Abstract
ATP-sensitive potassium (KATP ) channels were first discovered in the heart 30 years ago1. Reconstitution of KATP channel activity by coexpression of members of the pore-forming inward rectifier gene family (Kir6.1, KCNJ8, and Kir6.2 KCNJ11) with sulfonylurea receptors (SUR1, ABCC8, and SUR2, ABCC9) of the ABCC protein sub-family, has led to the elucidation of many details of channel gating and pore properties. In addition, the essential roles of Kir6.x and SURx subunits in generating cardiac and vascular KATP2 and the detrimental consequences of genetic deletions or mutations in mice have been recognised3. However, despite this extensive body of knowledge, there has been a paucity of defined roles of KATP subunits in human cardiovascular diseases, although there are reports of association of a single Kir6.1 variant with the J-wave syndrome in the electrocardiogram, and two isolated studies have reported association of loss of function mutations in SUR2 with atrial fibrillation and heart failure. Two new studies convincingly demonstrate that mutations in the SUR2 gene are associated with Cantu syndrome, a complex multi-organ disorder characterized by hypertrichosis, craniofacial dysmorphology, osteochondrodysplasia, patent ductus arteriosus, cardiomegaly, pericardial effusion, and lymphoedema. As we discuss, this realization of previously unconsidered consequences provides significant insight into the roles of the KATP channel in the cardiovascular system and suggests novel therapeutic possibilities.
Keywords: SUR2, Kir6.1, Kir6.2, Cantu, vasodilation, edema, arrhythmia
KATP channel structure and molecular regulation
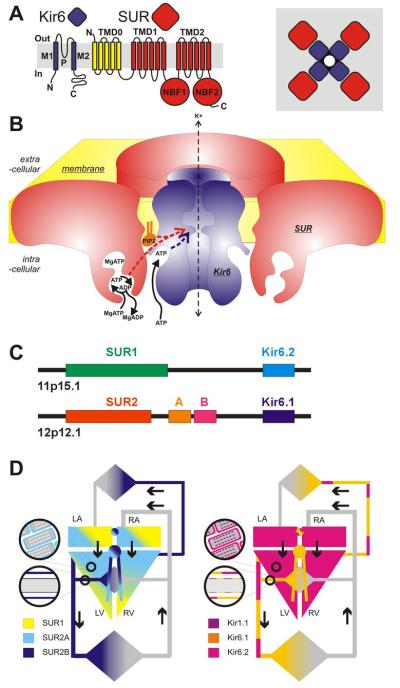
Canonical KATP channels are heterooctameric complexes of pore-forming Kir6 channel-forming subunits associated with regulatory SUR subunits, members of the ATP binding cassette (ABC) family of membrane proteins (Fig. 1). Two Kir6-encoding genes, KCNJ8 (Kir6.1) and KCNJ11 (Kir6.2)4,5, and two SUR genes, ABCC8 (SUR1) and ABCC9 (SUR2)5-7 encode mammalian KATP subunits, but alternative RNA splicing can give rise to multiple SUR protein variants (e.g. SUR2A and SUR2B) that confer distinct physiological and pharmacological properties on the channel complex8,9. Interestingly (Fig. 1C), the genes for Kir6.2 and SUR1 are located next to each other on human chromosome 11p15.15 suggesting an as yet unconsidered co-regulation at the gene level. In addition, the genes for Kir6.1 and SUR2 are also adjacent to one another on chromosome 12p12.17,10, implicating an evolutionary duplication. In heterologous expression systems, both Kir6.2 and SUR1 subunits co-assemble in a 4:4 stoichiometry5 (Fig. 1A,B) to generate the functional KATP channel11-13. Similarly, biochemical studies demonstrate that the SUR2 protein variants, SUR2A and SUR2B, can also coassemble with Kir6 subunits4,14-16, presumably in a similar octameric arrangement.
Fig. 1.
Cardiovascular KATP channels (A) Kir6 subunits generate the channel pore, SUR subunits serve the regulatory role, each channel being a functional octamer of 4 Kir6 subunits and 4 SUR subunits. (B) The metabolically controlled gate of the channel is located at the cytoplasmic end of the inner cavity. ATP binds to Kir6 subunits and this provides the energetic push to channel closure. MgATP binds to the ATP-binding sites (ABSs) formed at the NBF1-NBF2 interface on SUR subunits. ATP hydrolysis results in a conformational ‘activated’ state that is transduced to ‘over-ride’ ATP inhibition. The ‘activated state’ persists through ADP dissociation, and can be maintained by ADP rebinding. In addition, PIP2 interaction at a site near the ATP inhibitory site also provides an energetic pull to open channels, and sulfonylureas (SU) or K channel openers (KCO), interacting with the SUR subunit within the membrane, respectively cause channel closure or opening. (C) Human KATP gene structure. ABCC8 (SUR1) and KCNJ11 (Kir6.2) are immediately adjacent on chromosome 11p, whereas ABCC9 (SUR2) and KCNJ8 (Kir6.1) are immediately adjacent on chromosome 12. (D) KATP channel subunit distribution in the cardiovascular system.
Crystallographic studies of bacterial and eukaryotic Kir channels17,18] demonstrate a conserved architecture of Kir channels with two transmembrane helices (M1, M2) bridged by an extracellular loop that generates the narrow portion of the pore and controls ion selectivity (Fig. 1A). As with other ABCC family members, SURs contain two six-helix transmembrane domains, TMD1 and TMD2, but SURs also have an additional N-terminal TMD0 domain consisting of 5 transmembrane helices (Fig. 1A), critical for Kir6.x trafficking and gating19. SURs also contain one nucleotide binding fold (NBF1) between TMD1 and TMD2, and a second (NBF2) after TMD2 in the cytoplasmic loops (Fig. 1A). NBFs from bacterial ABC proteins crystallize as ‘head-to-tail’ dimers, and this is likely the functional arrangement between NBF1 and NBF2 in SUR (Fig. 1B)20. How the Kir6 and SUR subunits are physically connected remains unknown, but electron micrography and intersubunit FRET studies of complete KATP complexes suggest an intimate packing of 4 SUR and 4 Kir6.x subunits21,22 (Fig. 1A).
The key regulatory features of KATP channels are rapid and reversible closure by cytoplasmic ATP, and activation by nucleotide tri- and diphosphates20 (Fig. 1B). In the absence of other nucleotides, the free ATP concentration that causes half-maximal channel inhibition is in the micromolar range. Since cellular levels of cytosolic ATP concentration are in the millimolar range (1-5 mM) and change little with metabolism, [ATP] is probably always sufficient to almost fully inhibit channel activity. Channel activation then arises from the activating effects of Mg-nucleotides, particularly MgADP, on the SUR subunit23. Nucleotide regulation is probably the key molecular regulator of KATP channel activities, although other second messenger systems and regulators24 may be involved in control of channel activity and in causing channel-dependent pathologies.
Cardiovascular tissue distribution of KATP channel subunits
Cardiac myocytes
Kir6.1 and Kir6.2, as well as SUR2A, SUR2B and SUR1, and additional potential splice variants of SUR1 and SUR2, are all expressed in the heart4,25,26. Given that any pair of SURx:Kir6.x tetramers can co-assemble when heterologously expressed4,5, and that even within a single channel more than one SUR isoform or Kir6 isoform can coexist27-32, determining the molecular makeup of the channel in specific cell types is a challenge. There is now good evidence that in mouse hearts, SUR1 and Kir6.2 are major constituents of the atrial myocyte sarcolemmal KATP, whereas SUR2A and Kir6.2 generate ventricular KATP33,34. However, in hearts of larger animals, including humans, both SUR1 and SUR2A subunits probably contribute to sarcolemmal channels in both atrial and ventricular myocytes35 (Fig. 1D). The situation may be more complex in critical sub-regions of the heart, including nodal and conduction cells. KATP channel currents have been detected throughout the pacemaking and conduction systems36-38, but KATP single channel conductances in rabbit SA node cells and mouse conduction cells may be smaller than in ventricular myocytes36. This suggests a possible role for Kir6.1 in generating the channel pore, yet sarcolemmal KATP is abolished in Kir6.2−/− SA node cells39 indicating a necessary requirement for Kir6.2. The identity of the SUR component of KATP in these tissues is unknown, although KATP channels in these cell types do respond to the relatively SUR2-specific openers cromakalim and pinacidil, suggesting a major role for SUR2 in nodal KATP channels36-38.
Smooth muscle myocytes
KATP channel density is relatively low in vascular smooth muscle (VSM) compared to cardiac myocytes40,41 and the biophysical and the pharmacological properties are quite variable, reflecting variable expression of KATP subtypes in vascular beds42-49. There is considerable variation in reported single channel conductances45,46,50-54, although low-conductance channels (unitary conductances from 20-50 pS) may represent the predominant KATP channel subtype, with a more limited distribution of medium- and high conductance KATP channels (50-70 pS and >200 pS, respectively)55. Importantly, and unlike classic KATP channels of the heart4,56 or pancreas5,57, the predominant VSM KATP conductances are inactive in isolated membrane patches, and require nucleotide diphosphates (ADP, UDP, GDP) in the presence of Mg 2+ to open, leading to their functional designation as ‘nucleotide-dependent’ K+-channels, or KNDP channels47,48,53. Heterologously expressed Kir6.1/SUR2B channels recapitulate many of these biophysical properties of native VSM KATP/KNDP14,58-62. A subpopulation of VSM KATP in portal vein exhibits spontaneous activity in excised membrane patches, and displays high sensitivity to inhibitory ATP (K1/2 ATP = ~20 μM), and higher unitary conductance, reminiscent of Kir6.2/SUR2A-dependent KATP channels1,53,54,63. Thus the Kir6.1/SUR2B channel may represent the predominant VSM KATP, but other subtypes are also likely to be expressed in specific vascular beds, separately or in combination with Kir6.1/SUR2B subunits53 (Fig. 1D).
Vascular endothelium
KATP channels are also present in vascular endothelium64 and, by regulating endothelial electrical activity, they may affect release of vasoactive agents that in turn modulate smooth muscle function. Activation by KCOs and inhibition by glibenclamide has been demonstrated in coronary endothelium65 and in aortic endothelial cells66,67. The molecular composition of endothelial KATP channels remains largely unknown, but the presence of Kir6.1, Kir6.2, and SUR2B mRNA in guinea pig68 and in human coronary artery endothelial cells65 suggests that all three subunits may be involved in channel generation in these cells.
Mitochondrial KATP
A K+-selective, small conductance channel was first identified in rat liver mitochondria69, and reported to be reversibly inhibited by application of ATP, glibenclamide, and 4-aminopyridine (4-AP). These ‘mitoKATP’ channels were inhibited by acyl-coA and activated by GTP, GDP and diazoxide70,71. The pharmacology of heterologously expressed SUR1/Kir6.1 complexes appears to most closely resemble such properties72,73, yet ‘mitoKATP’ function is apparently unaffected in both Kir6.1−/− and Kir6.2−/− animals26,74 and efforts to determine whether specific SUR or Kir6 subunits are normally present in mitochondria have yielded inconsistent results73,75-79.
Chutkow et al. generated a SUR2 ‘knockout’ mouse in which the first nucleotide binding fold of SUR2 was disrupted by deletion of exons 12-1680. Experiments on these SUR2−/− mice revealed novel glibenclamide-insensitive channels in isolated sarcolemmal membrane patches, and antibodies raised against specific regions of the SUR2 protein suggested that the novel channels are formed of short SUR2 constructs that lack the first nucleotide binding fold (NBF1)81,82. Subsequent studies from the same group indicate that these proteins may be expressed in mitochondria83, and that SUR2−/− mice are protected against myocardial infarction resulting from global ischemia (as we also reported for SUR1−/− mice84), inconsistent with the generally accepted notion that opening of (SUR-dependent) sarcolemmal KATP channels is a protective mechanism in ischemia. A later study from the group indicated that re-expression of full-length SUR2A improved recovery from ischemia85, leading to the slightly convoluted argument that the improvement in the SUR2−/− animals over wild type is somehow the result of the short form SUR2 constructs. The possibility that these are increased in mitochondria might then explain improved mitochondrial energetics in these animals86.
Lack of confirmed presence of canonical SUR or Kir6 subunits in mitochondria has led to alternative hypotheses regarding ‘mitoKATP’ structure. In addition to opening KATP channels, diazoxide may inhibit succinate dehydrogenase87 and consistent with the idea that this key enzyme of both the Krebs cycle and electron transport chain might be a component of the ‘mitoKATP’ channel, Ardehali and colleagues identified a macromolecular complex that recapitulated ‘mitoKATP’ activity including diazoxide activation and 5-hydroxydecanoate inhibition88,89. The complex included succinate dehydrogenase, mitochondrial ATP-binding cassette protein-1 (mABC-1), ATP synthase, adenine nucleotide translocase, and phosphate carrier proteins, and it is not clear which component should be forming the channel pore, the reported records show only single channel activity over brief periods, and follow-up studies have not yet emerged.
Most recently, proteomic analysis of purified bovine mitochondrial inner membranes identified a short form (ROMK2) product of the KCNJ1 gene as containing an N-terminal mitochondrial targeting signal, and colocalization of a full-length epitope-tagged ROMK2 with mitochondrial ATP synthase β90. Additional experiments showed that tertiapin Q, a relatively specific ROMK blocker, inhibited functional assays of mitoK(ATP) activity in isolated mitochondria and inhibited the diazoxide-activated component of mitochondrial thallium uptake. While these studies await independent confirmation, they imply a role for ROMK2 (Kir1) subunits in generating the mitoKATP channel (Fig. 1D).
KATP and cardiovascular disease: The potential versus the genetic evidence
It has long been recognized that KATP channels provide a very large potential ionic conductance in the surface membranes of cardiac myocytes, as well as vascular smooth muscle and endothelium, and perhaps in the mitochondrial inner membrane of many cells. Under normal metabolic conditions, cardiac sarcolemmal KATP channels are predominantly closed, and they do not significantly contribute to cell excitability. However, these channels can open when exposed to a severe metabolic stress such as anoxia, metabolic inhibition or ischemia. In muscle cells, shortening the action potential reduces calcium entry and inhibits contractility91, thereby reducing energy consumption, potentially protecting the cell. Such a preservation ‘strategy’ is of course self-limiting, since if too many myocytes stop contracting, the heart will stop pumping and the animal will die, but it has always been a reasonable, if unproven, notion that temporary protection of a small number of cells, or region of the heart, against the damage of Caoverload during ischemia, is likely to be operable.
In the vasculature, inhibition of K+-channel activity will tend to cause depolarization of the membrane potential, activation of L-type voltage-sensitive Ca2+-channels, Ca2+-entry and vasoconstriction92. Conversely, activation of K+-channels will lead to membrane hyperpolarization, decrease in voltage-dependent Ca2+-entry and vasodilation92. The relationship between membrane potential and Ca2+-influx is especially steep in smooth muscle, with membrane depolarization or hyperpolarization of only a few millivolts causing several fold increases or decreases in [Ca2+]i respectively93,94. Endothelial cells lack voltage-dependent Ca channels and Ca entry through non-selective channels is enhanced at hyperpolarized voltages, in contrast to ‘excitable’ cells95,96. Activation of KATP channels will tend to hyperpolarize cells, leading to elevated [Ca2+]i, and elevated release of vasoactive agents, including EDHF and endothelin. Thus, gain- or loss-of K+-channel activity in either smooth muscle or endothelium could have profound pro-relaxant or pro-constrictive effects respectively on smooth muscle tone, a point we return to below.
Kir6 genes and disease
As discussed in detail below, genetic manipulation of KATP genes in mice can result in dramatic cardiovascular pathologies, yet until recently there has been little evidence for human cardiovascular disease resulting from KATP gene mutations (Table 1). KCNJ11 encodes the predominant KATP channel pore-forming subunit (Kir6.2) in both the pancreatic β-cell and in cardiac myocytes97. Gain- and loss-of function mutations in this gene are now very well understood to underlie neonatal diabetes and congenital hyperinsulinism, respectively98, but there is no published evidence for any cardiac problems in these patients.
Table 1.
REPORTED ASSOCIATION OF DISEASE WITH KATP CHANNEL MUTATIONS
| Gene | Clinical condition |
Features | # of affected individuals |
Refs |
|---|---|---|---|---|
| KCNJ8 (Kir6.1) | J-wave syndrome |
S422L mutation. Reportedly gain-offunction (GOF). Abnormalities in the Jpoint of the ECG, and including Brugada syndrome (BrS) and early repolarization syndrome (ERS), including VF and AF |
9 | 100-102 |
| SIDS | In-frame deletion (E332del) and lossof- function mutation (V346I). through as yet unexplained mechanisms. |
2 | 104 | |
| KCNJ11 (Kir6.2) | Neonatal diabetes |
Multiple GOF mutations cause inhibition of insulin secretion. No cardiovascular phenotype |
>100 | 182 |
| Type 2 diabetes | E23K variant, mild GOF, associated with T2DM, and potentially associated with HF |
30% Caucasians |
183-188 | |
| Congenital hyperinsulinism |
LOF mutations cause hypersecretion of insulin. No cardiovascular phenotype |
>10 | 98,182 | |
| ABCC8 (SUR1) | Neonatal diabetes |
Multiple GOF mutations cause inhibition of insulin secretion. No cardiovascular phenotype |
>100 | 182 |
| Congenital hyperin |
Multiple LOF mutations cause hypersecretion of insulin. No cardiovascular phenotype |
>100 | 98,182 | |
| ABCC9 (SUR2) | AF | Isolated case of LOF mutation assicated with AF originating in the vein of Marshal |
1 | 109 |
| Idiopathic dilated cardiomyopathy |
Two cases with distinct LOF mutations associated with heart failure due to idiopathic dilated cardiomyopathy |
2 | 108 | |
| Cantu syndrome | GOF mutations associated with complex multi-organ disease (See Table 2) |
25 | 110,113 |
KCNJ8 encodes Kir6.1, which is the main channel forming subunit expressed in smooth muscle and may also be expressed in some cardiac myocytes97,99 (Fig. 1D). Several recent studies have reported a single mutation, S422L, in the Kir6.1 protein to be associated with the ‘J-wave’ phenomenon, characterized by abnormalities in the J-point of the ECG, and including Brugada syndrome (BrS) and early repolarization syndrome (ERS). First reported by Haissaguerre et al100, J-point elevation in one patient with the S422L variant showed multiple (>100) recurrences of unresponsive ventricular fibrillation (VF), associated with accentuated early repolarization. Additional studies include that of Delaney et al101 who reported two (out of 325) atrial fibrillation (AF) probands with early repolarization, that of Medeiros-Domingo et al102, who reported one Brugada syndrome patient and one early repolarization syndrome patient carrying the same S422L variant out of 101 analyzed patients, and that of Barajas-Martinez et al., who reported 3 additional BrS and 1 ERS probands carrying the same variant103. The variant has not been identified in any control alleles. The latter two studies both reported enhanced channel activity for the S422L variant, arguing that gain-of-function in Kir6.1 channel activity is underlying the ERS and hence AF. Conversely, sequence analysis of DNA from necropsy tissue on 292 unrelated sudden infant death syndrome (SIDS) cases identified novel KCNJ8 variants in two individuals, an in-frame deletion (E332del) and a missense mutation (V346I), both in the distal C-terminus of Kir6.1. In this case, reduced channel activity was reported from recombinantly expressed mutant channels, leading the authors to conclude that loss-of-function mutations in Kir6.1may be one cause of SIDS104, through as yet unexplained mechanisms.
SUR genes and disease
ABCC8 encodes SUR1, which is the predominant regulatory sulfonylurea receptor (SUR1) in the pancreatic β-cell and is also present in the heart, predominantly in atria in rodents34, but potentially more widespread in humans35. Because of its involvement in the pancreatic KATP channel, gain- and loss-of function mutations in this gene also underlie neonatal diabetes and congenital hyperinsulinism respectively, but again there is no report of cardiac problems in these patients105-107. ABCC9 encodes the second SUR2 subunit, and this is likely to be the major SUR isoform in both cardiac and vascular muscle. There have been two reports of SUR2 loss of function mutations leading to cardiac disease, both from the group of Andre Terzic and colleagues108,109 (Table 1). In each case, the mutations are present in the C-terminal exons and will lead to a disruption of the second nucleotide binding fold of SUR2A, and hence reduce nucleotide stimulation of channel activity, without affecting SUR2B. In the first report, the single patient with the mutation presented with long-standing atrial fibrillation originating in the vein of Marshall, with normal cardiac morphology and contractile features109. The patient was successfully treated by radiofrequency ablation. In the second report, two individuals with two distinct mutations presented with heart failure due to idiopathic dilated cardiomyopathy108. There have been no subsequent reports of similar genetic defects in the intervening five years, and further evidence for causality of association of similar gene variants with disease in additional cases is lacking.
Two new papers reporting multiple different ABCC9 mutations, all associated with Cantu syndrome, a distinctive multi-organ disease (Table 2), now provide a clear picture of associated outcomes, and open up multiple new avenues of investigation. The first study110 involved genetic analysis of 14 individuals diagnosed with Cantu syndrome111, and ABCC9 coding mutations were identified in 11 of them. In six cases with no affected relatives, the mutations were de novo. Two families were also reported, one with an affected mother and two affected daughters, and one with an affected father and daughter, confirming that inheritance in this case is autosomal dominant. No analysis of recombinant channel function was made in this first study, but the conclusion that these mutations all lead to a gain-of channel function112 is cemented by the second study113, which identified ABCC9 coding mutations in an additional 14 of 16 identified patients. In that study, recombinant expression of mutant channel proteins clearly demonstrated a reduced sensitivity to ATP inhibition in 3 example mutants which, as discussed below, will lead to enhanced KATP channel activity wherever the channels are located.
Table 2.
MAJOR CLINICAL FEATURES OF CANTU SYNDROME
| Neonatal Features |
| Neonatal macrosomia |
| History of maternal polyhydramnios |
| Macrocephaly |
| Occasional slow postnatal growth and short stature later in life |
| Craniofacial dysmorphology |
| Coarse facial appearance (can be confused with a storage disoder) |
| Epicanthal folds |
| Broad nasal bridge |
| Anteverted nostrils |
| Long philtrum |
| Wide mouth with full lips |
| Macroglossia |
| High or narrow palate |
| Gingival hyperplasia |
| Anterior open bite |
| Hair |
| Congenital generalized hirsutism |
| Thick scalp hair |
| Thick and/or curly eyelashes |
| Excessive hair growth on forehead, face, back and limbs |
| Cardiovascular |
| Cardiomegaly |
| Concentric hypertrophy of the ventricles |
| Normal ventricular contractility |
| Pericardial effusion |
| Pulmonary hypertension |
| Partial pulmonary venous obstruction |
| Mitral valve regurgitation |
| Congenital anomalies |
| Patent ductus arteriosus |
| Bicuspid and/or stenotic aortic valve |
| Skeletal abnormalities |
| Thickened calvarium |
| Narrow shoulders and thorax |
| Pectus carinatum |
| Broad ribs |
| Platyspondyly and ovoid vertebral bodies |
| Hypoplastic ischium and pubic bones |
| Erlenmeyer-flask-like long bones with metaphyseal flaring |
| Narrow obturator foramen |
| Coxa vara |
| Scoliosis |
| Osteopenia |
| Delayed bone age |
| Hypoplastic ischium and pubic bones |
| Erlenmeyer-flask-like long bones with metaphyseal flaring |
| Narrow obturator foramen |
| Coxa vara |
| Scoliosis |
| Osteopenia |
| Delayed bone age |
| Skin and joints |
| Loose and/or wrinkled skin, especially in neonates |
| Deep palmar and plantar creases |
| Persistent fingertip pads |
| Hyperextensibility of joints |
| Lymphatic system |
| Lymphedema, onset usually in adolescence or adulthood |
| Gastrointestinal |
| Pyloric stenosis |
| Increased risk for upper gastrointestinal bleeding |
| Other reported features |
| Immune dysfunction or recurrent infections |
| Umbilical hernia |
| Renal anomalies |
| Genital anomalies |
Cantu Syndrome: Multiple tissue symptoms
Cantu syndrome (MIM 239850), or hypertrichosis-osteochondrodysplasia-cardiomegaly syndrome, was first described in 1982111. Subsequent reports112,114-121 have confirmed a constellation of features in ~30 patients (see Table 2). Congenital hypertrichosis is a constant feature, with thick scalp hair and excessive hair growth on the forehead, face, back and extremities. Generalized macrosomia is present in most cases, with large birth weights and lengths, although ultimate adult height is usually within the normal range. Macrocephaly is typically present at birth and usually persists. Multiple dysmorphic features (Table 2), including coarse facial appearance, skeletal abnormalities, and generalized osteopenia, as well as multiple additional clinical features have also been described. The cardiac features include cardiac enlargement, concentric hypertrophy of the ventricles, pulmonary hypertension and pericardial effusion. Yet, despite the enlargement of the heart with increased muscle mass, cardiac function is typically normal, with normal ventricular contractility on imaging studies112. Cardiac muscle biopsy in one patient showed mild myofibrillar disorganization but normal myofibers and mitochondria on electron microscopy, and in 2 other patients cardiac biopsy was reported as normal112,122. Pulmonary hypertension secondary to partial pulmonary venous obstruction has been reported in one case, and was associated with severe mitral valve regurgitation that spontaneously resolved116. Some patients have required pericardiocentesis and ultimately needed pericardial stripping to prevent reaccumulation of the pericardial effusion. A significant number of patients have had patent ductus arteriosus (PDA) requiring surgical closure, as well as bicuspid aortic valves with and without stenosis. Lymphedema involving the lower extremities may develop over time, and in one patient, lymphangiogram demonstrated dilated lymphatic vessels in the legs with delayed lymphatic drainage123.
Diazoxide, minoxidil and other related drugs have been used since the 1960s to treat severe refractory hypertension. Multiple reports of side effects of these drugs also include pronounced hypertrichosis, pericardial effusions, and edema in treated patients124 One report even noted coarsening of the facial features, reminiscent of Cantu syndrome, after 8 months treament with minoxidil125. It was subsequently recognized that one major action of minoxidil is opening of KATP channels97,126,127, and this led us to note the parallels between the symptoms of minoxidil exposure and the features of Cantu syndrome, and to suggest the possibility that Cantu syndrome might be the result of K channel hyperactivity112. Teratogenic effects of minoxidil, including marked hypertrichosis, dysmorphic facial features and low blood pressure have been reported in the offspring of a minoxidil-treated mother128. In additional reported cases of minoxidil teratogenicity, one infant had transposition of the great vessels and pulmonary bicuspid valvular stenosis leading to neonatal death, and another infant had hypertrichosis that resolved over the first 3 months of life129.
The two recent papers that describe specific mutations in the ABCC9 gene in a total of 25 of 31 Cantu syndrome patients110,113, definitively link the gene defect to the syndrome. All reported patients had the typical Cantu syndrome phenotype (Table 2), but 6 of 31 patients had no identifiable ABCC9 mutation suggesting that additional gene defects may be involved. Previous studies of Cantu syndrome patients have provided no definitive explanation of the underlying cause of the various features, and even now the realization of SUR2 mutations as causal does not immediately provide explanations for all features. There is strong evidence discussed below, for a physiologically important role of SUR2 in vascular relaxation, such that persistence of the PDA in Cantu syndrome patients may be readily explained as a consequence of maintained vessel dilation following birth. Patency of the ductus arteriosus is controlled by many factors, the most important of which are relatively low fetal oxygen tension, prostaglandin [PGE2] and prostacyclin [PGI2]) in the fetus. After birth, the abrupt increase in oxygen tension and falling PGE2 and PGI2 levels lead to inhibition of voltage-gated K channels and contraction of the smooth muscle fibers in the ductus, resulting in wall thickening and lumen obliteration. Mechanisms of persistent PDA are not clear130, but the enhancement of a K current in smooth muscle presents an obvious potential explanation in Cantu syndrome patients. Altered vascular tone may also underlie the edema and pericardial effusion, but the reason for cardiomegaly is not obvious. Cardiomegaly reported in most cases of Cantu Syndrome is due to increased myocardial mass (hypertrophy) with larger cardiac chambers but with normal systolic function, and this does not fit the diagnostic criteria of dilated or hypertrophic cardiomyopathy131. As we reported, cardiomegaly in two related Cantu syndrome cases has been associated with high output failure112 and may well be a secondary response to reduced vascular tone132. Similarly, the reason for osteochondrodysplasia and facial dysmorphology is not obvious and the mechanism by which minoxidil causes hair growth has remained controversial133. It has been speculated that by opening vascular K channels and dilation of blood vessels, the supply of oxygen, blood and nutrients to the hair follicle is increased, which cause follicles in the telogen phase to shed and be replaced by new thicker hairs in a new anagen phase. However, there is also evidence that SUR2 isoforms are present in follicular dermal papillae134 and while the new realization definitively ties the hair growth to an action on KATP channels, it does not immediately prove where the action is.
Cardiovascular disease and KATP mutations: Insights from genetically modified animals
Kir6.2 and SUR1 knockout animals exhibit complex cardiac phenotypes
Murine knockout models of each of the four KATP channel genes have been generated and extensively analyzed. Knockout of Kir6.2 results in a loss of glucose-dependent insulin secretion, modeling features of hyperinsulinism in humans135. Knockout of SUR1 reiterates essentially the same phenotype as Kir6.2−/−, and again the major effects are in the pancreas. Conversely (see next section), knockout of Kir6.1 or SUR2 leads to a vascular phenotype, presumably due to loss of KATP channel activity in either vascular smooth muscle or endothelium136,137.
Cardiac sarcolemmal KATP channels are predominantly closed and do not contribute significantly to the process of excitation-contraction coupling in physiological conditions (except perhaps under adrenergic stimulation, see below), since application of sulfonylureas generally has little or no effect on the cardiac action potential138. Accordingly, while Kir6.2 is the major cardiac Kir6 isoform, baseline ventricular action potential duration (APD) and contractile function are unaffected in isolated ventricular myocytes from Kir6.2−/− animals74,139,140. When metabolism is inhibited, the action potential can shorten markedly and contraction can be inhibited as a result of KATP activation91,141,142. KATP activation during ischemia is likely to be cardioprotective, since reduction of APD and contraction may preserve ATP stores that would otherwise be consumed during the contractile cycle. In support of this idea, treatment with the KATP opener pinacidil during ischemia increases cellular ATP and energy stored as creatine phosphate143. AP shortening is absent in Kir6.2−/− hearts, and the time to contractile failure is prolonged but the time to onset of rigor contracture is reduced74. Diastolic Ca2+ overload, myocardial damage, and increased mortality are also observed in isoproterenol challenged Kir6.2−/− myocytes144. In addition to highlighting the acute protective effect of KATP activation, Kir6.2−/− animals show increased mortality and exaggerated hypertrophy in response to pressure overload145,146, and to mineralocorticoid/salt challenge147. Together, these studies suggest that loss of KATP, by stopping the protective ‘unloading’ that KATP activation leads to, should tend to cause Ca overload and perhaps hasten the transition to heart failure under stressed conditions. However, two further studies seem to contradict a cardioprotective role. In these studies, from independent groups, both SUR2- (SUR2−/−) and SUR1-knockout (SUR1−/−) mice were found to be more tolerant of global ischemia-reperfusion than control mice, with reduced infarct sizes84,148. Since the SUR2−/− mice have a marked reduction of ventricular sarcolemmal KATP channels, the enhanced cardioprotection is opposite the expected phenotype (i.e. impaired protection). Cardioprotection in SUR2−/− mice might conceivably be due to the concomitant loss of the SUR2B component of vascular KATP channels, but similar cardioprotection in SUR1−/− mice84 could not be explained by such a mechanism.
As noted above, no cardiac problems have been reported for individuals with loss of function (LOF) or gain of function (GOF) mutations in Kir6.2 or SUR1, who suffer from profound pancreatic problems (hyperinsulinism or neonatal diabetes, respectively). In this regard, the lack of dramatic effects in both Kir6.2−/− and KATP overactive hearts (see below) is consistent and, while it still does not answer the question of why this large potential conductance is present in the heart, it really does seem to tell us that change of sarcolemmal KATP channels may not be so critical.
Kir6.1/SUR2 knockouts highlight vascular roles
Mouse models in which the Kir6.1 and SUR2 genes have been ‘knocked-out’ highlight the critical role of these subunits in the cardiovascular system, particularly in the coronary circulation26,137. The cardiovascular phenotypes of Kir6.1−/− and SUR2−/− mice are similar, and include baseline hypertension, coronary artery vasospasm and sudden cardiac death. Electrocardiograms from both animals show ST segment elevation and atrioventricular (AV) block, which may account for the sudden death. Importantly, SUR2−/− mice treated with the Ca channel blocker nifedipine exhibit a reduction in coronary artery vasospasm, implicating abnormally elevated [Ca2+]i due to loss of hyperpolarizing KATP current as causal in the hypercontractility137. Collectively, these KATP-null mice recapitulate clinical features of the human disorder of Prinzmetal (or variant) angina, but several studies have failed to demonstrate any association of human coronary vasospasm or hypertension with LOF mutations in Kir6.1 or SUR2149,150, even though linkage analysis indicates that there are associated genes within the same locus as Kir6.1 and SUR2151.
Kir6.1 transcripts are detected in heart, lung, brain, pancreas, and endothelium152 and SUR2 transcripts are found in multiple tissues, including cardiac and skeletal muscle (SUR2A)4,7, brain (SUR2A) and endothelium (SUR2B),60. Thus, the possibility exists that the cardiovascular phenotypes of Kir6.1−/− and SUR2−/− mice (or of Cantu syndrome patients), reflect loss (or gain) of KATP in smooth muscle or other tissues153. A role for non-smooth muscle KATP in cardiovascular homeostasis is supported by the finding that targeted suppression of endothelial KATP (Kir6.1/SUR2B) by transgenesis results in an increase in coronary perfusion pressure and a decrease in coronary blood flow64,68,152, a similar phenotype to that observed in Kir6.1−/− mice26. Interestingly, release of the vasoconstrictor endothelin-1 is increased by transgenic suppression of endothelial KATP, potentially implicating an elevated level of circulating endothelin-1 as causal in the vasoconstriction65. These studies raise the possibility of KATP-dependent paracrine signaling between endothelial cells and overlying vascular smooth myocytes, with the endothelial KATP regulating the release of endothelin-1. Transgenic restoration of VSM KATP currents by specific expression of the SUR2B isoform in VSM of SUR2−/− mice, does not resolve the coronary artery vasospasm, atrioventricular (AV) heart block, or sudden cardiac death exhibited by SUR2−/− animals154, providing further support for a potential role of non-VSM KATP in regulation of vascular tone.
Transgenic KATP GOF models
Given that sarcolemmal KATP channels are normally predominantly closed, we have long argued that gain-of-function mutations are as likely, if not more likely, to be key drivers of human disease as loss of function mutations155. To that end, we have generated multiple GOF mouse models. The first, modeling Kir6.2 GOF clearly revealed the potential for such GOF mutations to cause neonatal diabetes156 and led to the subsequent demonstration that such mutations are indeed causal in human neonatal diabetes157. In parallel studies, we have explored the potential for Kir6.2 GOF action in the heart, with considerably less emergent clarity158-160. Although we introduce channels that are very ATP-insensitive, they still remain closed under all but extreme circumstances, and cause no overt malfunction, mirroring the human Kir6.2 GOF condition – neonatal diabetes with no cardiac phenotype160. Curiously, we find that in ventricular myocytes from these animals there is a dramatically enhanced Ca current,158 which may be some compensatory response to an initial or local action potential shortening, and conceivably might be related to ‘high output’ heart failure that is seen in Cantu syndrome. These studies also reveal that overexpressing the SUR1 isoform the myocardium has an effect to prolong the PR interval161, and that when Kir6.2 GOF is expressed together with SUR1, second and third degree AV block, progressing to ventricular and supra-ventricular arrhythmias and sudden death follows161. This is accompanied in some cases by cardiac hypertrophy and in the most extreme cases, causes cardiac malformation at the very earliest stages of embryonic cardiac development162. In recombinant channels, SUR1-dependent channels are more sensitive to metabolic activation than SUR2A-dependent channels163, and we conclude that these pathologies are reflecting channel overactivity in some critical, but as yet unidentified, time-window or region of the heart. These results highlight that KATP overactivity in heart muscle can certainly be structurally and functionally detrimental, and may be modeling some of the cardiac consequences of SUR2 overactivity in Cantu syndrome, although cardiac hypertrophy and failure in Cantu syndrome patients is not obviously accompanied by arrhythmias or other cellular defects.
Following the same rationale of exploring GOF models, we have embarked on generation of a series of Kir6.1 and SUR2 GOF transgenic animals. Expression of Kir6.1 gain-of-function mutants in smooth muscle leads to a reduction of systolic and diastolic blood pressures (Li, A., Koster, J.C., Knutsen, R. and C.G. Nichols, unpublished), paralleling the effects of KCOs in human hypertensive patients. Conceivably, further study of these animals, as well as of SUR2 GOF transgenic animals will reveal additional features that model Cantu syndrome effects and permit testing of novel therapeutic approaches.
Potential for therapeutic modulation of cardiovascular KATP activity
There is tremendous potential for modulation of KATP channel activity in general and more importantly perhaps, in a tissue-specific manner, since there is already a rich pharmacology, not only of channel inhibitors but also channel openers (KCOs). KCOs have been used in two major clinical settings: (1) to block insulin secretion in conditions of hyperinsulinema, and (2) as antihypertensives. So far, clinical use of sulfonylureas has been limited to treatment of type 2 diabetes, and there has been debate about negative cardiovascular effects.
Minoxidil is reportedly the most active KCO at causing human hair growth, hence its commercial use in topical hair restoration products164,165, and as discussed above, it appears that most, if not all, of the effects of Cantu syndrome are replicated by high dose minoxidil, including hypertrichosis, facial dysmorphology, and pericardial effusion128. Such features have been reported for other KCOs; there is one report of pericardial effusion as a result of diazoxide therapy166 and although not attributed to the drug by the authors, another reported case of a patient on diazoxide who suffered from a pericardial effusion167. Interestingly, a clinical trial for the use of nicorandil, as a SUR2A specific activator, in the setting of acute MI actually reported lower rates of pericardial effusion than in untreated patients168.
Although there are certain dogmas in the literature regarding specificity of KCOs or inhibitors, careful binding analyses performed on cloned SURs have revealed complexities of binding and dependence on nucleotides which makes it difficult to predict in vivo efficacies at different SUR targets. In addition, it is very clear from intact cell and excised patch-clamp recordings that the ability of KCOs to activate KATP channel currents, depends critically on the metabolic state of the intracellular milieu, making direct comparison between different studies difficult169. The ability of diverse KCOs to lower blood pressure is well recognized, leading to their clinical use in acute and refractory hypertensive settings. Sulfonylureas inhibit KATP channels and have seen very widespread use as glucose lowering agents in the type 2 diabetes. There is a wide therapeutic range, and the main recognized side-effect is hypoglycemia, but there is a long-standing debate as to potential cardiovascular side-effects. KATP channel inhibitory drugs have not reached clinical acceptance in the cardiovascular arena, the expectation being that blockade of cardiac KATP channels may be detrimental in conditions of myocardial ischemia, during which these channels can open and are presumed protective as discussed above. This debate is still not resolved170,171.
Given the new realization of the SUR2-dependent basis of Cantu syndrome, the opportunity immediately presents itself for the use of KATP channel inhibitors as a potential ‘magic-bullet’ therapy, as they have proven in the treatment of Kir6.2- or SUR1-dependent neonatal diabetes172. It is generally accepted that most sulfonylureas are physiologically more potent inhibitors of SUR1-dependent KATP than SUR2A-dependent channels, although there has been no careful comparison of effect on SUR1-versus SUR2B-dependent channels. There has been a long-standing dogma that the drug HMR1098 is a cardiac specific KATP blocker173-176, although several studies including our recent direct head-to-head comparison confirm that it is also a more effective blocker of SUR1-dependent than SUR2A-dependent KATP channels34,177,178. Relative efficacies of HMR1098 versus sulfonylureas in specific physiological conditions may be important to understand, since it is conceivable that specific KATP inhibitors may counteract the symptoms of Cantu syndrome, without significantly affecting blood glucose control, a key issue if KATP channel inhibition is to be a viable treatment for the disease.
Further implications and future prospects
In spite of almost 30 years of research, we have remained ‘largely in the dark regarding the true physiological determinants, and relevance of sarcolemmal KATP activity’179, until very recently. We now realize that the subunit make-up of sarcolemmal KATP channels can be far more complex and labile than originally thought16, and together with the existence of mitoKATP, it may be reasonable to consider KATP channels as a family of channels180. The details of the involvement of sarcolemmal versus mitochondrial KATP channels in cardiovascular physiology and pathology remain unclear, but the growing association of Kir6.1 and SUR2 variants with specific electrical and contractile derangements and the new clear association with a complex syndrome firmly establish the importance of appropriate activity in normal function of the heart and vasculature. In addition to consideration of potential therapeutic implications of these new findings, we can also consider the broader mechanistic implications. As discussed above, key features of Cantu syndrome are consistent with activation of SUR2B-dependent KATP channels in the vasculature, leading to vasorelaxation. In this case, the likely associated Kir channel subunit is Kir6.1 (Fig. 1D) and we might reasonably suggest that GOF mutations in Kir6.1 should also be associated with these, if not all, symptoms of Cantu syndrome, paralleling the similar neonatal diabetic phenotypes of Kir6.2 and SUR1 GOF mutations. A purported GOF Kir6.1 mutation is already associated with the J-wave syndrome100-103, and this leads to a clear inconsistency: neither J-wave abnormalities nor other arrhythmias have been reported in Cantu syndrome patients, and none of the Cantu syndrome features have yet been reported for ERS patients. It remains conceivable that Cantu syndrome features are not due to enhanced cell membrane KATP activity, but instead are the result of Kir6-independent – i.e. mitochondrial - SUR2 activity. Also unexplained thus far is how opposing effects of LOF mutations108,109 versus GOF mutations110,113 in SUR2 could give rise to myocardial electrical derangements in the former case, but vascular derangements in the second.
Finally, we should recognize that the monogenic disease-associated KATP mutations, which cause relatively severe changes in channel function, are likely to represent only the ‘tip of the iceberg’ when it come to the disease-promoting effects of change in protein activity. Further studies of patients with some or all symptoms of Cantu syndrome will be facilitated by efforts to bring such patients together (www.cantusyndrome.org) and will no doubt reveal new mutations in the KATP subunits and perhaps in proteins that regulate KATP synthesis, trafficking, or location. We do not yet know which of the Cantu syndrome features are the most penetrant, and hence which of these features might appear in isolation, as the severity of the effect of a specific mutation is reduced. If one or other of the affected cardiovascular functions is the most sensitive to SUR2 GOF, then we may find far more cases of individuals with GOF variants linked to specific features such as PDA, pericardial effusion, and cardiomegaly with or without high output cardiac failure. It may require the detection of these patients with newer cardiac imaging modalities such as strain imaging to study the heterogeneity of myocardial fiber mass and orientation, or to detect abnormal electrical activation sequences at subclinical levels that may exist with SUR2 GOF mutations. We are only just beginning to recognize the cellular control mechanisms that regulate KATP channel subunit synthesis, trafficking and degradation181. Any alterations in such mechanisms, whether genetic or environmentally based, may also give rise to disease phenotypes similar to those resulting from the mutations discussed above, and may ultimately benefit therapeutically from the unique pharmacology of the sulfonylurea receptors.
Acknowledgments
Citation of financial support for the authors Our own experimental work has been supported by NIH grants HL45742 and HL95010 (to CGN).
Non-standard abbreviations
- KATP
ATP-sensitive potassium channel
- ABC
ATP-binding cassette family of proteins
- KCNJ
Inward rectifier K channel gene family
- Kir1, Kir6
Pore-forming subunits of KATP channels
- M1, M2
transmembrane helices of Kir6 subunits
- TMD1, TMD2
Transmembrane domains of SUR subunits
- NBF1, NBF2
Nucelotide binding folds of SUR subunits
- FRET
Fluorescence resonance energy transfer
- VSM
Vascular smooth muscle
- mitoKATP
Mitochondrial KATP channel
- mABC-1
mitochondrial ATP-binding cassette protein-1
- ROMK
protein product of the KCNJ1 gene
- BrS
Brugada syndrome
- ERS
Early repolarization syndrome
- AF
Atrial fibrillation
- APD
Action potential duration
- LOF
Loss of function
- GOF
Gain of function
- AV
Atrioventricular
- KCO
Potassium channel opener
Footnotes
Disclosures None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- 2.Flagg TP, Enkvetchakul D, Koster JC, Nichols CG. Muscle KATP channels: recent insights to energy sensing and myoprotection. Physiol Rev. 2010;90:799–829. doi: 10.1152/physrev.00027.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kane GC, Liu XK, Yamada S, Olson TM, Terzic A. Cardiac KATP channels in health and disease. Journal of Molecular & Cellular Cardiology. 2005;38:937–943. doi: 10.1016/j.yjmcc.2005.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inagaki N, Gonoi T, Clement JP, Wang CZ, Aguilar-Bryan L, Bryan J, Seino S. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–1017. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- 5.Inagaki N, Gonoi T, Clement JPt, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor [see comments] Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- 6.Aguilar-Bryan L, Nichols CG, Wechsler SW, Clement JPt, Boyd AE, 3rd, Gonzalez G, Herrera-Sosa H, Nguy K, Bryan J, Nelson DA. Cloning of the beta cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995;268:423–426. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- 7.Chutkow WA, Simon MC, Le Beau MM, Burant CF. Cloning, tissue expression, and chromosomal localization of SUR2, the putative drug-binding subunit of cardiac, skeletal muscle, and vascular KATP channels. Diabetes. 1996;45:1439–1445. doi: 10.2337/diab.45.10.1439. [DOI] [PubMed] [Google Scholar]
- 8.Shi Y, Wu Z, Cui N, Shi W, Yang Y, Zhang X, Rojas A, Ha BT, Jiang C. PKA phosphorylation of SUR2B subunit underscores vascular KATP channel activation by beta-adrenergic receptors. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1205–1214. doi: 10.1152/ajpregu.00337.2007.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chutkow WA, Makielski JC, Nelson DJ, Burant CF, Fan Z. Alternative splicing of sur2 Exon 17 regulates nucleotide sensitivity of the ATP-sensitive potassium channel. Journal of Biological Chemistry. 1999;274:13656–13665. doi: 10.1074/jbc.274.19.13656. [DOI] [PubMed] [Google Scholar]
- 10.Inagaki N, Gonoi T, Clement JP, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science (New York, N.Y.) 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- 11.Shyng S, Nichols CG. Octameric stoichiometry of the KATP channel complex. J Gen Physiol. 1997;110:655–664. doi: 10.1085/jgp.110.6.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clement JPt, Kunjilwar K, Gonzalez G, Schwanstecher M, Panten U, Aguilar-Bryan L, Bryan J. Association and stoichiometry of K(ATP) channel subunits. Neuron. 1997;18:827–838. doi: 10.1016/s0896-6273(00)80321-9. [DOI] [PubMed] [Google Scholar]
- 13.Inagaki N, Gonoi T, Seino S. Subunit stoichiometry of the pancreatic beta-cell ATP-sensitive K+ channel. FEBS Lett. 1997;409:232–236. doi: 10.1016/s0014-5793(97)00488-2. [DOI] [PubMed] [Google Scholar]
- 14.Yamada M, Isomoto S, Matsumoto S, Kondo C, Shindo T, Horio Y, Kurachi Y. Sulphonylurea receptor 2B and Kir6.1 form a sulphonylurea-sensitive but ATP-insensitive K+ channel. Journal of Physiology. 1997;499:715–720. doi: 10.1113/jphysiol.1997.sp021963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okuyama Y, Yamada M, Kondo C, Satoh E, Isomoto S, Shindo T, Horio Y, Kitakaze M, Hori M, Kurachi Y. The effects of nucleotides and potassium channel openers on the SUR2A/Kir6.2 complex K+ channel expressed in a mammalian cell line, HEK293T cells. Pflugers Archiv European Journal of Physiology. 1998;435:595–603. doi: 10.1007/s004240050559. [DOI] [PubMed] [Google Scholar]
- 16.Babenko AP, Gonzalez G, Aguilar-Bryan L, Bryan J. Reconstituted human cardiac KATP channels: functional identity with the native channels from the sarcolemma of human ventricular cells. Circ Res. 1998;83:1132–1143. doi: 10.1161/01.res.83.11.1132. [DOI] [PubMed] [Google Scholar]
- 17.Kuo A, Gulbis JM, Antcliff JF, Rahman T, Lowe ED, Zimmer J, Cuthbertson J, Ashcroft FM, Ezaki T, Doyle DA. Crystal structure of the potassium channel KirBac1.1 in the closed state. Science. 2003;300:1922–1926. doi: 10.1126/science.1085028. [DOI] [PubMed] [Google Scholar]
- 18.Tao X, Avalos JL, Chen J, MacKinnon R. Crystal structure of the eukaryotic strong inward-rectifier K+ channel Kir2.2 at 3.1 A resolution. Science. 2009;326:1668–1674. doi: 10.1126/science.1180310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bryan J, Vila-Carriles WH, Zhao G, Babenko AP, Aguilar-Bryan L. Toward linking structure with function in ATP-sensitive K+ channels. Diabetes. 2004;53(Suppl 3):S104–112. doi: 10.2337/diabetes.53.suppl_3.s104. [DOI] [PubMed] [Google Scholar]
- 20.Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–476. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- 21.Mikhailov MV, Campbell JD, de Wet H, Shimomura K, Zadek B, Collins RF, Sansom MS, Ford RC, Ashcroft FM. 3-D structural and functional characterization of the purified KATP channel complex Kir6.2-SUR1. Embo J. 2005;24:4166–4175. doi: 10.1038/sj.emboj.7600877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang S, Makhina EN, Masia R, Hyrc KL, Formanack ML, Nichols CG. Domain organization of the ATP-sensitive potassium channel complex examined by FRET. J Biol Chem. doi: 10.1074/jbc.M112.388629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nichols CG, Makhina EN, Pearson WL, Sha Q, Lopatin AN. Inward rectification and implications for cardiac excitability. Circ Res. 1996;78:1–7. doi: 10.1161/01.res.78.1.1. [DOI] [PubMed] [Google Scholar]
- 24.Beguin P, Nagashima K, Nishimura M, Gonoi T, Seino S. PKA-mediated phosphorylation of the human K(ATP) channel: separate roles of Kir6.2 and SUR1 subunit phosphorylation. EMBO Journal. 1999;18:4722–4732. doi: 10.1093/emboj/18.17.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrissey A, Rosner E, Lanning J, Parachuru L, Chowdhury PD, Han S, Lopez G, Tong X, Yoshida H, Nakamura TY, Artman M, Giblin JP, Tinker A, Coetzee WA. Immunolocalization of KATP channel subunits in mouse and rat cardiac myocytes and the coronary vasculature. BMC Physiology. 2005;5:1. doi: 10.1186/1472-6793-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miki T, Suzuki M, Shibasaki T, Uemura H, Sato T, Yamaguchi K, Koseki H, Iwanaga T, Nakaya H, Seino S. Mouse model of Prinzmetal angina by disruption of the inward rectifier Kir6.1. Nat Med. 2002;8:466–472. doi: 10.1038/nm0502-466. [DOI] [PubMed] [Google Scholar]
- 27.Cheng WW, Tong A, Flagg TP, Nichols CG. Random assembly of SUR subunits in K(ATP) channel complexes. Channels (Austin) 2008;2:34–38. doi: 10.4161/chan.2.1.6046. [DOI] [PubMed] [Google Scholar]
- 28.Chan KW, Wheeler A, Csanady L. Sulfonylurea receptors type 1 and 2A randomly assemble to form heteromeric KATP channels of mixed subunit composition. J Gen Physiol. 2008;131:43–58. doi: 10.1085/jgp.200709894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wheeler A, Wang C, Yang K, Fang K, Davis K, Styer AM, Mirshahi U, Moreau C, Revilloud J, Vivaudou M, Liu S, Mirshahi T, Chan KW. Coassembly of different sulfonylurea receptor subtypes extends the phenotypic diversity of ATP-sensitive potassium (KATP) channels. Mol Pharmacol. 2008;74:1333–1344. doi: 10.1124/mol.108.048355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cui Y, Giblin JP, Clapp LH, Tinker A. A mechanism for ATP-sensitive potassium channel diversity: Functional coassembly of two pore-forming subunits. Proc Natl Acad Sci U S A. 2001;98:729–734. doi: 10.1073/pnas.011370498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pountney DJ, Sun ZQ, Porter LM, Nitabach MN, Nakamura TY, Holmes D, Rosner E, Kaneko M, Manaris T, Holmes TC, Coetzee WA. Is the molecular composition of K(ATP) channels more complex than originally thought? Journal of Molecular & Cellular Cardiology. 2001;33:1541–1546. doi: 10.1006/jmcc.2001.1407. [DOI] [PubMed] [Google Scholar]
- 32.Kono Y, Horie M, Takano M, Otani H, Xie LH, Akao M, Tsuji K, Sasayama S. The properties of the Kir6.1-6.2 tandem channel co-expressed with SUR2A. Pflugers Archiv - European Journal of Physiology. 2000;440:692–698. doi: 10.1007/s004240000315. [DOI] [PubMed] [Google Scholar]
- 33.Glukhov AV, Flagg TP, Fedorov VV, Efimov IR, Nichols CG. Differential K(ATP) channel pharmacology in intact mouse heart. J Mol Cell Cardiol. 2009;48:152–160. doi: 10.1016/j.yjmcc.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flagg TP, Kurata HT, Masia R, Caputa G, Magnuson MA, Lefer DJ, Coetzee WA, Nichols CG. Differential structure of atrial and ventricular KATP: atrial KATP channels require SUR1. Circ Res. 2008;103:1458–1465. doi: 10.1161/CIRCRESAHA.108.178186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fedorov VV, Glukhov AV, Ambrosi CM, Kostecki G, Chang R, Janks D, Schuessler RB, Moazami N, Nichols CG, Efimov IR. Effects of KATP channel openers diazoxide and pinacidil in coronary-perfused atria and ventricles from failing and non-failing human hearts. J Mol Cell Cardiol. 2011;51:215–225. doi: 10.1016/j.yjmcc.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han X, Light PE, Giles WR, French RJ. Identification and Properties Of an Atp-Sensitive K+ Current In Rabbit Sino-Atrial Node Pacemaker Cells. Journal of Physiology. 1996;490:337–350. doi: 10.1113/jphysiol.1996.sp021148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kakei M, Noma A. Adenosine-5′-triphosphate-sensitive single potassium channel in the atrioventricular node cell of the rabbit heart. Journal of Physiology London. 1984;352:265–284. doi: 10.1113/jphysiol.1984.sp015290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Light PE, Cordeiro JM, French RJ. Identification and properties of ATP-sensitive potassium channels in myocytes from rabbit Purkinje fibres. Cardiovasc Res. 1999;44:356–369. doi: 10.1016/s0008-6363(99)00218-7. [DOI] [PubMed] [Google Scholar]
- 39.Fukuzaki K, Sato T, Miki T, Seino S, Nakaya H. Role of sarcolemmal ATP-sensitive K+ channels in the regulation of sinoatrial node automaticity: an evaluation using Kir6.2-deficient mice. J Physiol. 2008;586:2767–2778. doi: 10.1113/jphysiol.2007.148932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dart C, Standen NB. Adenosine-activated potassium current in smooth muscle cells isolated from the pig coronary artery. J Physiol. 1993;471:767–786. doi: 10.1113/jphysiol.1993.sp019927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nichols CG, Lederer WJ. The regulation of ATP-sensitive K+ channel activity in intact and permeabilized rat ventricular myocytes. Journal of Physiology London. 1990;423:91–110. doi: 10.1113/jphysiol.1990.sp018013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blanco-Rivero J, Gamallo C, Aras-Lopez R, Cobeno L, Cogolludo A, Perez-Vizcaino F, Ferrer M, Balfagon G. Decreased expression of aortic KIR6.1 and SUR2B in hypertension does not correlate with changes in the functional role of K(ATP) channels. Eur J Pharmacol. 2008;587:204–208. doi: 10.1016/j.ejphar.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 43.Cui Y, Tran S, Tinker A, Clapp LH. The molecular composition of K(ATP) channels in human pulmonary artery smooth muscle cells and their modulation by growth. Am J Respir Cell Mol Biol. 2002;26:135–143. doi: 10.1165/ajrcmb.26.1.4622. [DOI] [PubMed] [Google Scholar]
- 44.Standen NB, Quayle JM, Davies NW, Brayden JE, Huang Y, Nelson MT. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989;245:177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- 45.Miyoshi Y, Nakaya Y, Wakatsuki T, Nakaya S, Fujino K, Saito K, Inoue I. Endothelin blocks ATP-sensitive K+ channels and depolarizes smooth muscle cells of porcine coronary artery. Circ Res. 1992;70:612–616. doi: 10.1161/01.res.70.3.612. [DOI] [PubMed] [Google Scholar]
- 46.Ottolia M, Toro L. Reconstitution in lipid bilayers of an ATP-sensitive K+ channel from pig coronary smooth muscle. J Membr Biol. 1996;153:203–209. doi: 10.1007/s002329900123. [DOI] [PubMed] [Google Scholar]
- 47.Beech DJ, Zhang H, Nakao K, Bolton TB. K channel activation by nucleotide diphosphates and its inhibition by glibenclamide in vascular smooth muscle cells. Br J Pharmacol. 1993;110:573–582. doi: 10.1111/j.1476-5381.1993.tb13849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kajioka S, Kitamura K, Kuriyama H. Guanosine diphosphate activates an adenosine 5′-triphosphate-sensitive K+ channel in the rabbit portal vein. Journal of Physiology. 1991;444:397–418. doi: 10.1113/jphysiol.1991.sp018885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamouchi M, Kitamura K. Regulation of ATP-sensitive K+ channels by ATP and nucleotide diphosphate in rabbit portal vein. Am J Physiol. 1994;266:H1687–1698. doi: 10.1152/ajpheart.1994.266.5.H1687. [DOI] [PubMed] [Google Scholar]
- 50.Miyoshi Y, Nakaya Y. Angiotensin II blocks ATP-sensitive K+ channels in porcine coronary artery smooth muscle cells. Biochem Biophys Res Commun. 1991;181:700–706. doi: 10.1016/0006-291x(91)91247-a. [DOI] [PubMed] [Google Scholar]
- 51.Wakatsuki T, Nakaya Y, Inoue I. Vasopressin modulates K(+)-channel activities of cultured smooth muscle cells from porcine coronary artery. Am J Physiol. 1992;263:H491–496. doi: 10.1152/ajpheart.1992.263.2.H491. [DOI] [PubMed] [Google Scholar]
- 52.Furspan PB, Webb RC. Decreased ATP sensitivity of a K+ channel and enhanced vascular smooth muscle relaxation in genetically hypertensive rats. J Hypertens. 1993;11:1067–1072. doi: 10.1097/00004872-199310000-00010. [DOI] [PubMed] [Google Scholar]
- 53.Zhang HL, Bolton TB. Two types of ATP-sensitive potassium channels in rat portal vein smooth muscle cells. Br J Pharmacol. 1996;118:105–114. doi: 10.1111/j.1476-5381.1996.tb15372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cole WC, Malcolm T, Walsh MP, Light PE. Inhibition by protein kinase C of the K(NDP) subtype of vascular smooth muscle ATP-sensitive potassium channel. Circ Res. 2000;87:112–117. doi: 10.1161/01.res.87.2.112. [DOI] [PubMed] [Google Scholar]
- 55.Cole WC, Clement-Chomienne O. ATP-sensitive K+ channels of vascular smooth muscle cells. J Cardiovasc Electrophysiol. 2003;14:94–103. doi: 10.1046/j.1540-8167.2003.02376.x. [DOI] [PubMed] [Google Scholar]
- 56.Aguilar-Bryan L, Nichols CG, Rajan AS, Parker C, Bryan J. Co-expression of sulfonylurea receptors and KATP channels in hamster insulinoma tumor (HIT) cells. Evidence for direct association of the receptor with the channel. J Biol Chem. 1992;267:14934–14940. [PubMed] [Google Scholar]
- 57.Ashcroft FM, Harrison DE, Ashcroft SJ. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature. 1984;312:446–448. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- 58.Inagaki N, Gonoi T, Clement JPt, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- 59.Farzaneh T, Tinker A. Differences in the mechanism of metabolic regulation of ATP-sensitive K+ channels containing Kir6.1 and Kir6.2 subunits. Cardiovasc Res. 2008;79:621–631. doi: 10.1093/cvr/cvn138. [DOI] [PubMed] [Google Scholar]
- 60.Isomoto S, Kondo C, Yamada M, Matsumoto S, Higashiguchi O, Horio Y, Matsuzawa Y, Kurachi Y. A novel sulfonylurea receptor forms with BIR (Kir6.2) a smooth muscle type ATP-sensitive K+ channel. Journal of Biological Chemistry. 1996;271:24321–24324. doi: 10.1074/jbc.271.40.24321. [DOI] [PubMed] [Google Scholar]
- 61.Satoh E, Yamada M, Kondo C, Repunte VP, Horio Y, Iijima T, Kurachi Y. Intracellular nucleotide-mediated gating of SUR/Kir6.0 complex potassium channels expressed in a mammalian cell line and its modification by pinacidil. Journal of Physiology. 1998;511:663–674. doi: 10.1111/j.1469-7793.1998.663bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Babenko AP, Bryan J. A conserved inhibitory and differential stimulatory action of nucleotides on K(IR)6.0/SUR complexes is essential for excitation-metabolism coupling by K(ATP) channels. J Biol Chem. 2001;276:49083–49092. doi: 10.1074/jbc.M108763200. [DOI] [PubMed] [Google Scholar]
- 63.Trube G, Hescheler J. Inward-rectifying channels in isolated patches of the heart cell membrane: ATP-dependence and comparison with cell-attached patches. Pflugers Archiv European Journal of Physiology. 1984;401:178–184. doi: 10.1007/BF00583879. [DOI] [PubMed] [Google Scholar]
- 64.Janigro D, West GA, Gordon EL, Winn HR. ATP-sensitive K+ channels in rat aorta and brain microvascular endothelial cells. American Journal of Physiology. 1993 doi: 10.1152/ajpcell.1993.265.3.C812. [DOI] [PubMed] [Google Scholar]
- 65.Malester B, Tong X, Ghiu I, Kontogeorgis A, Gutstein DE, Xu J, Hendricks-Munoz KD, Coetzee WA. Transgenic expression of a dominant negative KATP channel subunit in the mouse endothelium: effects on coronary flow and endothelin-1 secretion. Faseb J. 2007 doi: 10.1096/fj.06-7821com. Published online March 6, 2007. [DOI] [PubMed] [Google Scholar]
- 66.Katnik C, Adams DJ. An ATP-sensitive potassium conductance in rabbit arterial endothelial cells. Journal of Physiology. 1995;485:595–606. doi: 10.1113/jphysiol.1995.sp020755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Katnik C, Adams DJ. Characterization of ATP-sensitive potassium channels in freshly dissociated rabbit aortic endothelial cells. American Journal of Physiology. 1997;272:H2507–2511. doi: 10.1152/ajpheart.1997.272.5.H2507. [DOI] [PubMed] [Google Scholar]
- 68.Mederos y, Schnitzler M, Derst C, Daut J, Preisig-Muller R. ATP-sensitive potassium channels in capillaries isolated from guinea-pig heart. J Physiol. 2000;525(Pt 2):307–317. doi: 10.1111/j.1469-7793.2000.t01-1-00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Inoue I, Nagase H, Kishi K, Higuti T. ATP-sensitive K+ channel in the mitochondrial inner membrane. Nature. 1991;352:244–247. doi: 10.1038/352244a0. [DOI] [PubMed] [Google Scholar]
- 70.Paucek P, Yarov-Yarovoy V, Sun X, Garlid KD. Inhibition of the mitochondrial KATP channel by long-chain acyl-CoA esters and activation by guanine nucleotides. J Biol Chem. 1996;271:32084–32088. doi: 10.1074/jbc.271.50.32084. [DOI] [PubMed] [Google Scholar]
- 71.Garlid KD, Paucek P, Yarov-Yarovoy V, Sun X, Schindler PA. The mitochondrial KATP channel as a receptor for potassium channel openers. J Biol Chem. 1996;271:8796–8799. doi: 10.1074/jbc.271.15.8796. [DOI] [PubMed] [Google Scholar]
- 72.Liu Y, Ren G, O’Rourke B, Marban E, Seharaseyon J. Pharmacological comparison of native mitochondrial K(ATP) channels with molecularly defined surface K(ATP) channels. Mol Pharmacol. 2001;59:225–230. [PubMed] [Google Scholar]
- 73.Hu H, Sato T, Seharaseyon J, Liu Y, Johns DC, O’Rourke B, Marban E. Pharmacological and histochemical distinctions between molecularly defined sarcolemmal KATP channels and native cardiac mitochondrial KATP channels. Mol Pharmacol. 1999;55:1000–1005. [PubMed] [Google Scholar]
- 74.Suzuki M, Sasaki N, Miki T, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, Seino S, Marban E, Nakaya H. Role of sarcolemmal K(ATP) channels in cardioprotection against ischemia/reperfusion injury in mice. J Clin Invest. 2002;109:509–516. doi: 10.1172/JCI14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cuong DV, Kim N, Joo H, Youm JB, Chung JY, Lee Y, Park WS, Kim E, Park YS, Han J. Subunit composition of ATP-sensitive potassium channels in mitochondria of rat hearts. Mitochondrion. 2005;5:121–133. doi: 10.1016/j.mito.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 76.Zhou M, Tanaka O, Sekiguchi M, He HJ, Yasuoka Y, Itoh H, Kawahara K, Abe H. ATP-sensitive K(+)-channel subunits on the mitochondria and endoplasmic reticulum of rat cardiomyocytes. Journal of Histochemical Cytochemistry. 2005 Jun 27; doi: 10.1369/jhc.5A6736.2005. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Singh H, Hudman D, Lawrence CL, Rainbow RD, Lodwick D, Norman RI. Distribution of Kir6.0 and SUR2 ATP-sensitive potassium channel subunits in isolated ventricular myocytes.[see comment] Journal of Molecular & Cellular Cardiology. 2003;35:445–459. doi: 10.1016/s0022-2828(03)00041-5. [DOI] [PubMed] [Google Scholar]
- 78.Suzuki M, Kotake K, Fujikura K, Inagaki N, Suzuki T, Gonoi T, Seino S, Takata K. Kir6.1: a possible subunit of ATP-sensitive K+ channels in mitochondria. Biochemical & Biophysical Research Communications. 1997;241:693–697. doi: 10.1006/bbrc.1997.7891. [DOI] [PubMed] [Google Scholar]
- 79.Foster DB, Rucker JJ, Marban E. Is Kir6.1 a subunit of mitoK(ATP)? Biochem Biophys Res Commun. 2008;366:649–656. doi: 10.1016/j.bbrc.2007.11.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chutkow WA, Samuel V, Hansen PA, Pu J, Valdivia CR, Makielski JC, Burant CF. Disruption of Sur2-containing KATP channels enhances insulin-stimulated glucose uptake in skeletal muscle. Proc Natl Acad Sci U S A. 2001;98:11760–11764. doi: 10.1073/pnas.201390398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pu J, Wada T, Valdivia C, Chutkow WA, Burant CF, Makielski JC. Evidence of KATP channels in native cardiac cells without SUR. Biophysical Journal. 2001;80:625a–626a. [Google Scholar]
- 82.Pu JL, Ye B, Kroboth SL, McNally EM, Makielski JC, Shi NQ. Cardiac sulfonylurea receptor short form-based channels confer a glibenclamide-insensitive K(ATP) activity. Journal of Molecular & Cellular Cardiology. 2008;44:188–200. doi: 10.1016/j.yjmcc.2007.09.010. Epub 2007 Sep 2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ye B, Kroboth SL, Pu JL, Sims JJ, Aggarwal NT, McNally EM, Makielski JC, Shi NQ. Molecular identification and functional characterization of a mitochondrial sulfonylurea receptor 2 splice variant generated by intraexonic splicing. Circ Res. 2009;105:1083–1093. doi: 10.1161/CIRCRESAHA.109.195040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Elrod JW, Harrell M, Flagg TP, Gundewar S, Magnuson MA, Nichols CG, Coetzee WA, Lefer DJ. Role of sulfonylurea receptor type 1 subunits of ATP-sensitive potassium channels in myocardial ischemia/reperfusion injury. Circulation. 2008;117:1405–1413. doi: 10.1161/CIRCULATIONAHA.107.745539. [DOI] [PubMed] [Google Scholar]
- 85.Stoller DA, Fahrenbach JP, Chalupsky K, Tan BH, Aggarwal N, Metcalfe J, Hadhazy M, Shi NQ, Makielski JC, McNally EM. Cardiomyocyte sulfonylurea receptor 2-KATP channel mediates cardioprotection and ST segment elevation. Am J Physiol Heart Circ Physiol. 2010;299:H1100–1108. doi: 10.1152/ajpheart.00084.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aggarwal NT, Pravdic D, McNally EM, Bosnjak ZJ, Shi NQ, Makielski JC. The mitochondrial bioenergetic phenotype for protection from cardiac ischemia in SUR2 mutant mice. Am J Physiol Heart Circ Physiol. 2010;299:H1884–1890. doi: 10.1152/ajpheart.00363.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hanley PJ, Mickel M, Loffler M, Brandt U, Daut J. K(ATP) channel-independent targets of diazoxide and 5-hydroxydecanoate in the heart. J Physiol. 2002;542:735–741. doi: 10.1113/jphysiol.2002.023960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ardehali H, Chen Z, Ko Y, Mejia-Alvarez R, Marban E. Multiprotein complex containing succinate dehydrogenase confers mitochondrial ATP-sensitive K+ channel activity. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:11880–11885. doi: 10.1073/pnas.0401703101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ardehali H, O’Rourke B, Marban E. Cardioprotective role of the mitochondrial ATP-binding cassette protein 1. Circulation Research. 2005;97:740–742. doi: 10.1161/01.RES.0000186277.12336.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Foster DB, Ho AS, Rucker JJ, Garlid AO, Chen L, Sidor A, Garlid KD, O’Rourke B. Mitochondrial ROMK Channel Is a Molecular Component of MitoKATP. Circulation Research. 2012;111:446–454. doi: 10.1161/CIRCRESAHA.112.266445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lederer WJ, Nichols CG, Smith GL. The mechanism of early contractile failure of isolated rat ventricular myocytes subjected to complete metabolic inhibition. Journal of Physiology. 1989;413:329–349. doi: 10.1113/jphysiol.1989.sp017657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. American Journal of Physiology. 1995;268:C799–822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- 93.Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol. 1990;259:C3–18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- 94.Nelson MT, Standen NB, Brayden JE, Worley JFd. Noradrenaline contracts arteries by activating voltage-dependent calcium channels. Nature. 1988;336:382–385. doi: 10.1038/336382a0. [DOI] [PubMed] [Google Scholar]
- 95.Daut J, Standen NB, Nelson MT. The role of the membrane potential of endothelial and smooth muscle cells in the regulation of coronary blood flow. J Cardiovasc Electrophysiol. 1994;5:154–181. doi: 10.1111/j.1540-8167.1994.tb01156.x. [DOI] [PubMed] [Google Scholar]
- 96.Coleman HA, Tare M, Parkington HC. Endothelial potassium channels, endothelium-dependent hyperpolarization and the regulation of vascular tone in health and disease. Clin Exp Pharmacol Physiol. 2004;31:641–649. doi: 10.1111/j.1440-1681.2004.04053.x. [DOI] [PubMed] [Google Scholar]
- 97.Miki T, Seino S. Roles of KATP channels as metabolic sensors in acute metabolic changes. Journal of Molecular & Cellular Cardiology. 2005;38:917–925. doi: 10.1016/j.yjmcc.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 98.Nichols CG, Koster JC, Remedi MS. beta-cell hyperexcitability: from hyperinsulinism to diabetes. Diabetes Obes Metab. 2007;9(Suppl 2):81–88. doi: 10.1111/j.1463-1326.2007.00778.x. [DOI] [PubMed] [Google Scholar]
- 99.Bao L, Kefaloyianni E, Lader J, Hong M, Morley G, Fishman GI, Sobie EA, Coetzee WA. Unique properties of the ATP-sensitive K channel in the mouse ventricular cardiac conduction system. Circ Arrhythm Electrophysiol. 2011;4:926–935. doi: 10.1161/CIRCEP.111.964643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Haissaguerre M, Chatel S, Sacher F, Weerasooriya R, Probst V, Loussouarn G, Horlitz M, Liersch R, Schulze-Bahr E, Wilde A, Kaab S, Koster J, Rudy Y, Le Marec H, Schott JJ. Ventricular fibrillation with prominent early repolarization associated with a rare variant of KCNJ8/KATP channel. J Cardiovasc Electrophysiol. 2009;20:93–98. doi: 10.1111/j.1540-8167.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- 101.Delaney JT, Muhammad R, Blair MA, Kor K, Fish FA, Roden DM, Darbar D. A KCNJ8 mutation associated with early repolarization and atrial fibrillation. Europace. 14:1428–1432. doi: 10.1093/europace/eus150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Medeiros-Domingo A, Tan BH, Crotti L, Tester DJ, Eckhardt L, Cuoretti A, Kroboth SL, Song C, Zhou Q, Kopp D, Schwartz PJ, Makielski JC, Ackerman MJ. Gain-of-function mutation S422L in the KCNJ8-encoded cardiac K(ATP) channel Kir6.1 as a pathogenic substrate for J-wave syndromes. Heart Rhythm. 2010;7:1466–1471. doi: 10.1016/j.hrthm.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Barajas-Martinez H, Hu D, Ferrer T, Onetti CG, Wu Y, Burashnikov E, Boyle M, Surman T, Urrutia J, Veltmann C, Schimpf R, Borggrefe M, Wolpert C, Ibrahim BB, Sanchez-Chapula JA, Winters S, Haissaguerre M, Antzelevitch C. Molecular genetic and functional association of Brugada and early repolarization syndromes with S422L missense mutation in KCNJ8. Heart Rhythm. doi: 10.1016/j.hrthm.2011.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tester DJ, Tan BH, Medeiros-Domingo A, Song C, Makielski JC, Ackerman MJ. Loss-of-function mutations in the KCNJ8-encoded Kir6.1 K(ATP) channel and sudden infant death syndrome. Circ Cardiovasc Genet. 4:510–515. doi: 10.1161/CIRCGENETICS.111.960195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Babenko AP, Polak M, Cave H, Busiah K, Czernichow P, Scharfmann R, Bryan J, Aguilar-Bryan L, Vaxillaire M, Froguel P. Activating mutations in the ABCC8 gene in neonatal diabetes mellitus.[see comment] New England Journal of Medicine. 2006;355:456–466. doi: 10.1056/NEJMoa055068. [DOI] [PubMed] [Google Scholar]
- 106.Proks P, Arnold AL, Bruining J, Girard C, Flanagan SE, Larkin B, Colclough K, Hattersley AT, Ashcroft FM, Ellard S. A heterozygous activating mutation in the sulphonylurea receptor SUR1 (ABCC8) causes neonatal diabetes. Hum Mol Genet. 2006;15:1793–1800. doi: 10.1093/hmg/ddl101. [DOI] [PubMed] [Google Scholar]
- 107.Ellard S, Flanagan SE, Girard CA, Patch AM, Harries LW, Parrish A, Edghill EL, Mackay DJ, Proks P, Shimomura K, Haberland H, Carson DJ, Shield JP, Hattersley AT, Ashcroft FM. Permanent neonatal diabetes caused by dominant, recessive, or compound heterozygous SUR1 mutations with opposite functional effects. Am J Hum Genet. 2007;81:375–382. doi: 10.1086/519174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bienengraeber M, Olson TM, Selivanov VA, Kathmann EC, O’Cochlain F, Gao F, Karger AB, Ballew JD, Hodgson DM, Zingman LV, Pang YP, Alekseev AE, Terzic A. ABCC9 mutations identified in human dilated cardiomyopathy disrupt catalytic KATP channel gating. Nature Genetics. 2004;36:382–387. doi: 10.1038/ng1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Olson TM, Alekseev AE, Moreau C, Liu XK, Zingman LV, Miki T, Seino S, Asirvatham SJ, Jahangir A, Terzic A. KATP channel mutation confers risk for vein of Marshall adrenergic atrial fibrillation. Nature Clinical Practice Cardiovascular Medicine. 2007;4:110–116. doi: 10.1038/ncpcardio0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.van Bon BW, Gilissen C, Grange DK, Hennekam RC, Kayserili H, Engels H, Reutter H, Ostergaard JR, Morava E, Tsiakas K, Isidor B, Le Merrer M, Eser M, Wieskamp N, de Vries P, Steehouwer M, Veltman JA, Robertson SP, Brunner HG, de Vries BB, Hoischen A. Cantu syndrome is caused by mutations in ABCC9. Am J Hum Genet. 2012;90:1094–1101. doi: 10.1016/j.ajhg.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cantu JM, Garcia-Cruz D, Sanchez-Corona J, Hernandez A, Nazara Z. A distinct osteochondrodysplasia with hypertrichosis-Individualization of a probable autosomal recessive entity. Hum Genet. 1982;60:36–41. doi: 10.1007/BF00281261. [DOI] [PubMed] [Google Scholar]
- 112.Grange DK, Lorch SM, Cole PL, Singh GK. Cantu syndrome in a woman and her two daughters: Further confirmation of autosomal dominant inheritance and review of the cardiac manifestations. Am J Med Genet A. 2006;140:1673–1680. doi: 10.1002/ajmg.a.31348. [DOI] [PubMed] [Google Scholar]
- 113.Harakalova M, van Harssel JJ, Terhal PA, van Lieshout S, Duran K, Renkens I, Amor DJ, Wilson LC, Kirk EP, Turner CL, Shears D, Garcia-Minaur S, Lees MM, Ross A, Venselaar H, Vriend G, Takanari H, Rook MB, van der Heyden MA, Asselbergs FW, Breur HM, Swinkels ME, Scurr IJ, Smithson SF, Knoers NV, van der Smagt JJ, Nijman IJ, Kloosterman WP, van Haelst MM, van Haaften G, Cuppen E. Dominant missense mutations in ABCC9 cause Cantu syndrome. Nat Genet. 2012;44:793–796. doi: 10.1038/ng.2324. [DOI] [PubMed] [Google Scholar]
- 114.Scurr I, Wilson L, Lees M, Robertson S, Kirk E, Turner A, Morton J, Kidd A, Shashi V, Stanley C, Berry M, Irvine AD, Goudie D, Turner C, Brewer C, Smithson S. Cantu syndrome: report of nine new cases and expansion of the clinical phenotype. Am J Med Genet A. 155A:508–518. doi: 10.1002/ajmg.a.33885. [DOI] [PubMed] [Google Scholar]
- 115.Garcia-Cruz D, Mampel A, Echeverria MI, Vargas AL, Castaneda-Cisneros G, Davalos-Rodriguez N, Patino-Garcia B, Garcia-Cruz MO, Castaneda V, Cardona EG, Marin-Solis B, Cantu JM, Nunez-Reveles N, Moran-Moguel C, Thavanati PK, Ramirez-Garcia S, Sanchez-Corona J. Cantu syndrome and lymphoedema. Clin Dysmorphol. 20:32–37. doi: 10.1097/MCD.0b013e32833d015c. [DOI] [PubMed] [Google Scholar]
- 116.Kobayashi D, Cook AL, Williams DA. Pulmonary hypertension secondary to partial pulmonary venous obstruction in a child with Cantu syndrome. Pediatr Pulmonol. 45:727–729. doi: 10.1002/ppul.21215. [DOI] [PubMed] [Google Scholar]
- 117.Engels H, Bosse K, Ehrbrecht A, Zahn S, Hoischen A, Propping P, Bindl L, Reutter H. Further case of Cantu syndrome: exclusion of cryptic subtelomeric chromosome aberrations. Am J Med Genet. 2002;111:205–209. doi: 10.1002/ajmg.10560. [DOI] [PubMed] [Google Scholar]
- 118.Lazalde B, Sanchez-Urbina R, Nuno-Arana I, Bitar WE, de Lourdes Ramirez-Duenas M. Autosomal dominant inheritance in Cantu syndrome (congenital hypertrichosis, osteochondrodysplasia, and cardiomegaly) Am J Med Genet. 2000;94:421–427. doi: 10.1002/1096-8628(20001023)94:5<421::aid-ajmg15>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 119.Concolino D, Formicola S, Camera G, Strisciuglio P. Congenital hypertrichosis, cardiomegaly, and osteochondrodysplasia (Cantu syndrome): a new case with unusual radiological findings. Am J Med Genet. 2000;92:191–194. [PubMed] [Google Scholar]
- 120.Robertson SP, Kirk E, Bernier F, Brereton J, Turner A, Bankier A. Congenital hypertrichosis, osteochondrodysplasia, and cardiomegaly: Cantu syndrome. Am J Med Genet. 1999;85:395–402. [PubMed] [Google Scholar]
- 121.Rosser EM, Kaariainen H, Hurst JA, Baraitser M, Hall CM, Clayton P, Leonard JV. Three patients with the osteochondrodysplasia and hypertrichosis syndrome--Cantu syndrome. Clin Dysmorphol. 1998;7:79–85. doi: 10.1097/00019605-199804000-00001. [DOI] [PubMed] [Google Scholar]
- 122.Nevin NC, Mulholland HC, Thomas PS. Congenital hypertrichosis, cardiomegaly and mild osteochondrodysplasia. Am J Med Genet. 1996;66:33–38. doi: 10.1002/(SICI)1096-8628(19961202)66:1<33::AID-AJMG8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 123.Garcia-Cruz D, Mampel A, Echeverria MI, Vargas AL, Castaneda-Cisneros G, Davalos-Rodriguez N, Patino-Garcia B, Garcia-Cruz MO, Castaneda V, Cardona EG, Marin-Solis B, Cantu JM, Nunez-Reveles N, Moran-Moguel C, Thavanati PK, Ramirez-Garcia S, Sanchez-Corona J. Cantu syndrome and lymphoedema. Clin Dysmorphol. 2011;20:32–37. doi: 10.1097/MCD.0b013e32833d015c. [DOI] [PubMed] [Google Scholar]
- 124.Pennisi AJ, Takahashi M, Bernstein BH, Singsen BH, Uittenbogaart C, Ettenger RB, Malekzadeh MH, Hanson V, Fine RN. Minoxidil therapy in children with severe hypertension. J Pediatr. 1977;90:813–819. doi: 10.1016/s0022-3476(77)81260-2. [DOI] [PubMed] [Google Scholar]
- 125.Mehta PK, Mamdani B, Shansky RM, Mahurkar SD, Dunea G. Severe hypertension. Treatment with minoxidil. JAMA. 1975;233:249–252. [PubMed] [Google Scholar]
- 126.Meisheri KD, Cipkus LA, Taylor CJ. Mechanism of action of minoxidil sulfate-induced vasodilation: a role for increased K+ permeability. J Pharmacol Exp Ther. 1988;245:751–760. [PubMed] [Google Scholar]
- 127.Winquist RJ, Heaney LA, Wallace AA, Baskin EP, Stein RB, Garcia ML, Kaczorowski GJ. Glyburide blocks the relaxation response to BRL 34915 (cromakalim), minoxidil sulfate and diazoxide in vascular smooth muscle. J Pharmacol Exp Ther. 1989;248:149–156. [PubMed] [Google Scholar]
- 128.Kaler SG, Patrinos ME, Lambert GH, Myers TF, Karlman R, Anderson CL. Hypertrichosis and congenital anomalies associated with maternal use of minoxidil. Pediatrics. 1987;79:434–436. [PubMed] [Google Scholar]
- 129.Rosa FW, Idanpaan-Heikkila J, Asanti R. Fetal minoxidil exposure. Pediatrics. 1987;80:120. [PubMed] [Google Scholar]
- 130.Schneider DJ, Moore JW. Patent ductus arteriosus. Circulation. 2006;114:1873–1882. doi: 10.1161/CIRCULATIONAHA.105.592063. [DOI] [PubMed] [Google Scholar]
- 131.Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O’Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I, Martin I, Nordet P. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation. 1996;93:841–842. doi: 10.1161/01.cir.93.5.841. [DOI] [PubMed] [Google Scholar]
- 132.Mehta PA, Dubrey SW. High output heart failure. QJM. 2009;102:235–241. doi: 10.1093/qjmed/hcn147. [DOI] [PubMed] [Google Scholar]
- 133.Rossi A, Cantisani C, Melis L, Iorio A, Scali E, Calvieri S. Minoxidil use in dermatology, side effects and recent patents. Recent Pat Inflamm Allergy Drug Discov. 6:130–136. doi: 10.2174/187221312800166859. [DOI] [PubMed] [Google Scholar]
- 134.Shorter K, Farjo NP, Picksley SM, Randall VA. Human hair follicles contain two forms of ATP-sensitive potassium channels, only one of which is sensitive to minoxidil. FASEB J. 2008;22:1725–1736. doi: 10.1096/fj.07-099424. [DOI] [PubMed] [Google Scholar]
- 135.Seino S, Iwanaga T, Nagashima K, Miki T. Diverse roles of K(ATP) channels learned from Kir6.2 genetically engineered mice. Diabetes. 2000;49:311–318. doi: 10.2337/diabetes.49.3.311. [DOI] [PubMed] [Google Scholar]
- 136.Miki T, Suzuki M, Shibasaki T, Uemura H, Sato T, Yamaguchi K, Koseki H, Iwanaga T, Nakaya H, Seino S. Mouse model of Prinzmetal angina by disruption of the inward rectifier Kir6.1. Nat Med. 2002;8:466–472. doi: 10.1038/nm0502-466. [DOI] [PubMed] [Google Scholar]
- 137.Chutkow WA, Pu J, Wheeler MT, Wada T, Makielski JC, Burant CF, McNally EM. Episodic coronary artery vasospasm and hypertension develop in the absence of Sur2 K(ATP) channels.[see comment] Journal of Clinical Investigation. 2002;110:203–208. doi: 10.1172/JCI15672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Faivre JF, Findlay I. Effects of tolbutamide, glibenclamide and diazoxide upon action potentials recorded from rat ventricular muscle. Biochim Biophys Acta. 1989;984:1–5. doi: 10.1016/0005-2736(89)90334-9. [DOI] [PubMed] [Google Scholar]
- 139.Li RA, Leppo M, Miki T, Seino S, Marban E. Molecular basis of electrocardiographic ST-segment elevation. Circ Res. 2000;87:837–839. doi: 10.1161/01.res.87.10.837. [DOI] [PubMed] [Google Scholar]
- 140.Suzuki M, Li RA, Miki T, Uemura H, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, Ogura T, Seino S, Marban E, Nakaya H. Functional roles of cardiac and vascular ATP-sensitive potassium channels clarified by Kir6.2-knockout mice. Circ Res. 2001;88:570–577. doi: 10.1161/01.res.88.6.570. [DOI] [PubMed] [Google Scholar]
- 141.Cole WC, McPherson CD, Sontag D. ATP-regulated K+ channels protect the myocardium against ischemia/reperfusion damage. Circ Res. 1991;69:571–581. doi: 10.1161/01.res.69.3.571. [DOI] [PubMed] [Google Scholar]
- 142.Venkatesh N, Lamp ST, Weiss JN. Sulfonylureas, ATP-sensitive K+ channels, and cellular K+ loss during hypoxia, ischemia, and metabolic inhibition in mammalian ventricle. Circ Res. 1991;69:623–637. doi: 10.1161/01.res.69.3.623. [DOI] [PubMed] [Google Scholar]
- 143.McPherson CD, Pierce GN, Cole WC. Ischemic cardioprotection by ATP-sensitive K+ channels involves high-energy phosphate preservation. Am J Physiol. 1993;265:H1809–1818. doi: 10.1152/ajpheart.1993.265.5.H1809. [DOI] [PubMed] [Google Scholar]
- 144.Zingman LV, Hodgson DM, Bast PH, Kane GC, Perez-Terzic C, Gumina RJ, Pucar D, Bienengraeber M, Dzeja PP, Miki T, Seino S, Alekseev AE, Terzic A. Kir6.2 is required for adaptation to stress. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:13278–13283. doi: 10.1073/pnas.212315199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yamada S, Kane GC, Behfar A, Liu XK, Dyer RB, Faustino RS, Miki T, Seino S, Terzic A. Protection conferred by myocardial ATP-sensitive K+ channels in pressure overload-induced congestive heart failure revealed in KCNJ11 Kir6.2-null mutant. J Physiol. 2006;577:1053–1065. doi: 10.1113/jphysiol.2006.119511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hu X, Xu X, Huang Y, Fassett J, Flagg TP, Zhang Y, Nichols CG, Bache RJ, Chen Y. Disruption of sarcolemmal ATP-sensitive potassium channel activity impairs the cardiac response to systolic overload. Circ Res. 2008;103:1009–1017. doi: 10.1161/CIRCRESAHA.107.170795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kane GC, Behfar A, Dyer RB, O’Cochlain DF, Liu XK, Hodgson DM, Reyes S, Miki T, Seino S, Terzic A. KCNJ11 gene knockout of the Kir6.2 KATP channel causes maladaptive remodeling and heart failure in hypertension. Hum Mol Genet. 2006;15:2285–2297. doi: 10.1093/hmg/ddl154. [DOI] [PubMed] [Google Scholar]
- 148.Stoller D, Kakkar R, Smelley M, Chalupsky K, Earley JU, Shi NQ, Makielski JC, McNally EM. Mice lacking sulfonylurea receptor 2 (SUR2) ATP-sensitive potassium channels are resistant to acute cardiovascular stress. Journal of Molecular & Cellular Cardiology. 2007;43:445–454. doi: 10.1016/j.yjmcc.2007.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ellis JA, Lamantia A, Chavez R, Scurrah KJ, Nichols CG, Harrap SB. Genes controlling postural changes in blood pressure: comprehensive association analysis of ATP-sensitive potassium channel genes KCNJ8 and ABCC9. Physiol Genomics. 2009;40:184–188. doi: 10.1152/physiolgenomics.00173.2009. [DOI] [PubMed] [Google Scholar]
- 150.Duan R, Cui W, Wang H. Mutational analysis of the Kir6.1 gene in Chinese hypertensive patients treated with the novel ATP-sensitive potassium channel opener iptakalim. Exp Ther Med. 2:757–760. doi: 10.3892/etm.2011.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Harrap SB, Cui JS, Wong ZY, Hopper JL. Familial and genomic analyses of postural changes in systolic and diastolic blood pressure. Hypertension. 2004;43:586–591. doi: 10.1161/01.HYP.0000118044.84189.44. [DOI] [PubMed] [Google Scholar]
- 152.Yoshida H, Feig JE, Morrissey A, Ghiu IA, Artman M, Coetzee WA. K ATP channels of primary human coronary artery endothelial cells consist of a heteromultimeric complex of Kir6.1, Kir6.2, and SUR2B subunits. J Mol Cell Cardiol. 2004;37:857–869. doi: 10.1016/j.yjmcc.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 153.Gendron ME, Thorin E, Perrault LP. Loss of endothelial KATP channel-dependent, NO-mediated dilation of endocardial resistance coronary arteries in pigs with left ventricular hypertrophy. Br J Pharmacol. 2004;143:285–291. doi: 10.1038/sj.bjp.0705937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kakkar R, Ye B, Stoller DA, Smelley M, Shi NQ, Galles K, Hadhazy M, Makielski JC, McNally EM. Spontaneous coronary vasospasm in KATP mutant mice arises from a smooth muscle-extrinsic process. Circ Res. 2006;98:682–689. doi: 10.1161/01.RES.0000207498.40005.e7. [DOI] [PubMed] [Google Scholar]
- 155.Nichols CG, Koster JC. Diabetes and insulin secretion: whither KATP? Am J Physiol Endocrinol Metab. 2002;283:E403–412. doi: 10.1152/ajpendo.00168.2002. [DOI] [PubMed] [Google Scholar]
- 156.Koster JC, Marshall BA, Ensor N, Corbett JA, Nichols CG. Targeted overactivity of beta cell K(ATP) channels induces profound neonatal diabetes. Cell. 2000;100:645–654. doi: 10.1016/s0092-8674(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 157.Gloyn AL, Pearson ER, Antcliff JF, Proks P, Bruining GJ, Slingerland AS, Howard N, Srinivasan S, Silva JM, Molnes J, Edghill EL, Frayling TM, Temple IK, Mackay D, Shield JP, Sumnik Z, van Rhijn A, Wales JK, Clark P, Gorman S, Aisenberg J, Ellard S, Njolstad PR, Ashcroft FM, Hattersley AT. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. New England Journal of Medicine. 2004;350:1838–1849. doi: 10.1056/NEJMoa032922. [DOI] [PubMed] [Google Scholar]
- 158.Flagg TP, Charpentier F, Manning-Fox J, Remedi MS, Enkvetchakul D, Lopatin A, Koster J, Nichols C. Remodeling of excitation-contraction coupling in transgenic mice expressing ATP-insensitive sarcolemmal KATP channels. American Journal of Physiology - Heart & Circulatory Physiology. 2004;286:H1361–1369. doi: 10.1152/ajpheart.00676.2003. [DOI] [PubMed] [Google Scholar]
- 159.Rajashree R, Koster JC, Markova KP, Nichols CG, Hofmann PA. Contractility and ischemic response of hearts from transgenic mice with altered sarcolemmal K(ATP) channels. Am J Physiol Heart Circ Physiol. 2002;283:H584–590. doi: 10.1152/ajpheart.00107.2002. [DOI] [PubMed] [Google Scholar]
- 160.Koster JC, Knopp A, Flagg TP, Markova KP, Sha Q, Enkvetchakul D, Betsuyaku T, Yamada KA, Nichols CG. Tolerance for ATP-insensitive K(ATP) channels in transgenic mice. Circ Res. 2001;89:1022–1029. doi: 10.1161/hh2301.100342. [DOI] [PubMed] [Google Scholar]
- 161.Flagg TP, Patton B, Masia R, Mansfield C, Lopatin AN, Yamada KA, Nichols CG. Arrhythmia susceptibility and premature death in transgenic mice overexpressing both SUR1 and Kir6.2[DeltaN30,K185Q] in the heart. Am J Physiol Heart Circ Physiol. 2007;293:H836–845. doi: 10.1152/ajpheart.00011.2007. [DOI] [PubMed] [Google Scholar]
- 162.Toib A, Zhang HX, Broekelmann TJ, Hyrc KL, Guo Q, Chen F, Remedi MS, Nichols CG. Cardiac specific ATP-sensitive K(+) channel (K(ATP)) overexpression results in embryonic lethality. J Mol Cell Cardiol. 53:437–445. doi: 10.1016/j.yjmcc.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Masia R, Enkvetchakul D, Nichols C. Differential nucleotide regulation of K(ATP) channels by SUR1 and SUR2A. Journal of Molecular & Cellular Cardiology. 2005;39:491–501. doi: 10.1016/j.yjmcc.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 164.Goldberg MR. Clinical pharmacology of pinacidil, a prototype for drugs that affect potassium channels. J Cardiovasc Pharmacol. 1988;12(Suppl 2):S41–47. doi: 10.1097/00005344-198812002-00008. [DOI] [PubMed] [Google Scholar]
- 165.Buhl AE, Waldon DJ, Conrad SJ, Mulholland MJ, Shull KL, Kubicek MF, Johnson GA, Brunden MN, Stefanski KJ, Stehle RG, et al. Potassium channel conductance: a mechanism affecting hair growth both in vitro and in vivo. J Invest Dermatol. 1992;98:315–319. doi: 10.1111/1523-1747.ep12499788. [DOI] [PubMed] [Google Scholar]
- 166.Avatapalle B, Banerjee I, Malaiya N, Padidela R. Echocardiography monitoring for diazoxide induced pericardial effusion. BMJ Case Rep. 2012 doi: 10.1136/bcr.03.2012.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shanti B, Silink M, Bhattacharya K, Howard NJ, Carpenter K, Fietz M, Clayton P, Christodoulou J. Congenital disorder of glycosylation type Ia: Heterogeneity in the clinical presentation from multivisceral failure to hyperinsulinaemic hypoglycaemia as leading symptoms in three infants with phosphomannomutase deficiency. J Inherit Metab Dis. 2009 doi: 10.1007/s10545-009-1180-2. [DOI] [PubMed] [Google Scholar]
- 168.Ito H, Taniyama Y, Iwakura K, Nishikawa N, Masuyama T, Kuzuya T, Hori M, Higashino Y, Fujii K, Minamino T. Intravenous nicorandil can preserve microvascular integrity and myocardial viability in patients with reperfused anterior wall myocardial infarction. J Am Coll Cardiol. 1999;33:654–660. doi: 10.1016/s0735-1097(98)00604-4. [DOI] [PubMed] [Google Scholar]
- 169.Koster JC, Sha Q, Nichols CG. Sulfonylurea and K(+)-channel opener sensitivity of K(ATP) channels. Functional coupling of Kir6.2 and SUR1 subunits. J Gen Physiol. 1999;114:203–213. doi: 10.1085/jgp.114.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Schramm TK, Gislason GH, Vaag A, Rasmussen JN, Folke F, Hansen ML, Fosbol EL, Kober L, Norgaard ML, Madsen M, Hansen PR, Torp-Pedersen C. Mortality and cardiovascular risk associated with different insulin secretagogues compared with metformin in type 2 diabetes, with or without a previous myocardial infarction: a nationwide study. Eur Heart J. 32:1900–1908. doi: 10.1093/eurheartj/ehr077. [DOI] [PubMed] [Google Scholar]
- 171.Gore MO, McGuire DK. Resolving drug effects from class effects among drugs for type 2 diabetes mellitus: more support for cardiovascular outcome assessments. Eur Heart J. 32:1832–1834. doi: 10.1093/eurheartj/ehr019. [DOI] [PubMed] [Google Scholar]
- 172.Pearson ER, Flechtner I, Njolstad PR, Malecki MT, Flanagan SE, Larkin B, Ashcroft FM, Klimes I, Codner E, Iotova V, Slingerland AS, Shield J, Robert JJ, Holst JJ, Clark PM, Ellard S, Sovik O, Polak M, Hattersley AT. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N.Engl.J.Med. 2006;355:467–477. doi: 10.1056/NEJMoa061759. [DOI] [PubMed] [Google Scholar]
- 173.Billman GE, Englert HC, Scholkens BA. HMR 1883, a novel cardioselective inhibitor of the ATP-sensitive potassium channel. Part II: effects on susceptibility to ventricular fibrillation induced by myocardial ischemia in conscious dogs. J Pharmacol Exp Ther. 1998;286:1465–1473. [PubMed] [Google Scholar]
- 174.Wirth KJ, Uhde J, Rosenstein B, Englert HC, Gogelein H, Scholkens BA, Busch AE. K(ATP) channel blocker HMR 1883 reduces monophasic action potential shortening during coronary ischemia in anesthetised pigs. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:155–160. doi: 10.1007/s002109900166. [DOI] [PubMed] [Google Scholar]
- 175.Manning Fox JE, Kanji HD, French RJ, Light PE. Cardioselectivity of the sulphonylurea HMR 1098: studies on native and recombinant cardiac and pancreatic K(ATP) channels. Br J Pharmacol. 2002;135:480–488. doi: 10.1038/sj.bjp.0704455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Weyermann A, Vollert H, Busch AE, Bleich M, Gogelein H. Inhibitors of ATP-sensitive potassium channels in guinea pig isolated ischemic hearts. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:374–381. doi: 10.1007/s00210-004-0882-0. [DOI] [PubMed] [Google Scholar]
- 177.Glukhov AV, Flagg TP, Fedorov VV, Efimov IR, Nichols CG. Differential K(ATP) channel pharmacology in intact mouse heart. J Mol Cell Cardiol. 48:152–160. doi: 10.1016/j.yjmcc.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhang HX, Akrouh A, Kurata HT, Remedi MS, Lawton JS, Nichols CG. HMR 1098 is not an SUR isotype specific inhibitor of heterologous or sarcolemmal K ATP channels. J Mol Cell Cardiol. 2010;50:552–560. doi: 10.1016/j.yjmcc.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Flagg TP, Nichols CG. Sarcolemmal K(ATP) channels: what do we really know? J Mol Cell Cardiol. 2005;39:61–70. doi: 10.1016/j.yjmcc.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 180.Flagg TP, Nichols CG. “Cardiac KATP”: a family of ion channels. Circ Arrhythm Electrophysiol. 2011;4:796–798. doi: 10.1161/CIRCEP.111.968081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Neagoe I, Schwappach B. Pas de deux in groups of four--the biogenesis of KATP channels. J Mol Cell Cardiol. 2005;38:887–894. doi: 10.1016/j.yjmcc.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 182.Flanagan SE, Clauin S, Bellanne-Chantelot C, de Lonlay P, Harries LW, Gloyn AL, Ellard S. Update of mutations in the genes encoding the pancreatic beta-cell K(ATP) channel subunits Kir6.2 (KCNJ11) and sulfonylurea receptor 1 (ABCC8) in diabetes mellitus and hyperinsulinism. Hum Mutat. 2009;30:170–180. doi: 10.1002/humu.20838. [DOI] [PubMed] [Google Scholar]
- 183.Villareal DT, Koster JC, Robertson H, Akrouh A, Miyake K, Bell GI, Patterson BW, Nichols CG, Polonsky KS. Kir6.2 variant E23K increases ATP-sensitive K+ channel activity and is associated with impaired insulin release and enhanced insulin sensitivity in adults with normal glucose tolerance. Diabetes. 2009;58:1869–1878. doi: 10.2337/db09-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Schwanstecher C, Meyer U, Schwanstecher M. K(IR)6.2 polymorphism predisposes to type 2 diabetes by inducing overactivity of pancreatic beta-cell ATP-sensitive K(+) channels. Diabetes. 2002;51:875–879. doi: 10.2337/diabetes.51.3.875. [DOI] [PubMed] [Google Scholar]
- 185.Gloyn AL, Hashim Y, Ashcroft SJ, Ashfield R, Wiltshire S, Turner RC. Association studies of variants in promoter and coding regions of beta- cell ATP-sensitive K-channel genes SUR1 and Kir6.2 with Type 2 diabetes mellitus (UKPDS 53) Diabet Med. 2001;18:206–212. doi: 10.1046/j.1464-5491.2001.00449.x. [DOI] [PubMed] [Google Scholar]
- 186.Hani EH, Boutin P, Durand E, Inoue H, Permutt MA, Velho G, Froguel P. Missense mutations in the pancreatic islet beta cell inwardly rectifying K+ channel gene (KIR6.2/BIR): a meta-analysis suggests a role in the polygenic basis of Type II diabetes mellitus in Caucasians. Diabetologia. 1998;41:1511–1515. doi: 10.1007/s001250051098. [DOI] [PubMed] [Google Scholar]
- 187.Inoue H, Ferrer J, Warren-Perry M, Zhang Y, Millns H, Turner RC, Elbein SC, Hampe CL, Suarez BK, Inagaki N, Seino S, Permutt MA. Sequence variants in the pancreatic islet beta-cell inwardly rectifying K+ channel Kir6.2 (Bir) gene: identification and lack of role in Caucasian patients with NIDDM. Diabetes. 1997;46:502–507. doi: 10.2337/diab.46.3.502. [DOI] [PubMed] [Google Scholar]
- 188.Sakura H, Wat N, Horton V, Millns H, Turner RC, Ashcroft FM. Sequence variations in the human Kir6.2 gene, a subunit of the beta-cell ATP-sensitive K-channel: no association with NIDDM in while Caucasian subjects or evidence of abnormal function when expressed in vitro. Diabetologia. 1996;39:1233–1236. doi: 10.1007/BF02658512. [DOI] [PubMed] [Google Scholar]