Abstract
Defining the role of high-dose therapy with autologous stem cell rescue in the therapeutic algorithm of follicular lymphoma remains a major challenge. In contrast to the acknowledged poor outcome associated with cyclophosphamide/total body irradiation conditioning in heavily pretreated patients, the prognostic impact of the number of previous therapy lines in patients treated with the chemotherapy-only containing regimen, BEAM, is unknown. From 1997 to 2008 80 patients (41 males, 39 females; median age, 51 years; range, 31-67) received high-dose therapy with autologous stem cell rescue with BEAM for relapsed follicular lymphoma at our center. Overall survival and time-to-progression were analyzed according to the number of prior treatment lines. The median number of previous treatment lines was three, with 61% of the patients having received more than three lines (including rituximab in 47%). After a median follow-up of 76 months (range, 14-160), three patients developed secondary myelodysplastic syndrome. The 5-year overall survival rate was 71% and 5-year time-to-progression was 44%. There were no differences in time-to-progression or overall survival according to the number of previous treatment lines or episodes of disease. In conclusion, high-dose therapy with autologous stem cell rescue with BEAM appears to be equally effective in second or third remission of follicular lymphoma.
Introduction
Several studies1-3 have demonstrated a prolonged and sustained progression free-survival in patients with relapsed follicular lymphoma (FL) treated with high-dose therapy (HDT) and autologous stem cell rescue (ASCR), supporting the notion that a subset of patients with FL might be cured with HDT-ASCR. The difficulty lies in determining the best timing for this procedure, given its toxicity, especially long term.3 This task has become even more difficult since the advent of alternative therapeutic options. Maintenance with rituximab in second remission results in prolonged progression-free survival and, in some studies, improved overall survival,4-7 regardless of whether patients received rituximab as part of the salvage treatment or not. However, there is no plateau in the progression-free survival curves and hence no indication that this strategy will result in the cure of FL (this notwithstanding the impact that rituximab has made on the outcome of patients with FL). In parallel, the advent of reduced-intensity conditioning regimens has significantly decreased the treatment-related mortality associated with allogeneic transplants and has, therefore, broadened the indications for this approach, generally regarded as the only potentially curative treatment for patients with FL so far.8
The best results reported for HDT-ASCR have been achieved in patients treated in first or second remission.1,3 However, some patients might not accept the potential toxicity of this procedure so early in the course of the disease, particularly in view of the reported success of maintenance therapy with rituximab. The main reasons for proceeding to HDT-ASCR in second remission rather than at a later response are to decrease the amount of therapy received by patients prior to HDT-ASCR and to perform the procedure at a time when a good response has been achieved, which might not be possible when the subsequent recurrence happens. Patients with chemosensitive recurrent FL who received HDT with a total-body irradiation (TBI)-conditioning regimen had a significantly prolonged response duration in comparison with historical controls,1 but this was offset by an increased risk of secondary malignancies, especially secondary myelodysplastic syndromes (MDS), likely to be related (at least partially) to the use of TBI.3 This prompted some centers to abandon TBI-conditioning regimens in favor of chemotherapy-only containing regimens such as BEAM (BCNU, etoposide, cytarabine, melphalan). The number of prior treatment lines also seemed to have an impact on the outcome in patients treated with a TBI-conditioning regimen, with a shorter overall survival for patients treated beyond second response or having received three or more previous lines of treatment.1 Indeed, this provides the rationale for favoring an allogeneic transplant over HDT/ASCR in patients in third remission who have not received HDT-ASCR. It is not known, however, whether the number of previous treatment lines also impairs the outcome of patients treated with BEAM, a theoretically less leukemogenic regimen, but this information might be important to decide the best timing for such a procedure. This is relevant at a time when patients with FL who did not receive immunochemotherapy as first-line treatment are presenting with disease progression.
Design and Methods
Eligibility for high-dose therapy
Patients had to be fit (Eastern Cooperative Oncology Group score of 0-1 with normal lung function tests and normal ejection fraction) with chemosensitive disease at the time of HDT-ASCR (at least partial response) to be eligible for this procedure. The management of patients with transformed FL at St Bartholomew's hospital includes HDT-ASCR to consolidate remission at the first episode of transformation, with the exception of chemotherapy-naïve patients at transformation (i.e. patients who present histological transformation having been managed expectantly or treated only with local radiotherapy), in which case they are treated with an anthracycline-containing regimen (with rituximab since its approval) and only those with an incomplete response or progression receive HDT-ASCR.
Patients' characteristics
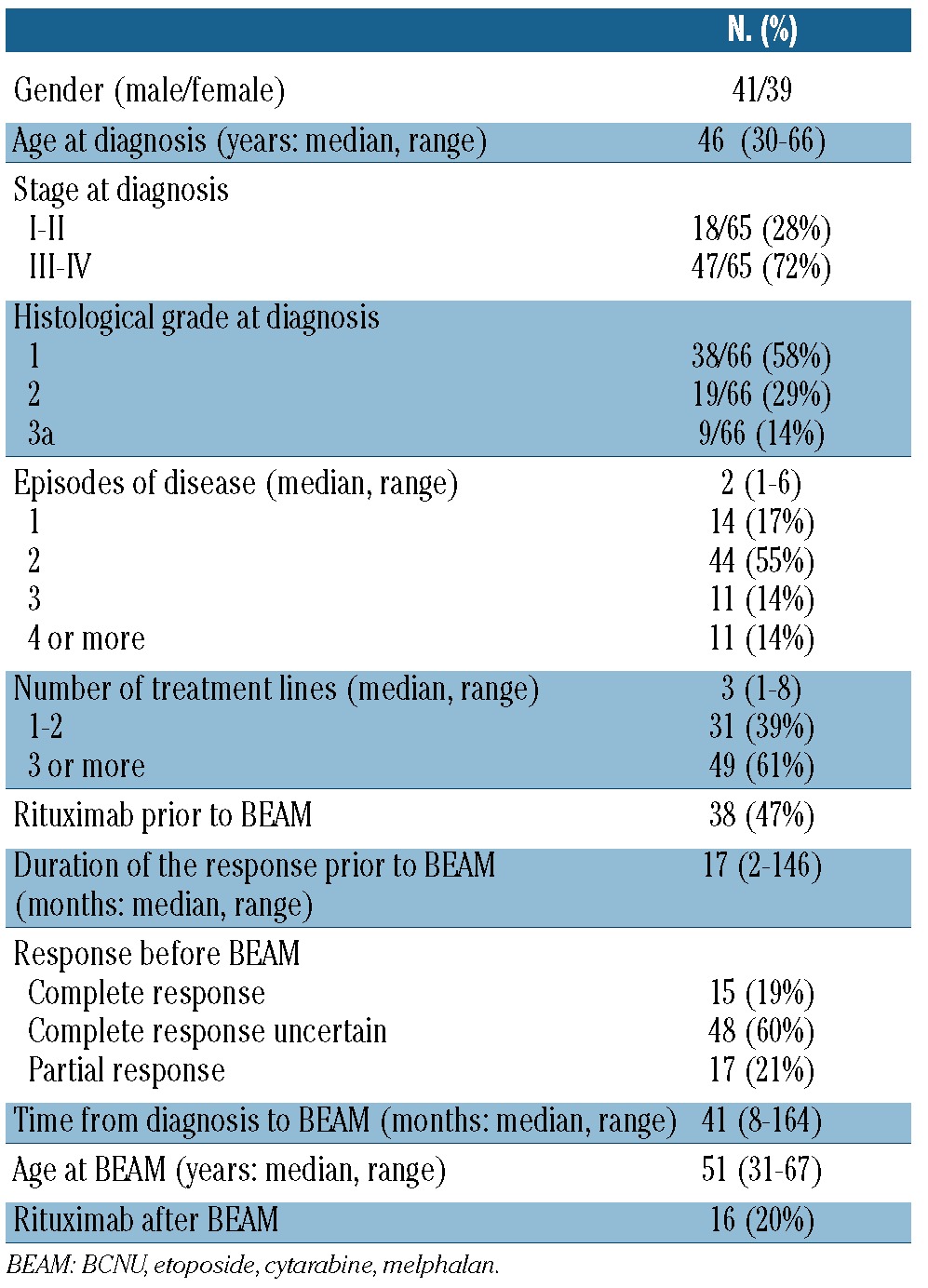
From January 1997 to September 2008, 80 patients with FL (median age, 51 years; range, 31-67) received HDT-ASCR at St Bartholomew's Hospital, with a chemotherapy-only conditioning regimen. The main characteristics of the patients are described in Table 1. Thirty patients had transformed FL diagnosed by a tissue biopsy at the episode of disease leading to HDT. High-dose therapy was administered in 14 patients (17%) in first remission: in 11 because more than one line of treatment was required to attain at least a partial response and in three because of histological transformation after initial expectant management. The remainder of the patients had HDT in second or subsequent remission. Thirty-eight patients (47%) had received rituximab prior to HDT, in one as part of the first-line therapy, in 28 as part of the salvage regimen before BEAM, and in four as part of the conditioning regimen for in vivo purging before BEAM (in the setting of a clinical trial). There was a trend toward a higher use of rituximab before BEAM in patients who had HDT for FL (56%) in comparison with those who had BEAM for transformed FL (33%, P=0.05).
Table 1.
Main characteristics of the patients.
High-dose therapy
All patients received the chemotherapy-only conditioning regimen BEAM as previously described.9 Among 46 patients for whom data were available, stem cell collection was performed after granulocyte colony-stimulating factor priming in 17 cases and after chemotherapy priming in 29 (with cyclophosphamide or cytrabine in 8 cases each, and following salvage chemotherapy with etoposide/cytarabine in 11 patients and R-ICE in 2). Following prior reports demonstrating the presence of chromosomal abnormalities pre-HDT in patients developing secondary MDS/acute myeloid leukemia,10 a triple fluorescent in-situ hybridization (FISH) assay with probes for the commonest abnormalities seen in secondary MDS/acute myeloid leukemia (5q31, 13q14 and 7q22) was implemented as part of the pre-HDT investigations from the year 2000. Thus, triple FISH analysis was performed in 38 patients and showed loss of 5q31 in one patient; the remaining 37 cases showed a diploid complement of all three probes tested. The source of stem cells was peripheral blood, with a median of 3x106/kg CD34+ cells infused (range, 0.97-27.6) in 72 patients with available data. The patients received supportive therapy according to standard protocols.
Definitions and statistical analysis
Response was categorized according to local criteria used at the time.11,12 Complete response was defined as in the consensus statement,13 ‘good partial remission’ is broadly comparable to complete response uncertain and ‘poor partial remission’ to partial response. An ‘episode’ of disease was defined as recurrence (in patients in complete remission) or progression (in patients in partial remission) after achievement of best response – irrespective of the number of lines of treatment needed. Overall survival was measured from the date of HDT to the date of last follow-up or death13 and time to progression was defined as the time from HDT until documented lymphoma progression or death as a result of lymphoma.14
Survival analysis was performed by the Kaplan-Meier15 method using the log-rank statistic16 to test for significant associations when appropriate. The risk of secondary MDS was calculated using cumulative incidence rates taking into account the competing risk structure. The median follow-up was calculated only for patients alive at the last follow up.
Informed consent and institutional review board approval was obtained to collect and store all the patients' information and perform the analyses.
Follow-up
Patients were followed-up monthly for the first 3 months, quarterly for 3 years, every 6 months for 2 years and annually thereafter. The follow up included the patient's history, physical examination, full blood count and serum biochemistry. All patients underwent surveillance investigations (comprising annual computed tomography scans and unilateral bone marrow aspirates and trephine biopsies) until 2008, when it was demonstrated that surveillance investigations did not result in improved survival in patients treated with cyclophosphamide-TBI.17 The date of relapse/progression was defined as the date of the scan or bone marrow biopsy that identified a surveillance recurrence or the date of the radiological investigation or biopsy that confirmed a clinical relapse/progression.
Results
Relapse and time to progression
After a median follow-up of 76 months (range, 14-160), disease progression has occurred in 43 patients at a median time of 12 months (range, 3-77). The median time to progression was significantly longer for patients who had FL (16 months) than for those who had transformed FL at the recurrence immediately prior to HDT (7 months, P=0.0003). A biopsy was performed at relapse after BEAM in 38 patients and demonstrated FL in 19 cases, transformed FL in 15 cases and non-Hodgkin's lymphoma (non-specified subtype) in four. Table 2 summarizes the results of the biopsies after BEAM.
Table 2.
Histology at relapse after BEAM according to the histology at the time of BEAM.
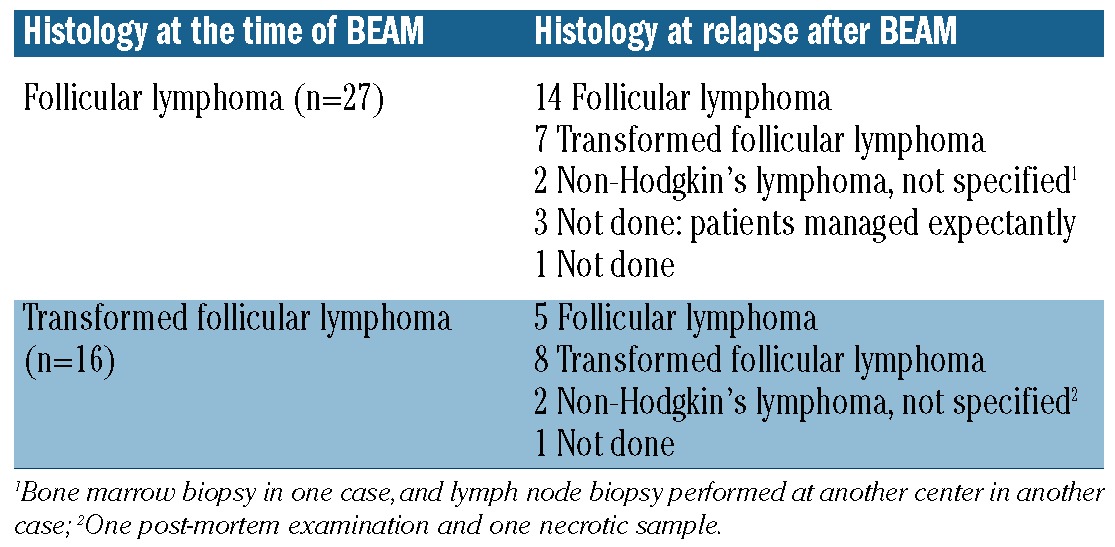
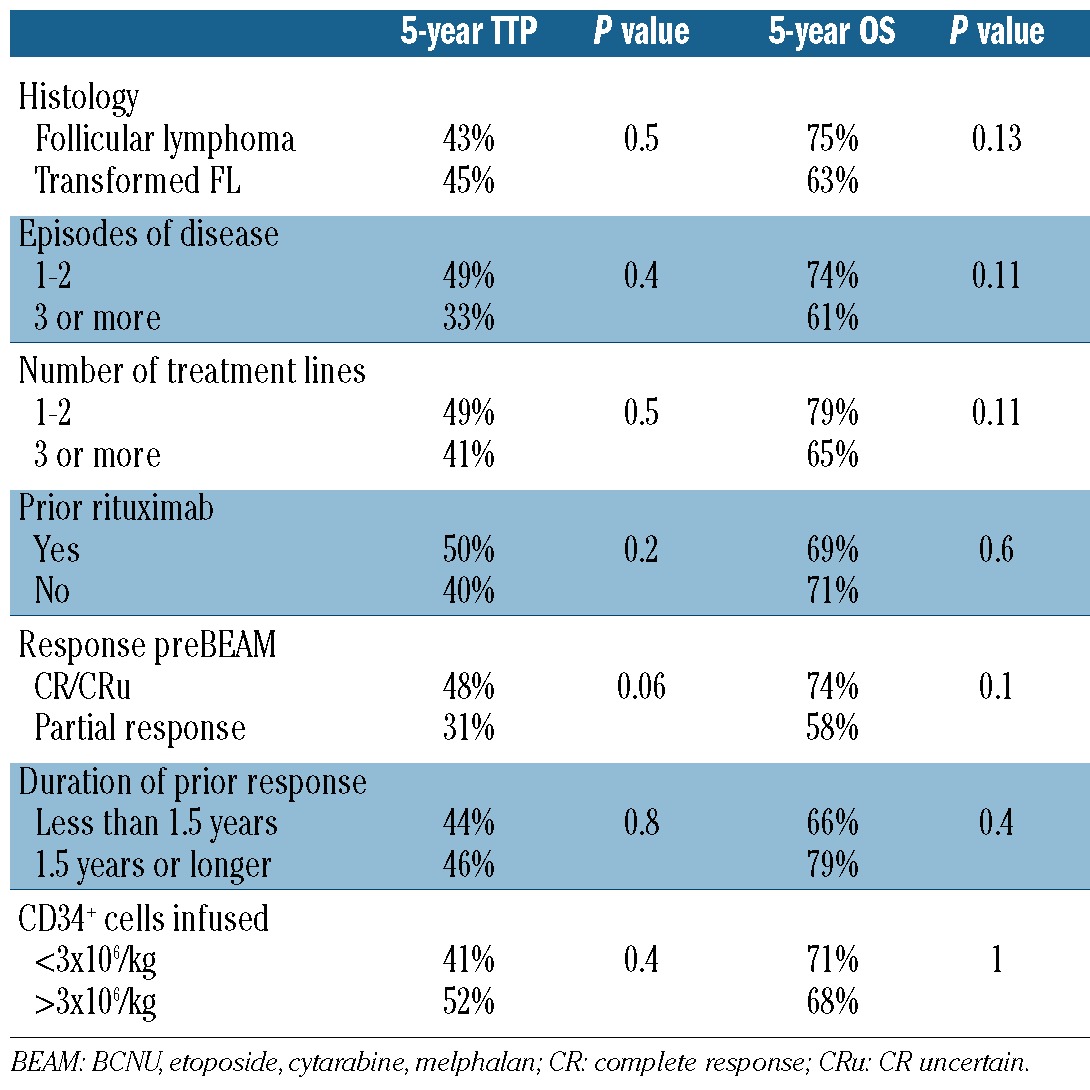
The 5-year time-to-progression was 44% [95% confidence interval (95% CI): 33-55%] (Figure 1). The 5-year time-to-progression for patients who had received one or two lines of treatment with BEAM was 49% (95% CI: 30-66%), in comparison to 41% (95%CI: 27-55%) for those who had received three or more prior lines of treatment (P=NS) (Figure 2). There were no statistically significant differences in time-to-progression according to the duration of the response prior to BEAM (5-year time-to-progression: 44% versus 46% for patients with a response lasting <1.5 years and >1.5 years, respectively, P=NS). The time-to-progression according to other potential prognostic factors is shown in Table 3.
Figure 1.
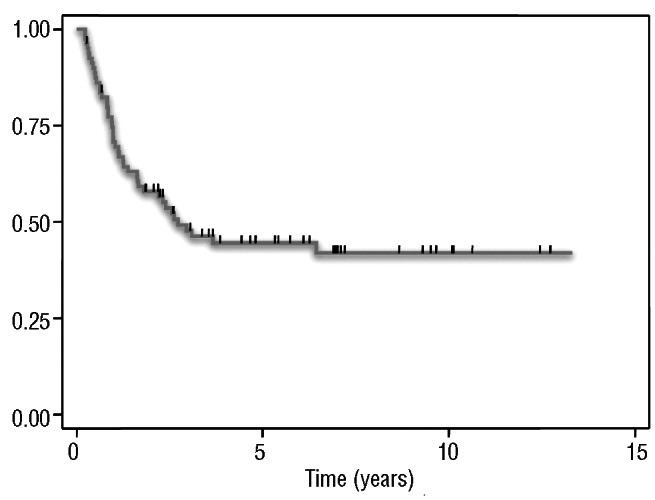
Time-to-progression in 80 patients treated with BEAM for relapsed FL.
Figure 2.
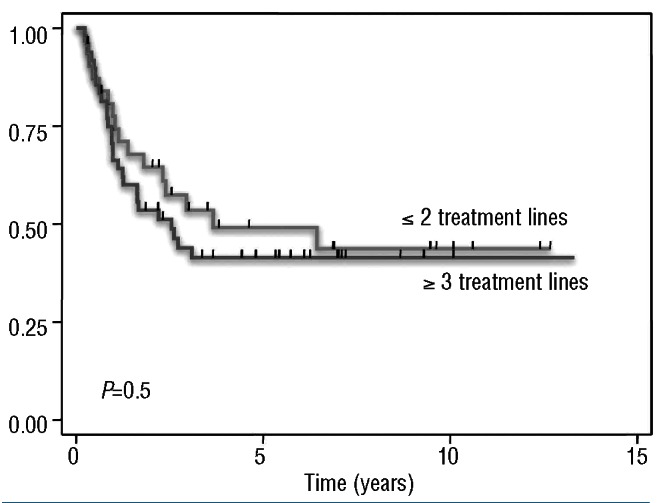
Time-to-progression according to the number of previous treatment lines in patients who received BEAM.
Table 3.
Prognostic factors for time-to-progression (TTP) and overall survival (OS).
Secondary myelodysplastic syndromes
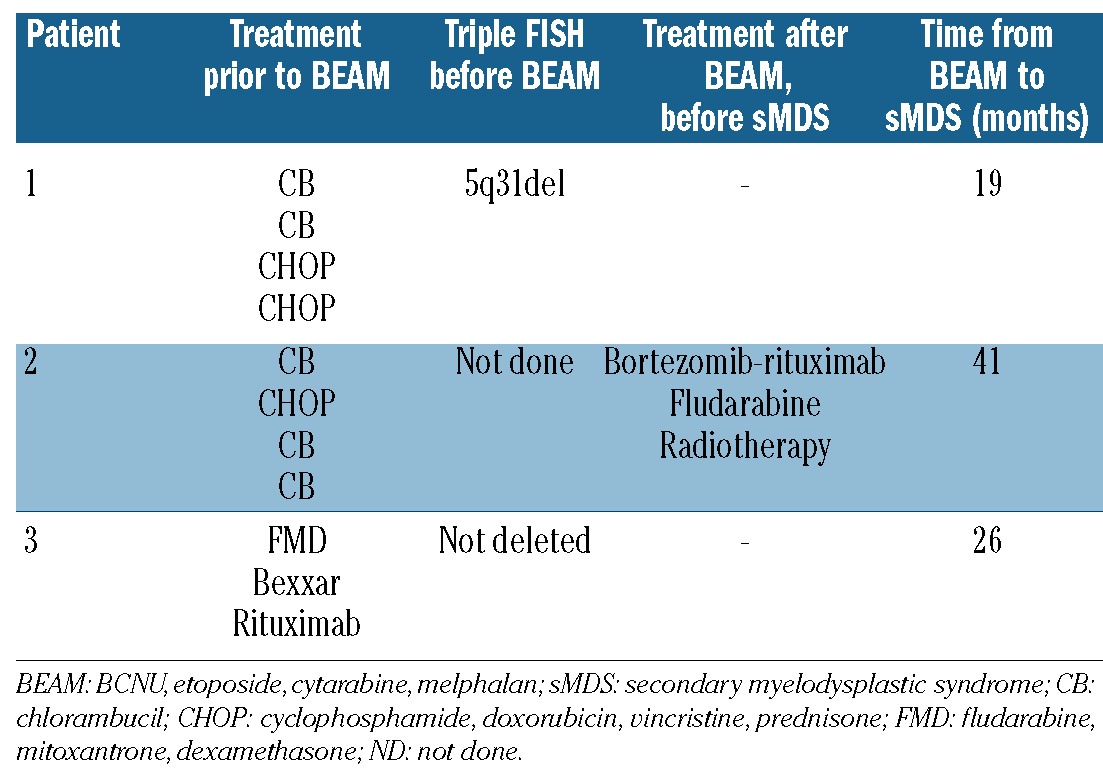
Three patients were diagnosed with secondary MDS after BEAM, the cumulative incidence of secondary MDS being 5% at 5 and 10 years. Table 4 shows the results of triple FISH pre-HDT and the treatments the patients received prior to the diagnosis of secondary MDS.
Table 4.
Triple FISH prior to HDT and treatments prior to the diagnosis of secondary myelodysplastic syndrome (sMDS)
Causes of death and overall survival
Twenty-eight patients have died at a median time of 32 months (range, 3-163) after BEAM. Among the 28 patients who have died, there was a trend towards a longer median time to death for patients who had BEAM for FL (40 months) than for patients who had BEAM for transformed FL (12 months, P=0.09). The causes of death were disease progression in 22 patients, treatment-related in one patient (pancytopenia, 3 months after HDT-ASCR), secondary MDS in two patients, and other causes in three (possible bronchiolitis obliterans in 1 case, and unknown cause in 2 cases, 22 and 113 months after their transplants).
The overall survival rate at 5 and 10 years was 71% (95% CI: 58-80) and 56% (95% CI: 40-69), respectively. The 5-year overall survival rate for patients who had received one or two lines of therapy before BEAM was 79% (95% CI: 59-90), in comparison with 65% (95% CI: 49-77%) for patients who had received three or more lines of treatment prior to BEAM (P=NS), as shown in Figure 3. Table 3 shows overall survival according to other potential risk factors.
Figure 3.
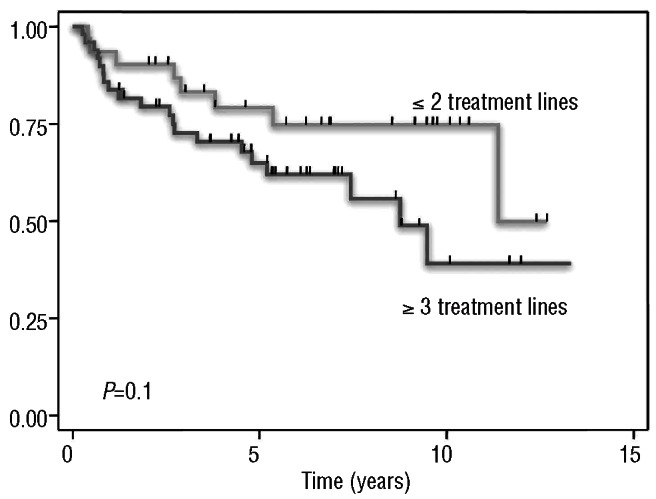
Overall survival according to the number of previous treatment lines in patients who received BEAM.
Discussion
This study confirms the promising results of HDT-ASCR in patients with chemosensitive recurrent FL with a 5-year time-to-progression of 44%. The advantage of HDT over conventional chemotherapy has been demonstrated when compared to historical controls1 and in randomized studies, both in first-line treatment18-21 and at relapse.22 Three recent retrospective studies with long follow-ups have shown that HDT in patients with relapsed FL results not only in prolonged progression free-survival but also in a sustained one,1-3 with a plateau in the progression free-survival curve, as can be seen in the present study (Figure 1), supporting the potential for cure of this approach.
However, for a disease that usually responds well to further therapy and is characterized by a prolonged survival such as FL, HDT is a relatively toxic treatment, especially with regards to long-term toxicity. Concerns about the high incidence of secondary MDS in patients receiving TBI-containing regimens were raised after reports with a long follow-up on this procedure were published.23 More recently, two randomized studies comparing HDT with conventional chemotherapy at first-line have demonstrated a significantly higher risk of secondary malignancies in patients receiving HDT (with a TBI conditioning regimen in both cases).20,24 A large registry study with a long follow-up has shown that TBI-containing regimens are associated with a higher risk of secondary malignancies, leading to a shorter overall survival.3 In addition to the type of conditioning regimen, the number of previous treatment lines3 and the type of therapy23 might also affect the risk of secondary malignancies and, subsequently, overall survival. In the current study, overall survival was not significantly different among patients who had received one or two treatment lines before BEAM and those more heavily pre-treated. In contrast, a prior study from our institution showed that a higher number of previous treatment lines correlated with a shorter overall survival in patients treated with cyclophosphamide-TBI.1 It can be hypothesized that BEAM is a less leukemogenic treatment than TBI-con-taining regimens and consequently that there is more allowance for additional treatment lines before this causes serious toxicity. In the current study too few patients had had four or more lines of treatment before BEAM to analyze the impact of this strategy on overall survival.
Notwithstanding the above, it is important to emphasize that the fact that proceeding to BEAM in third remission does not influence overall survival does not mean that the right timing for HDT in relapsed FL is in third remission: delaying BEAM until third remission carries the risk of losing patients who will not respond to the next salvage therapy or who will be too old to be considered for HDT at that time. The message is therefore not to recommend deferring HDT in patients in second response but that HDT with BEAM is safe in patients in third remission if they have not previously received HDT. Given the perceived increased risk of secondary MDS/acute myeloid leukemia following HDT with TBI in heavily pre-treated patients, it was the policy at St Bartholomew's Hospital, and other centers, to consider a reduced intensity conditioning allogeneic transplant, rather than HDT/ASCR in patients in third response. The current study supports that HDT with BEAM is safe in these circumstances. However, to safely recommend delaying HDT in patients in second response other prognostic factors should be taken into account and only patients with favorable risk factors should be offered alternative therapeutic options. In this regard, the finding that the duration of the previous response does not affect the outcome after BEAM is both reassuring and disappointing. On the one hand, patients with a short duration of response before HDT can still benefit from BEAM; on the other hand, the duration of the response before BEAM cannot be taken as an indicator of the potential success of HDT, but it should be considered in the light of other circumstances (i.e. short response duration following a rituximab-containing regimen) and prognostic factors to guide further therapy.
The authors acknowledge the limitations of this study, namely the relatively small number of patients, which might have hampered the ability of the study to detect significant differences, the follow-up not long enough to analyze the incidence of second malignancies and the heterogeneity of the study population. In this regard, the patients included in this study received HDT from 1997 onwards, when rituximab was introduced as part of the therapeutic options (in the setting of clinical trials) in the management of FL and, in fact, around half of them had received rituximab before having BEAM. This reflects the actual population of patients with relapsed FL at present in countries in which rituximab was not approved until relatively recently, still including a proportion of rituximab-naïve patients, and allows an analysis of the potential prognostic role of prior treatment with rituximab. In contrast with what has been reported in patients with aggressive lymphoma, in the current study patients with FL who had or had not received rituximab before HDT had a similar outcome after BEAM. Other series have confirmed the lack of a detrimental effect of prior rituximab before HDT for FL and have actually suggested that patients who receive both rituximab and HDT at relapse have a significantly better outcome than the remainder.25,26 This is reassuring as the population of patients candidate for HDT-ASCR who are rituximab-naïve is shrinking and most patients proceeding to HDT/ASCT have already received rituximab.
In summary, this study demonstrates that HDT with BEAM is still a safe procedure in patients in third remission of FL, as this does not compromise their outcome. In the current era of rituximab and reduced intensity conditioning regimens, the appropriate timing for HDT in relapsed FL remains to be defined. It might be that there is not an ‘appropriate time’ for HDT in FL, but that, in some specific situations or circumstances, HDT is the best option. The challenge lies in defining what these specific circumstances are. It is hoped that this question will be answered definitively by a randomized clinical trial, although we are pessimistic about this happening in the near future, given the economic costs and other significant difficulties involved. In this setting, this study adds important information that needs to be considered when offering HDT to patients with relapsed FL.
Supplementary Material
Acknowledgments
Funding
SM is kindly supported by grants from The Olivia Walduck Family and from The Mark Ridgwell Family Trust.
Footnotes
Authorship and Disclosures: Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Rohatiner AZ, Nadler L, Davies AJ, Apostolidis J, Neuberg D, Matthews J, et al. Myeloablative therapy with autologous bone marrow transplantation for follicular lymphoma at the time of second or subsequent remission: long-term follow-up. J Clin Oncol. 2007;25(18):2554-9 [DOI] [PubMed] [Google Scholar]
- 2.Kornacker M, Stumm J, Pott C, Dietrich S, Sussmilch S, Hensel M, et al. Characteristics of relapse after autologous stem-cell transplantation for follicular lymphoma: a long-term follow-up. Ann Oncol. 2009;20(4):722-8 [DOI] [PubMed] [Google Scholar]
- 3.Montoto S, Canals C, Rohatiner AZ, Taghipour G, Sureda A, Schmitz N, et al. Long-term follow-up of high-dose treatment with autologous haematopoietic progenitor cell support in 693 patients with follicular lymphoma: an EBMT registry study. Leukemia. 2007;21(11):2324-31 [DOI] [PubMed] [Google Scholar]
- 4.Ghielmini M, Schmitz SF, Cogliatti SB, Pichert G, Hummerjohann J, Waltzer U, et al. Prolonged treatment with rituximab in patients with follicular lymphoma significantly increases event-free survival and response duration compared with the standard weekly x 4 schedule. Blood. 2004;103(12):4416-23 [DOI] [PubMed] [Google Scholar]
- 5.Forstpointner R, Unterhalt M, Dreyling M, Bock HP, Repp R, Wandt H, et al. Maintenance therapy with rituximab leads to a significant prolongation of response duration after salvage therapy with a combination of rituximab, fludarabine, cyclophosphamide, and mitoxantrone (R-FCM) in patients with recurring and refractory follicular and mantle cell lymphomas: Results of a prospective randomized study of the German Low Grade Lymphoma Study Group (GLSG). Blood. 2006;108(13): 4003-8 [DOI] [PubMed] [Google Scholar]
- 6.Hochster H, Weller E, Gascoyne RD, Habermann TM, Gordon LI, Ryan T, et al. Maintenance rituximab after cyclophosphamide, vincristine, and prednisone prolongs progression-free survival in advanced indolent lymphoma: results of the randomized phase III ECOG1496 Study. J Clin Oncol. 2009;27(10):1607-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Oers MH, Van Glabbeke M, Giurgea L, Klasa R, Marcus RE, Wolf M, et al. Rituximab maintenance treatment of relapsed/resistant follicular non-Hodgkin's lymphoma: long-term outcome of the EORTC 20981 phase III randomized inter-group study. J Clin Oncol. 2010;28(17): 2853-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomson KJ, Morris EC, Milligan D, Parker AN, Hunter AE, Cook G, et al. T-cell-depleted reduced-intensity transplantation followed by donor leukocyte infusions to promote graft-versus-lymphoma activity results in excellent long-term survival in patients with multiply relapsed follicular lymphoma. J Clin Oncol. 2010;28(23): 3695-700 [DOI] [PubMed] [Google Scholar]
- 9.Ingram W, Devereux S, Das-Gupta EP, Russell NH, Haynes AP, Byrne JL, et al. Outcome of BEAM-autologous and BEAM-alemtuzumab allogeneic transplantation in relapsed advanced stage follicular lymphoma. Br J Haematol. 2008;141(2):235-43 [DOI] [PubMed] [Google Scholar]
- 10.Lillington DM, Micallef IN, Carpenter E, Neat MJ, Amess JA, Matthews J, et al. Detection of chromosome abnormalities pre-high-dose treatment in patients developing therapy-related myelodysplasia and secondary acute myelogenous leukemia after treatment for non-Hodgkin's lymphoma. J Clin Oncol. 2001;19(9):2472-81 [DOI] [PubMed] [Google Scholar]
- 11.Gallagher CJ, Gregory WM, Jones AE, Stansfeld AG, Richards MA, Dhaliwal HS, et al. Follicular lymphoma: prognostic factors for response and survival. J Clin Oncol. 1986;4(10):1470-80 [DOI] [PubMed] [Google Scholar]
- 12.Johnson PW, Rohatiner AZ, Whelan JS, Price CG, Love S, Lim J, et al. Patterns of survival in patients with recurrent follicular lymphoma: a 20-year study from a single center. J Clin Oncol. 1995;13(1):140-7 [DOI] [PubMed] [Google Scholar]
- 13.Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17(4): 1244-53 [DOI] [PubMed] [Google Scholar]
- 14.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579-86 [DOI] [PubMed] [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Am Stat Assoc. 1958;53:457-81 [Google Scholar]
- 16.Peto R, Pike MC. Conservatism of the approximation sigma (O-E)2-E in the logrank test for survival data or tumor incidence data. Biometrics. 1973;29(3):579-84 [PubMed] [Google Scholar]
- 17.Gerlinger M, Rohatiner AZ, Matthews J, Davies A, Lister TA, Montoto S. Surveillance investigations after high-dose therapy with stem cell rescue for recurrent follicular lymphoma have no impact on management. Haematologica. 2010;95(7): 1130-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenz G, Dreyling M, Schiegnitz E, Forstpointner R, Wandt H, Freund M, et al. Myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission prolongs progression-free survival in follicular lymphoma: results of a prospective, randomized trial of the German Low-Grade Lymphoma Study Group. Blood. 2004;104(9):2667-74 [DOI] [PubMed] [Google Scholar]
- 19.Sebban C, Mounier N, Brousse N, Belanger C, Brice P, Haioun C, et al. Standard chemotherapy with interferon compared with CHOP followed by high-dose therapy with autologous stem cell transplantation in untreated patients with advanced follicular lymphoma: the GELF-94 randomized study from the Groupe d'Etude des Lymphomes de l'Adulte (GELA). Blood. 2006;108(8):2540-4 [DOI] [PubMed] [Google Scholar]
- 20.Gyan E, Foussard C, Bertrand P, Michenet P, Le Gouill S, Berthou C, et al. High-dose therapy followed by autologous purged stem cell transplantation and doxorubicin-based chemotherapy in patients with advanced follicular lymphoma: a randomized multicenter study by the GOELAMS with final results after a median follow-up of 9 years. Blood. 2009;113(5):995-1001 [DOI] [PubMed] [Google Scholar]
- 21.Ladetto M, De Marco F, Benedetti F, Vitolo U, Patti C, Rambaldi A, et al. Prospective, multicenter randomized GITMO/IIL trial comparing intensive (R-HDS) versus conventional (CHOP-R) chemoimmunotherapy in high-risk follicular lymphoma at diagnosis: the superior disease control of R-HDS does not translate into an overall survival advantage. Blood. 2008;111(8):4004-13 [DOI] [PubMed] [Google Scholar]
- 22.Schouten HC, Qian W, Kvaloy S, Porcellini A, Hagberg H, Johnson HE, et al. High-dose therapy improves progression-free survival and survival in relapsed follicular non-Hodgkin's lymphoma: results from the randomized European CUP trial. J Clin Oncol. 2003;21(21):3918-27 [DOI] [PubMed] [Google Scholar]
- 23.Micallef IN, Lillington DM, Apostolidis J, Amess JA, Neat M, Matthews J, et al. Therapy-related myelodysplasia and secondary acute myelogenous leukemia after high-dose therapy with autologous hematopoietic progenitor-cell support for lymphoid malignancies. J Clin Oncol. 2000; 18(5):947-55 [DOI] [PubMed] [Google Scholar]
- 24.Lenz G, Dreyling M, Schiegnitz E, Haferlach T, Hasford J, Unterhalt M, et al. Moderate increase of secondary hematologic malignancies after myeloablative radiochemotherapy and autologous stem-cell transplantation in patients with indolent lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group. J Clin Oncol. 2004;22(24):4926-33 [DOI] [PubMed] [Google Scholar]
- 25.Sebban C, Brice P, Delarue R, Haioun C, Souleau B, Mounier N, et al. Impact of rituximab and/or high-dose therapy with autotransplant at time of relapse in patients with follicular lymphoma: a GELA study. J Clin Oncol. 2008;26(21):3614-20 [DOI] [PubMed] [Google Scholar]
- 26.Le Gouill S, De Guibert S, Planche L, Brice P, Dupuis J, Cartron G, et al. Impact of the use of autologous stem cell transplantation at first relapse both in naive and previously rituximab exposed follicular lymphoma patients treated in the GELA/GOELAMS FL2000 study. Haematologica. 2011;96(8): 1128-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


