Abstract
There are limited treatment options for older patients with acute myeloid leukemia and prognosis of these patients remains poor, thereby warranting development of novel therapies. We evaluated the efficacy and safety of azacitidine in combination with lenalidomide as front-line therapy for older patients with acute myeloid leukemia. Patients ≥60 years of age with untreated acute myeloid leukemia received azacitidine 75 mg/m2 for 7 days followed by escalating doses of lenalidomide daily for 21 days starting on day 8 of each cycle every 6 weeks. Patients received continued therapy until disease progression, unacceptable toxicity, or completion of 12 cycles. Forty-two patients (median age, 74 years) were enrolled with equal distribution according to European LeukemiaNet risk. The overall response rate was 40% (rate of complete remission with or without complete recovery of blood counts = 28%). The median time to complete remission with or without complete recovery of blood counts was 12 weeks, and duration of this status was 28 weeks (range, 4 - >104 weeks). Therapy-related acute myeloid leukemia and a high score on the Hematopoietic Cell Transplantation Comorbidity Index were negative predictors of response. Early death was noted in 17% of patients. Grades ≥ 3 toxicities were uncommon and most adverse events were gastrointestinal, fatigue and myelosuppression. In conclusion, a sequential combination of azacitidine plus lenalidomide has clinical activity in older patients with acute myeloid leukemia, and further studies of this combination are underway. This study is registered at www.clinicaltrials.gov as # NCT00890929.
Introduction
Two-thirds of patients with acute myeloid leukemia (AML) are over 60 years old at diagnosis. Since the introduction of anthracyclines combined with cytarabine as frontline therapy1 outcomes for these patients have remained poor. Adverse biological features, which are more common in older patients, coupled with decreased tolerance to conventional chemotherapy, are the main causes of these poor outcomes.2 Alternative treatment approaches for elderly patients with AML are needed.
Pathological epigenetic modifications, including aberrant DNA methylation, likely contribute to the pathogenesis of AML.3 Aberrant DNA methylation can be pharmacologically reversed by inhibitors of DNA methyltransferase (DNMT) enzymes, and a retrospective analysis suggests that AML patients with low blast counts derive a survival benefit following treatment with the DNMT inhibitor 5-azacitidine compared to conventional care.4
Lenalidomide, an immunomodulatory agent with a distinct and non-overlapping mechanism of action,5 possesses clinical activity against several myeloid malignancies including AML. Furthermore, preclinical data suggest that lenalidomide has, at least, additive activity when combined with cytosine analogs against AML cells.6 We, therefore, aimed to determine the efficacy and safety profile of the sequential combination of azacitidine followed by lenalidomide in elderly patients with untreated AML.
Design and Methods
Patients
From April 2009 to June 2011 (phase 1 from April 2009 through July 2010 and phase 2 from July 2010 through June 2011), 45 patients met the inclusion criteria for this study and were enrolled. All met World Health Organization criteria for the diagnosis of AML other than acute promyelocytic leukemia (and all had their diagnosis confirmed at Stanford University), were 60 years or older, not eligible for or unwilling to receive conventional induction chemotherapy, and had not received lenalidomide or prior AML treatment. Upon relapse or disease progression, none of the patients received conventional induction chemotherapy (7+3-like). Patients had an Eastern Cooperative Oncology Group performance status of ≤2, creatinine <1.5 mg/dL, total bilirubin <1.5 times the upper limit of normal and transaminase levels <2.5 times the upper limit of normal. The white blood cell count was required to be <10×109/L at the initiation of treatment, but use of hydroxyurea to attain this was permitted; no patients were ultimately excluded because of refractory elevated white blood cell counts. Patients were excluded for advanced malignancies, active opportunistic infections or concomitant chemotherapy. Baseline characteristics were used to calculate the Hematopoietic Cell Transplant-Comorbidity Index (HCT-CI).7 This study was approved by the Stanford University Research Compliance Office and informed consent was obtained from all subjects involved in the study. The clinical research was conducted in accordance with the Declaration of Helsinki. The trial was registered at www.ClinicalTrials.gov as # NCT00890929.
Study design
As previously shown, the first 16 patients were enrolled in the dose-finding part of the study following a typical ‘3+3’ dose escalation schema. All patients received azacitidine 75 mg/m2/day intravenously or subcutaneously for 7 days followed by escalating doses (5 mg, 10 mg, 25 mg and 50 mg) of lenalidomide orally for 21 days starting on day 8 of each cycle. This was followed by 14 days of observation, for a total 42-day cycle.8 Patients started in each cohort could not undergo intrapatient dose escalation. Thirty patients received the maximal tolerated dose of lenalidomide 50 mg for 21 days. Patients could receive up to 12 cycles; those with a complete remission (CR) or CR with incomplete recovery of blood counts (CRi), partial remission (PR) or stable disease continued treatment beyond their best response, provided they did not experience toxicity. A bone marrow biopsy and aspirate was performed within 28 days of enrollment and was repeated to assess responses after cycles 1, 3, 6 and 12. Following 12 cycles of therapy, those patients without evidence of progression were treated at the investigator's discretion.
Toxicities and adverse events were scored according to the National Cancer Institute's Common Terminology Criteria for Adverse Events, version 3.9 A serious adverse event was an adverse event that resulted in death or immediate risk of death, prolonged hospitalization or caused substantial disability. Dose reductions were recommended for grade 3 or 4 non-hematologic toxicities. Treatment cycles were individually customized based on response and toxicity as follows: (i) subjects with evidence of persistent AML (≥5% blasts) received repeated cycles of the combination. None of these patients had dose reductions; (ii) subjects with no morphological evidence of AML (<5% blasts), received repeated cycles of the combination. For those subjects without evidence of AML but who had grade 4 neutropenia (<0.5×109/L) lasting at least 14 days, the dose of lenalidomide was reduced to the previous dosing level for the subsequent cycles. A second dose reduction was permitted if the neutropenia occurred again following the initial dose reduction. One azacitidine dose reduction was permitted if the cytopenia persisted following the initial two lenalidomide dose reductions. Similarly, for subjects without evidence of AML but who had grade 3-4 non-hematologic malignancies, the lenalidomide dose was reduced to the previous dosing level for the subsequent cycles. Hematologic toxicity was not evaluable in patients with baseline neutropenia (<0.5×109/L) or thrombocytopenia (<20×109/L); these patients were administered 42-day treatment cycles with supportive care measures as needed. For patients with an absolute neutrophil count <0.5×109/L, a non-transfused platelet count of <20×109/L at baseline or those with peripheral blood count recovery after response to therapy, dose reductions were recommended after peripheral blood count recovery before initiating the next cycle. Growth factors were allowed, but not recommended, for subjects without evidence of AML with neutropenia.
Study assessments and statistical analysis
Responses were assessed according to the European LeukemiaNet (ELN) guidelines.10 Patients who received at least one dose of azacitidine were considered evaluable. In the phase 2 part of the study we enrolled 31 patients in a two-stage design. Using a null hypothesis of a 20% overall response rate and an alternative hypothesis of a 43% overall response rate, with a significance of 5.3%, an interim analysis was performed after eight patients were evaluable. Given that five responses had been noted, the trial was not stopped for futility and 22 more patients were accrued to a total of 30 patients. This design allowed an 84% power to reject the null hypothesis if the alternative hypothesis was correct, making the probability of stopping early 50% if the null hypothesis was correct and 8% if the alternative hypothesis was correct. Cytogenetic and molecular risk stratification also followed the ELN classification.11 Duration of response was measured only in responding patients as the length of time after achievement of a response (CR, CRi or PR) during which a patient survived without evidence of disease progression. Survival was measured from the first day of azacitidine treatment to death from any cause. Kaplan-Meier analysis was used to estimate response duration and overall survival. The impact of clinical characteristics on outcomes was assessed by Wilcoxon's test for continuous data or Fisher's exact test for categorical variables and was not adjusted for multiple comparisons. Log-rank tests were used to assess differences between survival curves. All tests were two-sided, with a level of statistical significance of 0.05. Confidence intervals (CI) were established at 95%.
Processing of biological samples and genomic profiling
Bone marrow aspirates were collected into tubes containing heparin or ethylenediaminetetraacetic acid. The samples were diluted four-fold with phosphate-buffered saline before the addition of Ficoll-Hypaque (Sigma-Aldrich, St Louis, MO, USA) density gradient. After centrifugation, the plasma and mononuclear layers were collected and stored at -80°C.
Screening for mutations in FMS-like tyrosine kinase 3 internal tandem duplication (FLT3-ITD) and D835 tyrosine kinase domain (FLT3-TKD), nucleophosmin (NPM1c), CCAAT enhancer binding protein alpha (CEBPA), isocitrate dehydrogenase 1 (IDH1; R132, R100 and IDH2; R172, R140) and ten-eleven translocation 2 (TET2) was performed as previously described.8
Results
Baseline characteristics
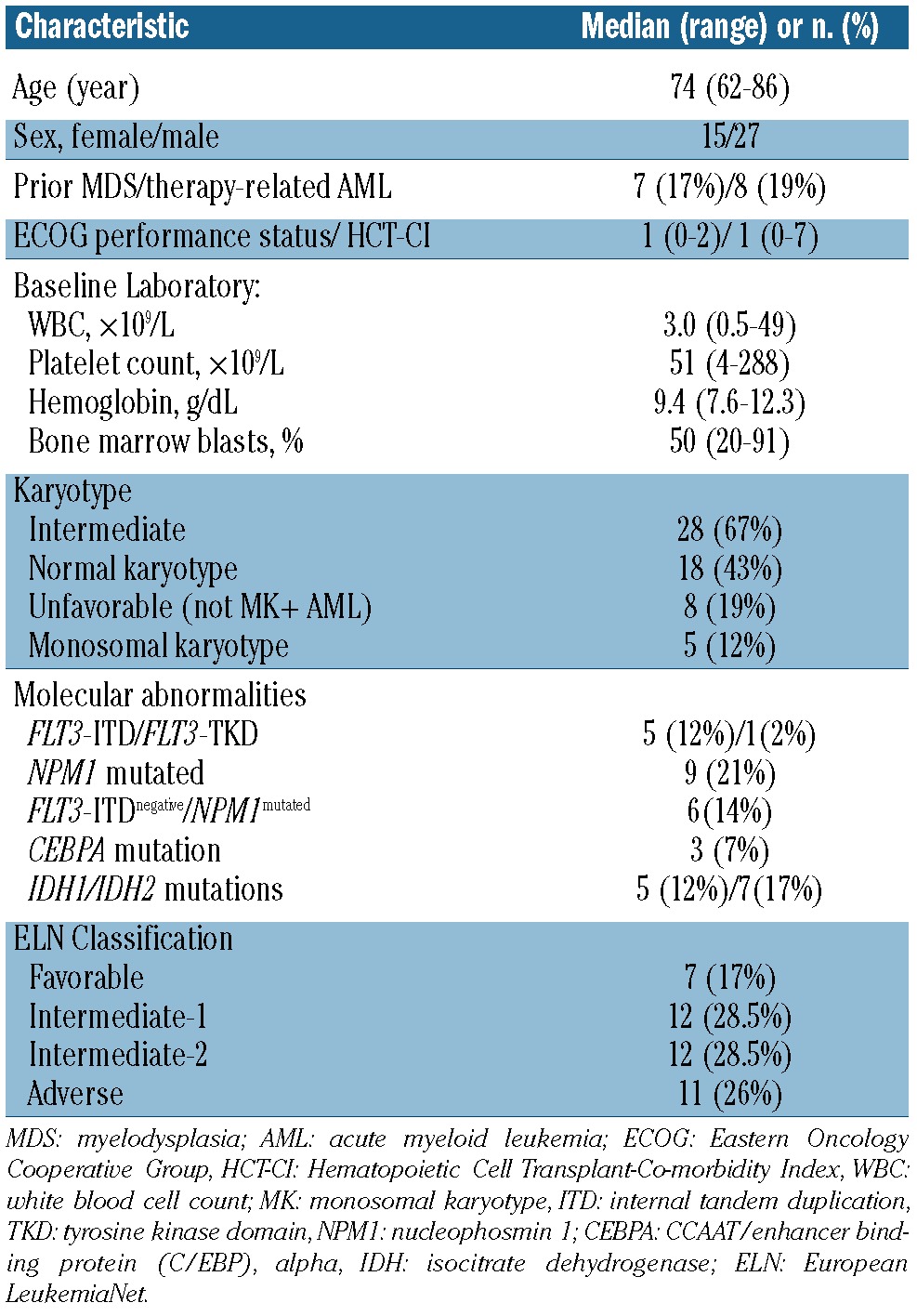
Of the 45 patients enrolled, four were excluded (one due to a diagnosis of chronic myelomonocytic leukemia-2 and three who died or withdrew consent before receiving a single dose of lenalidomide; 42 patients were therefore evaluable). The median age of the patients was 74 years (range, 62-86). Twenty-seven (66%) were male and 37 (90%) were non-Hispanic white individuals (Table 1). In 16 patients the AML was secondary: seven (17%) had a prior diagnosis of myelodysplastic syndrome and nine (22%) had therapy-related AML. The median HCT-CI was 1 (range, 0-7) and 32 (78%) patients had a performance status of 0 or 1. The median presenting white blood cell count was 3×109/L (range, 0.5 - 49×109/L), the median platelet count was 51×109/L (range, 4 - 288×109/L), and the bone marrow blast percentage was 50% (range, 20-91%). Twelve patients required prior hydroxyurea to maintain the white blood cell count 10×109/L; this treatment was given for a median of 6 days (range, 3-10 days). Five patients had received prior DNMT inhibitor therapy [azacitidine (n=4) or decitabine (n=1)] for prior myelodysplastic syndrome for a median of five cycles (range, 1-6). The remaining two patients with myelodysplastic sydndrome had previously only received growth factors. Sixteen patients were included in the phase 1 part of the study and have been previously reported;8 overall, 31 patients were treated at the maximal tolerated dose. According to the ELN classification, there were 7, 12, 12 and 11 patients in the favorable, intermediate-1, interme-diate-2 and adverse risk categories, respectively. Monosomal karyotype was noted in a lower number of patients than anticipated for this population (10%).11
Table 1.
Baseline characteristics of the study cohort (n=42).
Treatment outcomes
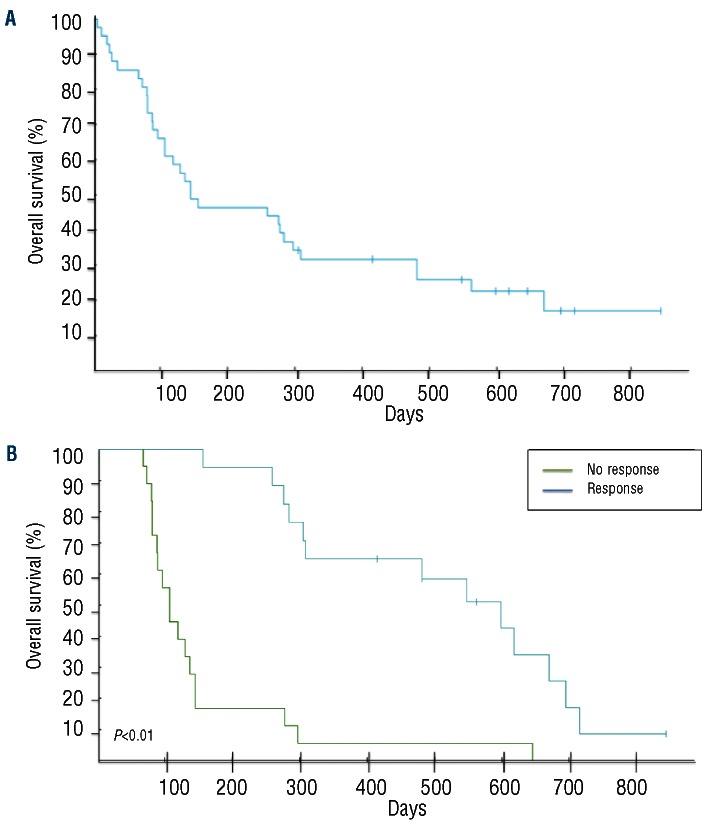
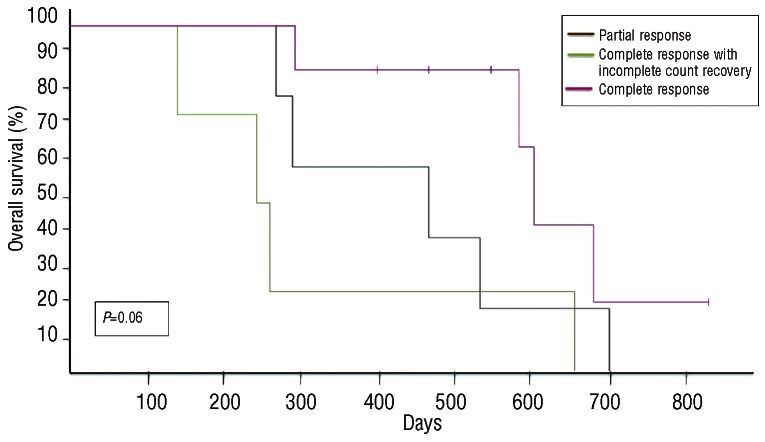
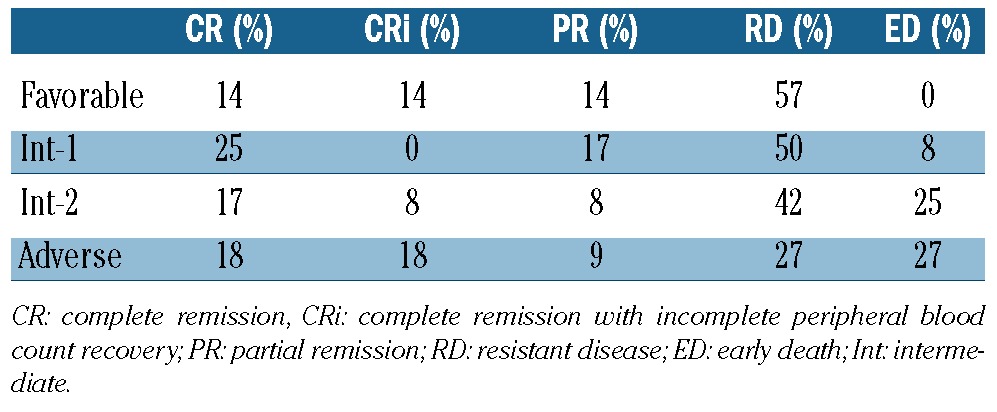
With a median follow up of 88 weeks (range, 1-120), nine (22%) patients remain alive (censored April 1st, 2012). Four patients remain on study and in first CR without evidence of disease progression, with follow-up times of 104, 71, 70 and 59 weeks. Three patients are in second CR following various salvage therapies [clofarabine followed by non-myeloablative stem cell transplantation (n=2) and temozolomide12 (n=1)], and two patients are receiving supportive care. The overall response rate (CR+CRi+PR) was 41% (CR = 19%, CRi = 9% and PR = 12%). The median time to CR/CRi was 12 weeks (range, 6-18) while that to PR was 6 weeks (range, 6-36). No differences in the overall response rate (P=0.95) or CR/CRi (P=0.92) were noted according to ELN classification. The median duration of response was 28 weeks (range, 6-104+). Eighteen (44%) patients had resistant disease while 13 of these patients (72%) had a decrease in bone marrow blasts. The median overall survival was 20 weeks (range, 1-121+) for the entire cohort and 69 weeks (range, 10-121+) for responders (Figure 1); responders had a better overall survival compared to non-responders (69 versus 15 weeks, P<0.01) (Figure 1B). Although the total number of responders is small, the overall survival did not differ between patients with a PR versus a CR/CRi (P=0.06) (Figure 2), or in those with resistant disease with or without blast reduction (P=0.12) or based on the presence of cytogenetic abnormalities (P=0.75) (data not shown). When analyzed according to ELN classification, patients with ELN adverse risk had a better median survival (38.5 weeks) compared to those in other categories (21, 32.5 and 17 weeks for favorable, intermediate-1 and intermediate-2, respectively) (P=0.012) (data not shown). Early death, defined as death within 28 days from the start of treatment13 occurred in seven subjects (17%), and in all cases was due to infectious complications or disease progression (Table 2). There were no grade 4 or 5 adverse events related to treatment.
Figure 1.
(A) Kaplan-Meier survival plot of untreated AML patients older than 60 years of age treated with sequential azacitidine plus lenalidomide. (B) Kaplan-Meier survival plot according to response in patients treated with sequential azacitidine plus lenalidomide.
Figure 2.
Kaplan-Meier survival plot of untreated AML patients older than 60 years of age treated with sequential azacitidine plus lenalidomide stratified by response.
Table 2.
Correlation between baseline ELN classification and response (n=42).
Adverse events and dose modifications
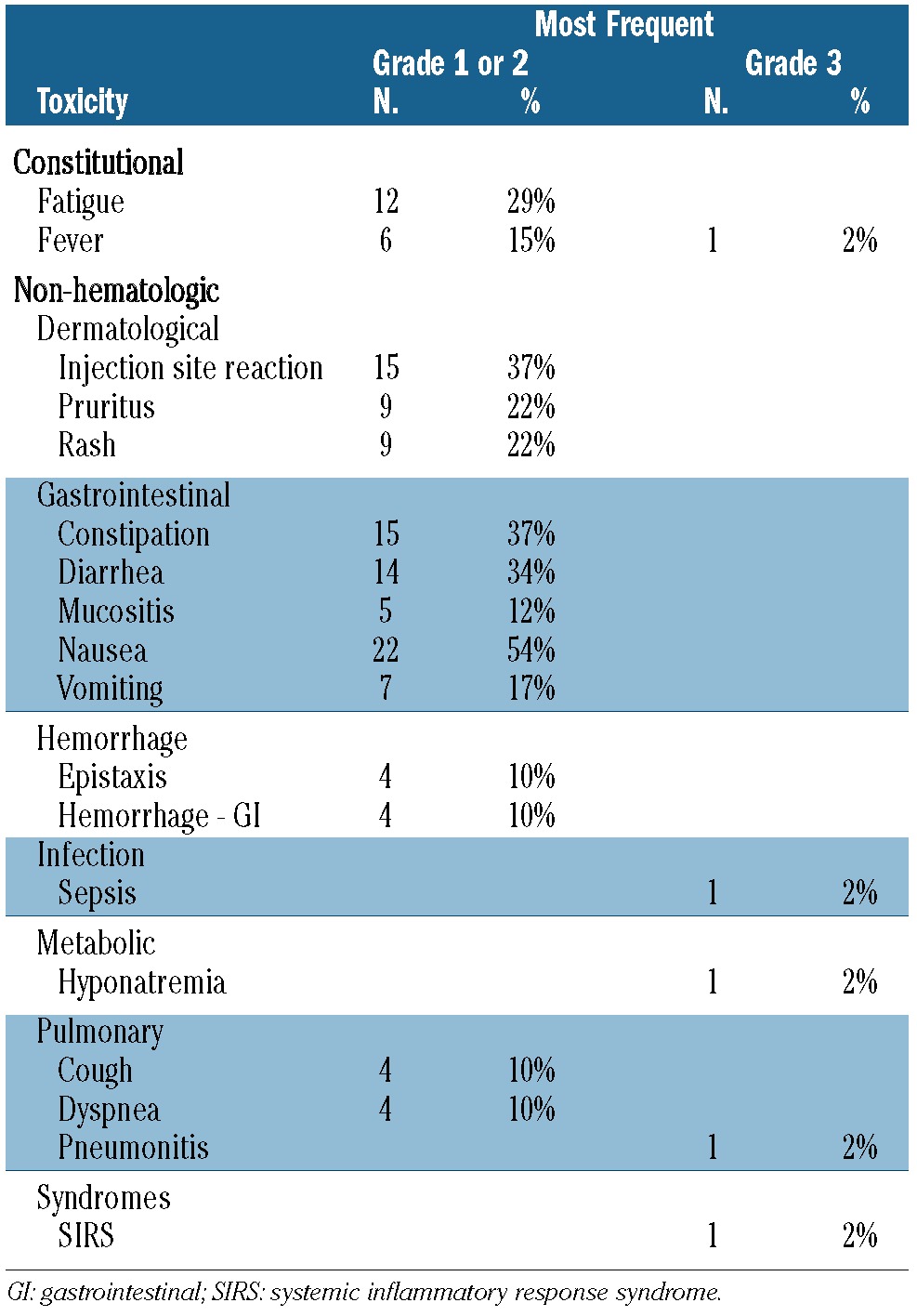
Overall, 196 cycles of the current combination of drugs were administered to 42 patients (mean 4.7 cycles/patient). The most common reason for removal from the study was disease progression (63%), followed by infectious complications in the absence of responses (22%). No patients were removed from study because of treatment-related toxicities. Table 3 summarizes the associated adverse events. Most adverse events were grade ≤2 with vomiting, reaction at the injection site and constipation being the most common toxicities. Grade 3 adverse events were relatively uncommon. After patients experienced responses, infectious complications or fevers were uncommon. Reductions of the lenalidomide dose due to neutropenia (45%), fatigue (27%) or rash (18%) were necessary in 11/17 responding patients after a median of 10, 5 and 2.5 cycles, respectively. Only one patient required an azacitidine dose reduction due to recurrent grade 4 neutropenia after five cycles. Deep venous thrombosis with an associated pulmonary embolism was noted in one patient upon platelet count recovery after achievement of first CR.
Table 3.
Drug-related adverse experiences (N=42).
Molecular and clinical predictors of response
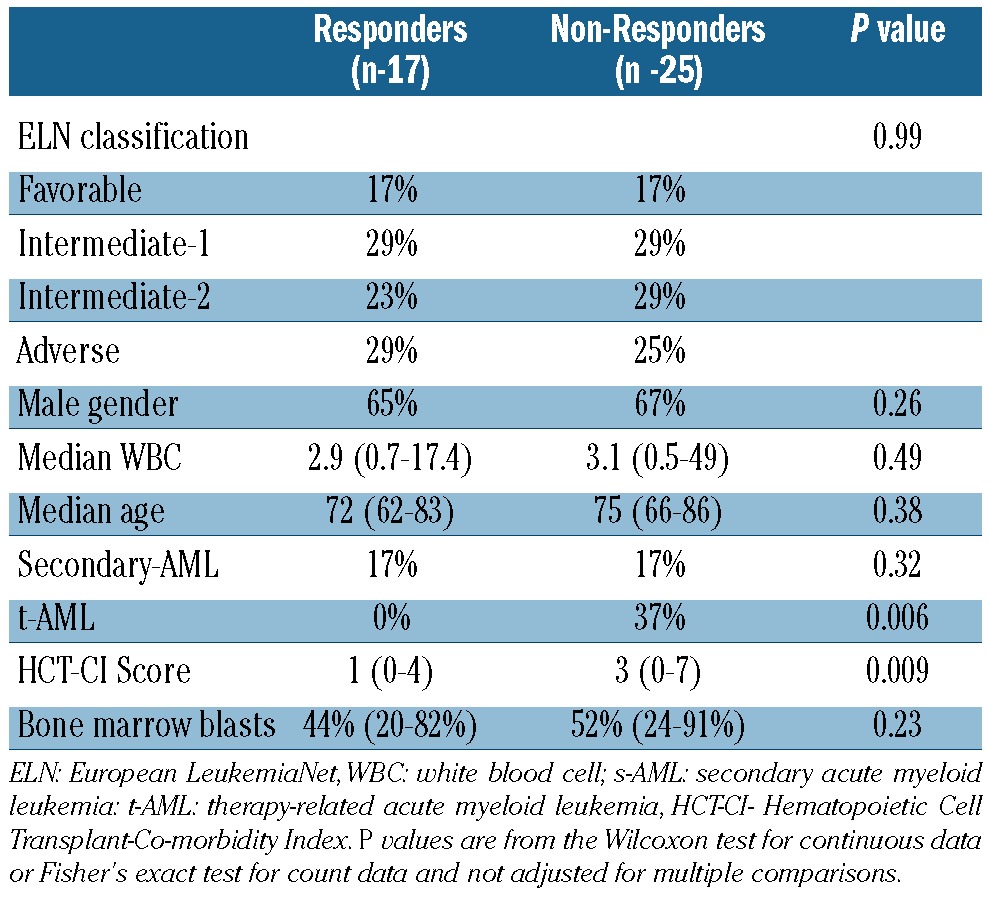
Baseline features associated with clinical responses were lower HCT-CI scores and de novo AML. No correlations between age at diagnosis, baseline white blood cell count, bone marrow blast percentage or ELN risk classification were noted (Table 4). Molecular abnormalities stratified by response demonstrated no associations between responses and recurrent genomic abnormalities (all P values >0.05) (data not shown).
Table 4.
Patients' characteristics and response to treatment.
Discussion
Population-based studies and clinical trials have consistently demonstrated that the outcomes for older patients with newly diagnosed AML receiving standard induction chemotherapy have not improved over the last five decades.14,15,16 The optimal treatment for these patients requires the consideration of multiple factors, such as patient-related and disease-related factors, which can help to determine the appropriate therapy. Both azacitidine and lenalidomide have single-agent activity in patients older than 60 years with untreated AML and have non-overlapping mechanisms of action that may complement each other.4,17 Our results suggest that sequential administration of azacitidine and lenalidomide is an outpatient-based alternative treatment option for untreated elderly AML patients, is associated with a higher overall response rate than seen with either agent alone, and is relatively well tolerated.
This treatment schedule is unique in several ways. First, both agents are given sequentially near the previously established maximal tolerated dose with minimal increase in toxicity and possibly increased efficacy. Preclinical data suggest that sequential administration of lenalidomide augmented the cytotoxic effects of the cytidine analog cytarabine, while concomitant exposure to these agents had antagonistic effects,6 although this observation was not confirmed in two recent clinical studies.18 Second, we used a different dose intensity schedule for lenalidomide (50 mg per os x 21 days given every 6 weeks) than previously reported.17,19 The maximal tolerated dose of lenalidomide was 50 mg daily for 21 out of every 28 days and most patients discontinued treatment due to disease progression after one cycle.20 A follow-up study using high-dose lenalidomide (50 mg daily) followed by maintenance doses (10 mg daily) had less than one-third of patients starting the third cycle of therapy.18 Single agent lenalidomide had minimal clinical activity and patients with AML with del(5q) had a short survival.20 In comparison, our patients received a median of about five cycles of treatment (∼30 weeks) with no dose reductions in 35% of the patients.
In AML, genomic abnormalities are likely the fundamental determinants of response to chemotherapy and survival.10,21 In fact, the recently proposed ELN risk classification is a robust prognostic method for relapse-free and overall survival.22 Remarkably, following the combination of azacitidine plus lenalidomide, the ELN risk classification had a minimal impact on outcomes, as patients with adverse risk genomic abnormalities had similar responses to those of lower-risk patients. These findings suggest that unknown genomic abnormalities mediate treatment sensitivity in these patients, a possibility reinforced by a recent report which proposed that mutations in DNMT3A may be the best predictor of response for patients treated with DNMT inhibitors.23 In addition, work previously reported by our group suggests that a specific methylation profile may predict benefit from this combination.8
Several questions remain. First, does sequential combination improve survival compared to either drug given alone? Should the combination be reserved for those patients not responding to single-agent therapy? Ongoing clinical trials have been designed to help answer these questions in patients with untreated AML, in patients with relapsed/refractory AML and in patients in whom initial DNMT inhibitor therapy failed. Here we show that azacitidine followed by lenalidomide can be safely given to elderly patients with untreated AML, and there is sufficient clinical activity to warrant further investigation, especially in those patients with a high risk of early death and those with adverse risk cytogenetic abnormalities.
Supplementary Material
Funding
This study was supported by a research funding from Celgene who provided lenalidomide, but did not participate in the design and conduct of the study, collection, management, analysis or interpretation of the data, or preparation or approval of the manuscript. DAP was supported by a Leukemia and Lymphoma Society Career Development Award, an ASCO Young Investigator Award, an ASH research training award and a NIH/NCRR CTSA KL2 award #RR025743.
Acknowledgments
The authors would like to thank the Stanford Oncore, Stanford Cancer Center Clinical Trials Office and Hematology Tissue bank for their support and assistance.
Footnotes
Authorship and Disclosures:Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Tefferi A, Letendre L. Going beyond 7 + 3 regimens in the treatment of adult acute myeloid leukemia. J Clin Oncol. 2012;30 (20):242558. [DOI] [PubMed] [Google Scholar]
- 2.Pollyea DA, Kohrt HE, Medeiros BC. Acute myeloid leukaemia in the elderly: a review. Br J Haematol. 2011;152(5):524-42 [DOI] [PubMed] [Google Scholar]
- 3.Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hyper-methylation. N Engl J Med. 2003;349(21): 2042-54 [DOI] [PubMed] [Google Scholar]
- 4.Fenaux P, Mufti GJ, Hellström-Lindberg E, Santini V, Gattermann N, Germing U, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28(4):562-9 [DOI] [PubMed] [Google Scholar]
- 5.Sekeres MA, List A F,, Cuthbertson D, Paquette R, Ganetzky R, Latham D, et al. Phase I combination trial of lenalidomide and azacitidine in patients with higher-risk myelodysplastic syndromes. J Clin Oncol. 2010;28(13):2253-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lancet JE, Komrokji RS, Yu D, Advani AS, Searles T, Sekeres MA, et al. A phase 1 study of sequential idarubicin + cytarabine, followed by lenalidomide, in patients with previously untreated acute myeloid leukemia (AML). Blood (ASH Annual Meeting Abstracts) 2011;118: Abstract 2600. [Google Scholar]
- 7.Sorror ML, Giralt S, Sandmaier BM, De Lima M, Shahjahan M, Maloney DG, et al. Hematopoietic cell transplantation specific comorbidity index as an outcome predictor for patients with acute myeloid leukemia in first remission: combined FHCRC and MDACC experiences. Blood. 2007;110(13): 4606-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollyea DA, Kohrt HE, Gallegos L, Figueroa ME, Abdel-Wahab O, Zhang B, et al. Safety, efficacy and biological predictors of response to sequential azacitidine and lenalidomide for elderly patients with acute myeloid leukemia. Leukemia. 2012;26(5): 893-901 [DOI] [PubMed] [Google Scholar]
- 9.National Cancer Institute Common Terminology Criteria for Adverse Events v3.0 (CTCAE). Bethesda, MD: National Cancer Institute, 2006. Available at http://ctep.info.nih.gov/reporting/ctc.html
- 10.Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453-74 [DOI] [PubMed] [Google Scholar]
- 11.Medeiros BC, Othus M, Fang M, Roulston D, Appelbaum FR. Prognostic impact of monosomal karyotype in young adult and elderly acute myeloid leukemia: the Southwest Oncology Group (SWOG) experience. Blood. 2010;116(13):2224-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medeiros BC, Kohrt HE, Gotlib J, Coutre SE, Zhang B, Arber DA, et al. Tailored temo-zolomide therapy according to MGMT methylation status for elderly patients with acute myeloid leukemia. Am J Hematol. 2012;87(1):45-50 [DOI] [PubMed] [Google Scholar]
- 13.Walter RB, Othus M, Borthakur G, Ravandi F, Cortes JE, Pierce SA, et al. Prediction of early death after induction therapy for newly diagnosed acute myeloid leukemia with pretreatment risk scores: a novel paradigm for treatment assignment. J Clin Oncol. 2011;29(33):4417-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juliusson G, Antunovic P, Derolf A. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113(18):4179-87 [DOI] [PubMed] [Google Scholar]
- 15.Kantarjian H, O'Brien S. Questions regarding frontline therapy of acute myeloid leukemia. Cancer. 2010;116(21):4896-901 [DOI] [PubMed] [Google Scholar]
- 16.Burnett AK, Milligan D, Goldstone A, Prentice A, McMullin M F,, Dennis M, et al. The impact of dose escalation and resistance modulation in older patients with acute myeloid leukaemia and high risk myelodysplastic syndrome: the results of the LRF AML14 trial. Br J Haematol. 2009;145(3): 318-32 [DOI] [PubMed] [Google Scholar]
- 17.Fehniger TA, Uy GL, Trinkaus K, Nelson AD, Demland J, Abboud CN, et al. A phase 2 study of high-dose lenalidomide as initial therapy for older patients with acute myeloid leukemia. Blood. 2011;117(6): 1828-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scherman E, Malak S, Perot C, Gorin NC, Rubio MT, Isnard F. Interest of the association azacitidine-lenalidomide as frontline therapy in high-risk myelodysplasia or acute myeloid leukemia with complex karyotype. Leukemia. 2012;26(4):822-4 [DOI] [PubMed] [Google Scholar]
- 19.Blum W, Garzon R, Klisovic RB, Schwind S, Walker A, Geyer S, et al. Clinical response and miR-29b predictive significance in older AML patients treated with a 10-day schedule of decitabine. Proc Natl Acad Sci USA. 2010;107(16):7473-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sekeres MA, Gundacker H, Lancet J, Advani A, Petersdorf S, Liesveld J, et al. A phase 2 study of lenalidomide monothera-py in patients with deletion 5q acute myeloid leukemia: Southwest Oncology Group Study S0605. Blood. 2011;118(3): 523-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marcucci G, Haferlach T, Döhner H. Molecular genetics of adult acute myeloid leukemia: prognostic and therapeutic implications. J Clin Oncol. 2011;29(5):475-86 [DOI] [PubMed] [Google Scholar]
- 22.Röllig C, Bornhäuser M, Thiede C, Taube F, Kramer M, Mohr B, et al. Long-term prognosis of acute myeloid leukemia according to the new genetic risk classification of the European LeukemiaNet recommendations: evaluation of the proposed reporting system. J Clin Oncol. 2011;29(20):2758-65 [DOI] [PubMed] [Google Scholar]
- 23.Metzeler KH, Walker A, Geyer S, Garzon R, Klisovic RB, Bloomfield CD, et al. DNMT3A mutations and response to the hypomethy-lating agent decitabine in acute myeloid leukemia. Leukemia. 2012;26(5): 1106-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.