Abstract
The low frequency of naturally occurring regulatory T cells (nTregs) in peripheral blood and the suboptimal protocols available for their ex vivo expansion limit the development of clinical trials based on the adoptive transfer of these cells. We have, therefore, generated a simplified, robust and cost-effective platform for the large-scale expansion of nTregs using a gas permeable static culture flask (G-Rex) in compliance with Good Manufacturing Practice. More than 109 putative Tregs co-expressing CD25 and CD4 molecules (92±5%) and FoxP3 (69±19%) were obtained within 21 days of culture. Expanded Tregs showed potent regulatory activity in vitro (80±13% inhibition of CD8+ cell division) and in vivo (suppression or delay of graft-versus-host disease in a xenograft mouse model) indicating that the cost-effective and simplified production of nTregs we propose will facilitate the implementation of clinical trials based on their adoptive transfer.
Introduction
Regulatory T cells (Tregs) are implicated in controling graftversus-host disease (GvHD) post allogeneic hematopoietic stem cell transplantation (HSCT)1 prompting the quest for novel therapies based on their adoptive transfer.
Initial studies to prevent or treat GvHD2,3 were based on the infusion of freshly isolated naturally occurring Tregs (nTregs) circulating in peripheral blood.4 Even though these studies established the overall safety of Treg-based therapies, they also clearly indicated that the low numbers of Tregs collected from the peripheral blood are inadequate for controlling GvHD.5 Given these limitations, protocols aimed at effectively selecting and expanding ex vivo fully functional nTregs in compliance with Good Manufacturing Practice (GMP) are badly needed.
Here we describe a simplified and cost-effective methodology that consistently and reproducibly expands nTregs that retain potent inhibitory function both in vitro and in vivo.
Design and Methods
Isolation of nTregs and culture conditions
Buffy coats were obtained from healthy volunteer donors (Gulf Coast Regional Blood Center, Houston, TX, USA) (IRB H-7634). Putative nTregs (CD4+CD25Bright) were enriched from peripheral blood mononuclear cells (PBMC) using positive selection, after labeling cells with a minimal amount of CD25-specific microbeads (2 μL/107 cells; Miltenyi Biotec Inc., Auburn, CA, USA).2,6 Immediately after selection (Day 1), CD25Bright cells (106 cells/mL) were resuspended in complete medium, consisting of RPMI1640 (Hyclone, Logan, UT, USA), 10% AB-human serum (Valley Biomedical, Winchester, VA, USA), 2 mM L-glutamine (BioWhittaker Inc., Walkersville, MD, USA), penicillin-streptomycin (BioWhittaker), and β-mercaptoethanol (50 μM) (Invitrogen, Carlsbad, CA, USA) and then activated with anti-CD3
(OKT3, Orthoclone, Cilag Ag Int., Zug, Switzerland) (1 μg/mL) and anti-CD28 monoclonal antibodies (mAb) (1 μg/mL)6 (BD Biosciences PharMingen, San Diego, CA, USA) in the presence of temsirolimus (LC Laboratories, Woburn, MA, USA) at a final concentration of 100 nM.7 On Day 7 (S1), cells were harvested, washed, counted and seeded in a G-Rex108 (Wilson Wolf Manufacturing, Saint Paul, MN, USA) (http://www.wilsonwolf.com/) and supplemented with soluble OKT3 (1 μg/mL), CD28 mAb (1 μg/mL), rIL-2 (50 IU/mL)6 (Proleukin; Chiron, Emeryville, CA, USA), temsirolimus (100 nM) and irradiated (40 Gy) allogeneic feeders obtained from at least two pooled CMV-seronegative donors meeting testing requirements for whole blood donation (1:5 Treg:feeders ratio). On Day 14 (S2), cells were seeded in the GRex1008 and supplemented with the same reagents used in S1. On Day 21 (S3), cells were harvested, counted and used for functional experiments. CD25 depleted cells (CD25Dep) expanded in parallel without temsirolimus were used as control cells. To further validate our approach for clinical use, putative nTregs were isolated using the CliniMACS device. Briefly, PBMC were labeled with clinical grade CD25-specific microbeads (18 μL buffer and 2 μL of beads for 1 × 107 cells). After incubation and washes, cell selection was then started according to E-cell System software version 3.2. At the end of the selection, cells were expanded as described for small-scale experiments. On Days 14 (S2) and 21 (S3), aliquots of expanded Tregs were cryopreserved in dimethyl sulfoxide (DMSO) according to standard procedure and stored in liquid nitrogen.
Results and Discussion
Naturally occurring Tregs undergo robust ex vivo expansion in the G-Rex device
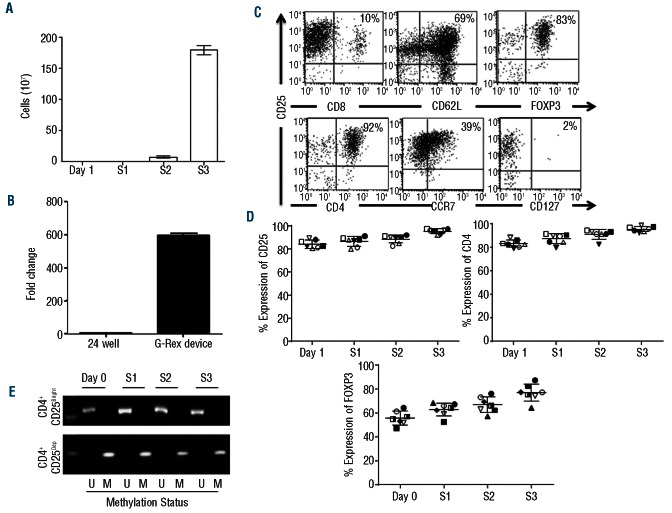
The methodology to isolate and expand nTregs is graphically summarized in the Online Supplementary Figure S1. To reduce the complexity of the process of selection from the peripheral blood, we isolated putative nTregs exclusively based on their bright expression of the CD25 molecule CD25Bright cells) without additional selection steps. Starting from 4.5×108±1×108 PBMC (obtained from 50 mL of buffy coat products), we recovered 3×106±1×106 cells. Upon selection, these cells consistently co-expressed CD4 and CD25 molecules (95%±5%), with limited contamination by CD8+ cells. On Day 7 (S1) and Day 14 (S2), (2.9×106±0.5×105 and 7.7×107±1.7×107 cells were obtained, respectively. After the third stimulation (Day 21, S3), we recovered 1.8×109±7.6×107 cells, corresponding to an over 600-fold expansion (Figure 1A). This degree of expansion was significantly higher than that obtained in parallel experiments (5-6 fold) in which isolated nTregs were grown using the same protocol but plated in conventional 24-well tissue culture plates (cells at Day 21 were 5.3×106±1.6×106 starting from 1×106) (Figure 1B). The percentage of CD4+CD25+ cells remained stable over three weeks of culture and was 92±5% by Day 21, with 69±19% of the cells expressing FoxP3 (Figure 1C and D). Expanded nTregs retained their expression of the lymph node homing molecules CD62L and CCR7 (69±4% and 39±3%, respectively), and lacked expression of the IL-7Rα (CD127) (2±1.2%), a known feature of Tregs.9 Of note, the percentage of FoxP3+ cells significantly increased from Day 1 to Day 21 of culture. FoxP3 promoter remained consistently unmethylated indicating the commitment of the expanded cells to the Treg state, despite some of them lacking FoxP3 protein expression by Day 21 of culture (Figure 1E).10 In contrast, the FoxP3 promoter of cultured CD25Dep control cells remained consistently methylated (Figure 1E). The phenotypic analysis of CD25Dep cells after three stimulations (S3) showed that CD4, CD25, FoxP3, CCR5 and CCR7 were expressed by 55±21%, 7±6.5%, 12±11%, 9±7%, and 11±5% of the cells, respectively.
Figure 1.
Robust ex vivo expansion of nTregs in the G-Rex device. (A) illustrates the number of nTregs obtained after 1, 2 or 3 (S1, S2 and S3) weeks of culture in the G-Rex device. Data illustrate average and standard deviations (SD) for 7 independent experiments. (B) Compares the fold expansions of nTregs cultured in 24-well plates or in the G-Rex device. Data show mean ± SD of 7 independent experiments. (C) Shows the flow cytometry plots of expanded nTregs on Day 21 (S3). The panels illustrate CD4, CD25, CCR7, CD127 and FoxP3 expression for one representative donor. (D) Illustrates the expression of CD25, CD4 and FoxP3 in expanded nTregs after each round of stimulation (S1, S2 and S3). Data summarize the results of 7 independent experiments. (E) Shows the methylation-specific semi-quantitative PCR of the FoxP3 promoter in nTregs and control CD25Dep cells obtained after each round of stimulation (S1, S2 and S3).
Ex vivo expanded nTregs maintain robust suppressive activity without undergoing senescence
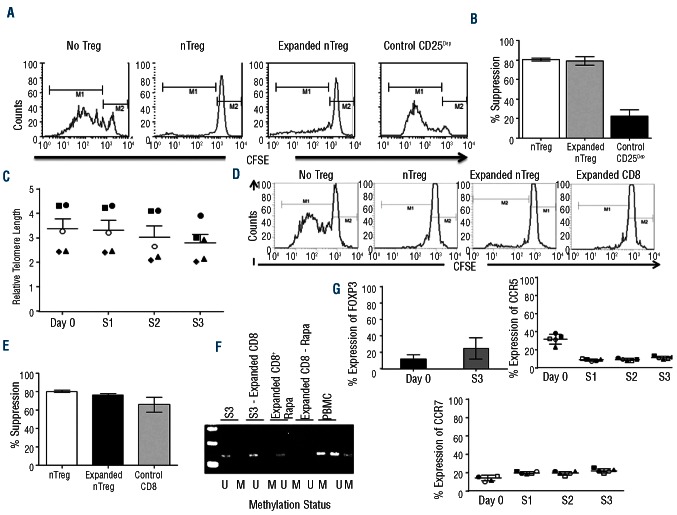
Using a CFSE-based suppression assay, we found equal suppression of T-cell divisions by either freshly isolated nTregs or expanded nTregs (S3) (80±10% and 80±13% suppression, respectively) (P<0.0001). No suppression was observed in the presence of expanded control CD25Dep cells (12±9% suppression) (Figure 2A and B). Importantly, the robust expansion of nTregs achieved in the G-Rex device was obtained without significantly compromising their telomere length. In freshly isolated nTregs (Day 1) and expanded nTregs at S1, S2 and S3, relative telomerase length (RTL) was 3.4±0.96%, 3±0.98%, 3±0.99%, 2.8±1.8%, respectively (Figure 2C), suggesting that, in addition to cell divisions, a significant preservation of cell viability contributes to the large number of cells expanded in the G-Rex device. Since by Day 21, expanded nTregs contained contaminating CD8+ cells (10±3.7%), we specifically assessed the functionality of these cells, as the infusion of functional CD8+ cells in the allogeneic HSCT setting may exacerbate GvHD and thus compromise the protective effects of Tregs. As illustrated in Figure 2D and E, T-cell divisions were suppressed equally well when either freshly isolated nTregs, expanded nTregs or selected CD8+ cells were added to the culture (80±8%, 76±8%, and 66±14% suppression, respectively) (P<0.001), suggesting that contaminating CD8+ cells likely acquired inhibitory properties during the ex vivo culture conditions. This inhibitory function of expanded CD8+ cells was corroborated by the detection of the unmethylated form of the FoxP3 promoter in these cells (Figure 2F). Even if we cannot exclude that some of the contaminating CD8+ cells have effector function, experiments in which the suppression assays were performed using different ratios of CD8+ cells and T-effector cells showed that their overall inhibitory function was significantly retained at 1:10 dilution (Online Supplementary Figure S2). The phenotypic analysis of these CD8+ cells showed that 25±13% of them expressed FoxP3 and 11±1.6% and 22±2.3% expressed CCR5 and CCR7, respectively (Figure 2G).
Figure 2.
Ex vivo expanded nTregs retain robust suppressive function without undergoing cell senescence. (A) The inhibitory activity of freshly isolated nTregs, expanded nTregs (S3), and expanded CD25Dep cells was assessed using a CFSE-based suppression assay. Panels illustrate the inhibitory activity of nTregs for a representative experiment. (B) The graph summarizes average and SD of the inhibitory function of nTregs for 7 independent experiments. (C) The graph illustrates the relative telomerase length (RTL) in freshly isolated nTregs (Day 1) and expanded nTregs at S1, S2 and S3. Data show mean ± SD for 7 independent experiments. (D) Contaminating CD8+ cells at Day 21 of culture were isolated and analyzed for their suppressive activity and compared to nTregs. Panels illustrate the inhibitory activity for one representa-tive experiment. (E) The graph summarizes average and SD of the inhibitory function of contaminating CD8+ cells and nTregs for 7 independent experiments. (F) Methylation-specific semi-quantitative PCR of FoxP3 promoter in cells obtained at the end of third round of stimulation (S3), nTregs obtained at the end of the third round of stimulation depleted of CD8 (S3-Expanded CD8), the expanded and purified CD8+ cells in S3 (Expanded CD8 + Rapa), CD25Dep population (CD8–Rapamycin), and PBMC. (G) Illustrates the expression of FoxP3 (after three stimulation, S3), and of CCR7 and CCR5 (after each stimulation, S1, S2, and S3) in the contaminating CD8+ cells. Data summarize the results of 5 independent experiments.
Expanded nTregs control GvHD in a xenogeneic mouse model
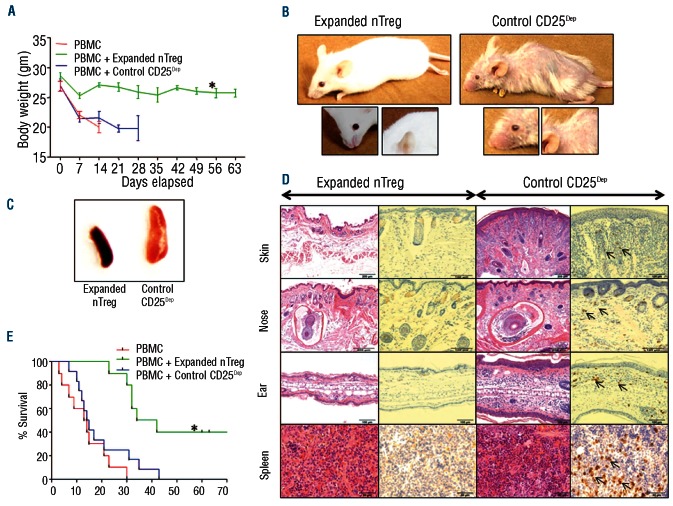
To investigate whether expanded nTregs retained their inhibitory function in vivo, we used a xenograft model of lethal GvHD.11 As illustrated in Figure 3A, the weight loss of control mice receiving PBMC and CD25Dep cells was significantly greater as compared to mice that received PBMC and expanded nTregs (7.2±1.9 g vs.1.9±1 g, respectively) (P=0.0045). In addition, by Day 60, mice receiving expanded nTregs had delayed occurrence or no signs of GvHD (Figure 3B), and showed normal sized spleen as compared to controls (Figure 3C). Finally, mice co-infused with expanded nTregs revealed no histopathological lesions compatible with GvHD in their skin, nose, or ear (Figure 3D), and showed significantly improved overall survival as compared to control mice (P<0.0003) (Figure 3E). As illustrated in the Online Supplementary Figure S3A, Tregs co-infused with PBMC did not abrogate the engraftment of PBMC, suggesting that in this model Tregs inhibit the expansion of T cells that cause the occurrence of GvHD. Finally, as previously demonstrated by others,12 nTregs expanded in the presence of rapamycin did not produce IL-17 (Online Supplementary Figure S3B).
Figure 3.
Expanded nTregs control GvHD in a xenogeneic mouse model. Irradiated NSG mice were infused with PBMC either alone or in combination with expanded nTregs (S3) or expanded control CD25Dep cells at 1:1 ratio. (A) The graph illustrates the measurements of the weight of the NSG mice. Data show mean ± SD of 12 animals for each group (*P=0.0001). (B) Images show that control mice developed signs of GvHD, such as hair loss and orbital tightening. (C) Spleen enlargements were observed in mice infused with control CD25Dep cells. (D) Representative H&E and immunohistochemistry staining for CD8+ cells in tissue sections obtained from skin, nose, ear, and spleen of treated mice. Control mice showed evidence of chronic dermatitis, with moderate diffuse epithelial hyperplasia, hyperkeratosis and marked multi-focal coalescing subcutaneous and dermal mononuclear inflammatory cell infiltrates. Arrows indicate infiltrating CD8+ cells. (E) Kaplan-Meier survival curve comparing NSG mice receiving human PBMC alone or in combination with expanded nTregs or expanded control CD25Dep cells (12 animals for each group) (*P=0.0003).
nTregs selected and expanded using the CliniMACS and G-Rex devices, respectively, maintain potent in vitro and in vivo suppressive function
To make our methodology GMP compliant, we adapted the selection using the CliniMACS system. CD25Bright cells (2.3×106 ± 0.7×106) were positively selected from PBMC (4.6×108 ± 0.7×108) of 3 buffy coats, using clinical grade anti-CD25 microbeads and expanded as described in the small scale experiments, resulting in 1.3×109±3×107 cells by Day 21 of culture (S3) (Online Supplementary Figure S4A) (547-fold expansion). These cells consistently co-expressed CD4, CD25 (97±2%) and FoxP3 (82±4%) (Online Supplementary Figure S4B and C), while the contaminating CD8+ cells were less than 1.2±1%. The expanded Tregs suppressed T-cell divisions in vitro (80±10% and 78±5% suppression for expanded and freshly isolated Tregs, respectively) (Online Supplementary Figure S4D), and maintained robust in vivo suppressive activity improving the overall survival of mice (P=0.0046) (Online Supplementary Figure S4E). Finally, because the infusion of freshly cultured Tregs is frequently impractical for clinical applications and a cryopreservation step is usually required for quality control tests, we evaluated whether expanded nTregs retained their functionality following cryopreservation and storage in liquid nitrogen. Expanded Tregs cryopreserved after Day 14 (FS2) or Day 21 (FS3) retained their suppressive activity both in vitro (Online Supplementary Figure S5A) and in vivo (Online Supplementary Figure S5B).
Our proposed strategy has significant advantages compared to other protocols.13,14 First, we have minimized the cell purification process to a single immune magnetic selection step based on their CD25 expression, which is sufficient to minimize the contamination by CD8+CD25+ cells in the final Treg products, provided that rapalogs are added to the cultures during the expansion phase. Importantly, we have observed that, in the presence of rapalogs, ‘contaminating’ CD8+CD25+ cells persisting at the end of the 21 days of culture show inhibitory properties and methylation of FoxP3 promoter. This is in accordance with previous observations showing that, in specific culture conditions, CD8+ cells may develop suppressive activity.10,15 Second, and most importantly, we have optimized a robust and cost-effective expansion protocol of Tregs. Stimulation of Tregs is obtained with anti-CD3/CD28 mAbs, now available as clinical grade reagents (Miltenyi Biotec Inc.), and feeder cells that meet GMP requirements.16,17 In addition, cells are easily accommodated, with minimal manipulation, in small gas permeable static culture flasks (G-Rex) that promote efficient gas exchange and availability of nutrients to the cells, while diluting waste products.
Remarkably, expanded Tregs had no significant shortening of their telomere lengths, indicating their potential capacity to undergo further divisions in vivo after adoptive transfer, and retained inhibitory function after freezing and thawing. These are important manufacturing aspects to be considered in clinical protocols of adoptive T-cell therapy as quality control tests of the produced cells are usually required. Hence, the cost-effective and simplified production of nTregs we propose will likely facilitate the implementation of clinical trials based on the infusion of these cells to control GvHD after allogeneic HSCT and graft rejection in solid organ transplant recipients, and to treat autoimmune diseases.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Roger Price for pathological evaluations, Dr. Cecilia Ljungberg for assistance with immunohistochemistry, and Reshma Kulkarni for the phenotypic analyses.
Funding: This work was supported in part by R01 CA142636 National Institutes of Health-NCI, W81XWH-10-10425 Department of Defense, Technology/Therapeutic Development Award and PACT (Production Assistance for Cell Therapy (PACT) NIH-NHLBI N01-HB-10-03.
Footnotes
Authorship and Disclosures; Information on authorship, contributions, and financial and other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
The online version of this article has a Supplementary Appendix.
References
- 1.Zorn E, Kim HT, Lee SJ, et al. Reduced frequency of FOXP3+ CD4+CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood. 2005;106 (8):2903-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang X, Lu L, Jiang S. Regulatory T cells: customizing for the clinic. Sci Transl Med. 2011;3(83):83ps–19. [DOI] [PubMed] [Google Scholar]
- 3.Leslie M. Immunology. Regulatory T cells get their chance to shine. Science. 2011; 332(6033):1020-1 [DOI] [PubMed] [Google Scholar]
- 4.Di Ianni M, Falzetti F, Carotti A, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117 (14):3921-8 [DOI] [PubMed] [Google Scholar]
- 5.Hippen KL, Riley JL, June CH, Blazar BR. Clinical perspectives for regulatory T cells in transplantation tolerance. Semin Immunol. 2011;23 (6):462-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakraborty R, Rooney C, Dotti G, Savoldo B. Changes in chemokine receptor expression of regulatory T cells after ex vivo culture. J Immunother. 2012;35 (4):329-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+ CD25+FoxP3+ regulatory T cells. Blood. 2005;105 (12):4743-8 [DOI] [PubMed] [Google Scholar]
- 8.Vera JF, Brenner LJ, Gerdemann U, et al. Accelerated production of antigen-specific T cells for preclinical and clinical applications using gas-permeable rapid expansion cultureware (G-Rex). J Immunother. 2010; 33 (3):305-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu W, Putnam AL, Xu-Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203 (7):1701-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyao T, Floess S, Setoguchi R, et al. Plasticity of Foxp3(+) T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity. 2012;36 (2):262-75 [DOI] [PubMed] [Google Scholar]
- 11.van Rijn RS, Simonetti ER, Hagenbeek A, et al. A new xenograft model for graft-versushost disease by intravenous transfer of human peripheral blood mononuclear cells in RAG2-/- gammac-/- double-mutant mice. Blood. 2003;102 (7):2522-31 [DOI] [PubMed] [Google Scholar]
- 12.Tresoldi E, Dell'Albani I, Stabilini A, et al. Stability of human rapamycin-expanded CD4+CD25+ T regulatory cells. Haematologica. 2011;96 (9):1357-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baecher-Allan C, Hafler DA. Suppressor T cells in human diseases. J Exp Med. 2004; 200 (3):273-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hippen KL, Merkel SC, Schirm DK, et al. Massive ex vivo expansion of human natural regulatory T cells (T(regs)) with minimal loss of in vivo functional activity. Sci Transl Med. 2011;3(83):83ra–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng SG, Wang JH, Koss MN, Quismorio F, Jr, Gray JD, Horwitz DA. CD4+ and CD8+ regulatory T cells generated ex vivo with IL-2 and TGF-b suppress a stimulatory graft-versus-host disease with a lupuslike syndrome. J Immunol. 2004;172 (3): 1531-9 [DOI] [PubMed] [Google Scholar]
- 16.Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. Generation of tumorinfiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother. 2003; 26 (4):332-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith CA, Ng CY, Heslop HE, et al. Production of genetically modified Epstein-Barr virus-specific cytotoxic T cells for adoptive transfer to patients at high risk of EBV-associated lymphoproleferative disease. J Hematother. 1995;4 (2):73-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.