Introduction
Chronic lymphocytic leukemia (CLL) is the most common leukemia in the Western world. An often indolent lymphoproliferative disorder, CLL is characterized by the progressive accumulation of monoclonal, small, mature-appearing CD5+ B-cells in the peripheral blood, bone marrow and secondary lymphoid organs.[1] With the notable exception of allogeneic stem cell transplantation, CLL is currently an incurable disease, despite the fact that good initial responses to chemoimmunotherapy are obtained, which prolong overall survival.[2] Current treatment modalities appear to eradicate malignant CLL cells less efficiently in the bone marrow and lymph nodes than in peripheral blood. Thus, patients who initially achieve a remission will eventually develop recurrent disease. Moreover, the differential response of the disease in different anatomic locations indicates a significant role of the tissue microenvironment in supporting CLL cell survival and enabling them to evade the toxic effects of chemotherapy.
Recent work has demonstrated that the trafficking, survival and proliferation of CLL cells is tightly regulated by the surrounding tissue microenvironment (Figure 1). This conclusion is bolstered by several lines of evidence. When cultured in vitro, CLL cells rapidly undergo apoptosis, but they can be temporarily rescued from programmed cell death by contact with stromal cells. Stromal cells are also known to confer a protective effect against chemotherapy-induced apoptosis.[3, 4] Additionally, there are differences in the characteristics of CLL cells in the various tissue compartments. CLL cells in the peripheral blood are arrested in G0/G1 phase of the cell cycle [5] and display features that are consistent with a defect in programmed cell death and prolonged in vivo survival [1]. Previously, this observation was thought to suggest that CLL is a malignancy of quiescent non-proliferating cells. However, recent data on telomere length[6] and in vivo measurement of CLL cell kinetics demonstrated that CLL cells exhibit a more prominent turnover than previously appreciated [7]. Patients with a higher proliferation rate are more likely to have active disease and clinical progression [7, 8]. This apparent discrepancy is due to the fact that proliferation of CLL cells takes place primarily in the secondary lymphoid tissues, although it also occurs to a lesser extent in the bone marrow, in areas with a vaguely nodular architecture termed ‘pseudofollicles’ or ‘proliferation centers’ [8, 9].
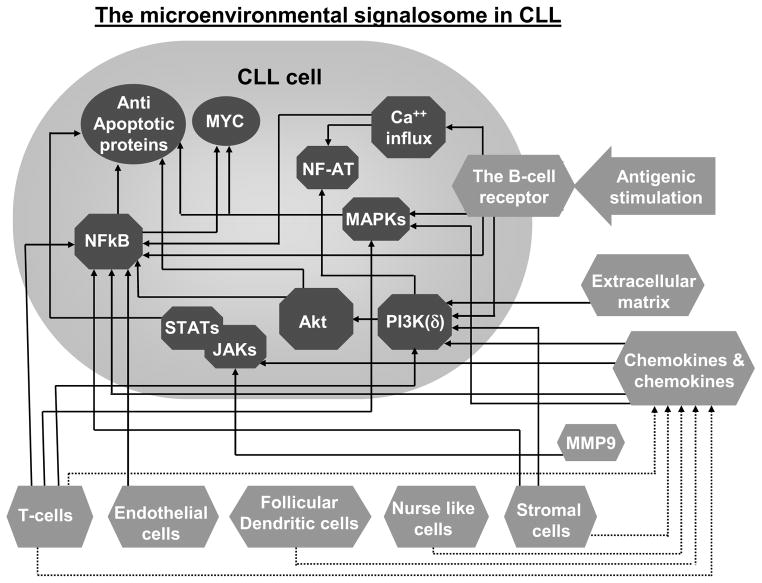
Figure 1.
The CLL microenvironmental signalosome: the convergence of microenvironmental-induced signaling responses into biochemical pathways within CLL cells. Microenvironmental elements (
 ) including cells (e.g. T-cells, nurse-like cells), the extracellular matrix (ECM) and enzymes (e.g. MMP9) stimulate CLL cells either directly (arrows) or via mediators such as cytokines and chemokines (dashed arrows). These extracellular triggers converge into an array of intracellular biochemical responses (
) including cells (e.g. T-cells, nurse-like cells), the extracellular matrix (ECM) and enzymes (e.g. MMP9) stimulate CLL cells either directly (arrows) or via mediators such as cytokines and chemokines (dashed arrows). These extracellular triggers converge into an array of intracellular biochemical responses (
 ), resulting in the up-regulation of MYC and anti-apoptotic proteins (
), resulting in the up-regulation of MYC and anti-apoptotic proteins (
 ), as well as additional cellular responses.
), as well as additional cellular responses.
The significant differences in the properties of the cells in the peripheral blood and lymphoid tissues are, at least in part, explained by antigenic stimulation and close interaction with various accessory cells as well as by exposure to different cytokines, chemokines, and extracellular matrix components (Figure 1). In the last decade there have been major advances in the understanding of the reciprocal interactions between CLL cells and the various microenvironmental compartments. Here, we will discuss the role of the microenvironment in the context of efforts to develop novel therapeutics that target the biology of CLL.
CLL cells in the context of the normal immune system
Normal B cells are programmed to rapidly respond to the environment, while causing little damage to normal tissues. They possess the ability to recognize, process and present foreign antigens to other components of the immune system, and to undergo maturation and eventually secrete antibodies directed at a specific antigen. They can undergo programmed cell death when their role is over. The reciprocal interaction of B cells with the surrounding environment leads to recruitment of cellular elements into specific tissue compartments. Furthermore, B cells migrate to various compartments that regulate their differentiation, proliferation, and survival or apoptosis. This normal immune response is achieved via multiple proteins that are produced by the B cell and the surrounding microenvironmental cells, leading to a well-orchestrated and tightly regulated sequence of events.
It is not surprising that CLL cells, the malignant counterpart of normal B cells, retain the ability to interact with their surrounding environment. However, the finely tuned orchestration and normal compartmentalization of the immune response is altered. The cause of this malignant transformation is most likely a combination of genetic predisposition and environmental triggers, leading to genetic and epigenetic changes resulting in exaggeration of positive signals and attenuation of inhibitory and pro-apoptotic mechanisms.
Interplay between tumor biology and the local microenvironment
Invasion of the primary and second lymphoid tissues by CLL cells disrupts the normal tissue architecture and physiology. The spleen and lymph nodes are diffusely infiltrated by CLL cells, while the bone marrow is involved in an interstitial, nodular and/or diffuse pattern. CLL cells retain the capacity to react to a variety of external stimuli and the tissue microenvironment provides supporting signals that may differ within the various anatomic sites.
CLL cells respond to the surrounding microenvironment in vivo as demonstrated by the activation of specific signaling pathways in the tumor cells in the tissue microenvironment resulting in changes in gene expression, cellular activation, proliferation, and apoptotic threshold [8, 10]. In a genome wide microarray study we found that purified CLL cells isolated concomitantly from peripheral blood, bone marrow, and lymph nodes show characteristic gene expression profiles that reflect differential activation of signaling pathways in the various anatomic compartments.[8] In particular, CLL cells in the lymph node upregulated > 100 genes responsive to BCR activation and NF-κB signaling and involved in proliferation. Several studies reported on comparative measurements of activation markers expressed on CLL cells and their proliferation rates in different anatomic compartments [8, 11–14]. The expression of activation markers such as CD38 and CD69 as well as proliferation is increased in CLL cells in the lymph node and bone marrow compared to circulating cells [8, 11–13]. Likewise, the antiapoptotic regulators BCL-XL, survivin and MCL1 are expressed at higher levels in CLL cells in lymph nodes compared to their counterparts in the peripheral blood [15]. In addition, “apoptotic priming”, which describes the proximity of a cell to the apoptotic threshold, is reduced in bone marrow resident CLL cells [10].
The microenvironment
Antigenic stimulation and B-cell receptor signaling
BCR signaling is a crucial component of normal B-cell development and plays an important role in differentiation, survival, proliferation, and antibody secretion. Conclusive experimental evidence established the importance of BCR signaling in the pathogenesis of activated B cell-like diffuse large B-cell lymphoma (ABC-DLBCL) [16, 17]. While the evidence is somewhat more circumstantial, BCR activation is now also emerging as a central stimulus in the pathogenesis of CLL [18–22]. This view is based on several lines of evidence including specific BCR structures indicating a restricted antigen specificity of CLL cells [23, 24], immunophenotypic characteristics shared with antigen-experienced B-cells [25–27], as well as phenotypic characteristics of anergic B-cells [28, 29], the demonstration of ongoing BCR signaling in vivo [8], and a correlation of increased BCR activation or reactivity with clinical outcome [30].
Based on the presence or absence of somatic mutations in the immunoglobulin heavy chain variable (IGHV) gene expressed by the clonal cells, CLL can be divided into two main subgroups.[31, 32] Expression of a mutated IGHV identifies a subtype that follows a stable or slowly progressive course, while expression of an unmutated IGHV gene is associated with progressive disease and inferior survival [31, 32]. Additionally, CLL cells use a restricted repertoire of IGHV genes, which encode part of the antigen interacting domains of the BCR. Thus, preferential usage of certain IGHV genes indicates a role for antigen selection in the development of the disease [23, 24]. Furthermore, some cases express virtually identical BCRs, so-called “stereotyped BCRs”, that recognize shared antigens [33–35]. These antigens remain incompletely defined but in many cases may be the target antigens of so called polyreactive or natural antibodies, including microbial antigens and autoantigens expressed by dying cells [36–38]. In the case of unmutated CLL, it is thought that the particular molecular motifs involved in tumor development are autoantigens; this view is supported by the observation that the majority of CLL clones exhibiting stereotyped BCRs also demonstrate unmutated IGHV genes, as well as several studies of soluble Igs demonstrating polyautoreactivity in unmutated CLL [39–42]. In contrast, stimulation via foreign antigen is likely to underlie the pathogenesis of mutated CLL, which exhibits less structural restriction of the BCR [41]. It is important to note that at present we have an incomplete understanding of where B-cells encounter antigen. Regardless, the secondary lymphoid tissues are likely to be the major anatomic site for BCR-antigen interaction. Antigens arriving through the lymph flow are sequestered and immobilized by cellular elements in the lymph nodes [43], thus providing an optimal setting for B-cell receptor stimulation. This is consistent with our observation of stronger BCR activation on CLL cells on the lymph nodes as compared to blood or bone marrow [8].
BCR signaling can be broadly divided into two main types; one that is antigen independent or “tonic” [44] and another that is antigen-mediated (Figure 2). “Tonic” signaling is mediated via PI3Kα and PI3Kδ, whereas antigen-dependent signaling involves activation of PI3Kδ in addition to several tyrosine kinases and adapter molecules. Antigen-dependent signaling is initiated by the tyrosine kinases LYN and SYK. In vitro studies have shown that BCR engagement on CLL cells triggers an intracellular signaling cascade leading to calcium mobilization, activation of the MEK/ERK, AKT/mTOR, and NF-κB pathways and upregulation of the anti-apoptotic proteins MCL1, BCL-XL and XIAP (Figure 1) [45–49].
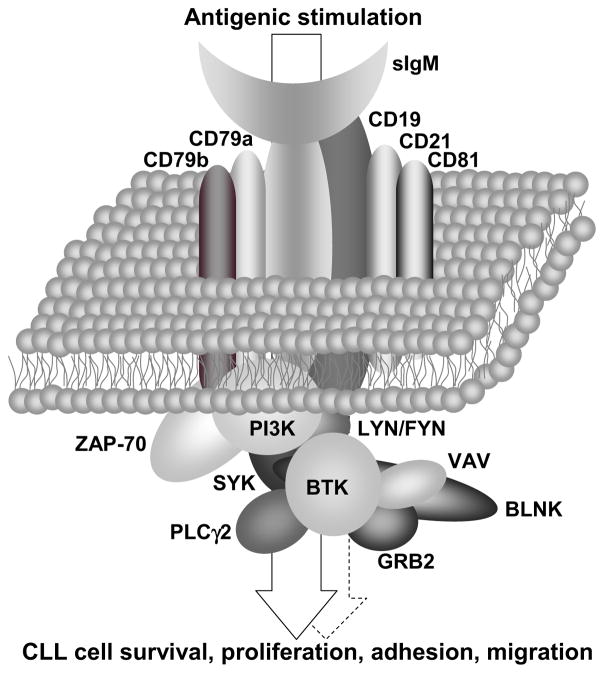
Figure 2.
The B-cell receptor – a signaling complex that delivers microenvironmental-derived information into the CLL cell. The sIgM serves as the backbone of the BCR, and is associated with other transmembrane molecules (e.g. CD19, CD21). The transmembrane components of the BCR associate with a variety of enzymes (e.g. SYK, BTK) and scaffold proteins (e.g. BLNK) to form a signaling complex. This complex translates extracellular cues, predominantly antigenic stimulation, into CLL cellular responses including survival, proliferation, adhesion and migration (arrow). Tonic or cell-autonomous activation (dashed arrow) does not require extracellular stimuli.
Recently a type of BCR signaling that appears to be unique to CLL cells has been described. Specifically, Duhren-von Minden and colleagues showed that epitopes in the framework region of surface immunglobulins (sIg) expressed on CLL cells serve as autoantigens, raising the possibility of auto-stimulation of the leukemic cells [50]. Building upon previous work demonstrating that the pre-BCR is able to induce ligand-independent cell-autonomous signaling by binding to an intrinsic pre-BCR glycosylation site [51], the group found that the BCRs of CLL cells constitutively signal even in the absence of any added antigen. Intriguingly, this type of signaling was equally demonstrated for BCRs derived from mutated and unmutated CLLs, and appeared to be cell-autonomous in that it was demonstrable on isolated individual cells [50]. Notably, such autonomous activation was not found with BCRs derived from multiple myeloma or other lymphoma cells. The heavy-chain complementarity-determining region (HCDR3) of the BCR was identified as the crucial interacting unit, since its insertion into a non-autonomously active BCR resulted in autonomously driven signaling. The authors then showed that the HCDR3 interacts with an intrinsic motif in the framework region 2 (FR2) of the sIg’s VH region [50]. Mutations in this internal FR2 epitope abrogated any autonomous signaling demonstrating that this epitope acts as an autoantigen binding to CLL BCRs. This finding does not in and of itself negate the important role for the classical model of extrinsic antigen in the pathogenesis of CLL. Further study is warranted to define the respective roles of the two modes of BCR signaling in CLL.
The responsiveness of CLL cells to BCR activation in vitro is heterogeneous.[52] IGHV unmutated CLL cells are typically BCR signaling competent whereas IGHV mutated CLL cells respond weakly or not at all to BCR crosslinking induced by anti IgM antibodies [30]. The Zeta-associated protein of 70-Kd (ZAP-70), a transducing signaling kinase downstream of the T-cell receptor, is expressed in most cases of IGHV unmutated CLL but less frequently in IGHV mutated CLL [25, 53–55]. Expression of ZAP-70, akin to IGHV mutation status, serves as a powerful prognostic marker and correlates with a more aggressive disease course [54–57]. ZAP-70 expression is associated with increased BCR signaling in vitro [46]. The non-responsiveness to BCR activation in some CLL cells is reminiscent of anergized B-cells and suggests that these CLL cells are chronically stimulated by antigen in vivo [29, 30]. Consistent with this view is the low expression of surface IgM in CLL compared to normal B-cells and the recovery of BCR responsiveness after prolonged in vitro culture [28]. Furthermore, CLL cells that do not respond to surface IgM crosslinking respond to anti-IgD or anti-CD79a antibodies, indicating that the intracellular signaling pathway is functional [30]. Nevertheless, it has become clear that the presence of ZAP-70 enhances BCR responsiveness [58]. Interestingly, this effect of ZAP-70 is independent of the kinase domain, but requires recruitment of ZAP-70 to the BCR [59, 60]. The demonstration of decreased internalization of an activated BCR in a B-cell line that was engineered to express ZAP-70 provides a link between decreased IgM expression that correlates with absence of ZAP-70 and an anergic phenotype [60]. Thus, one effect of ZAP-70 might be to interfere with anergy by maintaining higher IgM expression.
In vitro, BCR crosslinking protects CLL cells from apoptosis primarily through the PI3k/Akt pathway and increased expression of MCL1 [45, 48, 61]. BCR triggering also up-regulates adhesion and costimulatory molecules, and increases CLL cell migration in response to the chemokines CXCL12 and CXCL13 [62]. Moreover, BCR signaling likely plays a central role in promoting CLL cell proliferation. In vitro engagement of the BCR in CLL cells induces expression of MYC, cyclin D2 and cyclin-dependent kinase 4 (CDK4). Interestingly, although BCR activation promotes G1 cell cycle progression, cell division is not induced [49, 63]. Presumably additional co-stimulatory signals such as CD40L and IL4, provided in the tissue microenvironment, are required.
Cell-cell interactions
Most studies exploring the cellular interactions in CLL have been performed using peripheral blood cells. Experimental methods relying on the investigation of circulating CLL cells in isolation lack the ability to appropriately mimic the complex cellular interactions occurring in the lymphatic niche. In spite of this limitation, many in vitro observations have helped to elucidate the in vivo crosstalk between CLL cells and non-malignant cells [64, 65]. In the tissue microenvironment, CLL cells reside in close contact with T-lymphocytes, stromal cells, endothelial cells, follicular dendritic cells and macrophages. Interactions between these components regulate CLL cell trafficking, survival and proliferation in a manner that may be partly dependent on direct physical cell–to-cell contact or mediated through the exchange of soluble factors (Figure 1).
T-cells
The interaction between CLL cells and T cells is an important component of the malignant process. First, T-cells are important for CLL cell proliferation.[66, 67]. This has been directly demonstrated in a xenograft murine model of CLL where activated CD4+ T-cells were required for CLL cell proliferation [66]. In CLL patients, T-cells, predominantly of the CD4+ type, often make up a substantial fraction of the lymphoid infiltrate in the bone marrow and lymph nodes [68], where they are located both around and within proliferation centers [11, 69]. CD40, a key regulator of B-cell-T-cell interaction, is stimulated by CD4+ T-cells expressing CD154 [70], the ligand for CD40, that are preferentially colocalized with CLL cells in pseudofollicular proliferation centers [9]. CLL cells activated in vitro via CD40, alone or in combination with IL-4, enter into cell cycle [71, 72] and are rescued from both spontaneous [73] and drug-induced apoptosis [74]. CD40 signaling in CLL cells induces anti-apoptotic molecules such as MCL1, BCL-XL, BFL1 and Survivin [9, 75, 76]. Promotion of CLL cell survival and proliferation by CD40 signaling is mediated through the PI3K/AKT, MEK/ERK [72, 76] and NF-κB pathways [72–74]. Survivin, a member of the family of inhibitor of apoptosis proteins (IAP's), that is preferentially expressed in the large proliferating CLL cells interspersed with T-cells in lymph node pseudofollicles, integrates apoptosis resistance and proliferation [9, 77].
In addition to interactions mediated through direct cell-cell contact, T-cells also secrete soluble factors that may contribute to CLL cell growth and survival. IL-4 inhibits spontaneous and drug induced apoptosis in CLL cells via a mechanism involving BCL-2 upregulation [78]. Interestingly, both IL-2 [79] and TNFα [80, 81] variably induce CLL cell proliferation. Similarly, IFNγ [82], IFNα [83] and IL-13 [84] were also shown to support CLL cell survival.
Secondly, CLL cells can modify the cellular immune system to evade immune surveillance. Mechanisms are most likely multi-factorial including production of immune-suppressing cytokines such as tumor growth factor-β [85], and IL-10 [86], and expression of reduced levels of adhesion and co-stimulatory molecules [87], as well as increased numbers and altered function of regulatory T-cells [88, 89]. Gene expression profiling of purified T-cells from CLL patients revealed changes in genes involved mainly in cell differentiation, cytoskeleton and vesicle formation, trafficking and cytotoxicity that contribute to decreased immune response [90]. Accordingly, CD4+ and CD8+ T cells in CLL show impaired ability to form immunological synapses which is induced by direct cell contact with CLL cells [91] and is mediated through tumoral expression of CD200, CD270, CD274, and CD276 [92].
Stromal cells
Mesenchymal stromal cells (MSC) are another important cellular component of the tissue microenvironment. Early studies exploring CLL-stromal cell interactions relied upon bone marrow-derived stromal cells [3, 93]. These cells consist of a heterogeneous population of cells that provide structural and functional support for normal hematopoiesis. Later on, other types of human and murine MSCs have been shown to exhibit similar effects on CLL cells [94]. Stromal cells produce and secrete various cytokines, chemokines, proangiogenic factors, and extracellular matrix components, and also express surface receptors that predominantly regulate CLL cell migration and survival. The CLL-MSC crosstalk is bidirectional; thus, tumor cells are not only being supported by stromal cells but also are capable of activating and inducing stromal cell proliferation and secretion of mediators that sustain and intensify the malignant process [95–98]. In the lymphoid tissues of CLL patients, stromal cells are diffusely located throughout the tissue and in perivascular areas where they admix with CLL cells [99]. The stromal cells are highly productive of SDF-1 [100] and are also markedly positive for α-smooth muscle actin (αSMA), a marker induced in myofibroblasts that have been activated by tumor stromal specific growth factors [99]. Interestingly, CLL cells cocultured with bone marrow stromal cells are rescued from both spontaneous [3, 93, 101] and drug-induced apoptosis [3, 101], in a mechanism dependent on direct cell-cell contact [3, 4]. Murine fibroblast cell lines have been shown to protect CLL cells from apoptosis by maintaining expression of the antiapoptotic proteins BCL-XL, XIAP and FLIPL in the leukemic cells. Furthermore, cell-cell interactions activate the NF-κB pathway in a PI3K-dependent manner [72]. CXCL12, secreted from stromal cells, guides CLL cell migration towards the stromal layer and promotes penetration beneath it, a phenomenon called pseudoemperipolesis [101].
Both cell surface receptors and extracellular matrix elements were reported to be responsible for the enhanced survival of CLL cells that are in contact with the stroma layer. Adherence of CLL cells to stromal cells is mediated simultaneously through integrins β1 and β2 [93]. MSC highly express vascular cell adhesion molecule 1 (VCAM-1) [102]. Binding of α4β1 integrin (CD49d/CD29, or VLA-4) to either VCAM-1 or to the extracellular matrix component fibronectin rescues CLL cells from both spontaneous apoptosis and fludarabine induced apoptosis [93, 103], through PI3K/AKT signaling and BCL-XL upregulation [104].
Another aspect of CLL cell interaction with the stroma layer involves metalloproteinase-9 (MMP9), vascular endothelial growth factor (VEGF) and endothelial cells. MMP-9 is the major MMP produced by CLL cells that promotes their extravasation and lymphoid tissue infiltration through proteolytic degradation of basement membranes and extracellular matrix components [105]. Independently of its proteolytic activity, MMP-9 also partially mediates CLL cell survival in bone marrow derived stromal cell coculture [106]. Binding of MMP9 to α4β1 and CD44v in CLL cells results in LYN and STAT3 activation and induction of MCL1 [104]. Expression of MMP9 in CLL cells is regulated through α4β1 integrins and CXCL12 [105]. In this respect, CLL cells in the bone marrow and lymph nodes acquire and express higher levels of surface MMP-9 than that which could be attributed to tumor cell activation in tissue microenvironment or derived from their adjacent accessory cells [104]. The pro-angiogenic molecule VEGF also decreases spontaneous or drug-induced apoptosis of CLL cells, through upregulation of MCL1, XIAP, and STAT3 signaling [107, 108]. In coculture of CLL cell with bone marrow derived stromal cells, vast amounts of VEGF appear to be secreted form the stromal cells and VEGF blockade results in decreased CLL cell survival [109]. CLL cells cocultured with human vascular endothelial cells are also protected from apoptosis in a mechanism involving NF-κB mediated upregulation of BCL2, MCL1, and BCL-XL [110]. Endothelial cells further increase expression of CD38 and CD49 in CLL cells in a NF-κB dependent mechanism [110]. In addition, activation of CD44 on CLL cells by extracellular matrix components such as hyaluronic acid can promote CLL cell survival through activation of the PI3K pathway [111].
Follicular dendritic cells
The literature exploring the role of follicular dendritic cells (FDC) in CLL is relatively limited. FDC are accessory cells within normal germinal centers that retain intact antigen-antibody complexes on their cell surface and present these antigens to B-cells [112]. Normal germinal center B-cells that bind to the immune complexes survive and differentiate into either memory B-cells or plasma cells [112]. FDC are normally detected in secondary lymphoid tissue but not in the bone marrow [113]. In CLL, FDC are seen in the lymph nodes, pseudofollicles [69, 114, 115], and in bone marrows of patients with nodular involvement.[116] FDC secretes several important prosurvival factors and growth factors (e.g. BAFF and IL-15) and express other important adhesion molecules such as VCAM-1, ICAM-1, plexin B1 and CD44 [69, 112]. The effect of FDC on CLL cells was studied using a FDC cell line (HK cells); the HK cells were shown to rescue CLL cells from spontaneous and drug induced apoptosis in a manner that was dependent on direct cell- cell contact and associated with an increase in MCL1 [117].
Tissue macrophages, monocytes, and Nurse-like cells
An intriguing example of the distinct ability of CLL cells to affect normal cellular elements, leading to the loss of normal compartmentalization and spatial control of the immune response, is the recently described phenomenon of the interaction between CLL cells and “nurse like cells” (NLC). NLCs are an in vitro model thought to represent a counterpart of tissue associated macrophages in vivo. In long term cultures of peripheral blood mononuclear cells from CLL patients, large, round, occasionally bi-nucleated CD68 expressing cells grow out [118, 119]. Because CLL cells surround these cells and gain a survival advantage, they were termed “nurse like cells” (NLCs). Cells with similar phenotype are also detected in vivo in secondary lymphoid tissues of CLL patients [119], thus strengthening their biological relevance; yet, their numbers in the tissues are probably low [99]. NLCs actually differentiate from monocytes and their differentiation is dependent on cell-cell contact with CLL cells [119]. Monocytes obtained from normal donors cocultured with purified CLL cells also differentiate into NLCs [119] but normal B-cells do not induce differentiation of monocytes into NLCs [119]. Co-culturing CLL cells with NLCs protects CLL cells from spontaneous and drug induced apoptosis [101, 118] in a mechanism that is partially mediated through an increase in MCL1 expression [120]. NLCs produce and secrete chemokines and growth factors including CXCL12 and CXCL13 as well as B-cell activating factor of tumor necrosis factor family (BAFF) and a proliferation-inducing ligand (APRIL), which attract CLL cells into the tissue compartment and support their survival and proliferation.
BAFF and APRIL are TNF superfamily members that are important for B cell differentiation and survival [121–123]. CLL cells themselves also express BAFF and APRIL and their receptors [124, 125]. However, NLCs express higher levels of BAFF and APRIL than CLL cells [120]. BAFF binds to BAFF receptor (BAFF-R), B-cell maturation antigen (BCMA), transmembrane activator calcium modulator and cyclophilin ligand interactor (TACI), while APRIL binds only to the latter two receptors. BAFF and/or APRIL decrease both spontaneous and drug-induced apoptosis of CLL cell cells [120, 124, 125]. Co-culturing CLL cells with NLCs in the presence of a decoy receptor that binds both BAFF and APRIL partially abolishes the protective effect of the NLCs on the viability of the CLL cells [120]. Recently, it has been shown that BAFF in cooperation with MYC can lead to the development of a CLL-like lymphoproliferation in mice [126]. Notably, MYC and its target genes are up-regulated in CLL cells in the lymph node [8] and MYC is upregulated by BAFF [126] and by BCR engagement in vitro [127]. Interestingly, CLL cells that highly express c-MYC are more susceptible to apoptosis and can be rescued by BAFF [126].
Additional interactions between CLL cells and accessory cells in the tissue microenvironment involve the ligation of CD38 to CD31 and of CD100 to Plexin B1. CD38 levels on the surface of CLL cells are variable and high CD38 expression is a poor prognostic factor in CLL [31]. In proliferation centers, CD38 is upregulated in CLL cells exposed to activated T-cells expressing CD40L [11]. CD31, the ligand for CD38, is expressed on endothelial cells and NLCs. CD31 induces proliferation and prolongs the survival of CD38+ CLL cells [128]. CD100, a transmembrane protein that belongs to the semaphorin family, is expressed on CLL cells [129]. The high affinity receptor for CD100, Plexin B1, is expressed on bone marrow stromal cells, follicular dendritic cells, nurse like cells and activated T-cells [129]. It has been demonstrated that engagement of CD100 by PlexinB1 increases CLL cell proliferation and prolongs survival [128, 129].
Trafficking and homing of CLL cells into lymphoid tissues
Chemokines consist of two major subgroups; one group of homeostatic chemokines is constitutively produced and secreted within the tissue microenvironment and serves to maintain physiological trafficking. The second group includes inflammatory chemokines which are primarily induced in inflamed tissues to recruit effector cells [130]. Serum levels of some of the chemokines or their cognate receptors are highly elevated in CLL and a more effective chemotatic response is a characteristic of more aggressive subtypes of CLL cells. The responsiveness of circulating CLL cells to chemokine stimulation might facilitate the trafficking, homing and invasion of the leukemic cells into the “nourishing” tissue microenvironment.
An example of a chemokine that critically regulates CLL cell migration is CXCL12, a homeostatic chemokine that plays a critical role in normal trafficking and homing. CXCL12 is constitutively secreted at high levels by stromal cells and in vitro by NLCs [118]. CXCR4 (CD184), the receptor for CXCL12, is highly expressed on circulating CLL cells [131–133], which migrate more efficiently towards CXCL12 than normal B-lymphocytes. [131, 132]. CLL cells expressing CD38 and/or ZAP70 show stronger intracellular signaling and better chemotaxis in response to CXCL12 than cells with no CD38 or ZAP70 expression [133–135].
CXCL13 is another homeostatic chemokine which along with its cognate receptor, CXCR5, plays a central role in the recruitment of B-cells into the B-cell zone of secondary lymphoid organs [136]. CXCL13 is constitutively secreted by stromal cells in the B-cell areas of the secondary lymphoid tissues [137]. CXCL13 is expressed in vitro by NLCs as well as in vivo in the CD68+ macrophages present in CLL lymph nodes [112, 137]. Serum CXCL13 levels are higher in CLL patients compared to healthy individuals [137] and CXCR5 is also highly expressed in CLL cells [137, 138].
CCL19 and CCL21 are chemokines that regulate the recruitment of lymphocytes into the T-cell zone areas of the secondary lymphoid tissues through ligation to their cognate receptor CCR7 [139]. CCL19 and CCL21 are detected in the stroma and in the high endothelial venules (HEV) of lymph nodes in CLL, and the latter are an important route of lymphocyte entry into secondary lymphoid tissue [140]. Circulating CLL cells express high levels of CCR7 [133, 141] which are higher in ZAP-70+ CLL cells and in patients with prominent lymphadenopathy [133, 140]. CLL cells that are ZAP70+ or from patients with marked lymphadenopathy migrate more efficiently towards these chemokines [133, 140, 142].
CLL cells are not only capable of responding to cellular elements in the tissue microenvironment but also actively recruit cells from the microenvironment to their immediate vicinity. This involves CLL cell secretion of chemokines such as CCL17, CCL22, CCL3 and CCL4. CCL22 and CCL17 are T-cell attracting chemokines induced in CD40 activated CLL cells in the lymph nodes and bone marrow [70]. Thus, CLL cells can attract CD4+ T cells to the bone marrow and lymph nodes that augment tumor cell proliferation and survival and further induce release of CCL22, creating a positive feedback loop that further promotes the malignant process [70].
CCL3 and CCL4 are pro-inflammatory chemokines crucial for the response to infection, the mediation of inflammation and the recruitment of monocytes, T-cells from the blood into the tissue compartments [143]. CLL cells secrete these two chemokines in response to activation of the BCR and CD38 as well as during coculture with NLCs. Expression of CCL3 and CCL4 in CLL cells during coculture with NLCs positively correlates with ZAP-70 positivity [144]. CLL3 and CCL4 are also overexpressed in CD38+CD49d+ CLL cells more than those that are CD38−/CD49d− [145]. Both CCR1 and CCR5, which are the cognate receptors for CCL3 and CCL4, are expressed on monocytes and macrophages and induce their migration.[145]. Consistently, higher numbers of tumor infiltrating CD68+ macrophages were detected in the bone marrows of patients with CD38+CD49d+ CLL [145]. CCL3 may also indirectly protect CLL cells from apoptosis via induction of VCAM-1 (CD106) in endothelial cells [145]. Plasma levels of CCL3 and CCL4 are elevated in CLL patients compared to healthy individuals [144] and high CCL3 levels correlate with poor prognosis in CLL [146].
Signaling pathways activated in the tissue microenvironment
Given that CLL cells respond in vitro to a wide variety of external stimuli (Figure 1), it is a great challenge to determine which signaling pathways are the most relevant in vivo. Clearly, engagement of the BCR, via either autonomous or extrinsic mechanisms provides a signal. The PI3K/AKT, MAPK/ERK, NF-κB, WNT, JAK/STAT and NOTCH signaling pathways have been reported to mediate survival and/or proliferation of CLL in vitro. In particular the PI3K/AKT, MEK/ERK and NF-κB pathways have been shown to be activated in the tissue microenvironment. Because the composition of the microenvironment can vary between tissues different signaling pathways may be engaged in different locations. This may be in particular the case for the lymph nodes and for the so-called proliferation centers. The activity of many intracellular signals appears to be stronger and/or more sustained in patients with more aggressive subtypes of the disease and correlates with enhanced tumor proliferation and more rapid disease progression [8, 147]. In particular, PI3K/AKT signaling regulates CLL cell survival and trafficking [148]. The PI3k/AKT pathway mediates the chemotactic response of CLL cells towards CXCL12 [131, 132, 135], CXCL13 [137], CCL19, and CCL21 [133, 140, 149] and promotes CLL cell survival in response to a variety of external stimuli including BCR activation [45], CD40L [72], VCAM-1 [104], CCL19, CCL21 and many others. The inhibition of apoptosis in CLL cells through PI3K/AKT signaling is partially dependent on activation of NF-κB and upregulation of anti-apoptotic genes (e.g. BCL-XL and BFL-1). The classical NF-κB pathway has been shown to be activated in CLL cells in the lymph node more than in those derived from peripheral blood. A variety of in vitro stimuli induce NFκB activity in CLL cells including engagement of the BCR [48] or CD40 [72–74], exposure to cytokines such as BAFF or APRIL [120] and coculture with stromal cells [72] or endothelial cells [110]. Compared with normal B cells, CLL cells overexpress anti-apoptotic proteins such as BCL-2 and MCL1 [150, 151] and the resistance to apoptosis is further enhanced in the lymphoid tissues via upregulation of BCL-2 family molecules including MCL1 BCL-XL and Survivin [10, 15]. MCL1 expression in CLL cells is commonly regulated through PI3K/AKT signaling [45]. BCL-XL can be upregulated in CLL cells through BCR [45], and CD40 signaling [75] or through VCAM-1 [104], stromal cells [72] and endothelial cells [110]. The MAPK/ERK pathway also transmits pro-survival signals in CLL cells, as demonstrated in response to stimulation with CXCL12, CXCL13, CCL19 and CCL21. In addition, the MEK/ERK pathway is an important regulator of cell cycle progression and proliferation. MEK1/2 activity is important for MYC expression and S-phase entry of CLL cells. MEK/ERK mediates MYC expression in response to engagement of the BCR and Toll-like receptor 9 (induced in vitro by CpG-ODN) and with BAFF stimulation. Accordingly, both phosphorylated ERK and MYC are mostly expressed in large proliferating CLL cells confined to the proliferation centers within the lymph nodes [127]. MYC can contribute to genomic instability by selecting cells with defective DNA damage response, and its expression may be a driver of clonal evolution [152].
Cell proliferation is regulated by D-type cyclins that bind to CDK4 and CDK6, resulting in the phosphorylation of the retinoblastoma protein and the G1-S phase transition of the cell cycle. Cyclin D2 is overexpressed in CLL cells[153], especially in cells residing in the LN [8]. IgM ligation induces cyclin D2 and CDK4 in CLL cells.[63] The down-regulation of the cell cycle inhibitor p27 and progression of CLL cells into S phase are probably dependent on additional costimulatory signals such as CD40 ligand and IL-4 that are provided mainly by T-helper lymphocytes in the proliferation centers of the lymph node [154]. Accordingly, within CLL cells in the proliferation centers cyclin D2 is highly expressed and p27 is down regulated [155]. Cyclin D2 expression is regulated either directly through NF-κB or indirectly by c-Myc. Consistent with this finding is the observation that NF-κB activity is increased in the LN and particularly enhanced in CLL cells within the proliferation centers [8, 155].
Models of the CLL microenvironment
Modeling tumor-host interactions is an area of intense investigation. Such models are of particular interest given the fact that tissue resident CLL cells are not readily available. Currently, the most widely utilized in vivo model for CLL is the transgenic TCL1 mouse, in which the human TCL1 gene is expressed under the control of the immunoglobulin heavy chain variable region promoter and enhancer.[159] TCL1 is an oncogene commonly activated in mature T-cell lymphomas that enhances AKT signaling. Onset of disease is late in life and the tumor cells in TCL1 transgenic mice are relatively large lymphoid cells, expressing unmutated IGHV genes [159]. There is evidence for a role of BCR signaling in this model and a dysregulation of the T-cell compartment similar to what has been described in human CLL [160]. The TCL1 transgenic model has also been used successfully to study novel therapeutic approaches (discussed below). In contrast to the transgenic model, New Zealand Black (NZB) mice early in life spontaneously develop autoimmunity and B-cell hyperactivity, while a CLL-like disease manifests later in life. The late-onset clonal disease is of the IGVH unmutated type that is also ZAP70 positive.[156] NZB mice were found to harbor a point mutation in the 3'-flanking sequence of the pre–mir-16-1, which results in decreased levels of miR-16 in lymphoid tissues [157]. This is reminiscent of the most common chromosomal lesion in human CLL; a deletion of the 13q14 chromosomal region containing the mir-15a/16-1 and DLEU2 genes [158]. Recently Klein and colleagues showed that the deletion of the 13q14-minimal deleted region (MDR) harboring the DLEU2/miR-15a/16-1 cluster in mice results in development of a condition that resembles human CLL [161]. The leukemic cells of these mice express unmutated IGHV genes and some of them even present with BCRs showing stereotypical antigen binding regions [161]. Other transgenic mice models of CLL include (NZB×NZW)F1 mice programmed to express IL5 [162], mice overexpressing both BCL2 and a tumor necrosis factor receptor-associated factor [163] and myc/Baff transgenic mice [126].
A complementary approach has been to xenograft the Mec-1 cell line[165] or primary CLL cells[66,164] into immune-compromised mice. Recently, Bagnara et al. reported that peripheral blood mononuclear cells (PBMCs) from CLL patients xenografted into NOD/scid/γc null (NSG) mice localized and proliferated primarily in the murine spleen. These investigators found that proliferation of CLL cells in vivo was dependent on co-engrafted human T-cells.[66] Furthermore, by comparing CLL cells isolated from spleens of xenografted mice to CLL cells from human blood and LN, Sun et al showed that the murine spleen microenvironment supports CLL cell proliferation and activation to a similar degree as the human lymph node, which notably includes activation of BCR and NF-κB signaling in the xenografted cells. The model was then used to test the in vivo effects of ibrutinib, a Bruton’s tyrosine kinase inhibitor in clinical development. Ibrutinib inhibited BCR and NF-κB signaling induced by the microenvironment, decreased proliferation, induced apoptosis, and reduced the tumor burden in vivo.[167] Thus, these data indicate that the spleen of xenografted NSG mice can sufficiently model the role of the human microenvironment on CLL cells to make this a valid model for investigations of tumor microenvironment interactions and the evaluation of possible novel treatment approaches.
Targeting the microenvironment in CLL
The increasing appreciation of the role of the microenvironment in supporting CLL cell proliferation and survival as well as its contribution to chemoresistance has informed novel therapeutic approaches. Accordingly, a major effort is underway to find efficient ways to “chemically dissect” CLL cells from the microenvironmental signals, by either blocking extracellular triggers or abrogating intracellular signaling (Figure 3). In recent years, different compounds have been developed that are able to antagonize surface receptors or cytokines including small molecules that target signaling kinases and anti-apoptotic proteins. Clinically, impressive responses characterized by diminished lymphadenopathy and splenomegaly have been observed with some of these novel agents.
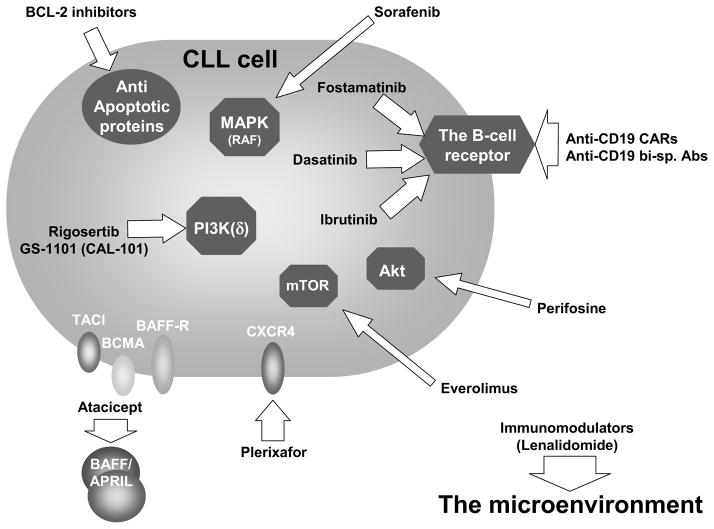
Figure 3.
Therapeutic targeting of microenvironmental-induced signaling in CLL. Current and experimental CLL therapeutics (arrows) target the various components of the microenvironment-CLL milieu and its associated signaling network. Thus the BCR and its associated components are targeted by antibodies (anti-CD19) or small molecules (e.g. SYK (e.g. fostamatinib) or BTK inhibitors (e.g. ibrutinib)). Small molecules are also utilized to inhibit mTOR, Akt, PI3K(δ) and the MAPK cascades. Extracellular inhibitors such as plerixafor or atacicept can block the association of SDF-1 or BAFF/APRIL, respectively, with their receptors on the CLL cell. Both the microenviroment (e.g. the immune system) and the outcome of its signaling responses in the CLL cells (e.g. upregulation of BCL-2) are avenues for therapeutic targeting.
CXCR4 receptor antagonists
CLL cells in the peripheral blood express high levels of the surface receptor CXCR4, which signals for chemotaxis, polymerization of actin, and migration through the vascular endothelium [131]. Blockade of the CXCR4-CXCL12 axis using CXCR4 receptor antagonists such as plerixafor (AMD3100) or T140 can efficiently antagonize CXCL12-mediated signaling and chemotaxis as well as stroma mediated protection from both spontaneous and drug induced apoptosis [101, 169]. The clinical use of plerixafor in CLL is investigated in combination with rituximab. Preliminary results showed that this agent induces dose-dependent mobilization of CLL cells to the peripheral blood [170].
Targeting BAFF and APRIL
As previously mentioned, BAFF and APRIL signaling through their cognate receptors BAFF-R, TACI and BCMA are important for normal B-cell survival and may play a role in CLL. Atacicept is a recombinant soluble form of the extracellular binding domain of TACI. By acting as a molecular decoy, it neutralizes the effects of BAFF and APRIL, blocking the activation of TACI, BCMA, and the BAFF-receptor. A phase Ib study of Atacicept demonstrated that intravenous doses of up to 27 mg/kg were well tolerated in 21 patients with refractory or relapsed CLL with one PR (ORR 5%) in the highest dose cohort [171].
Targeting BCR signaling
The pivotal role of BCR signaling in CLL pathogenesis points to this pathway as an ideal target for novel anti-CLL therapy (Figure 3). Small-molecule drugs targeting SYK, Bruton's tyrosine kinase (BTK), or PI3K isoform p110delta (PI3Kδ) show impressive results in patients with relapsed/refractory as well as in treatment naïve CLL (reviewed in [20, 21]). In the first few weeks of treatment with these agents responses typically manifest with a substantial regression in lymphadenopathy that is frequently paralleled by a transient lymphocytosis [172–174]. The early increase in the circulating lymphocytes is assumed to reflect redistribution of the CLL cells from the lymphatic tissues into the circulation, as a consequence of disruption of mechanisms involved in migration and retention of the tumor cells in their protective tissue microenvironments [175, 176]. Continued treatment over the course of months results in gradual decrease in the lymphocytosis and a deepening of responses with a high rate of remission achieved with increasing duration of treatment [172]. The clinical responses seen with these kinase inhibitors are attributed to the combined effects of direct cell cytotoxicity, inhibition of survival pathways, and impairment of CLL cell trafficking and tissue retention.
LYN inhibitors
LYN, a SRC family non-receptor tyrosine kinase, plays an important role in initiation as well as in the termination of BCR signaling. This dual role of LYN is due to its propagation of the BCR signal via phosphorylation of SYK and its concurrent activation of inhibitory phosphatases that terminate the response. Dasatinib, which was originally approved for treatment of chronic myeloid leukemia, is an oral kinase inhibitor primarily targeting ABL and SRC kinases but it also inhibits other kinases, including BTK [177]. In preclinical studies dasatinib has been shown to induce apoptosis of CLL cells that was associated with reduction in MCL1 and BCL-XL expression [178–180]. In a phase II trial in 15 patients with relapsed or refractory CLL, treatment with dasatinib (140mg/daily) achieved an overall response of 20% with a progression-free survival of 7.5 months. The major adverse reaction of the treatment was myelosuppression [181].
SYK inhibitors
SYK is a key protein kinase of proximal BCR signal transduction that is also involved in B-cell migration and adhesion independently of BCR activity [182]. Following BCR engagement, SYK is phosphorylated by LYN and amplifies the BCR signal through activation of downstream signaling pathways. In peripheral blood CLL cells SYK phosphorylation on the activating Y352 residue has been demonstrated [183]. In addition, CLL cells in the lymph node show increased phosphorylation of SYK compared to peripheral blood CLL cells, indicating activation of the kinase in the tissue microenvironment [8].
To date several SYK inhibitors have been studied in CLL in vitro and in vivo including fostamatinib (R788, the oral pro-drug of R406, the active metabolite), PRT318 and P505-15. In preclinical studies, treatment with SYK inhibitors resulted in inhibition of BCR activation, moderate apoptosis of CLL cells, reduced basal kinase activity of SYK, AKT, and ERK, and decreased MCL1 levels [183]. Furthermore, SYK inhibitors have been shown to antagonize exogenous prosurvival signals provided by stromal cell or NLC coculture [62, 183, 184], secretion of BCR regulated chemokines CCL3 and CCL4 [62, 183, 184], and migration towards CXCL12 and CXCL13 [62, 184]. In an Eμ-TCL1 transgenic mouse model, treatment with fostamatinib inhibited BCR signaling, reduced the proliferation and survival of the leukemic clone and extended the life of the treated mice [185].
Fostamatinib was the first SYK inhibitor introduced into clinical study [174]. In a phase 1/2 trial in patients with relapsed/refractory B-cell malignancy, fostamatinib was shown to be well tolerated with the most common adverse reactions being myelosuppression, fatigue and diarrhea. The highest overall response rate was achieved in patients with CLL/SLL (55%, 6 of 11, with a median progression free survival of 6.4 months), as compared to only 10–22% in the other NHLs [174]. The on-target effect of fostamatinib in CLL has been demonstrated by downregulation of BCR regulated target genes in tumor cells of CLL patients on fostamatinib [186]. Furthermore, fostamatinib inhibited CLL cell activation and proliferation. However, there was no correlation between the degree of inhibition of BCR signaling and clinical response suggesting that pathways bypassing BCR activation might play a role in shaping the response to such kinase inhibitors [186]. Fostamatinib is being tested in late stage clinical trials for rheumatoid arthritis[187] and in patients with diffuse large B cell lymphoma. Some novel SYK inhibitors have shown promising pre-clinical activity, and may have increased potency and specificity [184].
BTK inhibitors
BTK is a member of the TEC family of kinases that is critical for BCR signaling [188]. Mutations in BTK result in X-linked agammaglobulinemia, an inherited disorder manifesting with profound decrease in antibody production and severe defect in B-cell development [189]. Notably, BTK mRNA and protein expression levels are increased in CLL cells [190]. Ibrutinib (PCI-32765) is an orally administered irreversible and specific inhibitor of BTK that induces modest apoptosis in CLL cells irrespective of IGHV mutational status or interphase cytogenetics and overcomes prosurvival and proliferation signals provided by various tissue microenvironmental elements (such as CD40L, BAFF, IL-4, IL-6 and TNFα, fibronectin and stromal cells coculture and CpG oligonucleotide) [176, 190]. Ibrutinib abrogates CLL cell signaling, migration, and adhesion in response to tissue homing chemokines (such as CXCL12, CXCL13 and CCL19) and abrogates integrin α4β1-mediated adhesion to fibronectin and VCAM-1 [175, 176, 190]. Both in vitro and in vivo ibrutinib has been reported to inhibit CLL cell secretion of CLL3 and CCL4 [176]. At the molecular level, this agent inhibits BTK tyrosine phosphorylation following BCR or CD40 stimulation and abrogates activation of downstream signaling pathways including ERK, PI3K, and NF-κB in CLL cells [190]. Treatment with ibrutinib in the TCL1 mice model of CLL resulted in inhibition of disease progression [176].
A phase I open-label dose-escalation study evaluated the efficacy and tolerability of ibrutinib in patients with relapsed or refractory B-cell NHL and B-cell CLL [191]. Dose escalation proceeded to 12.5 mg/kg without dose-limiting side effects and with pharmacodynamic evidence for complete inhibition of BTK. 60% of all patients achieved an OR, with 16% achieving a CR; 16 patients with CLL/SLL were evaluated, with an objective response reported in 11 of these patients (69%). Notably, the observed responses were of marked duration, as the median progression-free survival for all patients reported at the time of data cutoff was 13.6 months. A subsequent phase 1b/2 study of ibrutinib in CLL patients who were either i) greater than 65 years of age and previously untreated or ii) diagnosed with relapsed or refractory disease, further demonstrated that ibrutinib was well tolerated with the most common side effects including diarrhea, fatigue and nausea. ORR was 71% for treatment naïve patients, 67% for relapsed or refractory patients, and 50% for high risk patients [172]. The estimated PFS at 26 months was 75% for the relapsed/refractory cohort and 96% for treatment naïve patients demonstrating a remarkable duration of response.
Targeting the PI3K/AKT/mTOR signaling pathway
The PI3K/AKT/mTOR signaling pathway is a critical intracellular signaling cascade controlling cell survival and proliferation in both malignant and non-malignant cells. PI3K is a pivotal “hub” connecting multiple extracellular signals to cellular responses. PI3K acts to convert phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2 (PIP2)) to PI(3,4,5)P3 (PIP3) which in turn forms a functional signaling complex with BTK and AKT. Several isoforms of PI3K have been characterized; the δ isoform of PI3K is selectively expressed in hematopoietic cells and functions to relay BCR, BAFF, CD30, and TLR signaling. CLL cells express the PI3Kδ and display increased PI3K activity [192]. Given the selective expression of PI3Kδ - in contrast to the more pervasive α and β isoforms - specific inhibition of the δ isoform is not expected to be toxic to normal tissues. Many PI3K inhibitors are currently being investigated in both pre-clinical and clinical settings. ON 01910.Na (Rigosertib), a multikinase phosphoinositide 3-kinase (PI3K) inhibitor in phase III trials for myelodysplastic syndrome, has demonstrated promising pre-clinical in vitro activity, inducing apoptosis in CLL cells that are cultured in contact with stromal cells via a dual mechanism of action involving both PI3K/AKT inhibition and induction of oxidative stress [193].
GS-1101 (CAL-101)
GS-1101 is an orally available highly selective PI3K-delta inhibitor that induces apoptosis in CLL cells [194]. The cytotoxic effect of GS-1101 is maintained despite the presence of various microenviromental components that normally support malignant CLL cells (including stromal cells, NLC, fibronectin, CD145 or TNFα BAFF and BCR stimulation). GS-1101 also inhibits the secretion of both anti-apoptotic and pro-inflammatory cytokines [192, 195]. GS-1101 sensitizes CLL cells to drug-induced apoptosis in the presence of stromal coculture [195] and inhibits CLL cell chemotaxis toward CXCL12 and CXCL13 [195]. The drug abrogates constitutive PI3K signaling in CLL cells as well as AKT and/or ERK activation by anti-IgM, soluble CD40 ligand and chemokines [195, 196]. In CLL patients treated with GS-1101 the serum levels of CCL3, CCL4, and CXCL13 were markedly reduced.[195]. A phase 1 study in 54 patients with previously treated CLL demonstrated the acceptable safety and promising clinical activity of GS-1101, with 26% of patients achieving an OR[173]. A >50% reduction in lymphadenopathy was observed in 80% of patients. Adverse events grade 3 or higher were minimal and included pneumonia and neutropenia, observed in less than a quarter of patients. Phase 2/3 clinical trials of GS-1101 are currently underway.
AKT Inhibitors
The serine/threonine kinase AKT is a key nodal regulator of cellular survival known to phosphorylate several cellular substrates including caspase 8[197], caspase 9[198], BAD [199], mTor [200], and the Forkhead family of transcription factors.[201] Furthermore, AKT activation is associated with resistance to chemotherapy. Early phase clinical trials are underway investigating the use of AKT inhibitors in several malignancies including CML, although experience with these agents in CLL is limited. MK-2206, an orally active allosteric AKT inhibitor has been shown to enhance the antitumor efficacy of other chemotherapeutic agents in vitro in several malignancies, although clinical use in CLL has not been reported [202]. Perifosine, another oral inhibitor of AKT, is undergoing phase II evaluation in patients with refractory and relapsed leukemia [203]. Pending the outcome of these and other clinical trials, further investigation is warranted to determine the utility of the use of AKT inhibitors is CLL.
mTOR inhibitors
Mammalian target of rapamycin (mTOR), a serine/threonine kinase of the PI3K/AKT/mTOR signaling network, is involved in cell growth, metabolism and proliferation and is commonly activated in B-cell neoplasms. Rapamycin (sirolimus, rapamune, Wyeth) is an immunosuppressive drug used to prevent rejection in organ transplantation. The drug profoundly blocks B-cell receptor mediated proliferation [204]. Preclinical studies in CLL have shown that rapamycin or its analog RAD001 block cell cycle progression by interfering with expression of critical cell cycle molecules [205]. Everolimus (RAD001, afinitor, Novartis), an orally available derivative of sirolimus, was evaluated in a phase II pilot trial in previously treated patients with CLL. The study was stopped early because of increased toxicity, although the drug showed modest clinical activity [206]. In another phase 2 study, everolimus (10mg/day) administered to patients with recurrent/refractory CLL achieved partial remissions in 18% of patients [207]. In a subset of these patients, treatment with everolimus was accompanied by an increase in lymphocytosis in parallel to reduction in lymphadenopathy.
Targeting the RAF/MEK/ERK signaling pathway
Sorafenib (BAY43-9006; nexavar) is an oral small molecule multi-kinase inhibitor approved for the treatment of advanced renal cell carcinoma [208] and unresectable hepatocellular carcinoma [209]. Sorafenib is a potent RAF serine/theronine kinase inhibitor targeting the RAF/MEK/ERK pathway and also inhibits other receptor tyrosine kinases involved in tumor progression and angiogenesis [210]. Sorafenib induces CLL cell death that is mediated via caspase activation and a decrease in MCL1 [211–213]. It overcomes apoptosis protection induced by NLC or stromal cell coculture and stromal-mediated chemoresistance [211–214]. The drug has been shown to block RAF/MEK/ERK signaling and the chemotaxis response induced by CXCL12 in CLL cells [211, 214]. It has also been shown to inhibit RAF and ERK activation by NLC or stromal cells [213, 214] and to interfere with VEFGR/STAT3 signaling induced by stromal cell coculture [213]. Sorafenib also abrogates BCR mediated signaling and survival in CLL cells. Interestingly, CLL cells derived from the lymph nodes are more sensitive to sorafenib than the cells found in the peripheral blood [214]. The clinical efficacy and tolerability of sorafenib in relapsed CLL is currently being evaluated in a phase II clinical trial.
Targeting anti-apoptotic proteins
The resistance of CLL cells to apoptosis is related to high expression of BCL-2 family anti-apoptotic proteins. The overexpression of these antiapoptotic proteins in CLL is endogenous as well as extrinsic and is in part regulated by signals derived from the tissue microenvironment [151]. Therefore, in recent years, several therapeutic strategies have been developed to target anti-apoptotic proteins in CLL, including antisense BCL-2 oligonucleotide, BH3 mimetics and others.
Oblimersen sodium is a synthetic BCL-2 antisense oligonucleotide that induces a decrease in BCL-2 mRNA and protein levels and apoptosis in CLL cells [215]. Oblimersen sodium when combined with different cytotoxic drugs increases CLL cell apoptosis. In a phase I/II trial, oblimersen sodium as a single agent, showed minimal activity in patients with relapsed/refractory CLL. Dosing was limited by development of a cytokine release syndrome. At the dosage of 3 mg/kg/d, two (8%) of 26 patients achieved a partial response [216]. A phase III study in patients with relapsed/refractory CLL showed that addition of oblimersen to fludarabine plus cyclophosphamide (FC) increased the CR/nPR rate compared to FC alone [217]. Accordingly, CR/nPR was achieved in 20 (17%) of 120 patients in the oblimersen group and eight (7%) of 121 patients in the FC-only group [217]. The combination of oblimersen-FC further resulted in increased survival in subsets of patients who achieved a least a partial response and in those who had fludarabine sensitive disease [217, 218].
Obatoclax mesylate (GX15-070) is a small-molecule pan-BCL-2 antagonist. This compound belongs to a class of BH3 mimetic agents that inhibit the activity of the anti-apoptotic BCL-2 members that antagonize the proapoptotic proteins BAX and BAK [219]. The BH3-only proteins BAX and BAK are directly sequestered and repressed by the antiapoptotic BCL-2 proteins. BH3 mimetic drugs BAX and BAK induce their release allowing them to oligomerize and trigger apoptosis via the formation of pores in the outer mitochondrial membrane. In a phase I trial, administration of obatoclax to heavily pretreated patients with advanced CLL resulted in a PR in one out of 26 patients. The major toxicities were neurologic including somnolence, ataxia and euphoria [220].
BH3 mimetics
The BH3 mimetics, ABT-737 and its orally active analog navitoclax, inhibit BCL-2, BCL-Xl and BCL-W. In pre-clinical studies ABT-737 has been shown to induce a rapid and potent proapoptotic activity in CLL cells independently of the common clinical and prognostic parameters in CLL [221, 222]. Addition of cytotoxic agents sensitizes CLL cells in vitro to ABT-737 [222]. In a phase I study in 29 relapsed/refractory CLL patients, navitoclax dosed at ≥100mg/d achieved durable partial responses in 35% of patients [223]. Navitoclax was also active in high-risk patients with fludarabine refractory disease, bulky lymphadenopathy and deletion of 17p [223]. The major dose-limiting toxicity was thrombocytopenia related to inhibition of BCL-XL [223].
AT-101 (gossypol isomer) is a small-molecule pan-BCL-2 antagonist. In preclinical studies, AT101 was shown to both induce CLL cell apoptosis and to overcome resistance mediated by stromal cell coculture, while sparing normal stromal cells [224]. A phase I trial of AT-101 in treatment naïve CLL patients with high risk disease demonstrated that the drug was well-tolerated [225]. Furthermore, 5 of 6 patients in this trial exhibited a decrease in lymphocyte count, while all patients demonstrated a reduction in lymphadenopathy.
XIAP inhibitors
X-linked inhibitor of apoptosis (XIAP) inhibits the proteolytic activity of caspase-3 via direct binding, is highly expressed in CLL cells, and plays an important role in TRAIL-induced apoptosis [226]. Since CLL cells have previously exhibited resistance to TRAIL-based treatments, novel inhibitors of XIAP have been developed in the hope of overcoming TRAIL-resistance in CLL [227]. One such novel small molecule inhibitor, compound A (CA), has been shown to render tumor cells from patients with 17p deletion, IGVH unmutated type CLL susceptible to TRAIL in vitro.
Immunomodulatory drugs
Lenalidomide, a derivative of thalidomide, is an immunomodulatory agent that has significant activity in 5q- myelodysplastic syndrome, multiple myeloma and other B-cell malignancies. In relapsed or refractory CLL, intermittent lenalidomide at 25 mg/d (3 weeks on/1 week off drug) was associated with acceptable toxicity, and achieved an overall response rate of 47%, with 9% of patients demonstrating a complete remission [228]. Another trial of relapsed/refractory CLL patients treated with daily lenalidomide at a dose of 10mg produced an overall response rate of 32%, with 7% CRs[229]. In a treatment-naïve setting, elderly patients with CLL treated with lenalidomide achieved an OR rate of 65%, including 10% CRs and an additional 5% CRs with residual cytopenias [230]. The activity of lenalidomide is irrespective of unfavorable genomic abnormalities such as unmutated IGHV or fludarabine-refractory disease status. However, at least 6 to 9 months of treatment may be needed to achieve the best possible response [229]. The most common toxicity of lenalidomine is myelosuppression [228, 229]. Lenalidomide is frequently associated with a cytokine release syndrome and tumor flare reaction (observed in 30–58% of patients) or tumor lysis syndrome[228, 231, 232]. Tumor flare is a unique immune mediated response to lenalidomide therapy characterized by painful lymph node enlargement that may be accompanied by fever and/or bone pain. It is generally managed with corticosteroids, while in more severe cases it may necessitate narcotics and hospitalization [229, 231]. Tumor flare may be predictive of clinical response to treatment with lenalidomide [233]. The optimal dosing schedule of lenalidomide is not well defined; and a dose of 25 mg once daily in relapsed CLL may be associated with unacceptable toxicity in some patients [231]. Furthermore, lenalidomide may be associated with an increased frequency of venous thromboembolism that may be related to endothelial dysfunction in the context of inflammatory cytokine secretion. Continuous low dose lenalidomide (10mg) is generally well tolerated and effective [229]. Importantly, the treatment is accompanied by increased serum immunoglobulin levels and typically reduces CCL3 and CCL4 plasma levels, which might indicate an inhibitory effect on intracellular signaling in CLL cells [230].
The mechanism of action of lenalidomide is not completely understood. In contrast to thalidomide, lenalidomide has weak antiangiogenic effects [229]. Lenalidomide induces transient immune activation including expression of costimulatory molecules such as CD40, CD80, and CD86 on CLL cells [231, 232], which may be mediated through PI3Kδ [194]. It also increases serum levels of interleukin IL-6, IL-10, IL-2R, IFNγ, and TNFα [229, 232]. Lenalidomide in part restores T-cell function by enhancing the formation of immunological synapses and promoting intracellular signaling [91, 160, 234]. In lymph node biopsies from CLL patients treated with lenalidomide a shift towards a Th-1 type immune reaction with production of IFNγ was documented, suggesting that lenalidomide might be able to restore anti-tumor immunity and immune surveillance in vivo [235]. Furthermore, lenalidomide enhances NK cell activity [236] and improves antibody-mediated cellular cytotoxicity directed by rituximab [237].
Summary and Outlook
Interactions of CLL cells with the surrounding microenvironment play a central role in the pathogenesis and the progression of the disease. The nature of these interactions is dependent on the properties of the CLL cell itself, but also likely depends upon properties of the specific patient's microenvironment, which may be shaped by the baseline expression levels of cytokines, the composition of various T cell subsets, stromal cell populations, and responses to antigenic stimulation. Major progress in the last decade has led to a much better understanding of both direct and indirect cellular interactions involved in CLL oncogenesis, and to the development of multiple new and promising therapies (Figure 3). Although there is still much to learn, it seems very possible that within a few years the improved understanding of key aspects of tumor biology and the development of novel therapeutic approaches will combine to change the natural history of CLL.
Key Points.
CLL is characterized by the accumulation of mature monoclonal B cells in the peripheral blood, bone marrow, spleen and lymph nodes.
Signals from the B cell receptor (BCR) and the tissue microenvironment converge on several key intracellular signaling pathways including the PI3K/AKT, MAPK/ERK, and NF-κB pathways and promote leukemic cell proliferation, survival, and resistance to chemotherapy.
Tissue sites provide a supportive microenvironment composed of T-cells, stromal cells, cytokines, chemokines and extracellular matrix components.
The lymph node is a pivotal site of CLL cell activation and proliferation through antigenic stimulation.
The dependence of CLL cells on signals from the BCR and tissue microenvironment presents opportunities for targeted therapy. Inhibitors of BCR signaling and therapeutic approaches to “chemically dissect” CLL cells from the supportive microenvironment have shown encouraging clinical results.
Acknowledgments
Research support: A.W. is supported by the Intramural Research Program of the National, Heart, Lung and Blood Institute, NIH
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Caligaris-Cappio F, Hamblin TJ. B-cell chronic lymphocytic leukemia: a bird of a different feather. J Clin Oncol. 1999;17(1):399–408. doi: 10.1200/JCO.1999.17.1.399. [DOI] [PubMed] [Google Scholar]
- 2.Hallek M, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164–74. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 3.Panayiotidis P, et al. Human bone marrow stromal cells prevent apoptosis and support the survival of chronic lymphocytic leukaemia cells in vitro. Br J Haematol. 1996;92(1):97–103. doi: 10.1046/j.1365-2141.1996.00305.x. [DOI] [PubMed] [Google Scholar]
- 4.Lagneaux L, et al. Adhesion to bone marrow stroma inhibits apoptosis of chronic lymphocytic leukemia cells. Leuk Lymphoma. 1999;35(5–6):445–53. doi: 10.1080/10428199909169609. [DOI] [PubMed] [Google Scholar]
- 5.Andreeff M, et al. Discrimination of human leukemia subtypes by flow cytometric analysis of cellular DNA and RNA. Blood. 1980;55(2):282–93. [PubMed] [Google Scholar]
- 6.Damle RN, et al. CD38 expression labels an activated subset within chronic lymphocytic leukemia clones enriched in proliferating B cells. Blood. 2007;110(9):3352–9. doi: 10.1182/blood-2007-04-083832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Messmer BT, et al. In vivo measurements document the dynamic cellular kinetics of chronic lymphocytic leukemia B cells. J Clin Invest. 2005;115(3):755–64. doi: 10.1172/JCI23409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herishanu Y, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011;117(2):563–74. doi: 10.1182/blood-2010-05-284984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Granziero L, et al. Survivin is expressed on CD40 stimulation and interfaces proliferation and apoptosis in B-cell chronic lymphocytic leukemia. Blood. 2001;97(9):2777–83. doi: 10.1182/blood.v97.9.2777. [DOI] [PubMed] [Google Scholar]
- 10.Davids MS, et al. Decreased mitochondrial apoptotic priming underlies stroma-mediated treatment resistance in chronic lymphocytic leukemia. Blood. 2012;120(17):3501–9. doi: 10.1182/blood-2012-02-414060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patten PE, et al. CD38 expression in chronic lymphocytic leukemia is regulated by the tumor microenvironment. Blood. 2008;111(10):5173–81. doi: 10.1182/blood-2007-08-108605. [DOI] [PubMed] [Google Scholar]
- 12.Ghia P, et al. The pattern of CD38 expression defines a distinct subset of chronic lymphocytic leukemia (CLL) patients at risk of disease progression. Blood. 2003;101(4):1262–9. doi: 10.1182/blood-2002-06-1801. [DOI] [PubMed] [Google Scholar]
- 13.Jaksic O, et al. CD38 on B-cell chronic lymphocytic leukemia cells has higher expression in lymph nodes than in peripheral blood or bone marrow. Blood. 2004;103(5):1968–9. doi: 10.1182/blood-2003-11-3890. [DOI] [PubMed] [Google Scholar]
- 14.Quijano S, et al. Association between the proliferative rate of neoplastic B cells, their maturation stage, and underlying cytogenetic abnormalities in B-cell chronic lymphoproliferative disorders: analysis of a series of 432 patients. Blood. 2008;111(10):5130–41. doi: 10.1182/blood-2007-10-119289. [DOI] [PubMed] [Google Scholar]
- 15.Smit LA, et al. Differential Noxa/Mcl-1 balance in peripheral versus lymph node chronic lymphocytic leukemia cells correlates with survival capacity. Blood. 2007;109(4):1660–8. doi: 10.1182/blood-2006-05-021683. [DOI] [PubMed] [Google Scholar]
- 16.Rui L, et al. Malignant pirates of the immune system. Nat Immunol. 2011;12(10):933–40. doi: 10.1038/ni.2094. [DOI] [PubMed] [Google Scholar]
- 17.Davis RE, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463(7277):88–92. doi: 10.1038/nature08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghia P, Chiorazzi N, Stamatopoulos K. Microenvironmental influences in chronic lymphocytic leukaemia: the role of antigen stimulation. J Intern Med. 2008;264(6):549–62. doi: 10.1111/j.1365-2796.2008.02030.x. [DOI] [PubMed] [Google Scholar]
- 19.Stevenson FK, et al. B-cell receptor signaling in chronic lymphocytic leukemia. Blood. 2011;118(16):4313–20. doi: 10.1182/blood-2011-06-338855. [DOI] [PubMed] [Google Scholar]
- 20.Wiestner A. Emerging role of kinase-targeted strategies in chronic lymphocytic leukemia. Blood. 2012;120(24):4684–91. doi: 10.1182/blood-2012-05-423194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woyach JA, Johnson AJ, Byrd JC. The B-cell receptor signaling pathway as a therapeutic target in CLL. Blood. 2012;120(6):1175–84. doi: 10.1182/blood-2012-02-362624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davids MS, Brown JR. Targeting the B cell receptor pathway in chronic lymphocytic leukemia. Leuk Lymphoma. 2012;53(12):2362–70. doi: 10.3109/10428194.2012.695781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fais F, et al. Chronic lymphocytic leukemia B cells express restricted sets of mutated and unmutated antigen receptors. J Clin Invest. 1998;102(8):1515–25. doi: 10.1172/JCI3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tobin G, et al. Subsets with restricted immunoglobulin gene rearrangement features indicate a role for antigen selection in the development of chronic lymphocytic leukemia. Blood. 2004;104(9):2879–85. doi: 10.1182/blood-2004-01-0132. [DOI] [PubMed] [Google Scholar]
- 25.Rosenwald A, et al. Relation of gene expression phenotype to immunoglobulin mutation genotype in B cell chronic lymphocytic leukemia. J Exp Med. 2001;194(11):1639–47. doi: 10.1084/jem.194.11.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein U, et al. Gene expression profiling of B cell chronic lymphocytic leukemia reveals a homogeneous phenotype related to memory B cells. J Exp Med. 2001;194(11):1625–38. doi: 10.1084/jem.194.11.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Damle RN, et al. B-cell chronic lymphocytic leukemia cells express a surface membrane phenotype of activated, antigen-experienced B lymphocytes. Blood. 2002;99(11):4087–93. doi: 10.1182/blood.v99.11.4087. [DOI] [PubMed] [Google Scholar]
- 28.Mockridge CI, et al. Reversible anergy of sIgM-mediated signaling in the two subsets of CLL defined by VH-gene mutational status. Blood. 2007;109(10):4424–31. doi: 10.1182/blood-2006-11-056648. [DOI] [PubMed] [Google Scholar]
- 29.Muzio M, et al. Constitutive activation of distinct BCR-signaling pathways in a subset of CLL patients: a molecular signature of anergy. Blood. 2008;112(1):188–95. doi: 10.1182/blood-2007-09-111344. [DOI] [PubMed] [Google Scholar]
- 30.Lanham S, et al. Differential signaling via surface IgM is associated with VH gene mutational status and CD38 expression in chronic lymphocytic leukemia. Blood. 2003;101(3):1087–93. doi: 10.1182/blood-2002-06-1822. [DOI] [PubMed] [Google Scholar]
- 31.Damle RN, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94(6):1840–7. [PubMed] [Google Scholar]
- 32.Hamblin TJ, et al. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94(6):1848–54. [PubMed] [Google Scholar]
- 33.Agathangelidis A, et al. Stereotyped B-cell receptors in one-third of chronic lymphocytic leukemia: a molecular classification with implications for targeted therapies. Blood. 2012;119(19):4467–75. doi: 10.1182/blood-2011-11-393694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Messmer BT, et al. Multiple distinct sets of stereotyped antigen receptors indicate a role for antigen in promoting chronic lymphocytic leukemia. J Exp Med. 2004;200(4):519–25. doi: 10.1084/jem.20040544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stamatopoulos K, et al. Over 20% of patients with chronic lymphocytic leukemia carry stereotyped receptors: Pathogenetic implications and clinical correlations. Blood. 2007;109(1):259–70. doi: 10.1182/blood-2006-03-012948. [DOI] [PubMed] [Google Scholar]
- 36.Chu CC, et al. Chronic lymphocytic leukemia antibodies with a common stereotypic rearrangement recognize nonmuscle myosin heavy chain IIA. Blood. 2008;112(13):5122–9. doi: 10.1182/blood-2008-06-162024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Binder M, et al. Stereotypical chronic lymphocytic leukemia B-cell receptors recognize survival promoting antigens on stromal cells. PLoS One. 2010;5(12):e15992. doi: 10.1371/journal.pone.0015992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herve M, et al. Unmutated and mutated chronic lymphocytic leukemias derive from self-reactive B cell precursors despite expressing different antibody reactivity. J Clin Invest. 2005;115(6):1636–43. doi: 10.1172/JCI24387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lanemo Myhrinder A, et al. A new perspective: molecular motifs on oxidized LDL, apoptotic cells, and bacteria are targets for chronic lymphocytic leukemia antibodies. Blood. 2008;111(7):3838–48. doi: 10.1182/blood-2007-11-125450. [DOI] [PubMed] [Google Scholar]
- 40.Catera R, et al. Chronic lymphocytic leukemia cells recognize conserved epitopes associated with apoptosis and oxidation. Mol Med. 2008;14(11–12):665–74. doi: 10.2119/2008-00102.Catera. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiorazzi N, Efremov DG. Chronic lymphocytic leukemia: A tale of one or two signals? Cell Res. 2012 doi: 10.1038/cr.2012.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chu CC, et al. Many chronic lymphocytic leukemia antibodies recognize apoptotic cells with exposed nonmuscle myosin heavy chain IIA: implications for patient outcome and cell of origin. Blood. 2010;115(19):3907–15. doi: 10.1182/blood-2009-09-244251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clark EA, Ledbetter JA. How B and T cells talk to each other. Nature. 1994;367(6462):425–8. doi: 10.1038/367425a0. [DOI] [PubMed] [Google Scholar]
- 44.Contri A, et al. Chronic lymphocytic leukemia B cells contain anomalous Lyn tyrosine kinase, a putative contribution to defective apoptosis. J Clin Invest. 2005;115(2):369–78. doi: 10.1172/JCI22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Longo PG, et al. The Akt/Mcl-1 pathway plays a prominent role in mediating antiapoptotic signals downstream of the B-cell receptor in chronic lymphocytic leukemia B cells. Blood. 2008;111(2):846–55. doi: 10.1182/blood-2007-05-089037. [DOI] [PubMed] [Google Scholar]
- 46.Chen L, et al. Expression of ZAP-70 is associated with increased B-cell receptor signaling in chronic lymphocytic leukemia. Blood. 2002;100(13):4609–14. doi: 10.1182/blood-2002-06-1683. [DOI] [PubMed] [Google Scholar]
- 47.Hivroz C, et al. Altered signal transduction secondary to surface IgM cross-linking on B-chronic lymphocytic leukemia cells. Differential activation of the phosphatidylinositol-specific phospholipase C. J Immunol. 1990;144(6):2351–8. [PubMed] [Google Scholar]
- 48.Petlickovski A, et al. Sustained signaling through the B-cell receptor induces Mcl-1 and promotes survival of chronic lymphocytic leukemia B cells. Blood. 2005;105(12):4820–7. doi: 10.1182/blood-2004-07-2669. [DOI] [PubMed] [Google Scholar]
- 49.Krysov S, et al. Surface IgM of CLL cells displays unusual glycans indicative of engagement of antigen in vivo. Blood. 2010;115(21):4198–205. doi: 10.1182/blood-2009-12-254847. [DOI] [PubMed] [Google Scholar]
- 50.Duhren-von Minden M, et al. Chronic lymphocytic leukaemia is driven by antigen-independent cell-autonomous signalling. Nature. 2012;489(7415):309–12. doi: 10.1038/nature11309. [DOI] [PubMed] [Google Scholar]
- 51.Ubelhart R, et al. N-linked glycosylation selectively regulates autonomous precursor BCR function. Nat Immunol. 2010;11(8):759–65. doi: 10.1038/ni.1903. [DOI] [PubMed] [Google Scholar]
- 52.Zupo S, et al. CD38 expression distinguishes two groups of B-cell chronic lymphocytic leukemias with different responses to anti-IgM antibodies and propensity to apoptosis. Blood. 1996;88(4):1365–74. [PubMed] [Google Scholar]
- 53.Wiestner A, et al. ZAP-70 expression identifies a chronic lymphocytic leukemia subtype with unmutated immunoglobulin genes, inferior clinical outcome, and distinct gene expression profile. Blood. 2003;101(12):4944–51. doi: 10.1182/blood-2002-10-3306. [DOI] [PubMed] [Google Scholar]
- 54.Crespo M, et al. ZAP-70 expression as a surrogate for immunoglobulin-variable-region mutations in chronic lymphocytic leukemia. N Engl J Med. 2003;348(18):1764–75. doi: 10.1056/NEJMoa023143. [DOI] [PubMed] [Google Scholar]
- 55.Rassenti LZ, et al. ZAP-70 compared with immunoglobulin heavy-chain gene mutation status as a predictor of disease progression in chronic lymphocytic leukemia. N Engl J Med. 2004;351(9):893–901. doi: 10.1056/NEJMoa040857. [DOI] [PubMed] [Google Scholar]
- 56.Orchard JA, et al. ZAP-70 expression and prognosis in chronic lymphocytic leukaemia. Lancet. 2004;363(9403):105–11. doi: 10.1016/S0140-6736(03)15260-9. [DOI] [PubMed] [Google Scholar]
- 57.Rassenti LZ, et al. Relative value of ZAP-70, CD38, and immunoglobulin mutation status in predicting aggressive disease in chronic lymphocytic leukemia. Blood. 2008;112(5):1923–30. doi: 10.1182/blood-2007-05-092882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen L, et al. ZAP-70 directly enhances IgM signaling in chronic lymphocytic leukemia. Blood. 2005;105(5):2036–41. doi: 10.1182/blood-2004-05-1715. [DOI] [PubMed] [Google Scholar]
- 59.Chen L, et al. ZAP-70 enhances IgM signaling independent of its kinase activity in chronic lymphocytic leukemia. Blood. 2008;111(5):2685–92. doi: 10.1182/blood-2006-12-062265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gobessi S, et al. ZAP-70 enhances B-cell-receptor signaling despite absent or inefficient tyrosine kinase activation in chronic lymphocytic leukemia and lymphoma B cells. Blood. 2007;109(5):2032–9. doi: 10.1182/blood-2006-03-011759. [DOI] [PubMed] [Google Scholar]
- 61.Bernal A, et al. Survival of leukemic B cells promoted by engagement of the antigen receptor. Blood. 2001;98(10):3050–7. doi: 10.1182/blood.v98.10.3050. [DOI] [PubMed] [Google Scholar]
- 62.Quiroga MP, et al. B cell antigen receptor signaling enhances chronic lymphocytic leukemia cell migration and survival: specific targeting with a novel Syk inhibitor, R406. Blood. 2009 doi: 10.1182/blood-2009-03-212837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deglesne PA, et al. Survival response to B-cell receptor ligation is restricted to progressive chronic lymphocytic leukemia cells irrespective of Zap70 expression. Cancer Res. 2006;66(14):7158–66. doi: 10.1158/0008-5472.CAN-06-0085. [DOI] [PubMed] [Google Scholar]
- 64.Burger JA. Nurture versus nature: the microenvironment in chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program. 2011;2011:96–103. doi: 10.1182/asheducation-2011.1.96. [DOI] [PubMed] [Google Scholar]
- 65.Dal Bo M, et al. Microenvironmental interactions in chronic lymphocytic leukemia: hints for pathogenesis and identification of targets for rational therapy. Curr Pharm Des. 2012;18(23):3323–34. doi: 10.2174/138161212801227078. [DOI] [PubMed] [Google Scholar]
- 66.Bagnara D, et al. A novel adoptive transfer model of chronic lymphocytic leukemia suggests a key role for T lymphocytes in the disease. Blood. 2011;117(20):5463–72. doi: 10.1182/blood-2010-12-324210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Devereux S. Two-faced T cells in CLL. Blood. 2011;117(20):5273–4. doi: 10.1182/blood-2011-03-342709. [DOI] [PubMed] [Google Scholar]
- 68.Pizzolo G, et al. Immunohistologic study of bone marrow involvement in B-chronic lymphocytic leukemia. Blood. 1983;62(6):1289–96. [PubMed] [Google Scholar]
- 69.Stevenson FK, Caligaris-Cappio F. Chronic lymphocytic leukemia: revelations from the B-cell receptor. Blood. 2004;103(12):4389–95. doi: 10.1182/blood-2003-12-4312. [DOI] [PubMed] [Google Scholar]
- 70.Ghia P, et al. Chronic lymphocytic leukemia B cells are endowed with the capacity to attract CD4+, CD40L+ T cells by producing CCL22. Eur J Immunol. 2002;32(5):1403–13. doi: 10.1002/1521-4141(200205)32:5<1403::AID-IMMU1403>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 71.Fluckiger AC, et al. Responsiveness of chronic lymphocytic leukemia B cells activated via surface Igs or CD40 to B-cell tropic factors. Blood. 1992;80(12):3173–81. [PubMed] [Google Scholar]
- 72.Cuni S, et al. A sustained activation of PI3K/NF-kappaB pathway is critical for the survival of chronic lymphocytic leukemia B cells. Leukemia. 2004;18(8):1391–400. doi: 10.1038/sj.leu.2403398. [DOI] [PubMed] [Google Scholar]
- 73.Furman RR, et al. Modulation of NF-kappa B activity and apoptosis in chronic lymphocytic leukemia B cells. J Immunol. 2000;164(4):2200–6. doi: 10.4049/jimmunol.164.4.2200. [DOI] [PubMed] [Google Scholar]
- 74.Romano MF, et al. Triggering of CD40 antigen inhibits fludarabine-induced apoptosis in B chronic lymphocytic leukemia cells. Blood. 1998;92(3):990–5. [PubMed] [Google Scholar]
- 75.Kitada S, et al. Bryostatin and CD40-ligand enhance apoptosis resistance and induce expression of cell survival genes in B-cell chronic lymphocytic leukaemia. Br J Haematol. 1999;106(4):995–1004. doi: 10.1046/j.1365-2141.1999.01642.x. [DOI] [PubMed] [Google Scholar]
- 76.Hallaert DY, et al. c-Abl kinase inhibitors overcome CD40-mediated drug resistance in CLL; Implications for therapeutic targeting of chemoresistant niches. Blood. 2008 doi: 10.1182/blood-2008-03-146704. [DOI] [PubMed] [Google Scholar]
- 77.Li F, et al. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature. 1998;396(6711):580–4. doi: 10.1038/25141. [DOI] [PubMed] [Google Scholar]
- 78.Dancescu M, et al. Interleukin 4 protects chronic lymphocytic leukemic B cells from death by apoptosis and upregulates Bcl-2 expression. J Exp Med. 1992;176(5):1319–26. doi: 10.1084/jem.176.5.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Trentin L, et al. Interleukin-15 promotes the growth of leukemic cells of patients with B-cell chronic lymphoproliferative disorders. Blood. 1996;87(8):3327–35. [PubMed] [Google Scholar]
- 80.Foa R, et al. Production of tumor necrosis factor-alpha by B-cell chronic lymphocytic leukemia cells: a possible regulatory role of TNF in the progression of the disease. Blood. 1990;76(2):393–400. [PubMed] [Google Scholar]
- 81.Reittie JE, et al. Interleukin-6 inhibits apoptosis and tumour necrosis factor induced proliferation of B-chronic lymphocytic leukaemia. Leuk Lymphoma. 1996;22(1–2):83–90. doi: 10.3109/10428199609051732. follow 186, color plate VI. [DOI] [PubMed] [Google Scholar]
- 82.Buschle M, et al. Interferon gamma inhibits apoptotic cell death in B cell chronic lymphocytic leukemia. J Exp Med. 1993;177(1):213–8. doi: 10.1084/jem.177.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Panayiotidis P, et al. Alpha-interferon (alpha-IFN) protects B-chronic lymphocytic leukaemia cells from apoptotic cell death in vitro. Br J Haematol. 1994;86(1):169–73. doi: 10.1111/j.1365-2141.1994.tb03269.x. [DOI] [PubMed] [Google Scholar]
- 84.Chaouchi N, et al. Interleukin-13 inhibits interleukin-2-induced proliferation and protects chronic lymphocytic leukemia B cells from in vitro apoptosis. Blood. 1996;87(3):1022–9. [PubMed] [Google Scholar]
- 85.Lotz M, Ranheim E, Kipps TJ. Transforming growth factor beta as endogenous growth inhibitor of chronic lymphocytic leukemia B cells. J Exp Med. 1994;179(3):999–1004. doi: 10.1084/jem.179.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fayad L, et al. Interleukin-6 and interleukin-10 levels in chronic lymphocytic leukemia: correlation with phenotypic characteristics and outcome. Blood. 2001;97(1):256–63. doi: 10.1182/blood.v97.1.256. [DOI] [PubMed] [Google Scholar]
- 87.Ranheim EA, Kipps TJ. Activated T cells induce expression of B7/BB1 on normal or leukemic B cells through a CD40-dependent signal. J Exp Med. 1993;177(4):925–35. doi: 10.1084/jem.177.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Beyer M, et al. Reduced frequencies and suppressive function of CD4+CD25hi regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine. Blood. 2005;106(6):2018–25. doi: 10.1182/blood-2005-02-0642. [DOI] [PubMed] [Google Scholar]
- 89.Biancotto A, et al. Phenotypic complexity of T regulatory subsets in patients with B-chronic lymphocytic leukemia. Mod Pathol. 2012;25(2):246–59. doi: 10.1038/modpathol.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gorgun G, et al. Chronic lymphocytic leukemia cells induce changes in gene expression of CD4 and CD8 T cells. J Clin Invest. 2005;115(7):1797–805. doi: 10.1172/JCI24176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ramsay AG, et al. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. J Clin Invest. 2008;118(7):2427–37. doi: 10.1172/JCI35017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ramsay AG, Gribben JG. The 3 Rs in CLL immune dysfunction. Blood. 2010;115(13):2563–4. doi: 10.1182/blood-2009-12-256909. [DOI] [PubMed] [Google Scholar]
- 93.Lagneaux L, et al. Chronic lymphocytic leukemic B cells but not normal B cells are rescued from apoptosis by contact with normal bone marrow stromal cells. Blood. 1998;91(7):2387–96. [PubMed] [Google Scholar]
- 94.Kurtova AV, et al. Diverse marrow stromal cells protect CLL cells from spontaneous and drug-induced apoptosis: development of a reliable and reproducible system to assess stromal cell adhesion-mediated drug resistance. Blood. 2009;114(20):4441–50. doi: 10.1182/blood-2009-07-233718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ding W, et al. Platelet-derived growth factor (PDGF)-PDGF receptor interaction activates bone marrow-derived mesenchymal stromal cells derived from chronic lymphocytic leukemia: implications for an angiogenic switch. Blood. 2010;116(16):2984–93. doi: 10.1182/blood-2010-02-269894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ding W, et al. Bi-directional activation between mesenchymal stem cells and CLL B-cells: implication for CLL disease progression. Br J Haematol. 2009;147(4):471–83. doi: 10.1111/j.1365-2141.2009.07868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ghosh AK, et al. Circulating microvesicles in B-cell chronic lymphocytic leukemia can stimulate marrow stromal cells: implications for disease progression. Blood. 2010;115(9):1755–64. doi: 10.1182/blood-2009-09-242719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schulz A, et al. Inflammatory cytokines and signaling pathways are associated with survival of primary chronic lymphocytic leukemia cells in vitro: a dominant role of CCL2. Haematologica. 2011;96(3):408–16. doi: 10.3324/haematol.2010.031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ruan J, et al. Magnitude of stromal hemangiogenesis correlates with histologic subtype of non-Hodgkin's lymphoma. Clin Cancer Res. 2006;12(19):5622–31. doi: 10.1158/1078-0432.CCR-06-1204. [DOI] [PubMed] [Google Scholar]
- 100.Orimo A, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121(3):335–48. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 101.Burger M, et al. Small peptide inhibitors of the CXCR4 chemokine receptor (CD184) antagonize the activation, migration, and antiapoptotic responses of CXCL12 in chronic lymphocytic leukemia B cells. Blood. 2005;106(5):1824–30. doi: 10.1182/blood-2004-12-4918. [DOI] [PubMed] [Google Scholar]
- 102.Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 103.de la Fuente MT, et al. Engagement of alpha4beta1 integrin by fibronectin induces in vitro resistance of B chronic lymphocytic leukemia cells to fludarabine. J Leukoc Biol. 2002;71(3):495–502. [PubMed] [Google Scholar]
- 104.Redondo-Munoz J, et al. Matrix metalloproteinase-9 promotes chronic lymphocytic leukemia b cell survival through its hemopexin domain. Cancer Cell. 2010;17(2):160–72. doi: 10.1016/j.ccr.2009.12.044. [DOI] [PubMed] [Google Scholar]
- 105.Redondo-Munoz J, et al. MMP-9 in B-cell chronic lymphocytic leukemia is up-regulated by alpha4beta1 integrin or CXCR4 engagement via distinct signaling pathways, localizes to podosomes, and is involved in cell invasion and migration. Blood. 2006;108(9):3143–51. doi: 10.1182/blood-2006-03-007294. [DOI] [PubMed] [Google Scholar]
- 106.Ringshausen I, et al. Constitutive activation of the MAPkinase p38 is critical for MMP-9 production and survival of B-CLL cells on bone marrow stromal cells. Leukemia. 2004;18(12):1964–70. doi: 10.1038/sj.leu.2403544. [DOI] [PubMed] [Google Scholar]
- 107.Lee YK, et al. VEGF receptor phosphorylation status and apoptosis is modulated by a green tea component, epigallocatechin-3-gallate (EGCG), in B-cell chronic lymphocytic leukemia. Blood. 2004;104(3):788–94. doi: 10.1182/blood-2003-08-2763. [DOI] [PubMed] [Google Scholar]
- 108.Lee YK, et al. VEGF receptors on chronic lymphocytic leukemia (CLL) B cells interact with STAT 1 and 3: implication for apoptosis resistance. Leukemia. 2005;19(4):513–23. doi: 10.1038/sj.leu.2403667. [DOI] [PubMed] [Google Scholar]
- 109.Gehrke I, et al. Bone marrow stromal cell-derived vascular endothelial growth factor (VEGF) rather than chronic lymphocytic leukemia (CLL) cell-derived VEGF is essential for the apoptotic resistance of cultured CLL cells. Mol Med. 2011;17(7–8):619–27. doi: 10.2119/molmed.2010.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Buggins AG, et al. Interaction with vascular endothelium enhances survival in primary chronic lymphocytic leukemia cells via NF-kappaB activation and de novo gene transcription. Cancer Res. 2010;70(19):7523–33. doi: 10.1158/0008-5472.CAN-10-1634. [DOI] [PubMed] [Google Scholar]
- 111.Herishanu Y, et al. Activation of CD44, a receptor for extracellular matrix components, protects chronic lymphocytic leukemia cells from spontaneous and drug induced apoptosis through MCL-1. Leuk Lymphoma. 2011;52(9):1758–69. doi: 10.3109/10428194.2011.569962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Park CS, Choi YS. How do follicular dendritic cells interact intimately with B cells in the germinal centre? Immunology. 2005;114(1):2–10. doi: 10.1111/j.1365-2567.2004.02075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chilosi M, et al. Routine immunofluorescent and histochemical analysis of bone marrow involvement of lymphoma/leukaemia: the use of cryostat sections. Br J Cancer. 1983;48(6):763–775. doi: 10.1038/bjc.1983.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ratech H, et al. Immunoarchitecture of the “pseudofollicles” of well-differentiated (small) lymphocytic lymphoma: a comparison with true follicles. Hum Pathol. 1988;19(1):89–94. doi: 10.1016/s0046-8177(88)80322-8. [DOI] [PubMed] [Google Scholar]
- 115.Schmid C, Isaacson PG. Proliferation centres in B-cell malignant lymphoma, lymphocytic (B-CLL): an immunophenotypic study. Histopathology. 1994;24(5):445–51. doi: 10.1111/j.1365-2559.1994.tb00553.x. [DOI] [PubMed] [Google Scholar]
- 116.Chilosi M, et al. Immunohistochemical demonstration of follicular dendritic cells in bone marrow involvement of B-cell chronic lymphocytic leukemia. Cancer. 1985;56(2):328–32. doi: 10.1002/1097-0142(19850715)56:2<328::aid-cncr2820560221>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 117.Pedersen IM, et al. Protection of CLL B cells by a follicular dendritic cell line is dependent on induction of Mcl-1. Blood. 2002;100(5):1795–801. [PubMed] [Google Scholar]
- 118.Burger JA, et al. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood. 2000;96(8):2655–63. [PubMed] [Google Scholar]
- 119.Tsukada N, et al. Distinctive features of “nurselike” cells that differentiate in the context of chronic lymphocytic leukemia. Blood. 2002;99(3):1030–7. doi: 10.1182/blood.v99.3.1030. [DOI] [PubMed] [Google Scholar]
- 120.Nishio M, et al. Nurselike cells express BAFF and APRIL, which can promote survival of chronic lymphocytic leukemia cells via a paracrine pathway distinct from that of SDF-1alpha. Blood. 2005;106(3):1012–20. doi: 10.1182/blood-2004-03-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schiemann B, et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293(5537):2111–4. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 122.Schneider P, et al. Maturation of marginal zone and follicular B cells requires B cell activating factor of the tumor necrosis factor family and is independent of B cell maturation antigen. J Exp Med. 2001;194(11):1691–7. doi: 10.1084/jem.194.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mackay F, et al. BAFF AND APRIL: a tutorial on B cell survival. Annu Rev Immunol. 2003;21:231–64. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 124.Kern C, et al. Involvement of BAFF and APRIL in the resistance to apoptosis of B-CLL through an autocrine pathway. Blood. 2004;103(2):679–88. doi: 10.1182/blood-2003-02-0540. [DOI] [PubMed] [Google Scholar]
- 125.Novak AJ, et al. Aberrant expression of B-lymphocyte stimulator by B chronic lymphocytic leukemia cells: a mechanism for survival. Blood. 2002;100(8):2973–9. doi: 10.1182/blood-2002-02-0558. [DOI] [PubMed] [Google Scholar]
- 126.Zhang W, et al. B-cell activating factor and v-Myc myelocytomatosis viral oncogene homolog (c-Myc) influence progression of chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2010;107(44):18956–60. doi: 10.1073/pnas.1013420107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Krysov S, et al. Surface IgM stimulation induces MEK1/2-dependent MYC expression in chronic lymphocytic leukemia cells. Blood. 2012;119(1):170–9. doi: 10.1182/blood-2011-07-370403. [DOI] [PubMed] [Google Scholar]
- 128.Deaglio S, et al. CD38 and CD100 lead a network of surface receptors relaying positive signals for B-CLL growth and survival. Blood. 2005;105(8):3042–50. doi: 10.1182/blood-2004-10-3873. [DOI] [PubMed] [Google Scholar]
- 129.Granziero L, et al. CD100/Plexin-B1 interactions sustain proliferation and survival of normal and leukemic CD5+ B lymphocytes. Blood. 2003;101(5):1962–9. doi: 10.1182/blood-2002-05-1339. [DOI] [PubMed] [Google Scholar]
- 130.Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat I. 2001;2(2):123–8. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 131.Burger JA, Burger M, Kipps TJ. Chronic lymphocytic leukemia B cells express functional CXCR4 chemokine receptors that mediate spontaneous migration beneath bone marrow stromal cells. Blood. 1999;94(11):3658–67. [PubMed] [Google Scholar]
- 132.Mohle R, et al. Overexpression of the chemokine receptor CXCR4 in B cell chronic lymphocytic leukemia is associated with increased functional response to stromal cell-derived factor-1 (SDF-1) Leukemia. 1999;13(12):1954–9. doi: 10.1038/sj.leu.2401602. [DOI] [PubMed] [Google Scholar]
- 133.Richardson SJ, et al. ZAP-70 expression is associated with enhanced ability to respond to migratory and survival signals in B-cell chronic lymphocytic leukemia (B-CLL) Blood. 2006;107(9):3584–92. doi: 10.1182/blood-2005-04-1718. [DOI] [PubMed] [Google Scholar]
- 134.Vaisitti T, et al. CD38 increases CXCL12-mediated signals and homing of chronic lymphocytic leukemia cells. Leukemia. 2010;24(5):958–69. doi: 10.1038/leu.2010.36. [DOI] [PubMed] [Google Scholar]
- 135.Deaglio S, et al. CD38 and ZAP-70 are functionally linked and mark CLL cells with high migratory potential. Blood. 2007 doi: 10.1182/blood-2007-06-094029. [DOI] [PubMed] [Google Scholar]
- 136.Gunn MD, et al. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt's lymphoma receptor-1. Nature. 1998;391(6669):799–803. doi: 10.1038/35876. [DOI] [PubMed] [Google Scholar]
- 137.Burkle A, et al. Overexpression of the CXCR5 chemokine receptor, and its ligand, CXCL13 in B cell chronic lymphocytic leukemia. Blood. 2007 doi: 10.1182/blood-2007-05-089409. [DOI] [PubMed] [Google Scholar]
- 138.Ticchioni M, et al. Homeostatic chemokines increase survival of B-chronic lymphocytic leukemia cells through inactivation of transcription factor FOXO3a. Oncogene. 2007 doi: 10.1038/sj.onc.1210519. [DOI] [PubMed] [Google Scholar]
- 139.Reif K, et al. Balanced responsiveness to chemoattractants from adjacent zones determines B-cell position. Nature. 2002;416(6876):94–9. doi: 10.1038/416094a. [DOI] [PubMed] [Google Scholar]
- 140.Till KJ, et al. The chemokine receptor CCR7 and alpha4 integrin are important for migration of chronic lymphocytic leukemia cells into lymph nodes. Blood. 2002;99(8):2977–84. doi: 10.1182/blood.v99.8.2977. [DOI] [PubMed] [Google Scholar]
- 141.Chunsong H, et al. CXC chemokine ligand 13 and CC chemokine ligand 19 cooperatively render resistance to apoptosis in B cell lineage acute and chronic lymphocytic leukemia CD23+CD5+ B cells. J Immunol. 2006;177(10):6713–22. doi: 10.4049/jimmunol.177.10.6713. [DOI] [PubMed] [Google Scholar]
- 142.Lopez-Giral S, et al. Chemokine receptors that mediate B cell homing to secondary lymphoid tissues are highly expressed in B cell chronic lymphocytic leukemia and non-Hodgkin lymphomas with widespread nodular dissemination. J Leukoc Biol. 2004;76(2):462–71. doi: 10.1189/jlb.1203652. [DOI] [PubMed] [Google Scholar]
- 143.Menten P, Wuyts A, Van Damme J. Macrophage inflammatory protein-1. Cytokine Growth Factor Rev. 2002;13(6):455–81. doi: 10.1016/s1359-6101(02)00045-x. [DOI] [PubMed] [Google Scholar]
- 144.Burger JA, et al. High-level expression of the T-cell chemokines CCL3 and CCL4 by chronic lymphocytic leukemia B cells in nurselike cell cocultures and after BCR stimulation. Blood. 2009;113(13):3050–8. doi: 10.1182/blood-2008-07-170415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zucchetto A, et al. CD38/CD31, the CCL3 and CCL4 chemokines, and CD49d/vascular cell adhesion molecule-1 are interchained by sequential events sustaining chronic lymphocytic leukemia cell survival. Cancer Res. 2009;69(9):4001–9. doi: 10.1158/0008-5472.CAN-08-4173. [DOI] [PubMed] [Google Scholar]
- 146.Sivina M, et al. CCL3 (MIP-1alpha) plasma levels and the risk for disease progression in chronic lymphocytic leukemia. Blood. 2011;117(5):1662–9. doi: 10.1182/blood-2010-09-307249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hewamana S, et al. Rel a is an independent biomarker of clinical outcome in chronic lymphocytic leukemia. J Clin Oncol. 2009;27(5):763–9. doi: 10.1200/JCO.2008.19.1114. [DOI] [PubMed] [Google Scholar]
- 148.Fruman DA, Bismuth G. Fine tuning the immune response with PI3K. Immunol Rev. 2009;228(1):253–72. doi: 10.1111/j.1600-065X.2008.00750.x. [DOI] [PubMed] [Google Scholar]
- 149.Cuesta-Mateos C, et al. Analysis of migratory and prosurvival pathways induced by the homeostatic chemokines CCL19 and CCL21 in B-cell chronic lymphocytic leukemia. Exp Hematol. 2010;38(9):756–64. 764 e1–4. doi: 10.1016/j.exphem.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 150.Hanada M, et al. bcl-2 gene hypomethylation and high-level expression in B-cell chronic lymphocytic leukemia. Blood. 1993;82(6):1820–8. [PubMed] [Google Scholar]
- 151.Kitada S, et al. Expression of apoptosis-regulating proteins in chronic lymphocytic leukemia: correlations with In vitro and In vivo chemoresponses. Blood. 1998;91(9):3379–89. [PubMed] [Google Scholar]
- 152.Balogh Z, et al. High rate of neoplastic cells with genetic abnormalities in proliferation centers of chronic lymphocytic leukemia. Leuk Lymphoma. 2011;52(6):1080–4. doi: 10.3109/10428194.2011.555889. [DOI] [PubMed] [Google Scholar]
- 153.Delmer A, et al. Overexpression of cyclin D2 in chronic B-cell malignancies. Blood. 1995;85(10):2870–6. [PubMed] [Google Scholar]
- 154.Solvason N, et al. Induction of cell cycle regulatory proteins in anti-immunoglobulin-stimulated mature B lymphocytes. J Exp Med. 1996;184(2):407–17. doi: 10.1084/jem.184.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Igawa T, et al. Cyclin D2 is overexpressed in proliferation centers of chronic lymphocytic leukemia/small lymphocytic lymphoma. Cancer Sci. 2011;102(11):2103–7. doi: 10.1111/j.1349-7006.2011.02046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Phillips JA, et al. The NZB mouse as a model for chronic lymphocytic leukemia. Cancer Res. 1992;52(2):437–43. [PubMed] [Google Scholar]
- 157.Raveche ES, et al. Abnormal microRNA-16 locus with synteny to human 13q14 linked to CLL in NZB mice. Blood. 2007;109(12):5079–86. doi: 10.1182/blood-2007-02-071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Calin GA, et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2002;99(24):15524–9. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bichi R, et al. Human chronic lymphocytic leukemia modeled in mouse by targeted TCL1 expression. Proc Natl Acad Sci U S A. 2002;99(10):6955–60. doi: 10.1073/pnas.102181599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gorgun G, et al. E(mu)-TCL1 mice represent a model for immunotherapeutic reversal of chronic lymphocytic leukemia-induced T-cell dysfunction. Proc Natl Acad Sci U S A. 2009;106(15):6250–5. doi: 10.1073/pnas.0901166106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Klein U, et al. The DLEU2/miR-15a/16-1 cluster controls B cell proliferation and its deletion leads to chronic lymphocytic leukemia. Cancer Cell. 2010;17(1):28–40. doi: 10.1016/j.ccr.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 162.Wen X, et al. Transgene-mediated hyper-expression of IL-5 inhibits autoimmune disease but increases the risk of B cell chronic lymphocytic leukemia in a model of murine lupus. Eur J Immunol. 2004;34(10):2740–9. doi: 10.1002/eji.200425267. [DOI] [PubMed] [Google Scholar]
- 163.Zapata JM, et al. TNF receptor-associated factor (TRAF) domain and Bcl-2 cooperate to induce small B cell lymphoma/chronic lymphocytic leukemia in transgenic mice. Proc Natl Acad Sci U S A. 2004;101(47):16600–5. doi: 10.1073/pnas.0407541101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Durig J, et al. A novel nonobese diabetic/severe combined immunodeficient xenograft model for chronic lymphocytic leukemia reflects important clinical characteristics of the disease. Cancer Res. 2007;67(18):8653–61. doi: 10.1158/0008-5472.CAN-07-1198. [DOI] [PubMed] [Google Scholar]
- 165.Bertilaccio MT, et al. A novel Rag2-/-gammac-/--xenograft model of human CLL. Blood. 2010;115(8):1605–9. doi: 10.1182/blood-2009-05-223586. [DOI] [PubMed] [Google Scholar]
- 166.Sun X, et al. The NSG - Human CLL Xenograft Model Recapitulates the Human Lymph Node Microenvironment in Regards to B-Cell Activation and Tumor Proliferation. ASH Annual Meeting Abstracts. 2011;118(21):973. [Google Scholar]
- 167.Herman SEM, et al. The Bruton Tyrosine Kinase Inhibitor, PCI-32765, Inhibits Activation and Proliferation of Human Chronic Lymphocytic Leukemia Cells in the NSG Xenograph Mouse Model of the Tissue Microenvironment. ASH Annual Meeting Abstracts. 2011;118(21):596. [Google Scholar]
- 168.Aydin S, et al. Investigating the role of CD38 and functionally related molecular risk factors in the CLL NOD/SCID xenograft model. Eur J Haematol. 2011;87(1):10–9. doi: 10.1111/j.1600-0609.2011.01626.x. [DOI] [PubMed] [Google Scholar]
- 169.Buchner M, et al. The microenvironment differentially impairs passive and active immunotherapy in chronic lymphocytic leukaemia - CXCR4 antagonists as potential adjuvants for monoclonal antibodies. Br J Haematol. 2010;151(2):167–78. doi: 10.1111/j.1365-2141.2010.08316.x. [DOI] [PubMed] [Google Scholar]
- 170.Andritsos L, et al. Preliminary Results From A Phase I Dose Escalation Study to Determine the Maximum Tolerated Dose of Plerixafor In Combination with Rituximab In Patients with Relapsed Chronic Lymphocytic Leukemia. ASH Annual Meeting Abstracts. 2010;116(21):2450. [Google Scholar]
- 171.Kofler DM, et al. Phase 1b trial of atacicept, a recombinant protein binding BLyS and APRIL, in patients with chronic lymphocytic leukemia. Leukemia. 2012;26(4):841–4. doi: 10.1038/leu.2011.286. [DOI] [PubMed] [Google Scholar]
- 172.Byrd JC, et al. The Bruton's Tyrosine Kinase (BTK) Inhibitor Ibrutinib (PCI-32765) Promotes High Response Rate, Durable Remissions, and Is Tolerable in Treatment Naive (TN) and Relapsed or Refractory (RR) Chronic Lymphocytic Leukemia (CLL) or Small Lymphocytic Lymphoma (SLL) Patients Including Patients with High-Risk (HR) Disease: New and Updated Results of 116 Patients in a Phase Ib/II Study. ASH Annual Meeting Abstracts. 2012;120(21):189. [Google Scholar]
- 173.Coutre SE, BJ, Furman RR, et al. Phase I study of CAL-101, an isoform-selective inhibitor of phosphatidylinositol 3-kinase P110d, in patients with previously treated chronic lymphocytic leukemia. J Clin Oncol. 2011;29 [Google Scholar]
- 174.Friedberg JW, et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2010;115(13):2578–85. doi: 10.1182/blood-2009-08-236471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.de Rooij MF, et al. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood. 2012;119(11):2590–4. doi: 10.1182/blood-2011-11-390989. [DOI] [PubMed] [Google Scholar]
- 176.Ponader S, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119(5):1182–9. doi: 10.1182/blood-2011-10-386417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hantschel O, et al. The Btk tyrosine kinase is a major target of the Bcr-Abl inhibitor dasatinib. Proc Natl Acad Sci U S A. 2007;104(33):13283–8. doi: 10.1073/pnas.0702654104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Amrein L, et al. Dasatinib sensitizes primary chronic lymphocytic leukaemia lymphocytes to chlorambucil and fludarabine in vitro. Br J Haematol. 2008;143(5):698–706. doi: 10.1111/j.1365-2141.2008.07418.x. [DOI] [PubMed] [Google Scholar]
- 179.Veldurthy A, et al. The kinase inhibitor dasatinib induces apoptosis in chronic lymphocytic leukemia cells in vitro with preference for a subgroup of patients with unmutated IgVH genes. Blood. 2008;112(4):1443–52. doi: 10.1182/blood-2007-11-123984. [DOI] [PubMed] [Google Scholar]
- 180.McCaig AM, et al. Dasatinib inhibits B cell receptor signalling in chronic lymphocytic leukaemia but novel combination approaches are required to overcome additional pro-survival microenvironmental signals. Br J Haematol. 2011;153(2):199–211. doi: 10.1111/j.1365-2141.2010.08507.x. [DOI] [PubMed] [Google Scholar]
- 181.Amrein PC, et al. Phase II study of dasatinib in relapsed or refractory chronic lymphocytic leukemia. Clin Cancer Res. 2011;17(9):2977–86. doi: 10.1158/1078-0432.CCR-10-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mocsai A, Ruland J, Tybulewicz VL. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol. 2010;10(6):387–402. doi: 10.1038/nri2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gobessi S, et al. Inhibition of constitutive and BCR-induced Syk activation downregulates Mcl-1 and induces apoptosis in chronic lymphocytic leukemia B cells. Leukemia. 2009;23(4):686–97. doi: 10.1038/leu.2008.346. [DOI] [PubMed] [Google Scholar]
- 184.Hoellenriegel J, et al. Selective, novel spleen tyrosine kinase (Syk) inhibitors suppress chronic lymphocytic leukemia B-cell activation and migration. Leukemia. 2012 doi: 10.1038/leu.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Suljagic M, et al. The Syk inhibitor fostamatinib disodium (R788) inhibits tumor growth in the Emu- TCL1 transgenic mouse model of CLL by blocking antigen-dependent B-cell receptor signaling. Blood. 2010;116(23):4894–905. doi: 10.1182/blood-2010-03-275180. [DOI] [PubMed] [Google Scholar]
- 186.Herman SEM, et al. Fostamatinib Inhibits BCR Signaling, and Reduces Tumor Cell Activation and Proliferation in Patients with Relapsed Refractory Chronic Lymphocytic Leukemia. ASH Annual Meeting Abstracts. 2012;120(21):2882. [Google Scholar]
- 187.Kyttaris VC. Kinase inhibitors: a new class of antirheumatic drugs. Drug Des Devel Ther. 2012;6:245–50. doi: 10.2147/DDDT.S25426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Buggy JJ, Elias L. Bruton tyrosine kinase (BTK) and its role in B-cell malignancy. Int Rev Immunol. 2012;31(2):119–32. doi: 10.3109/08830185.2012.664797. [DOI] [PubMed] [Google Scholar]
- 189.Tsukada S, et al. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell. 1993;72(2):279–90. doi: 10.1016/0092-8674(93)90667-f. [DOI] [PubMed] [Google Scholar]
- 190.Herman SE, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117(23):6287–96. doi: 10.1182/blood-2011-01-328484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Advani RH, et al. Bruton Tyrosine Kinase Inhibitor Ibrutinib (PCI-32765) Has Significant Activity in Patients With Relapsed/Refractory B-Cell Malignancies. J Clin Oncol. 2012 doi: 10.1200/JCO.2012.42.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Herman SE, et al. Phosphatidylinositol 3-kinase-delta inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116(12):2078–88. doi: 10.1182/blood-2010-02-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Chapman CM, et al. ON 01910.Na is selectively cytotoxic for chronic lymphocytic leukemia cells through a dual mechanism of action involving PI3K/AKT inhibition and induction of oxidative stress. Clin Cancer Res. 2012;18(7):1979–91. doi: 10.1158/1078-0432.CCR-11-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Herman SE, et al. The role of phosphatidylinositol 3-kinase-delta in the immunomodulatory effects of lenalidomide in chronic lymphocytic leukemia. Blood. 2011;117(16):4323–7. doi: 10.1182/blood-2010-11-315705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hoellenriegel J, et al. The phosphoinositide 3'-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118(13):3603–12. doi: 10.1182/blood-2011-05-352492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lannutti BJ, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117(2):591–4. doi: 10.1182/blood-2010-03-275305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Plate JM. PI3-kinase regulates survival of chronic lymphocytic leukemia B-cells by preventing caspase 8 activation. Leuk Lymphoma. 2004;45(8):1519–29. doi: 10.1080/10428190410001683642. [DOI] [PubMed] [Google Scholar]
- 198.Cardone MH, et al. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282(5392):1318–21. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 199.Datta SR, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91(2):231–41. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 200.Hahn-Windgassen A, et al. Akt activates the mammalian target of rapamycin by regulating cellular ATP level and AMPK activity. J Biol Chem. 2005;280(37):32081–9. doi: 10.1074/jbc.M502876200. [DOI] [PubMed] [Google Scholar]
- 201.Brunet A, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96(6):857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 202.Hirai H, et al. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther. 2010;9(7):1956–67. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]
- 203.Gills JJ, Dennis PA. Perifosine: update on a novel Akt inhibitor. Curr Oncol Rep. 2009;11(2):102–10. doi: 10.1007/s11912-009-0016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kay JE, et al. Inhibition of T and B lymphocyte proliferation by rapamycin. Immunology. 1991;72(4):544–9. [PMC free article] [PubMed] [Google Scholar]
- 205.Decker T, et al. Rapamycin-induced G1 arrest in cycling B-CLL cells is associated with reduced expression of cyclin D3, cyclin E, cyclin A, and survivin. Blood. 2003;101(1):278–85. doi: 10.1182/blood-2002-01-0189. [DOI] [PubMed] [Google Scholar]
- 206.Decker T, et al. A pilot trial of the mTOR (mammalian target of rapamycin) inhibitor RAD001 in patients with advanced B-CLL. Ann Hematol. 2009;88(3):221–7. doi: 10.1007/s00277-008-0582-9. [DOI] [PubMed] [Google Scholar]
- 207.Zent CS, et al. The treatment of recurrent/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL) with everolimus results in clinical responses and mobilization of CLL cells into the circulation. Cancer. 2010;116(9):2201–7. doi: 10.1002/cncr.25005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Escudier B, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 209.Llovet JM, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 210.Wilhelm SM, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64(19):7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 211.Messmer D, et al. Chronic lymphocytic leukemia cells receive RAF-dependent survival signals in response to CXCL12 that are sensitive to inhibition by sorafenib. Blood. 2011;117(3):882–9. doi: 10.1182/blood-2010-04-282400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Huber S, et al. Sorafenib induces cell death in chronic lymphocytic leukemia by translational downregulation of Mcl-1. Leukemia. 2011;25(5):838–47. doi: 10.1038/leu.2011.2. [DOI] [PubMed] [Google Scholar]
- 213.Fecteau JF, et al. Sorafenib-induced apoptosis of chronic lymphocytic leukemia cells is associated with downregulation of RAF and myeloid cell leukemia sequence 1 (Mcl-1) Mol Med. 2012;18(1):19–28. doi: 10.2119/molmed.2011.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Lopez-Guerra M, et al. Sorafenib targets BCR kinases and blocks migratory and microenvironmental survival signals in CLL cells. Leukemia. 2012;26(6):1429–32. doi: 10.1038/leu.2011.364. [DOI] [PubMed] [Google Scholar]
- 215.Pepper C, et al. Antisense-mediated suppression of Bcl-2 highlights its pivotal role in failed apoptosis in B-cell chronic lymphocytic leukaemia. Br J Haematol. 1999;107(3):611–5. doi: 10.1046/j.1365-2141.1999.01726.x. [DOI] [PubMed] [Google Scholar]
- 216.O'Brien SM, et al. Phase I to II multicenter study of oblimersen sodium, a Bcl-2 antisense oligonucleotide, in patients with advanced chronic lymphocytic leukemia. J Clin Oncol. 2005;23(30):7697–702. doi: 10.1200/JCO.2005.02.4364. [DOI] [PubMed] [Google Scholar]
- 217.O'Brien S, et al. Randomized phase III trial of fludarabine plus cyclophosphamide with or without oblimersen sodium (Bcl-2 antisense) in patients with relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2007;25(9):1114–20. doi: 10.1200/JCO.2006.07.1191. [DOI] [PubMed] [Google Scholar]
- 218.O'Brien S, et al. 5-year survival in patients with relapsed or refractory chronic lymphocytic leukemia in a randomized, phase III trial of fludarabine plus cyclophosphamide with or without oblimersen. J Clin Oncol. 2009;27(31):5208–12. doi: 10.1200/JCO.2009.22.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Nguyen M, et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci U S A. 2007;104(49):19512–7. doi: 10.1073/pnas.0709443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.O'Brien SM, et al. Phase I study of obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist, in patients with advanced chronic lymphocytic leukemia. Blood. 2009;113(2):299–305. doi: 10.1182/blood-2008-02-137943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Vogler M, et al. Concurrent up-regulation of BCL-XL and BCL2A1 induces approximately 1000-fold resistance to ABT-737 in chronic lymphocytic leukemia. Blood. 2009;113(18):4403–13. doi: 10.1182/blood-2008-08-173310. [DOI] [PubMed] [Google Scholar]
- 222.Mason KD, et al. The BH3 mimetic compound, ABT-737, synergizes with a range of cytotoxic chemotherapy agents in chronic lymphocytic leukemia. Leukemia. 2009;23(11):2034–41. doi: 10.1038/leu.2009.151. [DOI] [PubMed] [Google Scholar]
- 223.Roberts AW, et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol. 2012;30(5):488–96. doi: 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Balakrishnan K, et al. AT-101 induces apoptosis in CLL B cells and overcomes stromal cell-mediated Mcl-1 induction and drug resistance. Blood. 2009;113(1):149–53. doi: 10.1182/blood-2008-02-138560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.James DF, Castro JE, OLoria CE, Prada RA, Aguillon TJ. Kipps, AT-101, a small molecule Bcl-2 antagonist, in treatment naïve CLL patients (pts) with high risk features; Preliminary results from an ongoing phase I trial. Journal of Clinical Oncology; ASCO Annual Meeting Proceedings Part I; 2006; 2006. Jun 20, Supplement. [Google Scholar]
- 226.Eckelman BP, Salvesen GS, Scott FL. Human inhibitor of apoptosis proteins: why XIAP is the black sheep of the family. EMBO Rep. 2006;7(10):988–94. doi: 10.1038/sj.embor.7400795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Frenzel LP, et al. Novel X-linked inhibitor of apoptosis inhibiting compound as sensitizer for TRAIL-mediated apoptosis in chronic lymphocytic leukaemia with poor prognosis. Br J Haematol. 2011;152(2):191–200. doi: 10.1111/j.1365-2141.2010.08426.x. [DOI] [PubMed] [Google Scholar]
- 228.Chanan-Khan A, et al. Clinical efficacy of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase II study. J Clin Oncol. 2006;24(34):5343–9. doi: 10.1200/JCO.2005.05.0401. [DOI] [PubMed] [Google Scholar]
- 229.Ferrajoli A, et al. Lenalidomide induces complete and partial remissions in patients with relapsed and refractory chronic lymphocytic leukemia. Blood. 2008;111(11):5291–7. doi: 10.1182/blood-2007-12-130120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Badoux XC, et al. Lenalidomide as initial therapy of elderly patients with chronic lymphocytic leukemia. Blood. 2011;118(13):3489–98. doi: 10.1182/blood-2011-03-339077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Andritsos LA, et al. Higher doses of lenalidomide are associated with unacceptable toxicity including life-threatening tumor flare in patients with chronic lymphocytic leukemia. J Clin Oncol. 2008;26(15):2519–25. doi: 10.1200/JCO.2007.13.9709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Aue G, et al. Lenalidomide-induced upregulation of CD80 on tumor cells correlates with T-cell activation, the rapid onset of a cytokine release syndrome and leukemic cell clearance in chronic lymphocytic leukemia. Haematologica. 2009;94(9):1266–73. doi: 10.3324/haematol.2009.005835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Chanan-Khan A, et al. Tumor flare reaction associated with lenalidomide treatment in patients with chronic lymphocytic leukemia predicts clinical response. Cancer. 2011;117(10):2127–35. doi: 10.1002/cncr.25748. [DOI] [PubMed] [Google Scholar]
- 234.Ramsay AG, et al. Multiple inhibitory ligands induce impaired T-cell immunologic synapse function in chronic lymphocytic leukemia that can be blocked with lenalidomide: establishing a reversible immune evasion mechanism in human cancer. Blood. 2012;120(7):1412–21. doi: 10.1182/blood-2012-02-411678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Aue G, et al. Correlates of Lenalidomide Induced Immune Stimulation and Response in CLL: Analysis in Patients on Treatment. ASH Annual Meeting Abstracts. 2011;118(21):979. [Google Scholar]
- 236.Wu L, et al. lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clin Cancer Res. 2008;14(14):4650–7. doi: 10.1158/1078-0432.CCR-07-4405. [DOI] [PubMed] [Google Scholar]
- 237.Masood A, et al. Downregulation of BCL2 by AT-101 enhances the antileukaemic effect of lenalidomide both by an immune dependant and independent manner. Br J Haematol. 2011 doi: 10.1111/j.1365-2141.2011.08984.x. [DOI] [PubMed] [Google Scholar]