Abstract
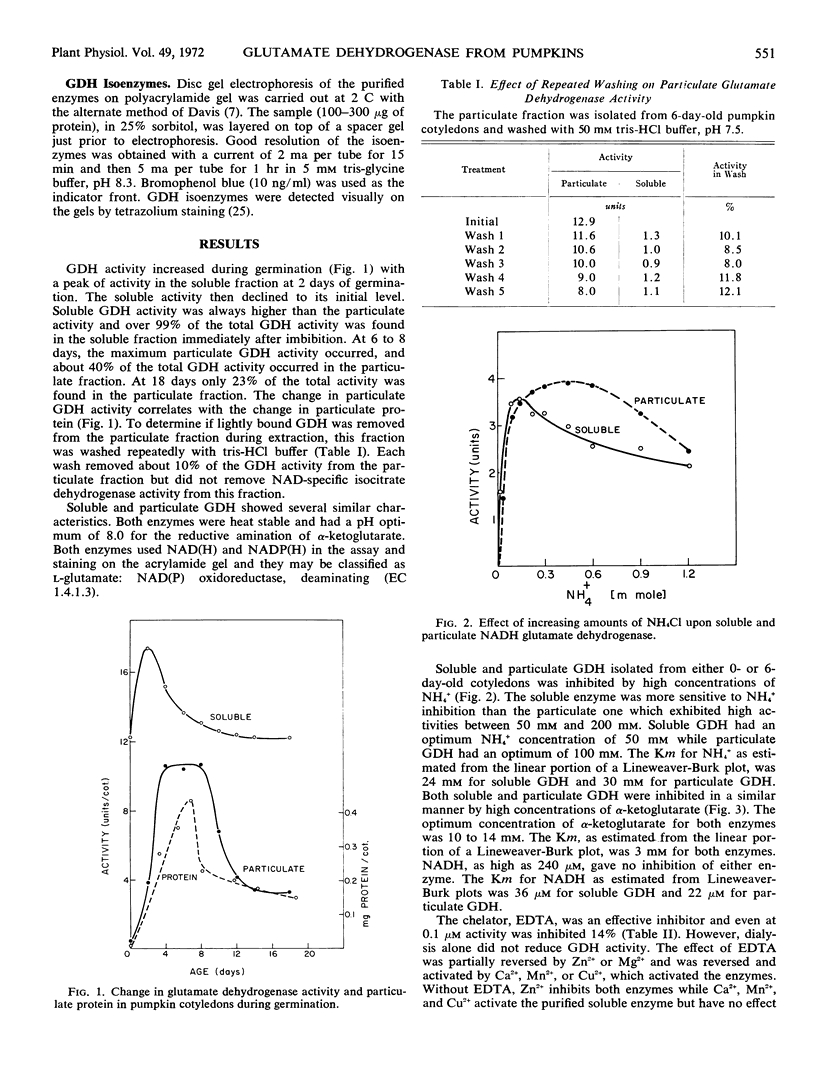
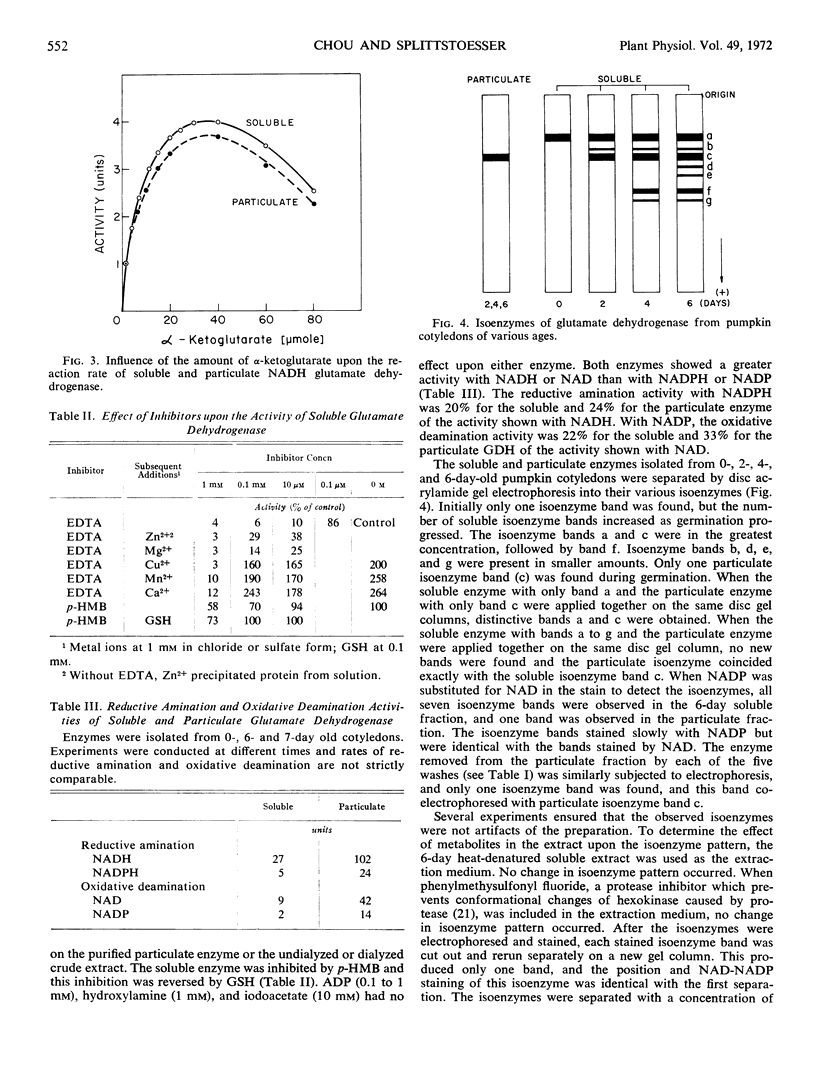
Glutamate dehydrogenase from pumpkin (Cucurbita moschata Pior. cultivar Dickinson Field) cotyledons was found in both soluble and particulate fractions with the bulk of the activity in the soluble fraction. Both enzymes used NAD(H) and NADP(H) but NAD(H) was favored. The enzymes were classified as glutamate-NAD oxidoreductase, deaminating (EC 1.4.1.3). Both enzymes were heat stable, had a pH optimum for reductive amination of 8.0, and were inhibited by high concentrations of NH4+ or α-ketoglutarate. The soluble enzyme was more sensitive to NH4+ inhibition and was activated by metal ions after ammonium sulfate fractionation while the solubilized particulate enzyme was not. Inhibition by ethylenediaminetetraacetate was restored by several divalent ions and inhibition by p-hydroxymercuribenzoate was reversed by glutathione. Particulate glutamate dehydrogenase showed a greater activity with NADP. The molecular weights of the enzymes are 250,000. Separation of the enzymes by disc gel electrophoresis showed that during germination the soluble isoenzymes increased from 1 to 7 in number, while only one particulate isoenzyme was found at any time. This particulate isoenzyme was identical with one of the soluble isoenzymes. A number of methods indicated that the soluble isoenzymes were not simply removed from the particulate fraction and that true isoenzymes were found.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARRATT R. W., STRICKLAND W. N. Purification and characterization of a TPN-specific glutamic acid dehydrogenase Neurospora crassa. Arch Biochem Biophys. 1963 Jul;102:66–76. doi: 10.1016/0003-9861(63)90321-7. [DOI] [PubMed] [Google Scholar]
- BONE D. H. Glutamic dehydrogenase of mung bean mitochondria. Nature. 1959 Sep 26;184(Suppl 13):990–990. doi: 10.1038/184990a0. [DOI] [PubMed] [Google Scholar]
- BULEN W. A. The isolation and characterization of glutamic dehydrogenase from corn leaves. Arch Biochem Biophys. 1956 May;62(1):173–183. doi: 10.1016/0003-9861(56)90100-x. [DOI] [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Mitochondria and glyoxysomes from castor bean endosperm. Enzyme constitutents and catalytic capacity. J Biol Chem. 1969 Jul 10;244(13):3507–3513. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- FRIEDEN C. GLUTAMATE DEHYDROGENASE. VI. SURVEY OF PURINE NUCLEOTIDE AND OTHER EFFECTS ON THE ENZYME FROM VARIOUS SOURCES. J Biol Chem. 1965 May;240:2028–2035. [PubMed] [Google Scholar]
- Hasson-Porath E., Poljakoff-Mayber A. Content of adenosine phosphate compounds in pea roots grown in saline media. Plant Physiol. 1971 Jan;47(1):109–113. doi: 10.1104/pp.47.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Keister D. L., Yike N. J. Energy-linked reactions in photosynthetic bacteria. II. The energy-dependent reduction of oxidized nicotinamide-adenine dinucleotide phosphate by reduced nicotinamide-adenine dinucleotide in chromatophores of Rhodospirillum rubrum. Biochemistry. 1967 Dec;6(12):3847–3857. doi: 10.1021/bi00864a031. [DOI] [PubMed] [Google Scholar]
- LéJohn H. B., Jackson S. G., Klassen G. R., Sawula R. V. Regulation of mitochondrial glutamic dehydrogenase by divalent metals, nucleotides, and alpha-ketoglutarate. Correlations between the molecular and kinetic mechanisms, and the physiological implications. J Biol Chem. 1969 Oct 10;244(19):5346–5356. [PubMed] [Google Scholar]
- OLSON J. A., ANFINSEN C. B. The crystallization and characterization of L-glutamic acid dehydrogenase. J Biol Chem. 1952 May;197(1):67–79. [PubMed] [Google Scholar]
- Pahlich E., Joy K. W. Glutamate dehydrogenase from pea roots: purification and properties of the enzyme. Can J Biochem. 1971 Jan;49(1):127–138. doi: 10.1139/o71-018. [DOI] [PubMed] [Google Scholar]
- REBEIZ C. A., CASTELFRANCO P., ENGELBRECHT A. H. FRACTIONATION AND PROPERTIES OF AN EXTRA-MITOCHONDRIAL ENZYME SYSTEM FROM PEANUTS CATALYZING THE BETA-OXIDATION OF PALMITIC ACID. Plant Physiol. 1965 Mar;40:281–286. doi: 10.1104/pp.40.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritenour G. L., Joy K. W., Bunning J., Hageman R. H. Intracellular localization of nitrate reductase, nitrite reductase, and glutamic Acid dehydrogenase in green leaf tissue. Plant Physiol. 1967 Feb;42(2):233–237. doi: 10.1104/pp.42.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze I. T., Colowick S. P. The modification of yeast hexokinases by proteases and its relationship to the dissociation of hexokinase into subunits. J Biol Chem. 1969 May 10;244(9):2306–2316. [PubMed] [Google Scholar]
- Splittstoesser W. E. Metabolism of arginine by aging and 7 day old pumpkin seedlings. Plant Physiol. 1969 Mar;44(3):361–366. doi: 10.1104/pp.44.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman D. A., Palin C., Laycock M. V. Isoenzymatic nature of L-glutamic dehydrogenase of higher plants. Nature. 1965 Jul 10;207(993):193–194. doi: 10.1038/207193a0. [DOI] [PubMed] [Google Scholar]
- Yue S. B. Isoenzymes of glutamate dehydrogenase in plants. Plant Physiol. 1969 Mar;44(3):453–457. doi: 10.1104/pp.44.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]



