Abstract
Chronic neuropathic pain is a significant consequence of spinal cord injury (SCI) that is associated with evoked pain, including allodynia and/or hyperalgesia. Allodynia is defined as a painful response to normally innocuous stimuli, and hyperalgesia occurs when there is an amplified pain response to normally noxious stimuli. We describe a model of a unilateral cervical level (C5) contusion injury where sensory recovery was assessed weekly for 6 weeks in 32 adult, female, Sprague-Dawley rats. Bilateral thermal hyperalgesia and tactile allodynia are detectable in the fore- and hindpaws as early as 7 days post-injury (dpi) and persist for at least 42 days. Paw withdrawal latency in response to a noxious thermal stimulus significantly intra-animal pre-operative values. Change in paw withdrawal latency plateaued at 21 dpi. Interestingly, bilateral forepaw allodynia develops in fewer than 40% of rats as measured by von Frey monofilament testing. Similar results occur in the hindpaws, where bilateral allodynia occurs in 46% of rats with SCI. The contralesional forepaw and both hindpaws of rats showed a slight increase in paw withdrawal threshold to tactile stimuli acutely after SCI, corresponding to ipsilesional forelimb motor deficits that resolve over time. That there is no difference among allodynic and non-allodynic groups in overall spared tissue or specifically of the dorsal column or ventrolateral white matter where ascending sensory tracts reside suggests that SCI-induced pain does not depend solely on the size or extent of the lesion, but that other mechanisms are in play. These observations provide a valid model system for future testing of therapeutic interventions to prevent the onset or to reduce the debilitating effects of chronic neuropathic pain after SCI.
Key words: central pain, mechanical allodynia, spinal cord injury, thermal hyperalgesia
Introduction
There is a growing population in the United States of more than 1.2 million persons living with spinal cord injury.1 More than 60% of these persons are considered quadriplegic, having obvious motor impairments of both the upper and lower limbs.2 These impairments are often coupled with unseen deficits in autonomic and sensory function in which SCI inflicts profound and chronic neuropathic pain in more than 70% of the injured population.3,4 This pain can be categorized by the dependence on perception of external stimuli. Spontaneous pain occurs intermittently without the application of any external stimuli and is described as burning, cutting, or piercing pain.5,6 Evoked pain occurs in response to a normally noxious (hyperalgesia) or innocuous (allodynia) stimulus.
After SCI, these aberrant and unpleasant sensations are defined further relative to the injury epicenter. Above-level pain occurs at dermatomes rostral to the SCI site; at-level pain occurs in dermatomes in close proximity to the injury epicenter; and below-level pain is localized to dermatomes caudal to the injury site. Neuropathic pain impinges on quality of life, persists over time, and is often refractory to treatment.7–14
To optimize treatments for SCI-induced neuropathic pain, it is imperative that we have valid and accurate platforms to investigate the mechanism(s) of its development and persistence. Rodent SCI models of neuropathic pain are attractive because they directly emulate the human condition, and it has been established that sensitive, accurate, and valid measures of evoked tactile and thermal sensation can be obtained throughout the period of recovery.6,15,16 After incomplete SCI, hypersensitive nocifensive behaviors correspond to anatomical and electrophysiological changes in supraspinal regions such as the ventral posterio-lateral (VPL) nucleus of the thalamus and the cortex,17–19 indicating that evoked stimulation is associated with supraspinal awareness and perception.
We present data pertaining to a moderate, unilateral C5 contusion injury as a model of chronic at- and below-level pain after SCI. We demonstrate the early temporal onset, bilateral nature, and persistence of both thermal hyperalgesia and tactile allodynia in response to this lesion. Finally, we show that chronic SCI-induced allodynia does not affect spontaneous locomotor recovery over time and does not correlate to less white matter sparing at the lesion epicenter.
Methods
Subjects and surgeries
Thirty-two adult, female, Sprague-Dawley rats (225–250 g; Charles River Laboratories) were housed two to three per cage in a controlled environment (12 h light-dark cycles) with food and water ad libidum. All experimental procedures were approved by the Drexel University Institutional Animal Care and Use Committee.
Spinal cord hemicontusion injuries were performed as described previously.20,21 Briefly, rats were anesthetized with ketamine (60 mg/kg), xylazine (6 mg/kg), and acepromazine (6 mg/kg) and given antibiotics (ampicillin, subcutaneously,100 mg/kg, daily for 7 days). After partial C5 laminectomy, the spinal column was stabilized in the Infinite Horizon Impact Device (Precision Systems and Instrumentation, Lexington, KY).22 A custom impactor probe measuring 0.6 mm in diameter was lowered to within 2 mm of the midportion of the right C5 spinal cord. The spinal cord was then rapidly contused with a force of 200 kilodynes (no dwell time), resulting in tissue displacement to a depth of 1600–1800 μm. The incision was closed in layers and 5 mL of lactated Ringer solution was administered subcutaneously to prevent dehydration.
Behavioral measures
Behavioral testing was conducted preoperatively to establish the baseline responses and then weekly after SCI. Because of the unilateral nature of our injury model, we evaluated the ipsilesional (right) and contralesional (left) fore- and hindlimbs separately.
Thermal hyperalgesia. Rats were habituated to the testing chamber for at least 7 days before pre-operative testing (20 min/day). Pre-operatively and once weight supported stepping occurred in the open field after SCI, we measured changes in thermal sensation using the Ugo Basile Plantar Heat test (Comerio VA, Italy) as first described by Hargraeves and colleagues.23 Briefly, rats were enclosed in a clear Plexiglas box with a glass bottom. After a 20 min acclimation period, a noxious infrared light beam was applied to the plantar surface of the paw, and paw withdrawal latency recorded in seconds. The infrared stimulus application automatically shut off at 30 sec to avoid tissue damage. Five trials were collected randomly for each paw with at least a 1 min delay between each trial. The trials for each paw were averaged to yield one score per paw.
As pain is a perception that requires cognitive (supraspinal) awareness of a stimulus, we observed supraspinal behaviors that occurred in response to application of the thermal stimulus, including licking or biting the paw, turning the head to look at the stimulus, moving away/escape from the stimulus, or vocalization on stimulus application.
Tactile allodynia
Rats were acclimated to the testing environment (Plexiglas chamber with a wire mesh bottom) for at least 7 days (20 min/day) before pre-operative testing. The up-down method for von Frey hair monofilaments (VFH, Stoelting Co., Wood Dale, IL) was used to measure the degree of tactile sensory changes after SCI.15,16 Post-injury assessment of nocifensive behavior was initiated only when there was evidence of weight support for a given limb. This ensured that the rat had suitable motor control to remove the paw from an unpleasant stimulus. A total of 10 VFH stimulus applications were collected for each paw for each day of testing, beginning with the 5.18 g VFH. Any supraspinally driven attention given to the tactile stimulus including vocalizing, licking, or guarding the stimulated paw was recorded. The response threshold was the lowest force (g) that produced a paw withdrawal and supraspinal behaviors in at least 50% of the applications. Paw testing order was determined randomly to minimize an order effect.
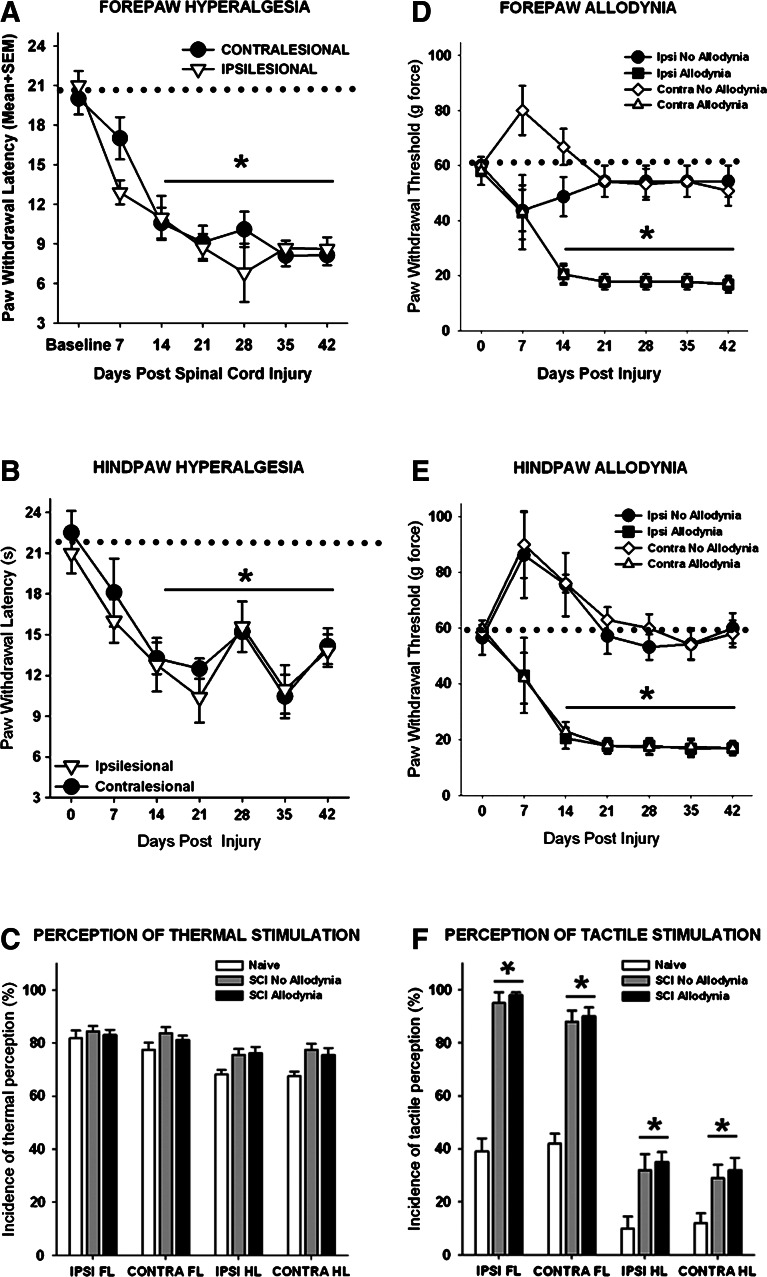
Spinal cord injured rats were discriminated post hoc as allodynic after SCI if they exhibited pain-related behaviors. Specifically, a rat must show a greater than 50% reduction in the paw withdrawal threshold to von Frey stimulation by 14 days after SCI that persisted for the duration of the study. Group means indicate that the SCI allodynia rats had a significantly lower paw withdrawal threshold than both their baseline paw withdrawal threshold and that of SCI rats, which maintained normal sensation and are considered non-allodynic (Fig. 1D and E, p<0.05). In addition, the rat must exhibit signs of supraspinal awareness including, vocalizing, licking, looking at, or guarding of the paw, or moving away from the stimulus after the application of the von Frey monofilament that elicits a paw withdrawal response.
FIG. 1.
Neuropathic pain behavior after unilateral C5 spinal cord injury (SCI). (A) Rats with SCI showed a decreased paw withdrawal latency of the ipsilesional and contralesional forepaws in response to a noxious thermal stimulus that plateaued at 21 days post-injury (dpi) compared with baseline values (represented as a dotted line; p<0.01 vs. baseline values). (B) Similar decrease in paw withdrawal latency occurred in both the ipsilesional and contralesional hindpaws after SCI. (C) At 42 dpi, the thermal stimulus was perceived because all rats that responded produced supraspinal behaviors after any of the four paws were stimulated. (D) Before injury, rats withdraw their forepaws from a tactile stimulus of 60 g, while moderate unilateral C5 SCI causes a significant reduction in both ipsilesional and contralesional paw withdrawal thresholds in a subset of rats that is established by 14 dpi and persists over time. (E) Both the ipsilesional and contralesional hindpaw exhibited a similar response to tactile stimulation. (F) At 42 dpi, rats produced supraspinal behaviors in response to tactile stimulation of each paw, regardless of the presence or absence of allodynia. After SCI, the forepaws and hindpaws showed significantly greater incidence of supraspinal behaviors regardless of the presence or absence of allodynia compared with normal (*p<0.01). Dotted line denotes normal, baseline paw withdrawal latencies to noxious thermal stimuli (A,B) and baseline paw withdrawal thresholds to tactile stimuil (D, E). SEM=standard error of the mean.
Open field locomotion
To confirm spinal cord injury, forelimb and hindlimb function was evaluated in an open field measuring 2.5×3 feet, and rats were observed for 4 min and scored by two trained persons blinded to injury condition/severity. To evaluate hindlimb locomotor performance, the Basso, Beattie, Bresnahan Locomotor Rating Scale (BBB) was used.24 The BBB is a 22-point semi-quantitative scale that scores hindlimb locomotion after SCI from complete paralysis (0) to normal and coordinated movement (21). Forelimb locomotor performance was assessed using the forelimb locomotor scale (FLS),25,26 which is an 18-point scale that ranks forelimb locomotion after cervical SCI based on a range of motion, degree of weight support, and paw placement.
Lesion analysis
Rats were euthanized (Euthasol, 390 mg/kg sodium pentobarbital, 50 mg/kg phenytoin, intraperitoneally) and perfused transcardially with 4% paraformaldehyde. Eight millimeters of cervical spinal cord spanning the rostrocaudal extent of the lesion (between C4 and C6) was removed, post-fixed in paraformaldehyde at 4°C overnight, and submersed in 30% sucrose for cryoprotection. A series of transverse sections 250 μm apart through the extent of this tissue block were mounted on slides and stained with cresyl violet (Sigma, St. Louis, MO) for Nissl substance and euriochrome cyanine (Sigma) for myelin. Sections were coverslipped with Vectashield mounting medium (Vector Laboratories, Burlingame, CA).
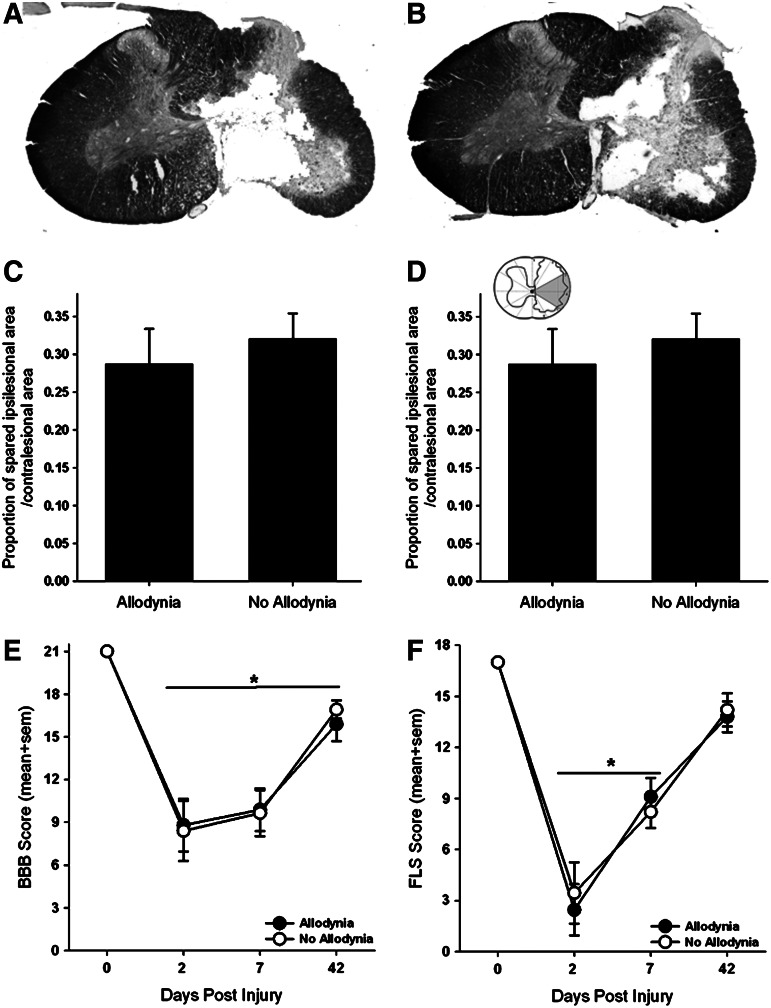
To determine the amount of spared tissue, the area of contralesional grey and white matter, spared grey and white matter on the ipsilesional side, and the lesion cavity were measured separately in 10 non-serial sections (250 μm apart) spanning the rostrocaudal extent of the lesion using the Cavalieri estimator method (Stereo Investigator, MicroBrightfield, Burlington, VT). We then determined the proportion of the spared tissue area on the ipsilesional side of the spinal cord to the tissue area on the contralesional (uninjured) spinal cord.20,21 To further delineate regions within the white matter that are important for relaying nociceptive information to supraspinal centers associated with pain, we used a pie-shaped overlay that had 12 equal wedges as described previously by Côté and colleagues21 (inset in Fig. 2D). The proportion of spared tissue for wedges three and four that includes lateral white matter was calculated, and the data were partitioned into groups based on the presence or absence of SCI-induced allodynia.
FIG. 2.
Anatomical and locomotor assessments in SCI rats with and without tactile allodynia. Representative transverse sections of the lesion epicenter stained for Nissl and myelin for a spinal cord injured rat with allodynia (A) and one with no allodynia (B). 200 kdyne impact produced a moderate, unilateral lesion with complete loss of grey matter and a spared rim of white matter on the right side of the spinal cord. Importantly, the grey and white matter of the contralesional spinal cord appears normal (scale bar=0.5 mm). Moderately injured rats with and without allodynia revealed no significant differences in tissue sparing at the lesion epicenter (C). Further analysis of tissue sparing in the area of the injured spinal cord containing the spinothalamic tract (grey area in inset) revealed no differences between either group (D). (E) Unilateral spinal cord injury (SCI) produced significant, bilateral deficits in hindlimb locomotor function as evaluated by the Basso, Beattie, Bresnahan (BBB) Locomotor Rating Scale (*p<0.01 vs. baseline). Importantly, there were no pain related differences in forelimb or hindlimb locomotion after SCI. (F) Assessment of the ipsilesional forelimb with the forelimb locomotor scale revealed significant deficits in ipsilesional forelimb function acutely after SCI that partially recovers over time (*p<0.01 vs baseline). sem=standard error of the mean.
Statistical analysis
Two-way repeated measure analyses of variance (ANOVAs) were performed for BBB, FLS, von Frey, and behavioral data (group×time) followed by Tukey post-hoc comparisons using the harmonic mean to correct for unequal group sizes. Hargreaves behavior data as well as epicenter sparing data were analyzed via one-way ANOVA with Bonferroni post-hoc analysis. Means and standard error of the mean are reported throughout.
Results
Thermal hyperalgesia
After unilateral C5 contusion, bilateral thermal hyperalgesia developed in both the fore- and hindpaws in all rats(Fig. 1A, 1B). Pre-operatively, the mean withdrawal latency to a noxious heat stimulus was 20.5±1.1 sec for the forepaws and 21.7±1.6 sec for the hindpaws. After unilateral C5 contusion, the fore- and hindpaws exhibited a progressive decline in paw withdrawal latency that reached a plateau by 21 dpi (p<0.05) and persisted for the duration of the study. At 7 dpi, the average latency of paw withdrawal was 12.9±0.9 sec and 17.0±1.6 sec for the ipsilesional and contralesional forepaws, respectively. By 21 dpi, the ipsilesional and contralesional forepaw withdrawal latencies were reduced to 9.12±1.3 sec and 8.73±1.0 sec (Fig. 1A). There were no significant differences in paw withdrawal latency between the ipsilesional and contralesional forelimbs at any time point. Similarly, the paw withdrawal latencies of both the ipsilesional and contralesional hindpaws were significantly decreased after unilateral C5 SCI (Fig. 1B), although there was some variation in the weekly scores between 21 and 42 dpi. All rats exhibited supraspinal behaviors in response to noxious thermal stimuli on all four paws in at least 65% of the trials (Fig. 1C).
Tactile allodynia
In contrast to the 100% response to thermal stimuli, only 38% of rats with a unilateral C5 contusion exhibited a >50% decrease in tactile sensory thresholds in the forepaws (Fig. 1D), and 46% of rats exhibited a >50% decrease in threshold of the hindpaws (Fig. 1E). Before SCI, the mean mechanical threshold to tactile stimuli was 56.3±5.1 g and 59.6±3.2 g for the fore- and hindpaws, respectively. Moderate unilateral C5 SCI causes a significant reduction (to 17.2±3.3 g) in both ipsilesional and contralesional forepaw withdrawal thresholds that was established by 14 dpi and persisted over the testing period (Fig. 1D). The ipsilesional and contralesional hindpaws displayed a similar response to tactile stimulation (Fig. 1E). Importantly, in both the fore- and hindpaws, transient hyposensitivity to tactile stimuli occurred for at least 7 dpi in rats in which allodynia did not develop. Positive paw withdrawal responses to tactile stimuli were often accompanied by hypervigilant behaviors including licking the stimulated paw as well as turning and looking at the stimulus, and occasionally moving away from the stimulus in animals with or without allodynia (Fig. 1F).
SCI-induced allodynia is not associated with differences in epicenter sparing or locomotor recovery
Contusive SCI produced a core lesion consisting of a cystic cavity surrounded by a rim of white matter that was confined to one side of the spinal cord, as previously described (Fig. 2A, 2B).20,21 Regardless of the presence or absence of allodynia, nearly all grey matter was eliminated, and only a small outer rim of densly stained white matter remained, predominantly in the lateral and ventral funiculi of the ipsilesional spinal cord. There was no evidence of tissue damage in the contralesional spinal cord. Tissue sparing at the lesion epicenter as a whole had no relationship to the presence of allodynic behavior (Fig. 2C). More detailed analysis of the lesioned tissue pertaining to the spinal thalamic tract also failed to show a relationship between the amount of tissue sparing to the development of allodynia (Fig. 2D).
The recovery of overground locomotion of this lesion model has been described extensively20,21 and was assessed in this study to confirm lesion severity. Examination of overground locomotion revealed that there were no differences in forelimb or hindlimb locomotor ability that corresponded to the presence of allodynia (Figs. 2E, 2F). Locomotor activity of both hindlimbs was impaired after SCI (Fig. 2E). At 2 dpi, rats could plantar step with both hindlimbs, but they were unable to coordinate forelimb and hindlimb movements (BBB score ≤11; *p<0.01 vs. baseline). By 42 dpi, deficits in paw placement, toe clearance, and trunk stability persisted in all rats (BBB score ≥15). The locomotor ability of the ipsilesional forelimb was assessed using the FLS (Fig. 2F). Unilateral C5 SCI produced deficits in forelimb locomotor function at 2 and 42 dpi compared with preoperative, normal forelimb function. Two days after SCI, rats exhibited extensive movement of one forelimb joint or slight movement of two forelimb joints (FLS score <3; p<0.01 vs. baseline). By 42 dpi, rats could continuously plantar step with the ipsilesional forepaw with parallel paw position and occasional toe clearance (FLS score ≥14).
Discussion
The current work establishes the onset and persistence of the sensory response of rats after moderate, unilateral C5 spinal cord contusion. We demonstrate that SCI induces differential aberration of thermal and tactile sensation at and below the level of SCI. While thermal hyperalgesia at and below the level of the SCI develops in all rats, at- and below-level allodynia that plateaus by 21d but persists through 42 dpi develops in only 40%. Importantly, early locomotor deficits associated with this lesion model allow for sensory testing for at- and below-level neuropathic pain to be examined as early as 7 dpi, providing a model to further study the acute pathology and underlying cellular and molecular mechanisms responsible for the development and/or maintenance of SCI-induced pain.
Of primary consideration, the high locomotor performance of rats with this type of unilateral and incomplete lesion allows experimenters to reliably test all paws early after injury. By 7 days post-injury, rats are able to bear weight on all four paws in the open field as well as in the von Frey and Hargreaves testing chambers. The fact that this lesion model allows valid and reliable testing at such an early time point indicates that it will be a valuable tool in the identification of factors responsible for the onset and development of chronic pain. Second, confounds of sensory testing for both at- and below-level neuropathic pain are minimized. This model allows for the same testing paradigms to be used in evaluating both at- and below-level pain, ensuring that the changes in sensation reported after SCI are authentic and do not represent variability introduced by testing on the glaborous versus the hair skin of the rat. Rather than testing at-level sensation on the trunk of the animal, as would be the case with a mid-thoracic injury, it can be evaluated on the plantar surface of the forepaw.
In human perceptual terms, the radiant heat source used in these experiments would be described as uncomfortably warm, but not scalding. This thermal stimulus elicited a heightened paw withdrawal response in all four paws that corresponded to hypervigilant and avoidance behaviors such as guarding or protecting the paws by careful placement of the tail between the paw and the glass or sustained flexion (paw withdrawal) after nearly every trial indicating that the stimulus was perceived as unpleasant. Interestingly, while all SCI rats were hypersensitive to thermal stimuli, only a subset (40%) exhibited tactile hypersensitivity of the paws. Those tactile forces that cause aversive withdrawal responses in a subset of rats with SCI are described as faint touch, emulating the feeling of a cat whisker against the skin and are considered to be innocuous.
In persons with SCI, evoked and spontaneous neuropathic pain is self-reported through valid questionnaires and semi-quantitative scales. In animal models of SCI, spontaneous neuropathic pain can be assessed by the incidence of autophagia or self-mutilation after injury. Several laboratories have reported the incidence of autophagia of the contralesional hindlimb after unilateral thoracic hemisection from 10% to more than 50% of the subjects.6,26–28 In the current study that utilized a unilateral C5 contusion, no rats displayed autophagia.
Behavioral measures, like von Frey and Hargreaves testing, assess a flexor withdrawal reflex that is evoked in response to noxious stimuli as a protective mechanism to prevent tissue damage. Previous work has shown that SCI produces heightened reflex responses and pain-like behavior when pathways involved in the perception and modulation of pain are severed.6,29,30 Importantly, this heightened paw withdrawal reflex occurs even in the absence of ascending or descending input. While we show heightened reflexes are important proxies of cognitive awareness, they do not directly correspond to the perception of pain. Reliance on parameters like paw withdrawal thresholds and latencies as indicators of pain perception is lessened with the added observations of supraspinally mediated behaviors. These behaviors—licking the paw, turning to glance at the paw, or vocalizing evoked in response to stimulus application—require supraspinal transmission and cognition. After SCI, the incidence of supraspinal behaviors elicited in response to both thermal and tactile stimulation was maintained or heightened and could easily be interpreted as the development of allodynia.
Several laboratories have reported anatomical and electrophysiological evidence for spared ascending sensory systems after incomplete SCI. In the normal spinal cord, tactile and temperature information are conveyed through the dorsal column/medial lemniscal or anterolateral spinothalamic tract, which are anatomically distinct pathways within the spinal cord. Other pathways can also subserve transmission of sensory information including the bilateral spinoreticular tracts, the spinomesencephalic tract that terminates in the tectum, parabrachial nucleus, and periaqueductal grey31–33 and/or decussating primary afferent fibers,34 or networks of long or short propriospinal interneurons.26 Indeed, the proposal of multisynaptic propriospinal matrices has been shown as a basis of recovery of locomotor function after bilateral staggered hemisections in both the rat36 and mouse.36 Basbaum26 showed that rats with staggered hemisections maintained the ability to acknowledge noxious stimulation of the hindlimb, indicating that the integrity of the spinothalamic tract was unnecessary for the perception of nocifensive stimuli.
Importantly, unilateral C5 contusion caused bilateral changes to sensory thresholds in sensory dermatomes of the forepaws near the injury epicenter (within two spinal segments) as well as of the hindpaws, which are far removed from the site of injury (by ∼20 spinal segments). That such robust behavioral changes occur suggests that damage to ascending first or second order neurons and to descending modulators of sensory pathways, rather than to the cell bodies within the dorsal horn, are necessary for appropriate sensory transmission. It is known that primary afferent fibers send collaterals into dorsal horn lamina up to three segments rostral and/or caudal to their entry zone, which may be sufficient to effectively convey tactile information.
The distribution and pattern of the different subtypes of afferent fibers within the dorsal horn of the spinal cord is critical to normal sensory function. The delineation of multimodal sensory changes and the development of robust allodynia of all four paws in only 40% of injured animals, as seen here after unilateral C5 contusion, could be a result of inappropriate sprouting between laminae of the different classes of primary afferent fibers. These changes in sensation and nociception may occur via central sensitization and reorganization of pain pathways along the sensory neuroaxis. Ab, Ad, and non-peptidergic c-fibers may sprout to form novel functional connections with neurons located in the different dorsal horn laminae as has been shown after peripheral nerve injury.37–39 Lu and Perl40 have shown that pain may be modulated by a small interneuron within the superficial dorsal horn that can augment or inhibit signals sent by Ab fibers that provide both innocuous and painful tactile information. Indeed, sprouting of peptidergic, small diameter afferents into deep dorsal horn laminae occurs in clinical41,42 as well as experimental SCI,43–49 and often correlates with unchecked hyperexcitability, as well as debilitating neuropathic pain,6,50 autonomic dysreflexia,44,47,48,51 and bladder dysfunction.52 Whether reorganization or plasticity of the Ab, Ad, or non-peptidergic c-fiber types occurs after SCI has not been determined yet.
Certainly, at- and below-level neuropathic pain are complex perceptions. It is unlikely that the primary damage to pathways that transmit and modulate sensation is the soul mediator of the development or persistence of pain after SCI. In fact, it is likely that the proximity to the lesion epicenter may play a significant role in the mechanisms underlying at- and below-level pain. In addition to the sprouting of peptidergic c fibers, SCI-induced pain has been associated with a robust inflammatory response,18,53 changes in intracellular signaling pathways associated with long-term potentiation,17,54,55 and changes in trophic support in dermatomes and spinal cord segments associated with neuropathic pain.56–58
Conclusion
This work establishes a moderate, unilateral C5 contusion injury as a model of SCI-induced neuropathic pain that is unique in that both at- and below-level neuropathic pain behaviors are easily monitored and the importance of the proximity to the lesion site can be further examined. Given the clinical importance of SCI-induced neuropathic pain, further study is needed to establish mechanisms underlying pain development and its persistence.
Acknowledgments
This study was supported by Paralyzed Veterans of America 2707 (MRD) and NIH Grant NS 026380 (JDH).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Christopher and Dana Reeve Foundation. Paralysis facts and figures. 2013. http://www.christopherreeve.org. [May]. http://www.christopherreeve.org 213.
- 2.University of Alabama Spinal Cord Injury Model System Information Network. 2013. http://www.uab.edu/medicine/sci/uab-scims-information/sci-infosheets. [May;2013 ]. http://www.uab.edu/medicine/sci/uab-scims-information/sci-infosheets
- 3.Siddall P.J. Molloy A.R. Walker S. Mather L.E. Rutkowski S.B. Cousins M.J. The efficacy of intrathecal morphin and clonidine in the treatment of pain after spinal cord injury. Anesth. Analg. 2000;91:1493–1498. doi: 10.1097/00000539-200012000-00037. [DOI] [PubMed] [Google Scholar]
- 4.Siddall P.J. Loeser J.D. Pain following spinal cord injury. Spinal Cord. 2001;39:63–73. doi: 10.1038/sj.sc.3101116. [DOI] [PubMed] [Google Scholar]
- 5.Davidoff G. Roth E.J. Pain and Central Nervous System Disease: The Central Pain Syndromes. Raven Press; New York: 1991. Clinical characteristics of central (dysesthetic) pain in spinal cord injury patients; pp. 77–83. [Google Scholar]
- 6.Christensen M.D. Everhart A.W. Pickelman J.T. Hulsebosch C.E. Mechanical and thermal allodynia in chronic central pain following spinal cord injury. Pain. 1996;68:97–107. doi: 10.1016/S0304-3959(96)03224-1. [DOI] [PubMed] [Google Scholar]
- 7.Finnerup N.B. Johannesen I.L. Bach F.W. Jensen T.S. Sensory function above lesion level in spinal cord injury patients with and without pain. Somatosens. Mot. Res. 2003;20:71–76. doi: 10.1080/0899022031000083843. [DOI] [PubMed] [Google Scholar]
- 8.Finnerup N.B. Johannesen I.L. Fuglsang-Frederiksen A. Bach F.W. Jensen T.S. Sensory function in spinal cord injury patients with and without central pain. Brain. 2003;126:57–70. doi: 10.1093/brain/awg007. [DOI] [PubMed] [Google Scholar]
- 9.Finnerup N.B. Sorensen L. Biering-Sorensen F. Johannesen I.L. Jensen T.S. Segmental hypersensitivity and spinothalamic function in spinal cord injury pain. Exp. Neurol. 2007;207:139–149. doi: 10.1016/j.expneurol.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Murray R.F. Asghari A. Egorov D.D. Rutkowski S.B. Siddall P.J. Soden R.J. Ruff R. Impact of spinal cord injury on self-perceived pre- and postmorbid cognitive, emotional and physical functioning. Spinal Cord. 2007;45:429–436. doi: 10.1038/sj.sc.3102022. [DOI] [PubMed] [Google Scholar]
- 11.Siddall P.J. McClelland J.M. Rutkowski S.B. Cousins M.J. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. 2003;103:249–257. doi: 10.1016/S0304-3959(02)00452-9. [DOI] [PubMed] [Google Scholar]
- 12.Warms C.A. Turner J.A. Marshall H.M. Cardenas D.D. Treatments for chronic pain associated with spinal cord injuries: many are tried, few are helpful. Clin. J. Pain. 2002;18:154–163. doi: 10.1097/00002508-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Widerström-Noga E. Biering-Sorensen F. Bryce T. Cardenas D.D. Finnerup N.B. Jensen M.P. Richards J.S. Siddall P.J. The international spinal cord injury pain basic data set. Spinal Cord. 2008;46:818–823. doi: 10.1038/sc.2008.64. [DOI] [PubMed] [Google Scholar]
- 14.Widerström-Noga E.G. Turk D.C. Types and effectiveness of treatments used by people with chronic pain associated with spinal cord injuries: influence of pain and psychosocial characteristics. Spinal Cord. 2003;41:600–609. doi: 10.1038/sj.sc.3101511. [DOI] [PubMed] [Google Scholar]
- 15.Detloff M.R. Clark L.M. Hutchinson K.J. Kloos A.D. Fisher L.C. Basso D.M. Validity of acute and chronic tactile sensory testing after spinal cord injury in rats. Exp. Neurol. 2010;225:366–376. doi: 10.1016/j.expneurol.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Detloff M.R. Fisher L.C. Deibert R.J. Basso D.M. Acute and chronic tactile sensory testing after spinal cord injury in rats. J. Vis. Exp. 2012;62:e3247. doi: 10.3791/3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crown E.D. Ye Z. Johnson K.M. Xu G.Y. McAdoo D.J. Westlund K.N. Hulsebosch C.E. Upregulation of the phosphorylated form of CREB in spinothalamic tract cells following spinal cord injury: relation to central neuropathic pain. Neurosci. Lett. 2005;384:139–144. doi: 10.1016/j.neulet.2005.04.066. [DOI] [PubMed] [Google Scholar]
- 18.Detloff M.R. Fisher L.C. McGaughy V. Longbrake E.E. Popovich P.G. Basso D.M. Remote activation of microglia and pro-inflammatory cytokines predict the onset and severity of below-level neuropathic pain after spinal cord injury in rats. Exp. Neurol. 2008;212:337–347. doi: 10.1016/j.expneurol.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerke M.B. Duggan A.W. Xu L. Siddall P.J. Thalamic neuronal activity in rats with mechanical allodynia following contusive spinal cord injury. Neuroscience. 2003;117:715–722. doi: 10.1016/s0306-4522(02)00961-2. [DOI] [PubMed] [Google Scholar]
- 20.Sandrow-Feinberg H.R. Izzi J. Shumsky J.S. Zhukareva V. Houle J.D. Forced exercise as a rehabilitation strategy after unilateral cervical spinal cord contusion injury. J. Neurotrauma. 2009;26:721–731. doi: 10.1089/neu.2008.0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Côté M.P. Detloff M.R. Wade R.E., Jr. Lemay M.A. Houlé J.D. Plasticity in ascending long propriospinal and descending supraspinal pathways in chronic cervical spinal cord injured rats. Front Physiol. 2012;3:330. doi: 10.3389/fphys.2012.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheff S.W. Rabchevsky A.G. Fugaccia I. Main J.A. Lumpp J.E., Jr. Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J. Neurotrauma. 2003;20:179–193. doi: 10.1089/08977150360547099. [DOI] [PubMed] [Google Scholar]
- 23.Hargreaves K. Dubner R. Brown F. Flores C. Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 24.Basso D.M. Beattie M.S. Bresnahan J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 25.Sandrow H.R. Shumsky J.S. Amin A. Houle J.D. Aspiration of a cervical spinal contusion injury in preparation for delayed peripheral nerve grafting does not impair forelimb behavior or axon regeneration. Exp. Neurol. 2008;210:489–500. doi: 10.1016/j.expneurol.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basbaum A.I. Conduction of the effects of noxious stimulation by short-fiber multisynaptic systems of the spinal cord in rat. Exp. Neurol. 1973;40:699–716. doi: 10.1016/0014-4886(73)90105-2. [DOI] [PubMed] [Google Scholar]
- 27.Xu X.J. Hao J.X. Aldskogius H. Seiger A. Wiesenfeld-Hallin Z. Chronic pain-related syndrome in rats after ischemic spinal cord lesion: a possible animal model for pain in patients with spinal cord injury. Pain. 1992;48:279–290. doi: 10.1016/0304-3959(92)90070-R. [DOI] [PubMed] [Google Scholar]
- 28.Levitt M. Levitt J.H. The deafferentation syndrome in monkeys: dysesthesias of spinal origin. Pain. 1981;10:129–147. doi: 10.1016/0304-3959(81)90190-1. [DOI] [PubMed] [Google Scholar]
- 29.Kloos A.D. Fisher L.C. Detloff M.R. Hassenzahl D.L. Basso D.M. Stepwise motor and all-or-none sensory recovery is associated with nonlinear sparing after incremental spinal cord injury in rats. Exp. Neurol. 2005;191:251–265. doi: 10.1016/j.expneurol.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 30.Lindsey A.E. LoVerso R.L. Tovar C.A. Hill C.E. Beattie M.S. Bresnahan J.C. An analysis of changes in sensory thresholds to mild tactile and cold stimuli after experimental spinal cord injury in the rat. Neurorehabil. Neural Repair. 2000;14:287–300. doi: 10.1177/154596830001400405. [DOI] [PubMed] [Google Scholar]
- 31.Willis W.D., Jr. Coggeshall R.E. 2nd. Plenum Press; New York: 1991. Sensory Mechanisms of the Spinal Cord; p. 401. [Google Scholar]
- 32.Craig A.D. Spinal distribution of ascending lamina I axons anterogradely labeled with Phaseolus vulgaris leucoagglutinin (PHA-L) in the cat. J. Comp. Neurol. 1991;313:377–393. doi: 10.1002/cne.903130212. [DOI] [PubMed] [Google Scholar]
- 33.Craig A.D. Distribution of brainstem projections from spinal lamina I neurons in the cat and the monkey. J. Comp. Neurol. 1995;361:225–248. doi: 10.1002/cne.903610204. [DOI] [PubMed] [Google Scholar]
- 34.Chung K. McNeill D.L. Hulsebosch C.E. Coggeshall R.E. Changes in dorsal horn synaptic disc numbers following unilateral dorsal rhizotomy. J. Comp. Neurol. 1989;283:568–577. doi: 10.1002/cne.902830410. [DOI] [PubMed] [Google Scholar]
- 35.Bareyre F.M. Kerschensteiner M. Raineteau O. Mettenleiter T.C. Weinmann O. Schwab M.E. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat. Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- 36.Courtine G. Song B. Roy R.R. Zhong H. Herrmann J.E. Ao Y. Qi J. Edgerton V.R. Sofroniew M.V. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat. Med. 2008;14:69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woolf C.J. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306:686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- 38.Woolf C.J. Shortland P. Coggeshall R.E. Peripheral nerve injury triggers central sprouting of myelinated afferents. Nature. 1992;355:75–78. doi: 10.1038/355075a0. [DOI] [PubMed] [Google Scholar]
- 39.Woolf C.J. Shortland P. Reynolds M. Ridings J. Doubell T. Coggeshall R.E. Reorganization of central terminals of myelinated primary afferents in the rat dorsal horn following peripheral axotomy. J. Comp. Neurol. 1995;360:121–134. doi: 10.1002/cne.903600109. [DOI] [PubMed] [Google Scholar]
- 40.Lu Y. Perl E.R. A specific inhibitory pathway between substantia gelatinosa neurons receiving direct C-fiber input. J. Neurosci. 2003;23:8752–8758. doi: 10.1523/JNEUROSCI.23-25-08752.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calancie B. Molano M.R. Broton J.G. Epidemiology and demography of acute spinal cord injury in a large urban setting. J. Spinal Cord Med. 2005;28:92–96. doi: 10.1080/10790268.2005.11753804. [DOI] [PubMed] [Google Scholar]
- 42.Kakulas B.A. Neuropathology: the foundation for new treatments in spinal cord injury. Spinal Cord. 2004;42:549–563. doi: 10.1038/sj.sc.3101670. [DOI] [PubMed] [Google Scholar]
- 43.Hagg T. Collateral sprouting as a target for improved function after spinal cord injury. J. Neurotrauma. 2006;23:281–294. doi: 10.1089/neu.2006.23.281. [DOI] [PubMed] [Google Scholar]
- 44.Krenz N.R. Weaver L.C. Changes in the morphology of sympathetic preganglionic neurons parallel the development of autonomic dysreflexia after spinal cord injury in rats. Neurosci. Lett. 1998;243:61–64. doi: 10.1016/s0304-3940(98)00101-3. [DOI] [PubMed] [Google Scholar]
- 45.Murray M. Goldberger M.E. Restitution of function and collateral sprouting in the cat spinal cord: the partially hemisected animal. J. Comp. Neurol. 1974;158:19–36. doi: 10.1002/cne.901580103. [DOI] [PubMed] [Google Scholar]
- 46.Ondarza A.B. Ye Z. Hulsebosch C.E. Direct evidence of primary afferent sprouting in distant segments following spinal cord injury in the rat: colocalization of GAP-43 and CGRP. Exp. Neurol. 2003;184:373–380. doi: 10.1016/j.expneurol.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 47.Weaver L.C. Marsh D.R. Gris D. Meakin S.O. Dekaban G.A. Central mechanisms for autonomic dysreflexia after spinal cord injury. Prog. Brain Res. 2002;137:83–95. doi: 10.1016/s0079-6123(02)37009-2. [DOI] [PubMed] [Google Scholar]
- 48.Weaver L.C. Verghese P. Bruce J.C. Fehlings M.G. Krenz N.R. Marsh D.R. Autonomic dysreflexia and primary afferent sprouting after clip-compression injury of the rat spinal cord. J. Neurotrauma. 2001;18:1107–1119. doi: 10.1089/08977150152693782. [DOI] [PubMed] [Google Scholar]
- 49.Zinck N.D. Rafuse V.F. Downie J.W. Sprouting of CGRP primary afferents in lumbosacral spinal cord precedes emergence of bladder activity after spinal injury. Exp. Neurol. 2007;204:777–790. doi: 10.1016/j.expneurol.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 50.Christensen M.D. Hulsebosch C.E. Spinal cord injury and anti-NGF treatment results in changes in CGRP density and distribution in the dorsal horn in the rat. Exp. Neurol. 1997;147:463–475. doi: 10.1006/exnr.1997.6608. [DOI] [PubMed] [Google Scholar]
- 51.Hou S. Duale H. Robchersky A.G. Intraspinal sprouting of unmyelinated pclvic afferents after complete spinal cord injury is correlated with autonomic dysreflexia induced by visceral pain. Neurosc. 2009;159:369–379. doi: 10.1016/j.neuroscience.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nagatomi J. Gloeckner D.C. Chancellor M.B. DeGroat W.C. Sacks M.S. Changes in the biaxial viscoelastic response of the urinary bladder following spinal cord injury. Ann. Biomed. Eng. 2004;32:1409–1419. doi: 10.1114/b:abme.0000042228.89106.48. [DOI] [PubMed] [Google Scholar]
- 53.Hains B.C. Waxman S.G. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J. Neurosci. 2006;26:4308–4317. doi: 10.1523/JNEUROSCI.0003-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Crown E.D. Gwak Y.S. Ye Z. Johnson K.M. Hulsebosch C.E. Activation of p38 MAP kinase is involved in central neuropathic pain following spinal cord injury. Exp. Neurol. 2008;213:257–267. doi: 10.1016/j.expneurol.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crown E.D. Ye Z. Johnson K.M. Xu G.Y. McAdoo D.J. Hulsebosch C.E. Increases in the activated forms of ERK 1/2, p38 MAPK, and CREB are correlated with the expression of at-level mechanical allodynia following spinal cord injury. Exp. Neurol. 2006;199:397–407. doi: 10.1016/j.expneurol.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 56.Sah D.W. Ossipov M.H. Rossomando A. Silvian L. Porreca F. New approaches for the treatment of pain: the GDNF family of neurotrophic growth factors. Curr. Top. Med. Chem. 2005;5:577–583. doi: 10.2174/1568026054367593. [DOI] [PubMed] [Google Scholar]
- 57.Wang R. Guo W. Ossipov M.H. Vanderah T.W. Porreca F. Lai J. Glial cell line-derived neurotrophic factor normalizes neurochemical changes in injured dorsal root ganglion neurons and prevents the expression of experimental neuropathic pain. Neuroscience. 2003;121:815–824. doi: 10.1016/s0306-4522(03)00491-3. [DOI] [PubMed] [Google Scholar]
- 58.Wang R. King T. Ossipov M.H. Rossomando A.J. Vanderah T.W. Harvey P. Cariani P. Frank E. Sah D.W. Porreca F. Persistent restoration of sensory function by immediate or delayed systemic artemin after dorsal root injury. Nat. Neurosci. 2008;11:488–496. doi: 10.1038/nn2069. [DOI] [PMC free article] [PubMed] [Google Scholar]