Abstract
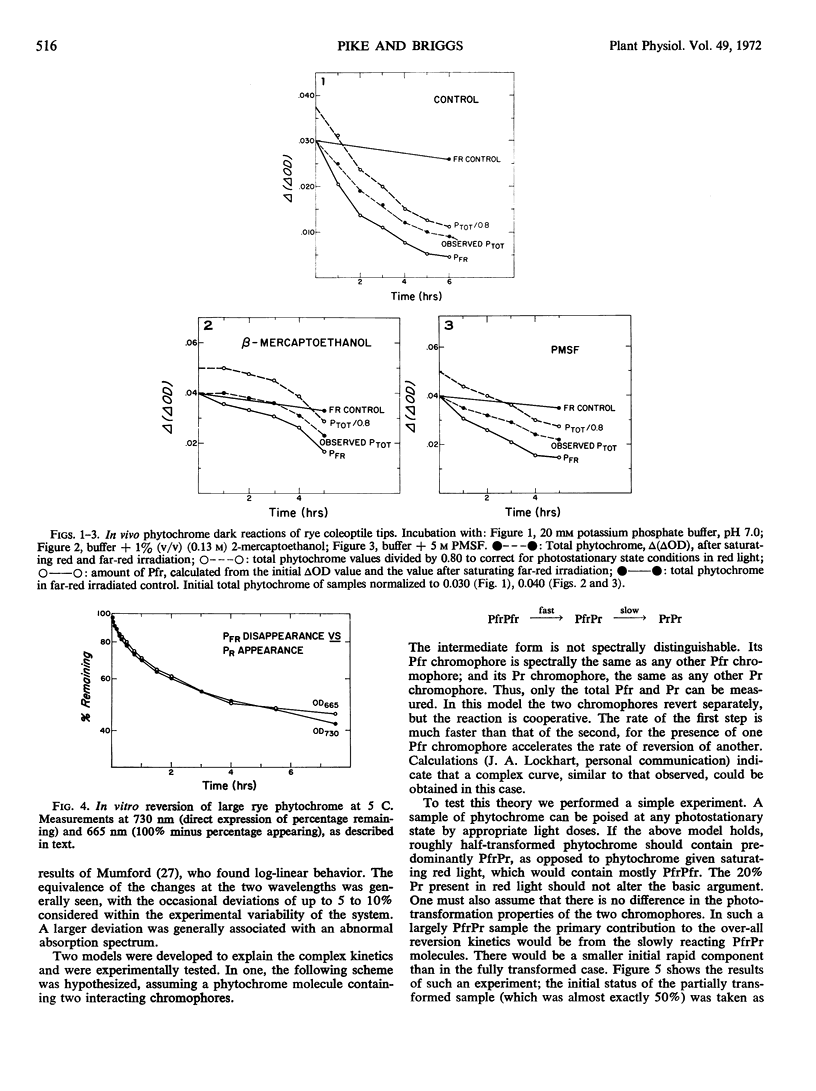
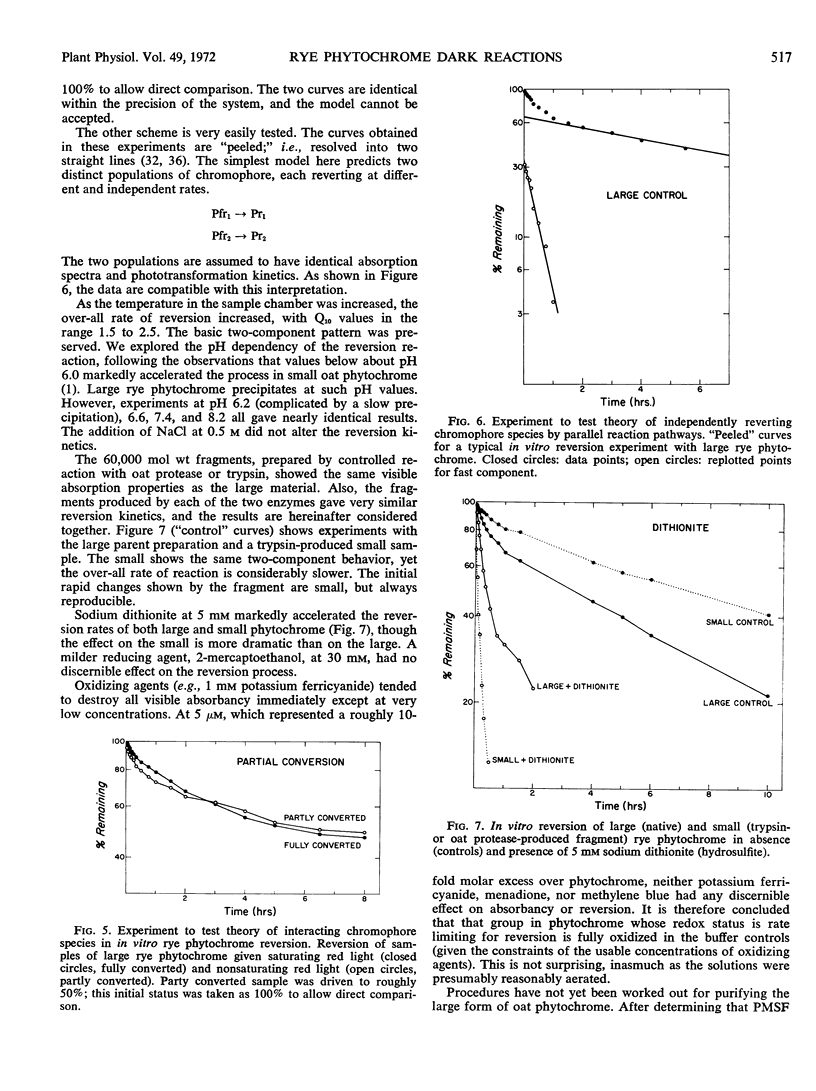
The dark reactions of Secale cereale L. cv. Balbo phytochrome have been investigated in coleoptile tips and in extensively purified extracts of large molecular weight phytochrome. Destruction, but not reversion, was detected in vivo. The effects of various inhibitors of an in vitro phytochrome-degrading protease did not support a view of proteolytic attack as the basis of in vivo destruction. In vitro, rye phytochrome (about 240,000 molecular weight) reverted extremely rapidly, even at 5 C. The reversion curves were resolved into two first order components. The previously studied 60,000 molecular weight species, obtained by controlled proteolysis of large rye phytochrome, showed a similar two-component pattern, but a much slower over-all reversion rate. This reduction in rate was caused mainly by the reversion of a greater percentage of the small phytochrome as the slow component. Sodium dithionite markedly accelerated the reversion rate of both large and small forms, but oxidants, at concentrations low enough to avoid chromophore destruction, had no effect. Both large and small crude Avena sativa L. phytochrome showed two-component reversion kinetics.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson G. R., Jenner E. L., Mumford F. E. Temperature and pH studies on phytochrome in vitro. Biochemistry. 1969 Mar;8(3):1182–1187. doi: 10.1021/bi00831a052. [DOI] [PubMed] [Google Scholar]
- BUTLER W. L., SIEGELMAN H. W., MILLER C. O. DENATURATION OF PHYTOCHROME. Biochemistry. 1964 Jun;3:851–857. doi: 10.1021/bi00894a022. [DOI] [PubMed] [Google Scholar]
- Briggs W. R., Siegelman H. W. Distribution of Phytochrome in Etiolated Seedlings. Plant Physiol. 1965 Sep;40(5):934–941. doi: 10.1104/pp.40.5.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. L., Lane H. C. Dark Transformations of Phytochrome in vivo. II. Plant Physiol. 1965 Jan;40(1):13–17. doi: 10.1104/pp.40.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. L., Lane H. C., Siegelman H. W. Nonphotochemical Transformations of Phytochrome in Vivo. Plant Physiol. 1963 Sep;38(5):514–519. doi: 10.1104/pp.38.5.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll D. L., Edwards J. L., Shropshire W., Jr Multiple chromophore species in phytochrome. Photochem Photobiol. 1968 Nov;8(5):465–475. doi: 10.1111/j.1751-1097.1968.tb05890.x. [DOI] [PubMed] [Google Scholar]
- Correll D. L., Steers E., Jr, Towe K. M., Shropshire W., Jr Phytochrome in etiolated annual rye. IV. Physical and chemical characterization of phytochrome. Biochim Biophys Acta. 1968 Sep 10;168(1):46–57. doi: 10.1016/0005-2795(68)90232-8. [DOI] [PubMed] [Google Scholar]
- Everett M. S., Briggs W. R. Kinetics of Phytochrome Phototransformation: A Re-examination. Plant Physiol. 1970 Jun;45(6):805–806. doi: 10.1104/pp.45.6.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya M., Hopkins W. G., Hillman W. S. Effects of metal-complexing and sulfhydryl compounds on nonphotochemical phytochrome changes in vivo. Arch Biochem Biophys. 1965 Oct;112(1):180–186. doi: 10.1016/0003-9861(65)90026-3. [DOI] [PubMed] [Google Scholar]
- Gardner G., Pike C. S., Rice H. V., Briggs W. R. "Disaggregation" of phytochrome in vitro-a consequence of proteolysis. Plant Physiol. 1971 Dec;48(6):686–693. doi: 10.1104/pp.48.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick R. E., Hillman W. S. Dark Reversion of Phytochrome in Sinapis alba L. Plant Physiol. 1970 Oct;46(4):596–598. doi: 10.1104/pp.46.4.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. M., Edsall P. C. Substitution of redox chemicals for radiation in phytochrome-mediated photomorphogenesis. Plant Physiol. 1966 Jun;41(6):949–952. doi: 10.1104/pp.41.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. J., Berns D. S. Protein aggregation. Studies of larger aggregates of C-phycocyanin. Biochem J. 1968 Dec;110(3):457–464. doi: 10.1042/bj1100457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur J. A., Briggs W. R. In vivo phytochrome reversion in immature tissue of the alaska pea seedling. Plant Physiol. 1971 Jul;48(1):46–49. doi: 10.1104/pp.48.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford F. E., Jenner E. L. Catalysis of the phytochrome dark reaction by reducing agents. Biochemistry. 1971 Jan 5;10(1):98–101. doi: 10.1021/bi00777a015. [DOI] [PubMed] [Google Scholar]
- Mumford F. E. Studies on the phytochrome dark reaction in vitro. Biochemistry. 1966 Feb;5(2):522–524. doi: 10.1021/bi00866a018. [DOI] [PubMed] [Google Scholar]
- Pike C. S., Briggs W. R. Partial Purification and Characterization of a Phytochrome-degrading Neutral Protease from Etiolated Oat Shoots. Plant Physiol. 1972 Apr;49(4):521–530. doi: 10.1104/pp.49.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt L. H., Briggs W. R. Photochemical and Nonphotochemical Reactions of Phytochrome in vivo. Plant Physiol. 1966 Mar;41(3):467–474. doi: 10.1104/pp.41.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves W. K., Briggs W. R. Kinetically distinguishable populations of phytochrome. Plant Physiol. 1968 Aug;43(8):1259–1263. doi: 10.1104/pp.43.8.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Taylor A. O. In vitro phytochrome dark reversion process. Plant Physiol. 1968 May;43(5):767–774. doi: 10.1104/pp.43.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Liew H. D. Graphic analysis of aggregates of linear and exponential processes. J Theor Biol. 1967 Jul;16(1):43–53. doi: 10.1016/0022-5193(67)90052-5. [DOI] [PubMed] [Google Scholar]



