Abstract
Evidence is mounting that a multi-gene kinase network is central to the regulation of renal Na+ and K+ excretion and that aberrant signalling through the pathway can result in renal sodium retention and hypertension. The kinase network minimally includes the Ste20-related proline-alanine-rich kinase, (SPAK), the With-No-Lysine (WNK) kinases, WNK4 and WNK1, and their effectors, the thiazide-sensitive NaCl cotransporter and the potassium secretory channel, ROMK. Available evidence indicates the kinase network normally functions as switch to change the mineralocorticoid hormone response of the kidney to either conserve sodium or excrete potassium, depending on whether aldosterone is induced by a change in dietary sodium or potassium. Recently, common genetic variants in the SPAK gene have been identified as hypertension susceptibility factors in the general population, suggesting that altered WNK-SPAK signaling plays an important role in essential hypertension. Here, we highlight recent breakthroughs in this emerging field and discuss areas of consensus and uncertainty.
Introduction
Hypertension (HTN) is a substantial public health problem, affecting over a billion people on the planet. It is a major independent risk factor for myocardial infarction, stroke, and end stage renal disease. The pathogenesis of essential HTN remains unknown, but epidemiological studies point to complex genetic and environmental factors. Genes play a major role in HTN susceptibility, with the heritability of blood pressure (BP) levels estimated to be 30–35%. Major environmental triggers include obesity and diet, especially high Na+ and low K+ dietary intake. The recent discovery of SPAK (Ste20-related proline-alanine-rich kinase, also known as serine threnione kinase 39 or STK39) as a HTN susceptibility gene in the general population1 together with the known involvement of With-No-Lysine (WNK) kinases in a rare familial disorder of HTN and hyperkalemia now casts light on the potential importance of a multi-gene kinase network in the genesis of HTN. In fact, a flood of recent studies are beginning to shed light on the mechanisms by which dietary salt intake influences renal Na+ transport and how mutations in these kinases and ion transport genes may lead to BP dysregulation. The objective of this minireview is to highlight recent breakthroughs and to discuss areas of consensus and uncertainty in this emerging field.
The Role of the Kidney in HTN
Physiologists have long appreciated the central role of renal salt excretion in the control of BP.2 Maintenance of a constant intravascular fluid volume and BP depends on the ability of the kidneys to regulate urinary Na+ excretion. The concerted action of several parallel Na+ transport mechanisms allows urinary Na+ excretion to match a wide range of Na+ dietary intakes, while minimizing fluctuations in the intravascular fluid volume and arterial BP. Although abnormal function of any one renal salt transport mechanism can usually be compensated for, a greater than normal increase in arterial BP must occur in order for urinary Na+ excretion to be brought into balance with an increased Na+ intake. Alfred Guyton, coined the term for this change in BP as the “set-point” for controlling Na+ excretion.2
Genetic Evidence Linking Renal Salt Handling to BP Regulation
Mutations in ~ 20 different genes have been linked with known single-gene forms of hereditary HTN or hypotension.3 Astonishingly, all of these genes encode molecules that control the ability of the kidney to maintain salt balance, reiterating the importance of this physiologic pathway in regulating BP. The distal Na+ channel ENaC and the thiazide-sensitive NaCl cotransporter (NCC) play a central role in BP regulation. Loss-of-function mutations in ENaC can cause the hypotension of Pseudohypoaldosteronism (PHA) Type I while gain-of-function mutations result in HTN (Liddle syndrome). Loss-of-function mutations in NCC cause Gitelman’s disease,4 an autosomal recessive salt losing nephropathy characterized by hypotension, hypokalemic metabolic alkalosis and altered divalent cation homeostasis. Conversely, HTN in PHA Type II (Gordon’s syndrome) ultimately results from a gain-in-function by NCC. This rare autosomal dominant disease of hypertension and hyperkalemia results from mutations in two With No (K) Lysine Kinase genes, WNK1 or WNK4.5 In the general population, rare heterozygous loss-of-function mutations in NCC produces clinically significant reductions in BP and protection from HTN.6 Moreover, studies from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), the largest clinical BP lowering trial in history, clearly demonstrated morbidity and mortality lowering benefits of inhibiting NCC in the treatment of essential HTN.7 Together, with recent genomic wide association studies identifying an important kinase regulator of renal salt transport, SPAK, as a HTN susceptibility gene1 it seems likely that essential HTN may be born out a combination of common and rare susceptibility alleles, that affect the ability the kidney to appropriately respond to dietary salt.
Sensitivity to Dietary Na+ and K+
As predicted by the Guyton model, dietary salt (NaCl) loading is well established to increase BP in humans. The best evidence comes from tightly controlled, dose-response trials in large populations of subjects. For example, the Dietary Approaches to Stop HTN trial (DASH) convincingly revealed a dose-dependent decrease in BP in response to Na+ reduction regardless of whether the subjects were hypertensive or non hypertensive.8 It should be pointed out that the reduction in BP is heterogenous,9 with individuals exhibiting a continuous spectrum of responses to a reduction in dietary Na+. In general, the effect of Na+ on BP is greatest in Blacks and individuals with chronic kidney disease or HTN. Thus, it is not just dietary Na+ that determines BP but genetic and other factors have an important impact.10 One of the most important of these other factors is dietary K+.11 High dietary K+ intake is associated with reduced BP. Furthermore, the rise in BP for a given increase in Na+ intake is blunted in the setting of a high K+ intake. For these reasons, recommended dietary regimens to reduce HTN, such as the DASH diet, provide a relatively high level of K+.10
The scientific basis for the response to K+ is not understood. However, the discovery that the WNK-SPAK kinase network regulates renal Na+ and K+ transport provides a clue that the impact of K+ diet on BP might be due to a physiological switch that alters the response of the kidney to either conserve Na+ or excrete K+, depending on whether secretion of the mineralocorticoid hormone, aldosterone, is induced by a change in dietary Na+ or K+. How renal Na+ reabsorption and K+ excretion are coordinately regulated has long been a puzzle because aldosterone controls both processes. In states of intravascular volume contraction (e.g. low Na+ diet), angiotensin II (AngII) stimulates aldosterone release to maximize renal NaCl reabsorption and restore BP. On the other hand, when aldosterone is released in response to a rise in plasma K+ (e.g. high K+ diet), it stimulates maximum K+ excretion without major effects on renal Na+ handling.
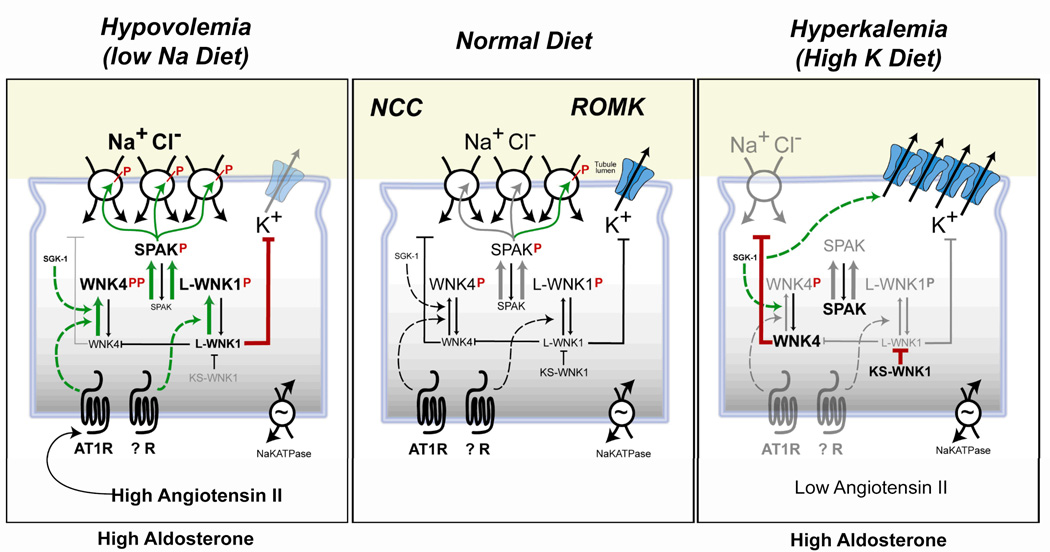
Studies of how PHAII causes both HTN and hyperkalemia have cast light onto how the kidney appropriately responds to aldosterone. Patients with the disease exhibit both excessive Na+ retention and impaired K+ excretion despite normal aldosterone and otherwise normal renal function. The discovery that mutations in WNK1 or WNK4 cause PHAII5 revealed a signaling system that may switch the balance between Na+ reabsorption and K+ excretion. In PHAII, mutations appear to aberrantly switch the kinases into the “hypovolemia” position and divorce the system from physiologic control, causing deleterious Na+ and K+ retention regardless of volume or K+ status. Although concepts about WNK signaling in the kidney are still emerging, present evidence suggests the WNK kinases converge with SPAK kinase to switch the mineralocorticoid hormone response of the kidney to be either Na+ retaining (antinatriuretic) or K+ excreting (kaliuretic). This likely occurs because the kinase pathway differently regulates the thiazide-sensitive Na+ chloride co-transporter, NCC12 and the K+ excretory channel, ROMK13 (Figure 1).
Figure 1. Model of the WNK/SPAK signal transduction system in the distal nephron.
switching the aldosterone response of the kidney to be antinatriuretic (left) or kaliuretic (right). Green arrow heads (activating pathways), red blunt end (inhibitory pathway). Left panel shows the pathway in the setting of low Na+ diet when Ang II and SGK-1 signaling leads to phosphorylation of WNK4. This stimulates phosphorylation of SPAK, which, in turn, phosphorylates NCC, activating Na+ transport to enhance conservation of Na+ in hypovolemia. Stimulation of unknown receptors are suspected to cause phosphorylation of L-WNK1 which can also stimulate SPAK phosphorylation. L-WNK1 has other functions: a) It blocks the NCC-inhibitory form of WNK4. b) It inhibits secretion of K+ via ROMK channels so as to, conserve K+ despite high aldosterone levels. Right panel shows the pathway in the setting of high dietary K+ intake, when aldosterone is stimulated and angiotensin is low. In the absence of sufficient AngII, ATR1 can not activate WNK4. This reduces SPAK activation and NCC phosphorylation. At the same time, dietary potassium loading increases the KS-WNK1 isoform to suppress the activity of L-WNK1. Consequently, the full inhibitory power of WNK4 on NCC becomes unleashed, blocking traffic of NCC to the apical membrane and thereby reducing NCC surface density. KS-WNK1 also blocks the effect of L-WNK1 on ROMK endocytosis, causing ROMK to increase at the apical membrane. In this way, K+ secretion in the DCT and CNT/CCD is maximized while NCC is suppressed. Aldosterone stimulation of ENaC (not shown) offsets the decreased Na+ reabsorption by NCC, allowing robust potassium secretion without changes in sodium balance. (Not shown for clarity are WNK3, which is believed to antagonize the inhibitory effects of WNK4 on NCC; and the possible WNK4 effects on ROMK).
Regulation of NCC by the WNK-SPAK Switch
NCC is the principal determinant of renal NaCl reabsorption in the distal convoluted tubule (DCT).14 It is tightly regulated by aldosterone and AngII, allowing Na+ reabsorption in the DCT to be modulated in accord with the demands of salt balance and intravascular pressure. Multiple studies suggest that members of the WNK kinase family converge with SPAK or possibly the related kinase, oxidative stress response kinase 1 (OSR1), to transmit and integrate the aldosterone and AngII response by controlling NCC transport activity.15,16 Work in model systems and genetically engineered mouse models revealed that wild-type WNK4 has the capacity to reduce NCC expression at the plasmalemma. Because PHAII missense mutations in WNK4 (WNK4PHAII) block this inhibitory activity,17;18 the apical expression and activity of the transporter are thought to increase in the disease. Moreover, a WNK4PHAII mouse model shows stimulation of SPAK/OSR1-dependent phosphorylation of NCC to enhance transport activity.19 Both factors -- increased apical membrane expression and increased activity of NCC—likely explain Na+ retention in PHAII. The effects of WNK4 on ROMK are somewhat more controversial20 (also see below), but early work in model systems indicated that WNK4PHAII mutations affect ROMK oppositely from NCC. Consequently, Kahle and collaborators postulated that WNK4 might physiologically shuttle between two states.21 They suggested that if WNK4 switched from its basal state to a mode that is mimicked by WNK4PHAII, it would tune the aldosterone response so that normally it restores intravascular volume without perturbing K+ balance.
The physiological mediators of the putative WNK4 switch have only begun to be identified but recent work highlights the importance of AngII. Since AngII levels are high in the hyperaldosterone-state of hypovolemia (or low salt diet) but are low in hyperkalemia, signaling through the AngII receptor, AT1R, has been a chief suspect in switching WNK4 into a mode that simultaneously activates NCC and inhibits ROMK. Consistent with this idea, AngII stimulates NCC22 and inhibits ROMK in vivo.23 Exciting recent studies reveal that AngII stimulation of NCC can be reconstituted in Xenopus oocytes when WNK4 and SPAK are included in the system.24 Together, with observations that AngII signaling increases phosphorylation of key sites on SPAK and NCC in a WNK4-dependent manner, it is likely that WNK4-SPAK are the chief arbiters of the signaling pathway between AT1R and NCC. According to this model, activation of the AT1R pathway converts WNK4 from an inhibiting mode to an NCC-activating form (Figure 1). The activating form of WNK4 induces a phosphorylation cascade, whereby WNK4 phosphorylates SPAK and then phospho-SPAK phosphorylates and activates NCC. The stimulatory effects of mutant WNK4PHAII are not augmented by AngII, consistent with the mutations having a gain-of-function effect resulting in constitutive activation of the signaling pathway.
Unlike WNK4, WNK1 is not able to mediate the AT1R signaling response.24 However, under certain conditions, such as osmotic stress, WNK1 can phosphorylate and activate SPAK.25;26 Based on these observations, it seems reasonable to hypothesize that a WNK1-dependent SPAK activation pathway may also be involved in stimulating NCC under certain physiological settings. Work in cell culture systems indicate the involvement of the PKB/PI3-kinase signaling pathway in activating WNK1.27;28 Such a pathway may be responsible for linking insulin to the regulation of NCC,29 but thus far suitable studies rigorously connecting these observations are lacking. To date, attention has largely focused on the ability of WNK1 isoforms to regulate NCC through their modulation of WNK4 (see below).
In fact, WNK4 appears be a focal point of regulation by other kinases as well,30–32 allowing the signal transduction pathway to be precisely tuned to meet different physiological demands of salt balance. For example, WNK3 enhances the transport activity of NCC31;33 via a SPAK-independent phosphorylation mechanism.34 Moreover, recent studies in heterologous expression systems and knockout mice indicate the aldosterone-induced kinase, SGK-1, modulates WNK4 activity so as to increase NCC abundance, phosphorylation and activity.30;35 The dual requirement of SGK-1 and ATR1 signaling for complete activation of WNK4 would provide a mechanism to integrate the effects of high aldosterone and AngII for appropriate activation NCC in hypovolemia.
It should be pointed out that an important detail of the model has recently been called into question. Based on the finding that NCC protein abundance is not augmented in genetically modified WNK4 mice, which bear a targeted deletion of exons 7–8, it has been argued that WNK4 may not usually function as an inhibitor of NCC in vivo.36 In fact, because these mice display reduced NCC phosphorylation and a reduced renal Na+ reabsorptive capacity, a case has been made for the idea that WNK4 only operates as an activator of NCC in vivo. According to this view, WNK4PHAII mutations only enhance the normal activity of the kinase.
While these new studies with the so-called hypomorphic WNK4 mice are very interesting and should not be dismissed, they are difficult to square with numerous reports that WNK4 can inhibit NCC.17;18;24;37–39 Even a modest over expression of two extra WT WNK4 transgenes leads to a dramatic decrease in NCC in vivo.40 Furthermore, RNAi-mediated knockdown of endogenous WNK4 in human embryonic kidney cells increases NCC.41 In our minds, the switch model shown in Figure 1 provides a way to reconcile the different findings but several key issues must be carefully examined. Chief among them, it remains to be tested what function is carried by exon 7–8. It is conceivable, for example, that this region of the kinase is not required for the inhibitory effects of WNK4. The switch model, points to another possibility. To date, only the NCC activation limb--low salt diet-- has been tested in the WNK4 hypomorphic mice. It will be important to learn if these mice also lose their ability to down regulate NCC in physiological settings such as in hyperkalemia35 as the model predicts.
WNK Regulation of K+ Balance
The physiologic uncoupling of aldosterone-dependent K+ secretion from Na+ reabsorption has been traditionally explained by the Na+ and flow-dependent nature of K+ excretion. In states of intravascular volume depletion, for example, stimulation of NCC at the DCT should diminish the supply of Na+ that can be delivered downstream to the connecting tubule (CNT) and cortical collecting duct (CCD) for efficient Na+/ K+ exchange, mediated by the epithelial Na+ channel, ENaC, and the K+ secretory channels, ROMK and BK.42 The textbook explanation is not completely satisfactory, however. It does not adequately explain how the kidney stimulates maximum K+ excretion without major effects on renal Na+ handling when aldosterone is elevated by increases in plasma K+. The classic teaching also overlooks that a significant fraction of K+ secretion likely occurs in the late DCT, especially when animals are fed a high- K+ diet. In fact, a K+ secretory pathway that is not dependent on ENaC has recently been described.43 Accumulating evidence favors the idea that a WNK signaling pathway may operate in parallel with the classic factors to achieve a robust kaliuretic response to a K+ load without altering Na+ balance.
The signaling response to K+ is just beginning to be unraveled. L-WNK1, WNK3 and WNK4 all down regulate ROMK at the cell surface in heterologous expression systems, likely by stimulating clathrin-dependent endocytosis. But, so far, only one WNK gene has emerged as a significant player in vivo. Studies in genetically modified WNK4 mice,19;40 for example, have been interpreted to indicate that WNK4 may not play a role in the physiological regulation of ROMK, but further studies with better, more specific ROMK antibodies and definitive tests of ROMK function are desperately required to test this hypothesis rigorously. WNK3 is not associated with PHAII and its physiological relevance in K+ balance has not been studied. By contrast, accumulating evidence indicates that products of the WNK1 gene are likely to operate as a key arbiter of the switch response to K+ diet.
Alternative promoter usage of the WNK1 gene produces a kidney-specific short form of WNK1,44;45 called KS-WNK1, and a more ubiquitous long form, called L-WNK1. Unlike L-WNK1, the kinase deficient KS-WNK1 form has no inhibitory effect on ROMK.46;47 Instead, KS-WNK1 negatively modulates L-WNK1 to suppress ROMK channel endocytosis. In fact, the apical surface expression of ROMK in the distal nephron is enhanced in transgenic mice that over express KS-WNK1.48 Most importantly, the relative abundance of the WNK1 isoforms is regulated by dietary K+. Because acute dietary K+ loading increases the relative abundance of KS-WNK1 while dietary K+ restriction increases the relative abundance of L-WNK1, the WNK1 isoform switch is well positioned to serve an important role in the physiologic regulation of ROMK apical surface density and K+ balance.46;47
Importantly, the two WNK1 isoforms regulate NCC differently than ROMK, providing a mechanism to maintain Na+ balance in the high aldosterone state of dietary K+ loading. Heterologous expression studies reveal that L-WNK1 upregulates NCC by blocking the inhibitory form of WNK4. KS-WNK1, on the other hand, antagonizes the effects of L-WNK1, and therefore indirectly inhibits NCC.38 Consequently, the full inhibitory power of WNK4 on NCC may become unleashed in states of dietary K+ loading, when KS-WNK1 expression is augmented and there is insufficient AngII to switch WNK4 into the SPAK-activating form. Down regulation of NCC in the hyperaldosterone-state of high dietary K+ together with inhibition of Na+ transport in the thick ascending limb,49 would counter balance enhanced ENaC-dependent Na+ reabsorption in the CNT and CCD, and help preserve Na+ homeostasis. Thus a chief prediction of the linkage between K+ and Na+ balance is that transport by NCC should be down regulated in response to dietary K+ loading as illustrated in Figure 1. Recent studies have indeed shown that the expression and phosphorylation of NCC are reduced by high K+ diet.35
Recent studies reveal a potential mechanism to explain how WNKs differently regulate NCC and ROMK, involving modulation of two distinct trafficking pathways. ROMK contains an unusual variant of the “NPXY” internalization signal, which serves as a docking site for the clathrin adaptor molecule, ARH.50 WNK-1 stimulates ROMK endocytosis through an ARH-dependent pathway. Unlike ROMK, NCC does not contain NPXY type signals and, therefore, does not have the capacity to engage ARH. In fact, Subramanya et al. recently discovered that WNK4 does not stimulate NCC endocytosis.41 Instead, WNK4 acts on newly synthesized NCC in the secretory pathway to stimulate interaction with the AP-3 clathrin adaptor and divert the transporter into the lysosomal pathway.
SPAK Regulation
SPAK was identified as a membrane-associated kinase that interacts with cation-chloride cotransporters and WNKs.51 Since this discovery in 2002, work in expression systems and model organisms have clearly established that SPAK directly regulates members of the SLC12 family, including NCC, as a part of an evolutionary conserved signaling pathway important for controlling electroneutral Na+ transport and osmotic cell volume regulation.52 The discovery of SPAK as a HTN susceptibility gene highlights its potential role in the regulation of renal Na+ transport for the control of BP. Based on recent studies, a working model for NCC activation in the kidney (Figure 1) envisages an upstream WNK (either WNK4 or WNK1) phosphorylating and activating SPAK, which in turn phosphorylates and activates the co-transporter. The model is largely based on biochemical studies in expression systems showing that WNK1 or WNK4 directly interact with SPAK and phosphorylate residues, T243 and S383 (mouse numbering).53 While the functional role of S383 phosphorylation is still not known, phosphorylation of T243 is absolutely required for SPAK activation.54 Furthermore, activation of the WNK4-SPAK signaling system in DCT cells in vitro by hypotonic stress or AngII induces phosphorylation of NCC.24 Obviously, future studies are required to test the validity of this model in vivo.
The exclusivity of SPAK in the proposed model has not been established. SPAK is closely related to OSR1 which, like SPAK, is widely expressed in many tissues including kidney.55 SPAK is believed to have evolved from OSR1 by gene duplication.52 OSR1 can also be activated by WNKs and phosphorylate NCC.25;26 While it might be imagined that OSR1 could substitute for SPAK, no change in OSR1 expression is found in SPAK null animals or when SPAK is knocked down by RNAi.56 Moreover, eliminating SPAK from cells that normally express both SPAK and OSR1 results in a distinct impairment of cotransporter function. Thus SPAK and OSR1 are regulated independently and may have distinct functional roles although the exact nature of their roles remains to be defined. In addition, there are reports that signaling via PKC isotypes can activate SPAK in other systems.57;58 While such a role in the kidney has not been evaluated, it is possible that renal SPAK activity is also regulated via non-WNK pathways.
Future Studies
While the scheme described in Figure 1 synthesizes much of the currently available data to present a unified mechanism for SPAK-WNK control of Na+-K balance and blood pressue, we consider it an evolving model, and only a framework for guiding future investigation. Certainly, much of the model needs to be rigorously tested, and much will be learned in the process. In our minds, a convergence of powerful approaches addressing this problem promises to rapidly yield a comprehensive and clinically useful understanding of multigene kinase control of BP in health and disease.
Central to this advance will be genetically modified mouse models that either eliminate or modify kinases of interest. While a critical and powerful approach, such studies need to be interpreted carefully since the kidney has a strong ability to compensate for defective or missing components. Thus, testing the physiological validity of findings from mouse models will require careful parallel studies in normal animals. Evaluation of the effects of altered Na+ and K+ diet on the status of switch elements together with measurements of Na+ and K+ transport should be particularly informative.
Although carefully performed animal studies are necessary, imporant and insightful, they may not offer detailed insights into the underlying cellular, biochemical and molecular mechanisms. Exhaustive mechanistic analysis will require further investigation in model systems, including oocytes and cultured cells. For such studies, it will be critical to complement and validate the standard kinase over-expression experiments with RNAi-mediated knock down approaches. It should be particularly instructive, for example, to replace endogenous components of the signaling pathway with physiological doses of strategically-designed mutants. Vigilant monitoring of the subcellular localization and phosphorylation status of the relevant parts of the signaling network will be crucial to guide interpretation.
Continued genetic analysis in humans promises to be a crucial arm of a multidisciplinary investigation, serving to identify additional variants that influence BP. This approach has been highly successful for rare alleles but association findings for common alleles have been much less consistent. Earlier genome-wide SNP arrays offered uneven and incomplete coverage of variants in genes in this network, particularly WNK4.59 Studies that focus on a single gene or a few genes often study different SNPs, making cross-validation a challenge. But the tide is rapidly turning. Now, newer arrays offer more complete coverage. Data from multiple studies can be more easily combined (>100,000 total subjects) or filled in through imputation, making it likely the next generation of GWAS will yield additional insights.60;61 Together with the application of new high throughput approaches to identify rare variants, assess copy number and look for epigenetic variables such DNA methylation, the field should soon have a more comprehensive view of the genetic basis of BP.
Each of these powerful approaches has both strengths and weaknesses but taken together they should continue to rapidly advance our understanding of the mechanisms underlying renal control of BP.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (DK086817, DK54231 and DK 63049 to PAW, R01HL088120 and R21DK084566 to YC, GM74771 to ED and DK32839 to JBW).
Footnotes
Disclosure
The authors declared no competing interests.
References
- 1.Wang Y, O'Connell JR, McArdle PF, et al. Whole-genome association study identifies STK39 as a hypertension susceptibility gene. Proc Natl Acad Sci U S A. 2009;106:226–231. doi: 10.1073/pnas.0808358106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guyton AC. Blood pressure control--special role of the kidneys and body fluids. Science. 1991;252:1813–1816. doi: 10.1126/science.2063193. [DOI] [PubMed] [Google Scholar]
- 3.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 4.Simon DB, Nelson-Williams C, Bia MJ, et al. Gitelman's variant of Bartter's syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet. 1996;12:24–30. doi: 10.1038/ng0196-24. [DOI] [PubMed] [Google Scholar]
- 5.Wilson FH, Disse-Nicodeme S, Choate KA, et al. Human hypertension caused by mutations in WNK kinases. Science. 2001;293:1107–1112. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- 6.Ji W, Foo JN, O'Roak BJ, et al. Rare independent mutations in renal salt handling genes contribute to blood pressure variation. Nat Genet. 2008;40:592–599. doi: 10.1038/ng.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 8.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 9.Obarzanek E, Proschan MA, Vollmer WM, et al. Individual blood pressure responses to changes in salt intake: results from the DASH-Sodium trial. HYPERTENSION. 2003;42:459–467. doi: 10.1161/01.HYP.0000091267.39066.72. [DOI] [PubMed] [Google Scholar]
- 10.Appel LJ, Brands MW, Daniels SR, et al. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. HYPERTENSION. 2006;47:296–308. doi: 10.1161/01.HYP.0000202568.01167.B6. [DOI] [PubMed] [Google Scholar]
- 11.Lichtenstein AH, Appel LJ, Brands M, et al. Summary of American Heart Association Diet and Lifestyle Recommendations revision 2006. Arterioscler Thromb Vasc Biol. 2006;26:2186–2191. doi: 10.1161/01.ATV.0000238352.25222.5e. [DOI] [PubMed] [Google Scholar]
- 12.Subramanya AR, Yang CL, McCormick JA, Ellison DH. WNK kinases regulate sodium chloride and potassium transport by the aldosterone-sensitive distal nephron. Kidney Int. 2006;70:630–634. doi: 10.1038/sj.ki.5001634. [DOI] [PubMed] [Google Scholar]
- 13.Welling PA, Ho K. A comprehensive guide to the ROMK potassium channel: form and function in health and disease. Am J Physiol Renal Physiol. 2009;297:F849–F863. doi: 10.1152/ajprenal.00181.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reilly RF, Ellison DH. Mammalian distal tubule: physiology, pathophysiology, and molecular anatomy. Physiol Rev. 2000;80:277–313. doi: 10.1152/physrev.2000.80.1.277. [DOI] [PubMed] [Google Scholar]
- 15.McCormick JA, Yang CL, Ellison DH. WNK kinases and renal sodium transport in health and disease: an integrated view. HYPERTENSION. 2008;51:588–596. doi: 10.1161/HYPERTENSIONAHA.107.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richardson C, Alessi DR. The regulation of salt transport and blood pressure by the WNK-SPAK/OSR1 signalling pathway. J Cell Sci. 2008;121:3293–3304. doi: 10.1242/jcs.029223. [DOI] [PubMed] [Google Scholar]
- 17.Wilson FH, Kahle KT, Sabath E, et al. Molecular pathogenesis of inherited hypertension with hyperkalemia: the Na-Cl cotransporter is inhibited by wild-type but not mutant WNK4. Proc Natl Acad Sci U S A. 2003;100:680–684. doi: 10.1073/pnas.242735399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang CL, Angell J, Mitchell R, Ellison DH. WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J Clin Invest. 2003;111:1039–1045. doi: 10.1172/JCI17443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang SS, Morimoto T, Rai T, et al. Molecular pathogenesis of pseudohypoaldosteronism type II: generation and analysis of a Wnk4(D561A/+) knockin mouse model. Cell Metab. 2007;5:331–344. doi: 10.1016/j.cmet.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Huang CL, Yang SS, Lin SH. Mechanism of regulation of renal ion transport by WNK kinases. Curr Opin Nephrol Hypertens. 2008;17:519–525. doi: 10.1097/MNH.0b013e32830dd580. [DOI] [PubMed] [Google Scholar]
- 21.Kahle KT, Wilson FH, Leng Q, et al. WNK4 regulates the balance between renal NaCl reabsorption and K+ secretion. Nat Genet. 2003;35:372–376. doi: 10.1038/ng1271. [DOI] [PubMed] [Google Scholar]
- 22.Sandberg MB, Riquier AD, Pihakaski-Maunsbach K, et al. ANG II provokes acute trafficking of distal tubule Na+-Cl(−) cotransporter to apical membrane. Am J Physiol Renal Physiol. 2007;293:F662–F669. doi: 10.1152/ajprenal.00064.2007. [DOI] [PubMed] [Google Scholar]
- 23.Wei Y, Zavilowitz B, Satlin LM, Wang WH. Angiotensin II inhibits the ROMK-like small conductance K channel in renal cortical collecting duct during dietary potassium restriction. J Biol Chem. 2007;282:6455–6462. doi: 10.1074/jbc.M607477200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.San Cristobal P, Pacheco-Alvarez D, Richardson C, et al. Angiotensin II signaling increases activity of the renal Na-Cl cotransporter through a WNK4-SPAK-dependent pathway. Proc Natl Acad Sci U S A. 2009;106:4384–4389. doi: 10.1073/pnas.0813238106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moriguchi T, Urushiyama S, Hisamoto N, et al. WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J Biol Chem. 2005;280:42685–42693. doi: 10.1074/jbc.M510042200. [DOI] [PubMed] [Google Scholar]
- 26.Zagorska A, Pozo-Guisado E, Boudeau J, et al. Regulation of activity and localization of the WNK1 protein kinase by hyperosmotic stress. J Cell Biol. 2007;176:89–100. doi: 10.1083/jcb.200605093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vitari AC, Deak M, Collins BJ, et al. WNK1, the kinase mutated in an inherited high-blood-pressure syndrome, is a novel PKB (protein kinase B)/Akt substrate. Biochem J. 2004;378:257–268. doi: 10.1042/BJ20031692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu BE, Stippec S, Lazrak A, et al. WNK1 activates SGK1 by a phosphatidylinositol 3-kinase-dependent and non-catalytic mechanism. J Biol Chem. 2005;280:34218–34223. doi: 10.1074/jbc.M505735200. [DOI] [PubMed] [Google Scholar]
- 29.Song J, Hu X, Riazi S, et al. Regulation of blood pressure, the epithelial sodium channel (ENaC), and other key renal sodium transporters by chronic insulin infusion in rats. Am J Physiol Renal Physiol. 2006;290:F1055–F1064. doi: 10.1152/ajprenal.00108.2005. [DOI] [PubMed] [Google Scholar]
- 30.Rozansky DJ, Cornwall T, Subramanya AR, et al. Aldosterone mediates activation of the thiazide-sensitive Na-Cl cotransporter through an SGK1 and WNK4 signaling pathway. J Clin Invest. 2009 doi: 10.1172/JCI38323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang CL, Zhu X, Ellison DH. The thiazide-sensitive Na-Cl cotransporter is regulated by a WNK kinase signaling complex. J Clin Invest. 2007;117:3403–3411. doi: 10.1172/JCI32033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yue P, Lin DH, Pan CY, et al. Src family protein tyrosine kinase (PTK) modulates the effect of SGK1 and WNK4 on ROMK channels. Proc Natl Acad Sci U S A. 2009;106:15061–15066. doi: 10.1073/pnas.0907855106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rinehart J, Kahle KT, de Los HP, et al. WNK3 kinase is a positive regulator of NKCC2 and NCC, renal cation-Cl- cotransporters required for normal blood pressure homeostasis. Proc Natl Acad Sci U S A. 2005;102:16777–16782. doi: 10.1073/pnas.0508303102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glover M, Zuber AM, O'Shaughnessy KM. Renal and brain isoforms of WNK3 have opposite effects on NCCT expression. J Am Soc Nephrol. 2009;20:1314–1322. doi: 10.1681/ASN.2008050542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vallon V, Schroth J, Lang F, et al. Expression and phosphorylation of the Na-Cl-cotransporter NCC in vivo is regulated by dietary salt, potassium and SGK1. Am J Physiol Renal Physiol. 2009;297:F704–F712. doi: 10.1152/ajprenal.00030.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohta A, Rai T, Yui N, et al. Targeted disruption of the Wnk4 gene decreases phosphorylation of Na-Cl cotransporter, increases Na excretion, and lowers blood pressure. Hum Mol Genet. 2009 doi: 10.1093/hmg/ddp344. [DOI] [PubMed] [Google Scholar]
- 37.Yang CL, Zhu X, Wang Z, et al. Mechanisms of WNK1 and WNK4 interaction in the regulation of thiazide-sensitive NaCl cotransport. J Clin Invest. 2005;115:1379–1387. doi: 10.1172/JCI22452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Subramanya AR, Yang CL, Zhu X, Ellison DH. Dominant-negative regulation of WNK1 by its kidney-specific kinase-defective isoform. Am J Physiol Renal Physiol. 2006;290:F619–F624. doi: 10.1152/ajprenal.00280.2005. [DOI] [PubMed] [Google Scholar]
- 39.San Cristobal P, Ponce-Coria J, Vazquez N, et al. WNK3 and WNK4 amino-terminal domain defines their effect on the renal Na+-Cl- cotransporter. Am J Physiol Renal Physiol. 2008;295:F1199–F1206. doi: 10.1152/ajprenal.90396.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lalioti MD, Zhang J, Volkman HM, et al. Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genet. 2006;38:1124–1132. doi: 10.1038/ng1877. [DOI] [PubMed] [Google Scholar]
- 41.Subramanya AR, Liu J, Ellison DH, et al. WNK4 Diverts the Thiazide-sensitive NaCl Cotransporter to the Lysosome and Stimulates AP-3 Interaction. J Biol Chem. 2009;284:18471–18480. doi: 10.1074/jbc.M109.008185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sansom SC, Welling PA. Two channels for one job. Kidney Int. 2007;72:529–530. doi: 10.1038/sj.ki.5002438. [DOI] [PubMed] [Google Scholar]
- 43.Frindt G, Palmer LG. K+ secretion in the rat kidney: Na+ channel-dependent and -independent mechanisms. Am J Physiol Renal Physiol. 2009;297:F389–F396. doi: 10.1152/ajprenal.90528.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delaloy C, Lu J, Houot AM, et al. Multiple promoters in the WNK1 gene: one controls expression of a kidney-specific kinase-defective isoform. Mol Cell Biol. 2003;23:9208–9221. doi: 10.1128/MCB.23.24.9208-9221.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Reilly M, Marshall E, Speirs HJ, Brown RW. WNK1, a gene within a novel blood pressure control pathway, tissue-specifically generates radically different isoforms with and without a kinase domain. J Am Soc Nephrol. 2003;14:2447–2456. doi: 10.1097/01.asn.0000089830.97681.3b. [DOI] [PubMed] [Google Scholar]
- 46.Lazrak A, Liu Z, Huang CL. Antagonistic regulation of ROMK by long and kidney-specific WNK1 isoforms. Proc Natl Acad Sci U S A. 2006;103:1615–1620. doi: 10.1073/pnas.0510609103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wade JB, Fang L, Liu J, et al. WNK1 kinase isoform switch regulates renal potassium excretion. Proc Natl Acad Sci U S A. 2006;103:8558–8563. doi: 10.1073/pnas.0603109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Z, Wang HR, Huang CL. Regulation of ROMK channel and K+ homeostasis by kidney-specific WNK1 kinase. J Biol Chem. 2009;284:12198–12206. doi: 10.1074/jbc.M806551200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stokes JB. Consequences of potassium recycling in the renal medulla. Effects of ion transport by the medullary thick ascending limb of Henle's loop. J Clin Invest. 1982;70:219–229. doi: 10.1172/JCI110609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fang L, Garuti R, Kim BY, et al. The ARH adaptor protein regulates endocytosis of the ROMK potassium secretory channel in mouse kidney. J Clin Invest. 2009;119:3278–3289. doi: 10.1172/JCI37950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piechotta K, Lu J, Delpire E. Cation chloride cotransporters interact with the stress-related kinases Ste20-related proline-alanine-rich kinase (SPAK) and oxidative stress response 1 (OSR1) J Biol Chem. 2002;277:50812–50819. doi: 10.1074/jbc.M208108200. [DOI] [PubMed] [Google Scholar]
- 52.Delpire E, Gagnon KB. SPAK and OSR1, key kinases involved in the regulation of chloride transport. Acta Physiol (Oxf) 2006;187:103–113. doi: 10.1111/j.1748-1716.2006.01565.x. [DOI] [PubMed] [Google Scholar]
- 53.Vitari AC, Deak M, Morrice NA, Alessi DR. The WNK1 and WNK4 protein kinases that are mutated in Gordon's hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases. Biochem J. 2005;391:17–24. doi: 10.1042/BJ20051180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gagnon KB, England R, Delpire E. Characterization of SPAK and OSR1, regulatory kinases of the Na-K-2Cl cotransporter. Mol Cell Biol. 2006;26:689–698. doi: 10.1128/MCB.26.2.689-698.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Piechotta K, Garbarini N, England R, Delpire E. Characterization of the interaction of the stress kinase SPAK with the Na+-K+-2Cl− cotransporter in the nervous system: evidence for a scaffolding role of the kinase. J Biol Chem. 2003;278:52848–52856. doi: 10.1074/jbc.M309436200. [DOI] [PubMed] [Google Scholar]
- 56.Geng Y, Hoke A, Delpire E. The Ste20 kinases Ste20-related proline-alanine-rich kinase and oxidative-stress response 1 regulate NKCC1 function in sensory neurons. J Biol Chem. 2009;284:14020–14028. doi: 10.1074/jbc.M900142200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Y, Hu J, Vita R, et al. SPAK kinase is a substrate and target of PKC theta in T-cell receptor-induced AP-1 activation pathway. EMBO J. 2004;23:1112–1122. doi: 10.1038/sj.emboj.7600125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith L, Smallwood N, Altman A, Liedtke CM. PKC delta acts upstream of SPAK in the activation of NKCC1 by hyperosmotic stress in human airway epithelial cells. J Biol Chem. 2008;283:22147–22156. doi: 10.1074/jbc.M801752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sober S, Org E, Kepp K, et al. Targeting 160 candidate genes for blood pressure regulation with a genome-wide genotyping array. PLoS One. 2009;4:e6034. doi: 10.1371/journal.pone.0006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Newton-Cheh C, Johnson T, Gateva V, et al. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet. 2009;41:666–676. doi: 10.1038/ng.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Levy D, Ehret GB, Rice K, et al. Genome-wide association study of blood pressure and hypertension. Nat Genet. 2009;41:677–687. doi: 10.1038/ng.384. [DOI] [PMC free article] [PubMed] [Google Scholar]