinib was then assessed in in vitro assays using primary material from 2 patients with JAK2 rearrangements, one patient with JAK2 amplification, 7 healthy controls and 3 CML controls. JAK2 amplification is very rare in hematologic malignancies and has not previously been described in myelodysplastic syndrome. CGH and FISH showed amplification of a region on chromosome 9 containing JAK2 (Figure 2A and B). The total number of copies of JAK2 was estimated at 6–7 per cell suggesting increased JAK2 signaling and a potential response to ruxolitinib. Cells from all 3 patients were grown for two weeks in methylcellulose in the presence of ruxolitinib. An overall reduction in colony growth was seen in patients compared with healthy controls (t-test, P<0.05) and CML controls (Figure 3A) which was progressive with increasing ruxolitinib concentration (Figure 3B and Online Supplementary Table S1). Colonies were plucked into fixative followed by FISH analysis using split-apart JAK2 probes to assess the proportions of JAK2-rearrangement positive and negative cells at each ruxolitinib concentration and these data were combined with colony counts to show the relative dynamics of the JAK2-rearrangement positive and negative fractions (Figure 3C and D; calculations are provided in Online Supplementary Table S2). In both cases 1 and 2, the reduction in JAK2-rearrangement positive cells occurs at lower ruxolitinib concentrations and is greater than that seen in JAK2-rearrangement negative cells at all concentrations. Both patients with JAK2 rearrangements showed an overall reduction in JAK2 rearranged colonies (&x003C7;2 test; case 1, P<0.02; case 2, P<0.05). In case 1, complete eradication of PCM1-JAK2 positive colonies at 500 nM ruxolitinib was seen. For case 3, only JAK2-amplified colonies were seen (65 and 11 colonies analyzed by FISH from 100 nM and 500 nM ruxolitinib-treated cultures, respectively) suggesting that the level of cells negative for the JAK2-amplification may be too low to allow detection of a differential effect using this assay.
Figure 2.
Amplification of JAK2 in case 3 with myelodysplastic syndrome. Comparative genomic hybridization using a NimbleGen CGH 6×630K Whole-Genome Tiling Array (NimbleGen Inc., Madison, WI, USA) (A) and fluorescence in situ hybridization (FISH) with probes flanking the JAK2 locus (Kreatech, Amsterdam, The Netherlands) (B) revealed a JAK2 amplification contained within a single chromosome (⇨) and a non-amplified JAK2 allele (→).
Figure 3.
Ruxolitinib inhibits colony growth of JAK2 rearranged or amplified primary cells. (A) Colony numbers of samples from 3 patients with JAK2 rearrangements or amplification, 7 healthy controls and 3 CML controls were counted at Days 7 and 14. The y-axis response index is the mean reduction in Days 7 and 14 colony counts in ruxolitinib-treated methylcellulose cultures compared to untreated cultures for all ruxolitinib concentrations. (B) Comparison of JAK2 cases and healthy controls showed a progressive reduction in colony numbers with increasing ruxolitinib concentrations. In cases 1 (C) and 2 (D) a subset of colonies were plucked on Day 14 for JAK2 FISH analysis allowing the size of the JAK2-rearrangement positive and negative fractions to be estimated. In both cases, the number of JAK2-rearrangement positive colonies fell at lower ruxolitinib concentrations than JAK2-rearrangement negative colonies.
In summary, we have shown by in vitro assays using both cell line models and primary patient material that ruxolitinib has significant activity against JAK2 activated by gene rearrangement and present evidence for potential activity against cells with JAK2 amplification. Since aberrant activation of JAK2 has also been demonstrated in lymphoid disorders, e.g. by JAK2 rearrangement or SOCS1 mutation in lymphoma7,16 and CRLF2, IL7R and JAK family mutations in acute lymphoblastic leukemia,17,18 it is possible that treatment with ruxolitinib will have wider potential applicability in addition to the treatment of patients with JAK2 rearrangement-positive MPN described here.
Supplementary Material
Footnotes
Funding
This work was supported by Leukaemia and Lymphoma Research, UK and Deutsche José Carreras Leukämie-Stiftung e.V. - DJCLS R09/29f and H11/03, Germany.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Ho JM, Beattie BK, Squire JA, Frank DA, Barber DL. Fusion of the ets transcription factor TEL to Jak2 results in constitutive Jak-Stat signaling. Blood. 1999;93(12):4354–64 [PubMed] [Google Scholar]
- 2.Ho JM, Nguyen MH, Dierov JK, Badger KM, Beattie BK, Tartaro P, et al. TEL-JAK2 constitutively activates the extracellular signal-regulated kinase (ERK), stress-activated protein/Jun kinase (SAPK/JNK), and p38 signaling pathways. Blood. 2002;100(4):1438–48 [PubMed] [Google Scholar]
- 3.Reiter A, Walz C, Watmore A, Schoch C, Blau I, Schlegelberger B, et al. The t(8;9)(p22;p24) is a recurrent abnormality in chronic and acute leukemia that fuses PCM1 to JAK2. Cancer Res. 2005;65(7):2662–7 [DOI] [PubMed] [Google Scholar]
- 4.Griesinger F, Hennig H, Hillmer F, Podleschny M, Steffens R, Pies A, et al. A BCR-JAK2 fusion gene as the result of a t(9;22)(p24;q11.2) translocation in a patient with a clinically typical chronic myeloid leukemia. Genes Chromosome Cancer. 2005;44(3):329–33 [DOI] [PubMed] [Google Scholar]
- 5.Peeters P, Raynaud SD, Cools J, Wlodarska I, Grosgeorge J, Philip P, et al. Fusion of TEL, the ETS-variant gene 6 (ETV6), to the receptor-associated kinase JAK2 as a result of t(9;12) in a lymphoid and t(9;15;12) in a myeloid leukemia. Blood. 1997;90(7):2535–40 [PubMed] [Google Scholar]
- 6.Najfeld V, Cozza A, Berkofsy-Fessler W, Prchal J, Scalise A. Numerical gain and structural rearrangements of JAK2, identified by FISH, characterize both JAK2617V>F-positive and -negative patients with Ph-negative MPD, myelodys-plasia, and B-lymphoid neoplasms. Exp Hematol. 2007;35(11):1668–76 [DOI] [PubMed] [Google Scholar]
- 7.Van Roosbroeck K, Cox L, Tousseyn T, Lahortiga I, Gielen O, Cauwelier B, et al. JAK2 rearrangements, including the novel SEC31A-JAK2 fusion, are recurrent in classical Hodgkin lymphoma. Blood. 2011;117(15):4056–64 [DOI] [PubMed] [Google Scholar]
- 8.Walz C, Cross NC, Van Etten RA, Reiter A. Comparison of mutated ABL1 and JAK2 as oncogenes and drug targets in myeloproliferative disorders. Leukemia. 2008;22(7):1320–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smedley D, Demiroglu A, Abdul-Rauf M, Heath C, Cooper C, Shipley J, et al. ZNF198-FGFR1 transforms Ba/F3 cells to growth factor independence and results in high level tyrosine phosphorylation of STATS 1 and 5. Neoplasia. 1999;1(4):349–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grand FH, Iqbal S, Zhang L, Russell NH, Chase A, Cross NC. A constitutively active SPTBN1-FLT3 fusion in atypical chronic myeloid leukemia is sensitive to tyrosine kinase inhibitors and immunotherapy. Exp Hematol. 2007;35(11):1723–7 [DOI] [PubMed] [Google Scholar]
- 11.Chase A, Grand FH, Cross NC. Activity of TKI258 against primary cells and cell lines with FGFR1 fusion genes associated with the 8p11 myeloproliferative syndrome. Blood. 2007;110(10):3729–34 [DOI] [PubMed] [Google Scholar]
- 12.Sohal J, Chase A, Goldman JM, Cross NC. Assignment of ZNF262 to human chromosome band 1p34-->p32 by in situ hybridization. Cytogenet Cell Genet. 1999;85(3–4):306–7 [DOI] [PubMed] [Google Scholar]
- 13.Parganas E, Wang D, Stravopodis D, Topham DJ, Marine JC, Teglund S, et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93(3):385–95 [DOI] [PubMed] [Google Scholar]
- 14.Gu TL, Goss VL, Reeves C, Popova L, Nardone J, Macneill J, et al. Phosphotyrosine profiling identifies the KG-1 cell line as a model for the study of FGFR1 fusions in acute myeloid leukemia. Blood. 2006;108(13):4202–4 [DOI] [PubMed] [Google Scholar]
- 15.Shi JG, Chen X, McGee RF, Landman RR, Emm T, Lo Y, et al. The pharmacokinetics, pharmacodynamics, and safety of orally dosed INCB018424 phosphate in healthy volunteers. J Clin Pharmacol. 2011;51(12):1644–54 [DOI] [PubMed] [Google Scholar]
- 16.Weniger MA, Melzner I, Menz CK, Wegener S, Bucur AJ, Dorsch K, et al. Mutations of the tumor suppressor gene SOCS-1 in classical Hodgkin lymphoma are frequent and associated with nuclear phospho-STAT5 accumulation. Oncogene. 2006;25(18):2679–84 [DOI] [PubMed] [Google Scholar]
- 17.Tasian SK, Loh ML. Understanding the biology of CRLF2-overexpressing acute lymphoblastic leukemia. Critical Reviews in Oncogenesis. 2011;16(1–2):13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Ding L, Holmfeldt L, Wu G, Heatley SL, Payne-Turner D, et al. The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature. 2012;481(7380):157–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.