Hosokawa et al.1 described 22 patients with myelodysplastic syndrome-unclassified (MDS-U) and del(13q) alone or in combination with other cytogenetic abnormalities. Overall, these patients showed a favorable outcome with immunosuppressive therapy and the authors proposed that del(13q) should not be categorized as intermediate risk cytogenetic abnormality in patients with bone marrow failure syndrome.
We retrospectively analyzed 353 patients diagnosed as aplastic anemia (AA) between 1973 and 2012 at our institution. For 86 patients cytogenetic analysis at any time during the disease course was available. We identified 6 patients (7%) with del(13q). Table 1 shows patients' characteristics. Median age at diagnosis was 50.5 years (range 22-71 years); 3 patients were male. One patient was diagnosed with very severe AA, 3 patients with severe AA (SAA) and 2 with non-severe AA according to Camitta et al.2 Deletion 13q was detected at diagnosis in 4 patients. One female patient (UPN 936) had a normal karyotype at diagnosis and developed del(13q) after five months and after a first course of antithymocyte globulin (ATG) and cyclosporine A (CYA). Two years later this patient relapsed and received a second course of ATG and CYA with disappearance of the del(13q) clone. She reached a partial response (PR) and remains under immunosuppression with CYA, independent of transfusions 86 months after last ATG.
Table 1.
Characteristics of patients with aplastic anemia and 13q deletion.
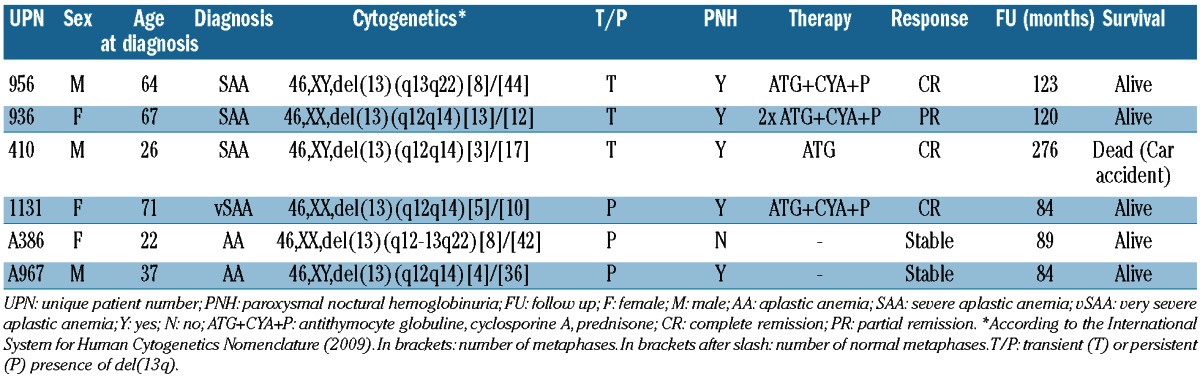
One patient diagnosed in 1987 with SAA (UPN 410) was treated with ATG in 1990; cytogenetic details at diagnosis were not available. This patient achieved a complete response. Del(13q) was documented 13 years after the diagnosis and ten years after ATG, respectively. He remained hematologically stable for 276 months until his death in a car accident.
In 2 patients, del(13q) was no longer detectable at last follow up. Interestingly, all but one patient presented a PNH clone by flow cytometry. All patients except one are still alive after a median follow up of 105 months (range 84-276 months). None of the patients showed progression to acute myeloid leukemia or MDS.
Our cohort with bone marrow failure and del(13q) shows similar characteristics and outcome as that reported by Hosokawa et al. The differential diagnosis between AA and MDS can be difficult due to overlapping morphological characteristics.3 In patients like these, and when a cytogenetic abnormality is present, proposing a diagnosis is a challenge. While cytogenetic abnormalities affecting chromosome 5 or 7 as well as complex cytogenetic abnormalities even in the absence of dysplasia favor the diagnosis of MDS, other cytogenetic findings are less conclusive. Thus, cytogenetic abnormalities in AA have been described; however, their significance and prognostic impact remains unclear.4-6 Del(13q) has been reported to occur in patients with MDS and other hematologic malignancies.7 Other groups have already reported cases of patients with bone marrow failure syndrome with del(13q) who showed good response to immunosuppressive therapy.8,9 Whether the high frequency of a PNH clone is just by chance, due to the small number of patients, or whether it represents a real characteristic of the AA with del(13q) remains an open question.
We diagnosed and treated the present cohort with bone marrow failure syndrome and del(13) as aplastic anemia. We agree with Hosokawa et al. that patients with bone marrow failure syndrome, presenting isolated del(13q) in the absence of dysplastic changes, should be better classified as aplastic anemia and treated accordingly since they represent a subgroup of patients with favorable long-term outcome.
Supplementary Material
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Hosokawa K, Katagiri T, Sugimori N, Ishiyama K, Sasaki Y, Seiki Y, et al. Favorable outcome of patients who have 13q deletion: a suggestion for revision of the WHO ‘MDS-U’ designation. Haematologica. 2012;97(12):1845-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camitta BM, Rappeport JM, Parkman R, Nathan DG. Selection of patients for bone marrow transplantation in severe aplastic anemia. Blood. 1975;45(3):355-63 [PubMed] [Google Scholar]
- 3.Rovo A, Tichelli A, Dufour C. Diagnosis of acquired aplastic anemia. Bone Marrow Transplant. Epub 2012 Nov 19. [DOI] [PubMed] [Google Scholar]
- 4.Keung YK, Pettenati MJ, Cruz JM, Powell BL, Woodruff RD, Buss DH. Bone marrow cytogenetic abnormalities of aplastic anemia. Am J Hematol. 2001;66(3):167-71 [DOI] [PubMed] [Google Scholar]
- 5.Maciejewski JP, Risitano A, Sloand EM, Nunez O, Young NS. Distinct clinical outcomes for cytogenetic abnormalities evolving from aplastic anemia. Blood. 2002;99(9):3129-35 [DOI] [PubMed] [Google Scholar]
- 6.Mikhailova N, Sessarego M, Fugazza G, Caimo A, De Filippi S, van Lint MT, et al. Cytogenetic abnormalities in patients with severe aplastic anemia. Haematologica. 1996;81(5):418-22 [PubMed] [Google Scholar]
- 7.Steensma DP, Dewald GW, Hodnefield JM, Tefferi A, Hanson CA. Clonal cytogenetic abnormalities in bone marrow specimens without clear morphologic evidence of dysplasia: a form fruste of myelodysplasia? Leuk Res. 2003;27(3):235-42 [DOI] [PubMed] [Google Scholar]
- 8.Ishiyama K, Karasawa M, Miyawaki S, Ueda Y, Noda M, Wakita A, et al. Aplastic anaemia with 13q-: a benign subset of bone marrow failure responsive to immunosuppressive therapy. Br J Haematol. 2002;117(3):747-50 [DOI] [PubMed] [Google Scholar]
- 9.Saitoh T, Saiki M, Kumagai T, Kura Y, Sawada U, Horie T. Spontaneous clinical and cytogenetic remission of aplastic anemia in a patient with del(13q). Cancer Genet Cytogenet. 2002;136(2):126-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


