Abstract
Different types of graft-versus-host disease prophylaxis have been proposed in the setting of reduced intensity and non-myeloablative allogeneic stem cell transplantation. An alternative combination with sirolimus and tacrolimus has recently been tested although comparative studies against the classical combination of a calcineurin inhibitor and mycophenolate mofetil or methotrexate are lacking. We describe the results of a prospective, multicenter trial using sirolimus + tacrolimus as immunoprophylaxis, and compare this approach with our previous experience using cyclosporine + mycophenolate in the setting of unrelated donor transplantation setting after reduced-intensity conditioning. Forty-five patients received cyclosporine + mycophenolate between 2002 and mid-2007, while the subsequent 50 patients, who were transplanted from late 2007, were given sirolimus + tacrolimus. No significant differences were observed in terms of hematopoietic recovery or acute graft-versus-host disease overall, although gastrointestinal acute graft-versus-host disease grade ≥2 was more common in the cyclosporine + mycophenolate group (55% versus 21%, respectively, P=0.003). The 1-year cumulative incidence of chronic graft-versus-host disease was 50% versus 90% for the patients treated with the sirolimus- versus cyclosporine-based regimen, respectively (P<0.001), while the incidence of extensive chronic disease was 27% versus 49%, respectively (P=0.043). The 2-year non-relapse mortality rate was 18% versus 38% for patients receiving the sirolimus- versus the cyclosporine-based regimen, respectively (P=0.02). The event-free survival and overall survival at 2 years were 53% versus 29% (P=0.028) and 70% versus 45% (P=0.018) among patients receiving the sirolimus- versus the cyclosporine-based regimen, respectively. In conclusion, in the setting of reduced intensity transplantation from an unrelated donor, promising results can be achieved with the combination of sirolimus + tacrolimus, due to a lower risk of chronic graft-versus-host disease and non-relapse mortality, which translates into better event-free and overall survival rates, in comparison with those achieved with cyclosporine + mycophenolate. This trial was registered at www.clinicaltrials.gov as 2007-006416-32 by GEL-TAMO/GETH.
Introduction
Many studies have described different conditioning regimens and different types of graft-versus-host disease (GVHD) prophylaxis in patients undergoing reduced intensity (RIC) and non-myeloablative allogeneic stem cell transplantation.1-8 One of the most widely used approaches is based on the combination of cyclosporine (CsA) and mycophenolate mofetil (MMF), which was initially developed by the Seattle group in the non-myeloablative transplant setting. Various studies have reported overall survival rates at 3-5 years in the range of 43-64% among patients who did not have advanced disease at the time of transplantation, with incidences of grades 2-4 acute and overall chronic GVHD of 11-63% and 44-90%, respectively.9-13 In our experience of using CsA-MMF as GVHD prophylaxis after RIC based on fludarabine plus busulphan or melphalan, the incidences of grades 2-4 acute GVHD and extensive chronic GVHD were 53% and 93%, respectively, in a series of patients receiving allogeneic stem cell transplants from unrelated donors.3
A more recent immunoprophylactic strategy consists of the combination of sirolimus plus tacrolimus (Si-Tac), which was mostly abandoned in the setting of myeloablative conditioning because of a high incidence of associated veno-occlusive disease and microangiopathy,14 although it gives promising results in the RIC setting. In this regard, overall survival rates of 62-72% at 1-3 years have been reported, with the incidence of grades 2-4 acute GVHD being 14-43% and that of chronic GVHD being 46-63%.6,15-18 Unfortunately, we lack data from multicenter trials using this approach and these results have not been confirmed by other groups. For example, Furlong et al.19 described a high incidence of toxicity, which led to 42% of patients withdrawing from the trial, with a high incidence of acute GVHD (77%).
Against this background, the primary objective of the current study was to evaluate the incidence of GVHD after RIC allogeneic transplantation from unrelated donors using a modified Si-Tac combination as GVHD prophylaxis, in a prospective multicenter trial started in 2007 (2007-006416-32 trial by GEL-TAMO/GETH). The results of this trial were compared with those from a previous trial in which patients, enrolled between 2002 and 2007, received the same RIC protocols, but were given CsA-MMF as GVHD prophylaxis (TNE-ANM 2001 from GETH).3 A comparative analysis of the overall outcomes from the two trials was a secondary objective of the current study.
Design and Methods
Patients' characteristics
From February 2007 to August 2010, 50 patients were included in the Si-Tac prospective multicenter trial. The study was approved by the Ethics Committees at each center, and each patient provided written, informed consent to participation after a detailed explanation in accordance with the Declaration of Helsinki. The patients' characteristics are shown in Table 1. The median age at the time of transplantation was 50 years (range, 23 to 64 years). Forty-eight per cent of the patients were in first or subsequent complete remission from their underlying disease, 30% showed a partial response, while 22% had active or progressive disease at the time of transplantation. Forty-four per cent of the patients had received a prior transplant at the time of their inclusion in the trial. The source of progenitor cells was peripheral blood in 90% of the patients, and 35% received stem cells from an HLA allele-mismatched donor (five cases had one, and three cases had two allele mismatches at HLA A, B, C and DRB1).
Table 1.
Patient and engraftment characteristics (percentages in parentheses).
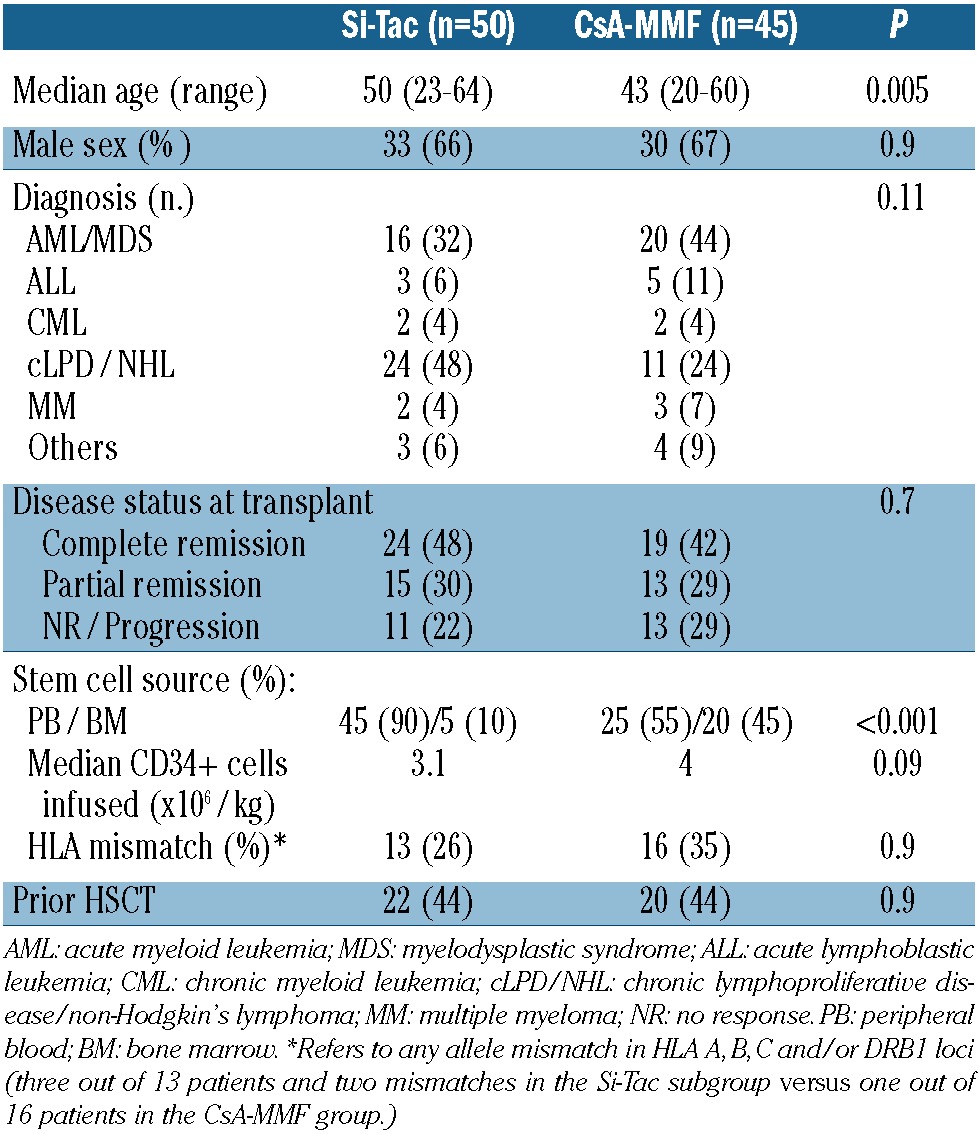
Compared with the 50 newly recruited patients, the 45 patients who received CsA-MMF as GVHD prophylaxis were significantly younger (P=0.005), and peripheral blood stem cells were used in 55% of cases (P<0.001). No other differences were found between the two subgroups (Table 1). More than 90% of the pairs (either the donor or recipient) in both subgroups were cytomegalovirus seropositive.
Conditioning regimen and graft-versus-host disease prophylaxis
Two RIC regimens were used, one that is recommended for lymphoid malignancies and the other for myeloid malignancies. The lymphoid RIC regimen consisted of fludarabine 30 mg/m2 administered intravenously on days -9 to -5, followed by melphalan 70 mg/m2 intravenously on days -3 and -2. The myeloid regimen consisted of the same doses of fludarabine together with oral busulphan 1 mg/kg for 10 doses (days -6 to -4, total 10 mg/kg), with phenytoin given as anticonvulsant prophylaxis.20-22 Hematopoietic stem cells from an unrelated donor were infused on day 0.
Patients transplanted from 2007 were included in the 2007-006416-32 GEL-TAMO/GETH trial and received GVHD prophylaxis consisting of sirolimus at a dose of 6 mg/day p.o. on day –6, followed by 4 mg/day p.o. from day –5; tacrolimus was started on day –3 at a dose of 0.02 mg/kg/day as a continuous i.v. infusion. Levels of both drugs were monitored from day –1 and doses were adjusted for target blood levels of 5-10 ng/mL for sirolimus and 5-10 ng/mL for tacrolimus. Drug levels were assessed onsite using immunoassays. In the absence of acute GVHD, we planned to taper the dose of tacrolimus by 5% each week starting on day +56 and stopping on day +180, and to taper the dose of sirolimus from day +180, stopping this on day +240.
Patients transplanted from 2002 to 2006 were included in the TNE-ANM 2001 trial; in this subset GVHD prophylaxis consisted of CsA plus MMF, as previously described.3 CsA was given at a dose of 1 mg/kg/day intravenously from days –7 to –2, and then 3 mg/kg/day intravenously or orally from day –1. Levels were maintained in the therapeutic range until tapering. MMF was given orally at a dose of 1 g every 8-12 h or intravenously at a dose of 15 mg/kg every 8-12 h. The dose was planned to be tapered starting on day +56 and stopping on day +100, although MMF was stopped as scheduled in only 10 of the 45 patients, while the remaining patients received this drug beyond day +100.
Antiviral, antibacterial and antifungal prophylaxis was given according to each institution's standard practice, with no differences between the two groups, except for the use of antifungal prophylaxis with triazols, which were not allowed for patients receiving Si-Tac per protocol because of the strong interactions between these drugs. Neither granulocyte colony-stimulating factor nor heparin prophylaxis was used.
Acute and chronic GVHD were graded by pre-defined criteria.23,24 In order to establish the diagnosis, all patients had biopsies of at least one of the organs involved. Since MMF can induce gastrointestinal toxicity, suspected cases of gastrointestinal GVHD had to be proven histologically. A clinical diagnosis of post-trans-plant thrombotic microangiopathy was established according to previously defined international criteria.25
Statistical analysis
Eight end-points were considered: acute GVHD grades 2-4 and 3-4, chronic GVHD, extensive chronic GVHD, relapse, nonrelapse mortality, overall survival and event-free survival. Acute GVHD, chronic GVHD, non-relapse mortality and relapse were analyzed and compared using the Gray test.26 The cumulative incidence was computed with the cmprsk package for R 2.14.0 soft-ware (R Development Core Team (2011); http://www.R-project.org/). Competing events were defined as follows. In the case of acute GVHD, chronic GVHD and relapse the competing event was death without the occurrence of the event of interest. For non- relapse mortality the competing event was relapse. Relapse was also considered as a competing event for GVHD. Overall survival and event-free survival were estimated by the Kaplan-Meier method, and groups were compared with the log-rank test using SPSS version 13.0 (SPSS Inc, Chicago, IL, USA).
Events were calculated from the time of transplantation as follows. Non-relapse mortality was defined as death from any cause (GHVD-related or other cause), without prior relapse or progression of the underlying disease. The relapse incidence was analyzed from the time of transplantation until the time of relapse in those patients in remission before or after the transplant. Eventfree survival was calculated from transplantation until disease progression or death, and those patients who did not achieve a disease response (complete or partial remission) any time after transplantation were considered as having had events on day 100, since this was the earliest date for complete disease evaluation. Overall survival was calculated from transplantation until death from any cause, and surviving patients were censored at their last follow-up. Patients who showed evidence of engraftment could be evaluated for acute GVHD, whereas patients who engrafted and survived more than 100 days could be evaluated for chronic GVHD. For both acute GVHD and chronic GVHD, the day of onset was analyzed as the time to event in evaluable patients.
Multivariate Cox regression was performed in SPSS to control for the effects of GVHD prophylaxis treatment and other prognostic factors, such as age, HLA disparity and status at transplantation, as well as the date of inclusion during the recruitment period. The proportional hazard assumption was tested for each variable analytically and graphically. Overall survival, event-free survival, non-relapse mortality and Cox regression models were compared with and without censoring the first cohort (CsA-MMF) at the longest follow-up of the second cohort (Si-Tac) in order to rule out the possibility of any bias related to differences in the median follow-up of the two subgroups.
Differences were considered to be statistically significant for two sided P-values <0.05.
Results
Regimen-related toxicity and graft-versus-host disease
Patients receiving Si-Tac reached blood counts of >1,000/mm3 granulocytes and >50,000 platelets/mm3 at a median of 20 and 25 days after transplantation, respectively. As far as regards non-hematologic toxicity, the regimen was well tolerated, with only six patients developing >grade 2 gastrointestinal toxicity and one patient experiencing neurological toxicity. These data are summarized in Table 2. Five patients developed reversible thrombotic microangiopathy, and there were no cases of veno-occlusive disease. Interestingly, four of the five patients with thrombotic microangiopathy received fluconazole as antifungal prophylaxis.
Table 2.
Extra-hematologic toxicity.
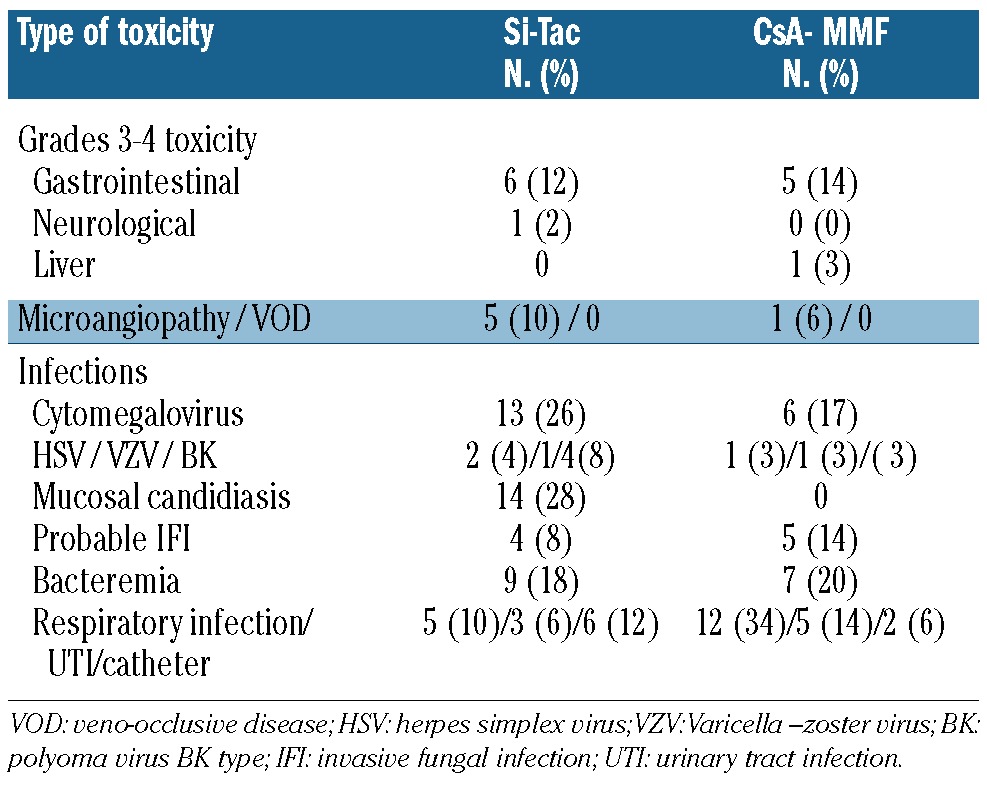
The incidence of cytomegalovirus reactivation was 26%, while 8% of patients developed a probable invasive fungal infection.
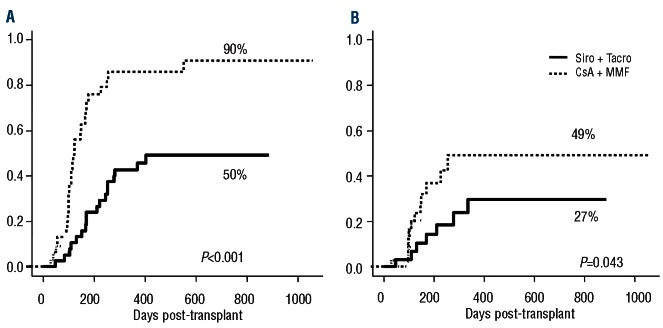
The 100-day cumulative incidences of grades 2-4 and 3-4 acute GVHD were 49% (95% CI 42-70%) and 16% (95% CI 7-28 %), among patients receiving Si-Tac versus 45% (95% CI 30-59%) and 20% (95% CI 10-33%) among patients receiving CsA-MMF in the previous trial (P=0.5 for both comparisons) (Figure 1). The median day of onset of acute GVHD was 31 days (range, 5-92 days) after transplantation versus 33 days (range, 2-103 days), respectively. The incidence of gastrointestinal acute GVHD ≥grade 2 was 21% (95% CI 11-39) in Si-Tac patients, compared with 55% (95% CI 38-72) in the CsA-MMF cohort (P=0.007). Moreover, 83% of patients receiving Si-Tac achieved complete remission after first-line treatment with steroids, compared with 56% of patients receiving CsA-MMF, while 10% and 7% had a partial response and no response, respectively, in the Si-Tac groups versus 24% and 20% in the CsA-MMF group (P=0.08). Chronic GVHD occurred at a median of 211 days (range, 84-487 days) after transplantation, compared with 184 days (range, 51-1057) in the CsA-MMF group. The 1-year cumulative incidence of overall and extensive chronic GVHD was 50% (95% CI 34-64%) and 27% (95% CI 14-41%), respectively, while these values were 90% (95% CI 69-97%) and 49% (95% CI 27-67%), respectively, for patients in the CsA-MMF cohort (P<0.001 for overall and P=0.043 for extensive chronic GVHD; Figure 2). The type of onset of chronic GVHD was de novo, quiescent and progressive in 75%, 6% and 19% of the patients in the Si-Tac trial, compared with 34%, 33% and 33% in patients treated with CsA-MMF, respectively (P=0.023). Finally, according to the National Institutes of Health score system, 48%, 39% and 13% patients were categorized as having mild, moderate and severe chronic GVHD, respectively, in the Si-Tac group, compared with 23%, 36% and 41%, respectively, among patients receiving CsA-MMF (P=0.025). Although it was planned that MMF would be withdrawn faster than sirolimus, this was not actually possible because of this higher incidence of chronic GVHD among patients in the CsA-MMF group. In this regard, comparing exposure to any systemic immunosuppressive drug, no differences were found between the two subgroups up to 2 years post-transplantation: 38% versus 35% of patients were off immunosuppression at 2 years among those receiving Si-Tac and CsA-MMF, respectively.
Figure 1.
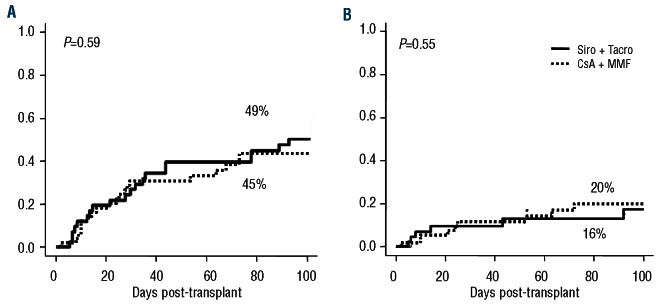
Cumulative incidence of grades 2-4 (A) and 3-4 (B) acute graft-versus-host disease.
Figure 2.
Cumulative incidence of overall (A) and extensive (B) chronic graft-versus-host disease.
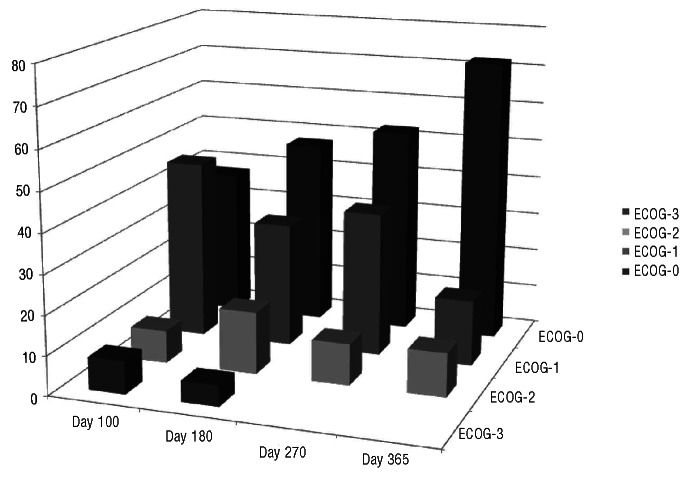
Figure 3 shows the performance status at 3, 6, 9 and 12 months post-transplant among patients included in the Si-Tac group. As shown, most evaluable patients (>90%) had a good performance status (ECOG=0 or 1) at these times, with improvement during the first year of follow-up.
Figure 3.
Performance scores from 3 months to 1 year post-transplant among patients receiving Si-Tac GVHD prophylaxis.
Disease response and outcome
The 2-year incidence of disease relapse in the Si-Tac and CsA-MMF groups was 18% (95% CI: 4-39%) versus 29% (95% CI: 14-46%), respectively (P=0.262) (Figure 4A), while the 2-year non-relapse mortality was 18% (95% CI: 8-31%) versus 38% (95% CI: 24-52%), respectively (P=0.02) (Figure 4B). The causes of non-relapse mortality are summarized in Table 3. After a mean follow-up of 504 days (95-898) among surviving patients receiving Si-Tac, the estimated 2-year event-free and overall survival rates were 53% (95% CI: 38-74%) versus 29% (95% CI: 18-48%) (P=0.028) and 70% (95% CI: 57-86%) versus 45% (95% CI: 31-64) (P=0.018), respectively (Figures 5A and 5B).
Figure 4.
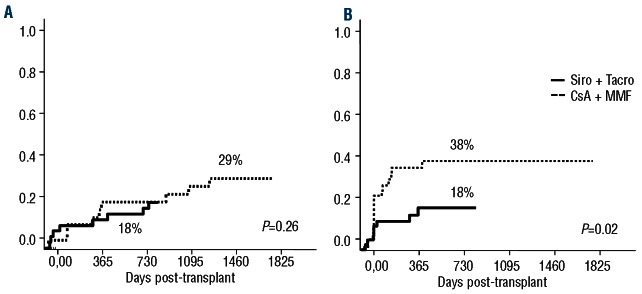
Cumulative incidence of relapse (A) and non-relapse mortality (B).
Table 3. Non-relapse mortality (percentages in parentheses, from total number of deaths).
Figure 5.
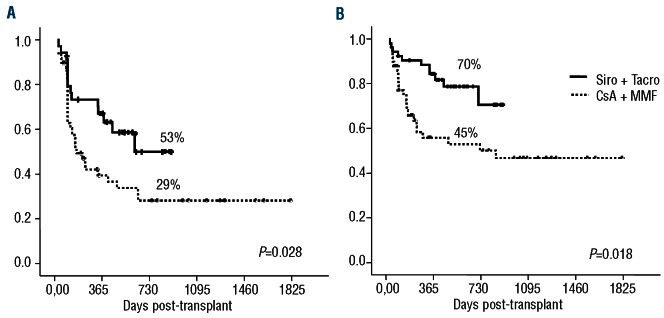
Overall (A) and event-free (B) survival.
In multivariate analysis of overall survival, neither age (HR=1.02, 95% CI: 0.98-1.05; P=0.208), HLA mismatch (HR=0.68, 95% CI: 0.31-1.47; P=0.332) or disease status at transplant (HR=1.01, 95% CI: 0.44-2.30; P=0.971) for active disease influenced the outcome. Most importantly, the year of transplantation during the recruitment period did not influence the outcome (HR=0.99, 95% CI: 0.74-1.32; P=0.974). In this regard, no change in the risk pattern before or after the introduction of Si-Tac was observed (HR=1, 95% CI: 0.12-8.39; P=0.998). Only the type of GVHD prophylaxis (Si-Tac versus CsA-MMF) had a significant association with outcome in the multivariate analysis (HR= 2.62, 95% CI: 1.26-5.42; P=0.009).
Finally, overall survival, non-relapse mortality, event-free survival and relapse rate did not differ when the follow-up of the patients receiving CsA-MMF was censored at the time of the longest follow-up among those receiving Sic-Tac (898 days). In fact, no events (deaths or relapses) occurred after 898 days in the first cohort of patients.
Discussion
The lack of comparative studies to identify the best approach for GVHD prophylaxis explains the absence of consensus and the large number of strategies used within the RIC setting.27,28 The current study is the first to evaluate the use of sirolimus plus tacrolimus without methotrexate among patients undergoing RIC allogeneic transplantation from an unrelated donor in a multicenter trial. Using this approach, the addition of low-dose methotrexate was not shown to have a clear benefit to overall outcomes in previous studies and, furthermore, it can result in delayed engraftment and greater than expected transplant-related toxicity (mainly causing renal dysfunction).8,15,18,19 We confirmed the previous findings from single-institution studies concerning toxicity, GVHD, overall survival and event-free survival.16-18,29-32 Considering the characteristics of the patients included in this trial (median age of 50 years; 52% with measurable or active disease at the time of transplantation) and of the donors (35% had at least one HLA mismatch), our results with respect to overall and event-free survival are very promising. Thus, like the findings reported by the Dana Farber's group,15 we found a low toxicity profile with a small number of patients displaying thrombotic microangiopathy, and no patients developing sinusoidal obstruction syndrome. In fact, all but one of the patients developing thrombotic microangiopathy received -azoles as antifungal prophylaxis, which was not allowed per protocol. This is in contrast to previous reports that described the use of Si-Tac in the myeloablative setting, and other studies in the non-myeloablative setting that found a high incidence of toxicity, leading to protocol withdrawal in a significant proportion of patients.19,29-31 It is worth mentioning that drug levels were measured onsite using immunoassays, rather than by high performance liquid chromatography. Remarkably, levels of toxicity and GVHD incidence were similar to those reported by Cutler et al.15
In the current study we used a lower loading dose of sirolimus, which was started on day –6 instead of day –3, as had previously been done.6,14,18,32 This schedule was planned in an attempt to obtain therapeutic levels before day +1 without increasing the toxicity profile. Interestingly, it was not associated with increased toxicity during the conditioning regimen and, considering the similar incidence of acute and chronic GVHD to those in previous studies in which sirolimus was started on day –3, both approaches appear to have similar efficacy.
The combination of CsA and MMF as GVHD prophylaxis is widely used in the RIC setting in many centers,33,34 based on the initial non-myeloablative protocol designed by the Seattle consortium.12 In the RIC setting, however, a high incidence of chronic GVHD has been observed in several studies,10,35,36 including our previous trial of unrelated donor transplantation,3 in which there was an incidence of extensive chronic GVHD of around 50% and a median duration of immunosuppressive treatment of 25 months. The current comparison of Si-Tac and CsA-MMF is not based on a randomized trial. However, the data emerge from two consecutive clinical trials with no differences in the RIC procedure or the supportive care other than the GVHD prophylaxis itself. Moreover, as far as the patients' characteristics are concerned, the only differences between the two subgroups would favor a higher mortality among the Si-Tac patients (higher median age) and a greater incidence of extensive chronic GVHD (higher proportion of transplants with peripheral blood as the source of stem cells).
Although the differences in acute GVHD were not statistically significant, it is worth mentioning the higher response rate for acute GVHD within the Si-Tac subgroup after first-line therapy. It is also noteworthy that, in this trial, the evaluation of patients' performance status was systematically documented. Such data have seldom been reported in previous studies.16 The favorable ECOG performance status reported after 1 year of transplantation (ECOG=0-1 in more than 90% of evaluable patients), the low incidence of CMV infection, the small number of invasive fungal infections observed and the low rate of infection-related mortality (6% at 2 years post-transplant) in the Si-Tac group can be attributed to the lower incidence of chronic GVHD. Remarkably, sirolimus has direct antineoplastic, antifungal and anti-cytomegalovirus activities with possible clinical implications, as recently suggested in two large studies by Marty et al.36 and Armand et al.38 In the current comparison, the lower incidence of GVHD in the Si-Tac group was not associated with a lower response or a higher relapse rate compared with that in patients receiving CsA-MMF. Considering that both subgroups received the same RIC, these data indicate that sirolimus allows the reduction of GVHD without hampering the efficacy of the procedure. Furthermore, the lower incidence of complications translates into a better survival. In conclusion, the current multicenter trial reveals promising outcomes for patients undergoing RIC allogeneic transplantation from an unrelated donor using sirolimus and tacrolimus as GVHD prophylaxis, without in vivo T-cell depletion. Compared with CsA-MMF, Si-Tac led to a lower incidence of chronic GVHD and a lower rate of non-relapse mortality, which translate into better survival.
Supplementary Material
Footnotes
Authorship and Disclosures; Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Storb R, Deeg HJ, Whitehead J, Appelbaum F, Beatty P, Bensinger W, et al. Methotrexate and cyclosporine compared with cyclo -sporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med. 1986;314(12):729-35 [DOI] [PubMed] [Google Scholar]
- 2.Slavin S, Nagler A, Naparstek E, Kapelushnik Y, Aker M, Cividalli G, et al. Non -myeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998;91(3):756-63 [PubMed] [Google Scholar]
- 3.Perez-Simon JA, Martino R, Caballero D, Valcarcel D, Rebollo N, Caballero D, et al. Reduced-intensity conditioning allogeneic transplantation from unrelated donors: evaluation of mycofenolate mofetil plus cyclosporin A as graft-versus-host disease prophylaxis. Biol Blood Marrow Transplant. 2008;14(6):664-71 [DOI] [PubMed] [Google Scholar]
- 4.Nash RA, Antin JH, Karanes C, Fay JW, Avalos BR, Yeager AM, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96(6):2062-8 [PubMed] [Google Scholar]
- 5.Deeg HJ, Flowers ME, Leisenring W, Appelbaum FR, Martin PJ, Storb RF. Cyclosporine (CSP) or CSP plus methylprednisolone for graft-versus-host disease prophylaxis in patients with high-risk lymphohemopoietic malignancies: long-term follow-up of a randomized trial. Blood. 2000;96(3):1194-5 [PubMed] [Google Scholar]
- 6.Cutler C, Kim HT, Hochberg E, Ho V, Alyea E, Lee SJ, et al. Sirolimus and tacrolimus without methotrexate as graft-versus-host disease prophylaxis after matched related donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2004;10(5):328-36 [DOI] [PubMed] [Google Scholar]
- 7.Ratanatharathorn V, Nash R.A, Przepiorka D, Devine SM, Klein JL, Weisdorf D, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998;92(7):2303-14 [PubMed] [Google Scholar]
- 8.Ho VT, Aldridge J, Kim HT, Cutler C, Koreth J, Armand P, et al. Comparison of tacrolimus and sirolimus (Tac/Sir) versus tacrolimus, sirolimus, and mini-methotrexate (Tac/Sir/MTX) as acute graft-versus-host disease prophylaxis after reduced-intensity conditioning allogeneic peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2009;15(7):844-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rotta M, Storer BE, Sahebi F, Shizuru JA, Bruno B, Lange T, et al. Long-term outcome of patients with multiple myeloma after autologous hematopoietic cell transplantation and nonmyeloablative allografting. Blood. 2009;113(14):3383-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez R, Parker P, Nademanee A, Smith D, O'Donnell M R,, Stein A, et al. Cyclosporine and mycofenolate mofetil prophylaxis with fludarabine and melphalan conditioning for unrelated donor transplantation: a prospective study of 22 patients with hematologic malignancies. Bone Marrow Transplant. 2004;33(11):1123-9 [DOI] [PubMed] [Google Scholar]
- 11.Nash RA, Johnston L, Parker P, McCune JS, Storer B, Slattery JT, et al. A phase I/II study of mycofenolate mofetil in combination with cyclosporine for prophylaxis of acute graft-versus-host disease after myeloablative conditioning and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2005;11(7):495-505 [DOI] [PubMed] [Google Scholar]
- 12.Gyurkocza B, Storb R, Storer BE, Chauncey TR, Lange T, Shizuru JA, et al. Non -myeloablative allogeneic hematopoietic cell transplantation in patients with acute myeloid leukemia. J Clin Oncol. 2010;28(17):2859-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giralt S. Update on non-myeloablative stem cell transplantation for hematologic malignancies. Int J Hematol. 2002;76(Suppl 1):368-75 [PubMed] [Google Scholar]
- 14.Rodriguez R, Nakamura R, Palmer JM, Parker P, Shayani S, Nademanee A, et al. A phase II pilot study of tacrolimus/sirolimus GVHD prophylaxis for sibling donor hematopoietic stem cell transplantation using 3 conditioning regimens. Blood. 2010;115(5):1098-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cutler C, Antin JH. Sirolimus for GVHD prophylaxis in allogeneic stem cell transplantation. Bone Marrow Transplant. 2004;34(6):471-6 [DOI] [PubMed] [Google Scholar]
- 16.Claxton DF, Ehmann C, Rybka W. Control of advanced and refractory acute myelogenous leukaemia with sirolimus-based nonmyeloablative allogeneic stem cell transplantation. Br J Haematol. 2005;130(2):256-64 [DOI] [PubMed] [Google Scholar]
- 17.Antin JH, Kim HT, Cutler C, Ho VT, Lee SJ, Miklos DB, et al. Sirolimus, tacrolimus, and low-dose methotrexate for graft-versus-host disease prophylaxis in mismatched related donor or unrelated donor transplantation. Blood. 2003;102(5):1601-5 [DOI] [PubMed] [Google Scholar]
- 18.Alyea EP, Li S, Kim HT, Cutler C, Ho V, Soiffer RJ, et al. Sirolimus, tacrolimus, and low-dose methotrexate as graft-versus-host disease prophylaxis in related and unrelated donor reduced-intensity conditioning allogeneic peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2008;14(8):920-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furlong T, Kiem HP, Appelbaum FR, Carpenter PA, Deeg HJ, Doney K, et al. Sirolimus in combination with cyclosporine or tacrolimus plus methotrexate for prevention of graft-versus-host disease following hematopoietic cell transplantation from unrelated donors. Biol Blood Marrow Transplant. 2008;14(5):531-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martino R, Caballero MD, Canals C, Simon JA, Solano C, Urbano-Ispizua A, et al. Allogeneic peripheral blood stem cell transplantation with reduced-intensity conditioning: results of a prospective multicentre study. Br J Haematol. 2001;115(3):653-9 [DOI] [PubMed] [Google Scholar]
- 21.Perez-Simon JA, Diez-Campelo M, Martino R, Sureda A, Caballero D, Cañizo C, et al. Impact of CD34+ cell dose on the outcome of patients undergoing reduced-intensityconditioning allogeneic peripheral blood stem cell transplantation. Blood. 2003;102(3):1108-13 [DOI] [PubMed] [Google Scholar]
- 22.Valcarcel D, Martino R, Caballero D, Martin J, Ferra C, Nieto JB, et al. Sustained remissions of high-risk acute myeloid leukemia and myelodysplastic syndrome after reduced-intensity conditioning allogeneic hematopoietic transplantation: chronic graft-versus-host disease is the strongest factor improving survival. J Clin Oncol. 2008;26(4):577-84 [DOI] [PubMed] [Google Scholar]
- 23.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825-8 [PubMed] [Google Scholar]
- 24.Sullivan KM. Chronic graft-versus-host disease. Cancer Treat Res. 1990;50:79-98 [DOI] [PubMed] [Google Scholar]
- 25.Batts ED, Lazarus HM. Diagnosis and treatment of transplantation-associated thrombotic microangiopathy: real progress or are we still waiting? Bone Marrow Transplant. 2007;40(8):709–19 [DOI] [PubMed] [Google Scholar]
- 26.Wang R, Lagakos SW, Gray RJ. Testing and interval estimation for two-sample survival comparisons with small sample sizes and unequal censoring. Biostatistics. 2010;11(4):676-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorror ML, Leisenring W, Deeg HJ, Martin PJ, Storb R. Twenty-year follow-up of a controlled trial comparing a combination of methotrexate plus cyclosporine with cyclosporine alone for prophylaxis of graftversus-host disease in patients administered HLA-identical marrow grafts for leukemia. Biol Blood Marrow Transplant. 2005;11(10):814-25 [DOI] [PubMed] [Google Scholar]
- 28.Storb R, Antin JH, Cutler C. Should methotrexate plus calcineurin inhibitors be considered standard of care for prophylaxis of acute graft-versus-host disease? Biol Blood Marrow Transplant. 2010;16(1 Suppl):S18-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cutler C, Stevenson K, Kim HT, Richardson P, Ho VT, Linden E, et al. Sirolimus is associated with veno-occlusive disease of the liver after myeloablative allogeneic stem cell transplantation. Blood. 2008;112(12):4425-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cutler C, Henry NL, Magee C, Li S, Kim HT, Alyea E, et al. Sirolimus and thrombotic microangiopathy after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11(7):551-7 [DOI] [PubMed] [Google Scholar]
- 31.Cutler C, Antin JH. Sirolimus immunosuppression for graft-versus-host disease prophylaxis and therapy: an update. Curr Opin Hematol. 2010;17(6):500-4 [DOI] [PubMed] [Google Scholar]
- 32.Cutler C, Li S, Ho VT, Koreth J, Alyea E, Soiffer RJ, et al. Extended follow-up of methotrexate-free immunosuppression using sirolimus and tacrolimus in related and unrelated donor peripheral blood stem cell transplantation. Blood. 2007;109(7):3108-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rezvani AR, Norasetthada L, Gooley T, Sorror M, Bouvier ME, Sahebi F, et al. Non-myeloablative allogeneic haematopoietic cell transplantation for relapsed diffuse large B-cell lymphoma: a multicentre experience. Br J Haematol. 2008;143(3):395-403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rezvani AR, Storer B, Maris M, Sorror ML, Agura E, Maziarz RT, et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in relapsed, refractory, and transformed indolent non-Hodgkin's lymphoma. J Clin Oncol. 2008;26(2):211-7 [DOI] [PubMed] [Google Scholar]
- 35.Flowers ME, Traina F, Storer B, Maris M, Bethge WA, Carpenter P, et al. Serious graftversus-host disease after hematopoietic cell transplantation following nonmyeloablative conditioning. Bone Marrow Transplant. 2005;35(3):277-82 [DOI] [PubMed] [Google Scholar]
- 36.Sorror M, Storer B, Sandmaier B, Maris M, Shizuru J, Maziarz R, et al. Five-year followup of patients with advanced chronic lymphocytic leukemia treated with allogeneic hematopoietic cell transplantation after non-myeloablative conditioning. Clin Oncol. 2012;26(30):4912-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marty FM, Bryar J, Browne SK, Schwarzberg T, Ho VT, Bassett IV, et al. Sirolimus=based graft-versus-host disease prophylaxis protects against cytomegalovirus reactivation after allogeneic hematopoietic stem cell transplantation: a cohort analysis. Blood. 2007;110(2):490-500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Armand P, Gannamaneni S, Kim HT, Cutler CS, Ho VT, Koreth J, et al. Improved survival in lymphoma patients receiving sirolimus for graft-versus-host disease prophylaxis after allogeneic hematopoietic stem-cell transplantation with reduced-intensity conditioning. J Clin Oncol. 2008;26(35):5767-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.