Abstract
Prior studies have investigated patients' characteristics, treatments, and outcomes for older adults with myelodysplastic syndromes, but most failed to distinguish chronic myelomonocytic leukemia. Recognizing potentially important differences between the diseases, we undertook a population-based comparison of baseline characteristics, treatments, and outcomes between older adults with chronic myelomonocytic leukemia and myelodysplastic syndromes. The patients' data were obtained from Surveillance Epidemiology and End Results registry data from 2001-2005, linked to Medicare claims. Baseline characteristics, treatment (red blood cell transfusions, hematopoietic growth factors, hypomethylating agents, chemotherapy or transplantation), progression to acute myeloid leukemia, and overall survival were compared using bivariate techniques. Multivariate logistic regression estimated differences in treatments received. Cox proportional hazard models estimated the effects of chronic myelomonocytic leukemia relative to myelodysplastic syndromes on progression-free survival. A larger proportion of patients with chronic myelomonocytic leukemia (n=792), compared to patients with myelodysplastic syndromes (n=7,385), failed to receive any treatment (25% versus 15%; P<0.0001), or only received red blood cell transfusions (19.8% versus 16.7%; P=0.037). A larger percentage of patients with chronic myelomonocytic leukemia progressed to acute myeloid leukemia (42.6% versus 15.5%, respectively; P<0.0001), with shorter time to progression. Chronic myelomonocytic leukemia patients had a shorter median survival (13.3 versus 23.3 months; P<0.0001) and lower 3-year survival rate (19% versus 36%; P<0.0001). Adjusted estimates, controlling for baseline characteristics and selected treatments, indicate that chronic myelomonocytic leukemia was associated with an increased risk of progression to acute myeloid leukemia or death (HR 2.22; P<0.0001), compared to myelodysplastic syndromes. In conclusion, chronic myelomonocytic leukemia is less frequently treated in older adults and is associated with worse outcomes, even after controlling for the patients' baseline characteristics and selected treatments. Our data suggest the need for continued evaluation of the biological differences between these diseases and clinical trials targeting chronic myelomonocytic leukemia.
Introduction
Chronic myelomonocytic leukemia (CMML) is a clonal stem cell disorder that displays features of both a myelodysplastic syndrome (MDS) and a myeloproliferative neoplasm. Diagnostic criteria for CMML include persistent peripheral blood monocytosis (>10ϗ109/L), absence of the Philadelphia chromosome and/or BCR-ABL1 fusion gene, absence of platelet-derived growth factor receptor or gene rearrangement, fewer than 20% blasts in the blood and the bone marrow, and dysplasia of one or more myeloid lineages.1 CMML was classified as a MDS in the French-American-British classification system in 1982,2 but was subsequently reclassified as a mixed myelodysplastic/myeloproliferative disorder by the World Health Organization (WHO) in 2001.3 CMML shares clinical and biological features with MDS, including development of cytopenias and bone marrow failure, risk of progression to acute myeloid leukemia (AML), and overlapping recurring cytogenetic abnormalities. Like MDS, it has a variable clinical course, with reported rates of transformation to AML of 15 to 52% and a median overall survival of 12 to 18 months.4-6 Treatment modalities for the two diseases are also similar, including hematopoietic growth factors (erythropoiesis-stimulating agents and granulocyte colony-stimulating factor), transfusion support, hypomethylating agents, and allogeneic hematopoietic stem cell transplantation.
As a result of its previous classification and its shared features with MDS, CMML has often been combined with MDS in both epidemiological and clinical studies, and few comparisons of the two diseases have been published. Patients with CMML were reported to have a worse 3-year survival than those with MDS on the basis of combined data from the North American Association of Central Cancer Registries (NAACCR) and Surveillance Epidemiology and End Results (SEER) databases (21% versus 45% for CMML and MDS, respectively),7 as well as data from the Veterans Administration population (21% versus 31%, respectively).8 While treatments for both diseases are similar, data on efficacy of therapeutic agents in CMML are often extrapolated from studies in which CMML was combined with MDS. Also, while the International Prognostic Scoring System has an established role in determining the prognosis of MDS and in choosing the appropriate treatment,9 there is considerably more debate over the best tools for predicting prognosis in CMML.10 As a result, there may be greater uncertainty, and hence more heterogeneity, in therapeutic approaches to CMML.
In this study, using the SEER-Medicare database, we compared patients' characteristics, treatments, progression to AML and progression-free survival (progression to AML or death) between older adults with CMML or MDS. Adjusted survival analyses allowed us to evaluate whether CMML is fundamentally a more aggressive disease than MDS, when controlling for patients' characteristics and treatments received.
Design and Methods
Data and samples
Patients were identified from the SEER-Medicare database, which combines SEER data on incident cancers from 17 state and regional cancer registries with Medicare enrollment and claims data. SEER includes information on cancer site, histology, selected demographic characteristics, date of diagnosis, and date and cause of death. Medicare enrollment data provide further details on demographics and enrollment over time by Parts A, B and Medicare Advantage Plans. Census tract-level data on person and household characteristics provided additional demographic information. Medicare Part A and B claims show detailed service-level information (dates of services, diagnoses, procedures and Medicare-allowed reimbursements and payments) for inpatient and outpatient hospital and physician services. Part B claims capture drugs administered by infusion or injection in a physician's office or other outpatient setting. Data on oral medications were not included, as SEER-Medicare did not report data on prescription drugs not covered by Medicare Part B during the period of our study.
The overall sample included cases of CMML and MDS newly diagnosed between 2001 and 2005, with claims from 2000 through 2007. Cases were identified based on International Classification of Disease for Oncology Third Edition (ICD-O-3) codes: 9945 for CMML and 9980 [refractory anemia (RA)], 9982 [RA with sideroblasts (RARS)], 9983 [RA with excess blasts (RAEB)], 9985 [refractory cytopenia with multilineage dysplasia (RCMD)], 9986 [MDS with 5q deletion (5qdel)], 9987 [therapy-related MDS (t-MDS)], and 9989 [MDS, not otherwise specified (NOS)] for MDS. Code 9984 (RA with excess blasts in transformation) was excluded because this entity is categorized as AML by the WHO criteria. The diagnosis was confirmed by histology in 87% of patients in both groups. To ensure completeness of Medicare claims records, patients were excluded if they had incomplete information regarding date of diagnosis or death or had any period without Medicare Parts A and B or with Health Maintenance Organization enrollment during the 12 months prior to, or the month of, diagnosis. Medicare Part C patients were not included because there were no claims data available. These criteria resulted in exclusion of 18.9% of the initial sample.
Patients' characteristics
Sociodemographic characteristics included the patients' age, race, sex, census tract-level median household income measured in quartile ranges, census tract-level measures of educational attainment, and year of diagnosis.
Risk stratification of myelodysplastic syndromes
MDS patients were classified into lower-risk (RA, RARS, RCMD and 5qdel), RAEB, t-MDS, and MDS-NOS, using the SEER indicators. Although WHO categories do not fully match International Prognostic Scoring System risk categories, their prognostic value has been documented.11
Baseline health status
The patients' health status was determined from a series of indicators of baseline acute or chronic conditions, based on the presence of ICD-9 CM diagnostic codes in Medicare claims in the 12 months prior to the diagnosis of MDS. To establish a diagnosis, we required one inpatient or two outpatient claims with the relevant diagnosis codes (with at least two claims 29 days apart but within 12 months, to limit inclusion of patients with rule-out diagnoses). The conditions that we identified included acute myocardial infarction, congestive heart failure or other ischemic heart disease, cardiac arrhythmias, stroke, renal disease (acute and chronic renal disease, congenital, nephritic syndrome, nephrotic syndrome and dialysis), hepatitis or other liver disease, venous thromboembolic events, Alzheimer's dementia, and severe mental illness (depression, schizophrenia, bipolar disorder). We also created claims-based indicators associated with poor baseline performance status, including prior period hospitalization, stay in a skilled nursing facility, admission to a nursing home, home oxygen, walking aids, and wheelchair.12 We included diagnoses of other cancers within the prior 5 years, and receipt of red blood cell transfusions prior to diagnosis.
Treatments and outcome measurements
Treatments were identified in the period from diagnosis until death or censoring using presence of claims for specific inpatient diagnosis-related groups and procedures (based on ICD-9 CM procedure codes, the Healthcare Common Procedure Coding System or National Drug Codes). Specific treatments included red blood cell transfusion, erythropoiesis-stimulating agents, granulocyte colony-stimulating factor, hypomethylating agents, chemotherapy, and transplantation.
Dates of death through 2007 are reported in the Medicare enrollment files. The onset of AML was identified based on the presence of either a new diagnosis in SEER or one inpatient or two outpatient diagnoses of AML in Medicare claims. Survival was calculated from the first day of the diagnosis month to the date of death. Follow-up was censored if patients were alive at the end of the study period or if they lost Medicare Part A or B or enrolled in a Medicare Advantage plan.
Statistical analysis
We used bivariate techniques (χ2 test, t-test) to compare characteristics and treatments between patients with CMML and MDS. Multivariate logistic regression models were used to estimate the effect of having CMML relative to MDS on the probability of receiving the most common treatments, controlling for patients' characteristics, baseline health status and year of diagnosis. Marginal probabilities were calculated to reflect the change in adjusted probability of the treatment when the diagnosis was CMML versus MDS. Kaplan-Meier estimates compared progression-free survival across the groups, and Cox proportional hazard models using the pooled CMML and MDS sample estimated the effects of CMML relative to MDS on survival, controlling for patients' characteristics, baseline health status, and selected treatments (erythropoiesis-stimulating agents, granulocyte colony-stimulating factor, hypomethylating agents). Within CMML, Cox proportional hazard models estimated the effects of patients' characteristics on progression-free survival. Estimates based on sample sizes of ten or fewer observations were suppressed to comply with the National Cancer Institute's requirements for data confidentiality. All analyses were completed using SAS® 9.2 (SAS, Cary, NC, USA) and Stata 10. The project was approved by the University of Maryland Baltimore Institutional Review Board.
Results
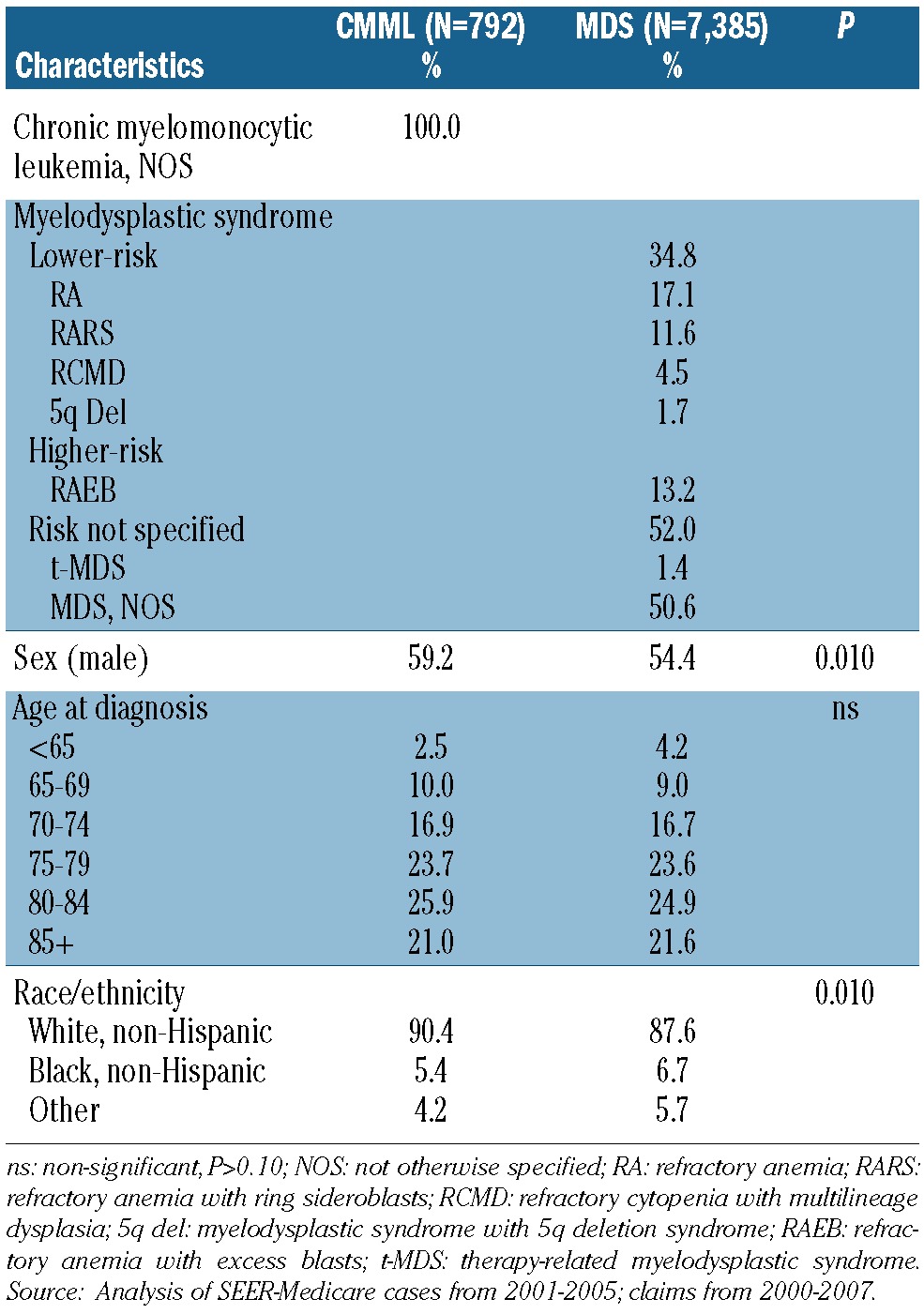
The cohort included 792 patients diagnosed with CMML and 7,385 patients diagnosed with MDS between 2001 and 2005. Selected baseline characteristics are compared in Table 1. Distributions were similar for age, education (not shown), income and diagnosis of other cancers within 5 years (Online Supplementary Table S1). The majority of patients were male, with greater male predominance in CMML than in MDS (59.2% versus 53.8%; P=0.01). There was a higher proportion of whites in the CMML category (90.42% versus 87.6%; P=0.01). Fewer CMML patients than MDS patients had chronic heart failure/ischemic heart disease (37.4% versus 47.1%; P<0.0001) or liver disease (2.8% versus 4.8%; P=0.01). Rates of other baseline comorbidities were similar. Hospital admissions and wheelchair claims were less frequent in CMML patients, while other baseline healthcare use was similar (Online Supplementary Table S1).
Table 1.
Characteristics of the patients with CMML or MDS.
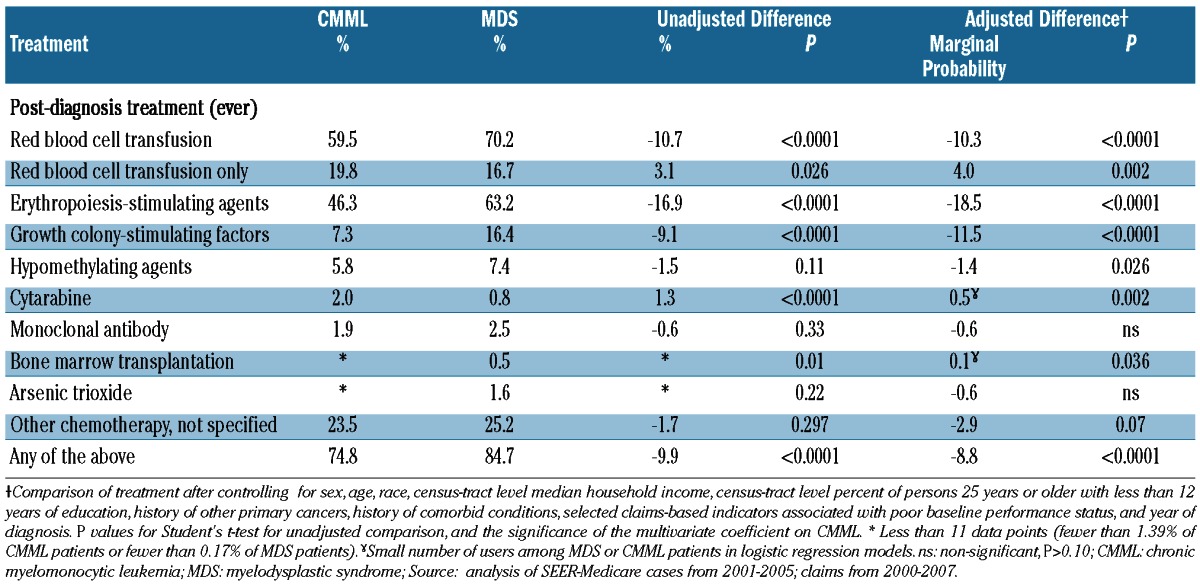
Treatments received are compared in Table 2. A larger proportion of CMML patients than MDS patients did not receive any treatment (25.2% versus 15.3%; P<0.0001), and CMML patients were also more likely to have been treated with red blood cell transfusions alone (19.8% versus 16.7%; P=0.037). CMML patients received erythropoiesis-stimulating agents (46.3% versus 63.2%; P<0.0001) and granulocyte colony-stimulating factor (7.3% versus 16.4%; P<0.0001) less frequently than MDS patients, but were treated more frequently with cytarabine, etoposide, and hematopoietic stem cell transplantation, although rates of treatment were very low in both diseases. After adjusting for baseline characteristics, differences in treatment were very similar in magnitude and significance to the unadjusted differences, with the exception of hypomethylating agents, for which the adjusted difference indicated lower rates for patients with CMML.
Table 2.
Comparison of treatments received by CMML and MDS patients.
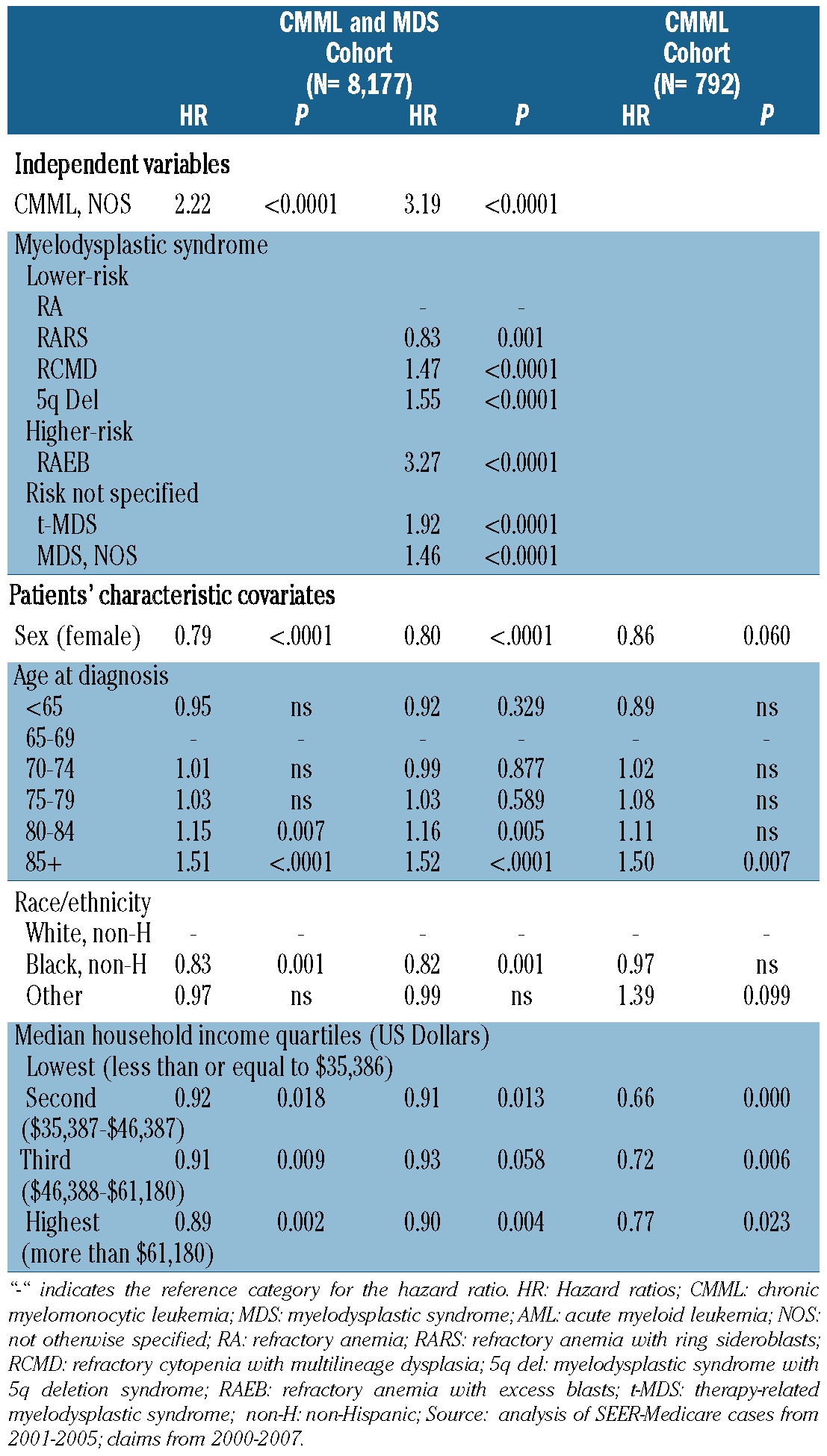
A significantly higher percentage of CMML patients progressed to develop AML [43% versus 16%; P<0.0001, hazard ratio (HR) 4.15, 95% confidence interval (95% CI): 3.67-4.69)] and among patients who progressed to AML, progression occurred earlier in CMML than in MDS patients (median 8 versus 32 weeks; P<0.0001; Figure 1A). CMML patients had a shorter median survival (13.3 versus 23.3 months; P<0.0001) (Figure 1B), and a lower survival probability at 1 year (51% versus 66%; P<0.0001) and at 3 years (19% versus 36%; P<0.0001). CMML patients also had a lower composite progression-free survival rate at 1 year (35% versus 62%; P<0.0001) and at 3 years (20% versus 45%; P<0.0001), compared with MDS patients (Figure 1C) (Table 3). Adjusted estimates of progression-free survival (Table 4) indicate that CMML was associated with an increased risk of AML progression or death, compared to MDS (HR 2.22, P<0.0001). Inclusion of treatment indicators in the model did not alter the adjusted effect of CMML (HR 2.14, P<0.0001, data not shown). When we compared patients with CMML and individual WHO subcategories of MDS to a reference group of patients with RA, the risk associated with CMML (HR 3.19, P<0.0001) was similar to that associated with RAEB (HR 3.27, P<0.0001), and remained so after controlling for treatments received (CMML: HR 3.03, P<0.0001) (RAEB: HR 3.28, P<0.0001) (data not shown). Increased age was associated with an increased risk of AML or death, while female sex, black race, and higher median household income were associated with decreased risk. Within the CMML cohort, increased age was associated with increased risk of AML or death, and higher median household income was associated with decreased risk (Online Supplementary Table S2).
Figure 1.
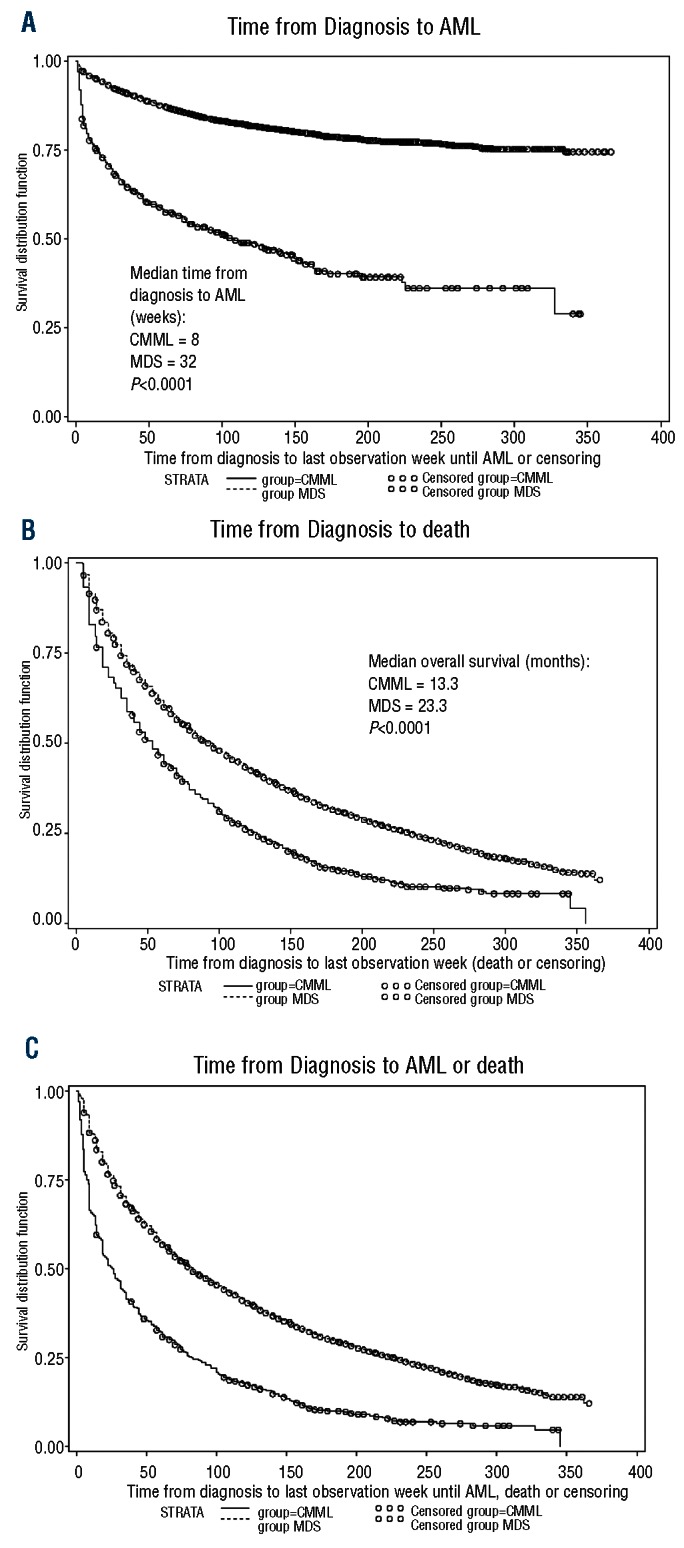
Comparisons between CMML and MDS patients. (A) Time (weeks) from diagnosis to AML for CMML and MDS patients. (B) Time (weeks) from diagnosis to death for CMML and MDS patients. (C) Time (weeks) from diagnosis to AML or death for CMML and MDS patients.
Table 3.
Progression to AML and/or death in CMML and MDS patients.
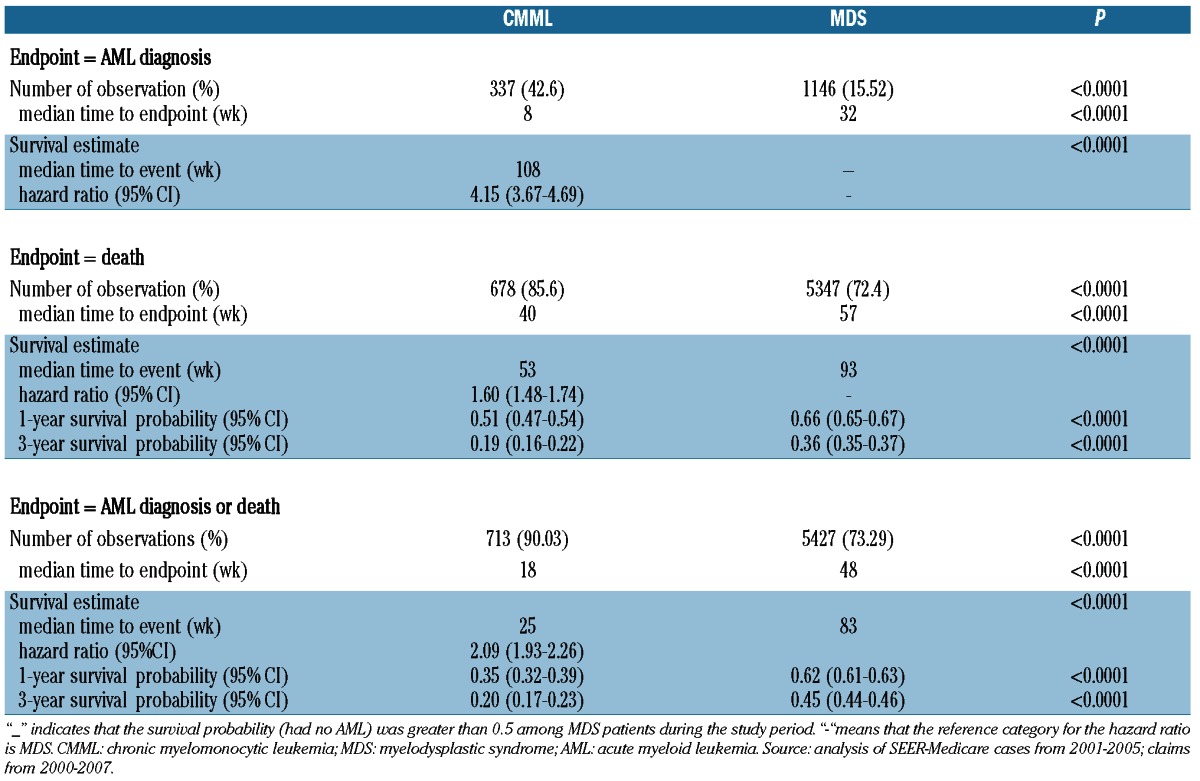
Table 4.
Hazard ratios (HR) for progression to acute myeloid leukemia or death.
Discussion
To our knowledge, this work represents one of the first extensive comparisons of CMML and MDS at the population level, comparing characteristics, treatments and outcomes of patients. We found that CMML was associated with a higher rate of progression to AML, more rapid progression, and shorter progression-free survival and overall survival. Importantly, we found that a higher proportion of CMML patients did not receive treatment, compared to the proportion of MDS patients. Despite lower rates of treatments and small differences in patients' characteristics, our adjusted analyses suggest that differences in biology between CMML and MDS may account for the substantially worse outcomes observed in the CMML cohort.
Differences in outcomes of malignancies may be explained by the complex interplay between characteristics of the population affected, treatment availability and tolerability, and biology of the disease. We found that there were relatively few differences in baseline characteristics between the cohorts. MDS patients had a higher prevalence of several baseline conditions, including chronic heart failure/ischaemic heart disease, arrhythmias and liver disease, and made greater use of healthcare services associated with poor performance status. MDS patients have been reported to have an increased prevalence of chronic heart failure and arrhythmias and a higher age-adjusted risk of cardiac-related events compared to the general Medicare population,13 but it is unclear why we observed these differences in co-morbid conditions between MDS and CMML. Interestingly, CMML patients had a higher male and white predominance, which where both independently associated with increased risk of progression to AML or death.
To our knowledge, our study provides the first comparison of treatment patterns for MDS and CMML patients. Overall, CMML patients were treated less frequently, compared to MDS patients. While our database does not provide much insight into the biological characteristics of the diseases that affected decisions about timing of treatment and use of different treatment modalities, there were relatively few differences in baseline health status and economic status, factors that can affect tolerability of treatment and access to treatment, respectively. Furthermore, differences in treatment patterns remained after adjusting for baseline characteristics, with MDS patients still more frequently receiving treatment of any sort. This suggests the need to reevaluate risk assessment and treatment algorithms used for CMML.
We observed relatively low rates for some treatments in both groups. For example, use of hypomethylating agents, which have been shown to be disease-modifying,14 was infrequent in both groups. This likely reflects Food and Drug Administration approval in the latter part of our study period (azacitidine in 2004 and decitabine in 2006); we would expect to see higher treatment rates in more recent years. We observed that more CMML patients were treated with allogeneic hematopoietic stem cell transplantation, even though Medicare restricts coverage of transplantation for MDS generally.15 However, the percentages of patients receiving this therapy were very low for both diseases, likely due to the older age of these patients and less frequent use of reduced-intensity transplantation in the time period studied.
Our analysis showed that more CMML patients progressed to AML and their overall survival was shorter, consistent with the results from prior population-based studies comparing MDS and CMML.7,8 In a unique extension, our adjusted analyses showed that differences in outcomes were not explained by patients' characteristics that we could measure using the SEER-Medicare data, nor by the use of the most common treatments. While we observed different use rates for some of the less common treatments (for example, cytarabine and allogeneic stem cell transplantation), these differences would be unlikely to explain the large differences in outcomes between the two groups. Our results suggest that more aggressive biology, rather than differences in treatment, may account for the worse outcomes observed in our CMML cohort. However, we acknowledge that there might be other patients' characteristics that are important determinants of outcomes. Furthermore, while there is some information on treatment efficacy that is specific to CMML,16-19 these patients are often combined with those with MDS in therapeutic clinical trials. Our findings also highlight the need for additional clinical trials to address the efficacy of treatments specifically in CMML patients.
In our database, 35% of MDS patients were considered at lower risk (RA, RARS, RCMD, 5qdel), 15% were considered at higher risk (RAEB, t-MDS) and 50% were classified as having MDS NOS. This distribution is similar to the distributions in the NAACCR and SEER and the Veterans Administration population studies.7,8 Given the high proportion of patients who had pathological confirmation of their diagnosis, we expect that the high proportion assigned to MDS-NOS reflects coding procedures that do not emphasize recording the risk group, rather than a failure to assess that information clinically. In the survival analysis that compared CMML to all MDS patients, our reference group reflects the pooled experience of the MDS cohort, many of whom are not assigned to a specific WHO category. When we addressed this limitation by comparing survival to that of patients with lower risk MDS (RA) only, we found that patients with CMML and higher-risk MDS (RAEB) have a similar increased risk of progression to AML or death. Further studies are needed to determine whether there are differences in outcomes between MDS and CMML patients with similar risk stratification. Analysis of differences in clinical parameters in a dataset such as that used to develop the International Prognostic Scoring System could be useful, but that specific dataset excluded CMML patients with proliferative disease.9
Our study has several potential limitations. It includes an elderly population, as only 2.5% and 4.17% of CMML and MDS patients, respectively, were younger than 65 years at diagnosis, and our findings cannot necessarily be extrapolated to younger patients. Also, population-based databases such as SEER-Medicare have limitations, including underreporting,20,21 but we have no reason to think that underreporting would affect MDS differently than CMML. While, as previously noted, the lack of data on prognostic factors including cytopenias, blast percentages, and cytogenetic findings is an important limitation of the analysis, the diagnoses were confirmed by histology in 87% of all cases, adding to the accuracy of this population-based study. Delays in treatment may lead to worse outcomes, but we did not analyze time from diagnosis to first treatment because of the lack of prognostic data, as we would have been unable to determine whether delays in starting treatment were appropriate. We also did not compare untreated CMML and MDS patients because we thought that there was likely to be treatment selection based on patients' characteristics that were not captured in our database. Furthermore, given that less is known about determinants of treatment efficacy specific to CMML, we were concerned that the unobserved factors could differ for the two diseases. As a result, we did not focus on the estimated effects of treatments, but noted that their inclusion did not affect the estimated effect of CMML relative to MDS on survival. Comparison of patients with diagnoses of other MDS/myeloproliferative neoplasms would also have been of interest, but the numbers of cases did not allow for meaningful comparisons.
Even with the above-noted limitations, our study represents a unique comparison of baseline characteristics, treatments and outcomes between CMML and MDS patients. Importantly we have observed that CMML patients were less frequently treated, but also had more frequent progression to AML and shorter survival even after controlling for baseline characteristics and treatments received. Our data support the continued need to study biological differences between MDS and CMML, evaluate the prognostic scoring systems and treatment algorithms used in CMML, further assess the efficacy of existing therapies and develop new treatments for CMML.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the Pharmaceutical Research Computing Center (PRC) for data management and analytic support. This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Funding: This work was supported by NIH 1RC1CA145831-01(ARRA Challenge Grant) (Davidoff, PI) and 2K24CA111717-06A1 (Gore, PI).
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures:Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Swerdlow S.H, Campo E, Harris N.L, Jaffe E.S, Pileri S. A, Stein H, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC: Lyon: 2008 [Google Scholar]
- 2.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol. 1982;51(2):189-99 [PubMed] [Google Scholar]
- 3.Emanuel PD. Mixed myeloproliferative and myelodysplastic disorders. Curr Hematol Malig Rep. 2007;2(1):9-12 [DOI] [PubMed] [Google Scholar]
- 4.Germing U, Kundgen A, Gattermann N. Risk assessment in chronic myelomonocytic leukaemia (CMML). Leuk Lymphoma. 2004;45(7):1311-8 [DOI] [PubMed] [Google Scholar]
- 5.Onida F, Kantarjian HM, Smith TL, Ball G, Keating MJ, Estey EH, et al. Prognostic factors and scoring systems in chronic myelomonocytic leukaemia: a retrospective analysis of 213 patients. Blood. 2002;99(3):840-9 [DOI] [PubMed] [Google Scholar]
- 6.Parikh SA, Tefferi A. Chronic myelomonocytic leukemia: 2012 update on diagnosis, risk stratification, management. Am J Hematol. 2012;87(6):610-9 [DOI] [PubMed] [Google Scholar]
- 7.Rollison DE, Howlader N, Smith MT, Strom SS, Merritt WD, Ries LA, et al. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001-2004, using data from the NAACCR and SEER programs. Blood. 2008;112(1):45-52 [DOI] [PubMed] [Google Scholar]
- 8.Komrokji RS, Matacia-Murphy GM, Al Ali NH, Beg MS, Safa MM, Rollison DE, et al. Outcome of patients with myelodysplastic syndromes in the Veterans administration population. Leuk Res. 2010;34(1):59-62 [DOI] [PubMed] [Google Scholar]
- 9.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079-88 [PubMed] [Google Scholar]
- 10.Bacher U, Haferlach T, Schnittger S, Kreipe H, Kroger N. Recent advances in diagnosis, molecular pathology and therapy of chronic myelomonocytic leukaemia. Br J Haematol. 2011;153(2):149-67 [DOI] [PubMed] [Google Scholar]
- 11.Malcovati L, Porta MG, Pascutto C, Invernizzi R, Boni M, Travaglino E, et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J Clin Oncol. 2005;23(30):7594-603 [DOI] [PubMed] [Google Scholar]
- 12.Davidoff AJ, Tang M, Seal B, Edelman M. Chemotherapy and survival benefit in elderly patients with advanced non-small cell lung cancer. J Clin Oncol. 2010;28(13): 2191-7 [DOI] [PubMed] [Google Scholar]
- 13.Goldberg SL, Chen E, Corral M, Guo A, Mody-Patel N, Pecora AL, et al. Incidence and clinical complications of myelodysplastic syndromes among United States Medicare beneficiaries. J Clin Oncol. 2010;28(17):2847-52 [DOI] [PubMed] [Google Scholar]
- 14.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Glagounidis A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomized, open-label, phase III study. Lancet Oncol. 2009;10(3):223-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.http://www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?NCDId=45&ncdver=5&bc=AgAAQAAAAAAA&
- 16.Gerhartz HH, Marcus R, Delmer A, Zwierzina H, Suciu S, Dardenne M, et al. A randomized phase II study of low-dose cytosine arabinoside (LD-AraC) plus granu-locyte-macrophage colony-stimulating factor (rhGM-CSF) in myelodysplastic syndromes (MDS) with high risk of developing leukaemia. EORTC leukaemia co-operative group. Leukaemia. 1994;8(1):16-23 [PubMed] [Google Scholar]
- 17.Beran M, Estey E, O'Brien S, Cortes J, Koller CA, Giles FJ, et al. Topotecan and cytarabine is an active combination regimen in myelodysplastic syndromes and chronic myelomonocytic leukemia. J Clin Oncol. 1999;17(9):2819-30 [DOI] [PubMed] [Google Scholar]
- 18.Zang DY, Deeg HJ, Gooley T, Anderson JE, Anasetti C, Sanders J, et al. Treatment of chronic myelomonocytic leukaemia by allogeneic marrow transplantation. Br J Haematol. 2000;110(1):217-22 [DOI] [PubMed] [Google Scholar]
- 19.Braun T, Itzykson R, Renneville A, de Renzis B, Dreyfus F, Laribi K, et al. Molecular predictors of response to decitabine in advanced chronic myelomonocytic leukemia: a phase 2 trial. Blood. 2011;118(14):3824-31 [DOI] [PubMed] [Google Scholar]
- 20.Craig BM, Rollison DE, List AF, Cogle CR. Diagnostic testing, treatment, cost of care, and survival among registered and non-registered patients with myelodysplastic syndromes. Leuk Res. 2011;35(11);1453-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Craig BM, Rollison DE, List AF, Cogle CR. Underreporting of myeloid malignancies by United States cancer registries. Cancer Epidemiol Biomarkers Prev. 2012;21(3):474-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


