Abstract
Following spinal cord injury (SCI), anatomical changes such as axonal sprouting occur within weeks in the vicinity of the injury. Exercise training enhances axon sprouting; however, the exact mechanisms that mediate exercised-induced plasticity are unknown. We studied the effects of exercise training after SCI on the intrinsic and synaptic properties of spinal neurons in the immediate vicinity (<2 segments) of the SCI. Male mice (C57BL/6, 9–10 weeks old) received a spinal hemisection (T10) and after 1 week of recovery, they were randomized to trained (treadmill exercise for 3 weeks) and untrained (no exercise) groups. After 3 weeks, mice were killed and horizontal spinal cord slices (T6–L1, 250 μm thick) were prepared for visually guided whole cell patch clamp recording. Intrinsic properties, including resting membrane potential, input resistance, rheobase current, action potential (AP) threshold and after-hyperpolarization (AHP) amplitude were similar in neurons from trained and untrained mice (n=67 and 70 neurons, respectively). Neurons could be grouped into four categories based on their AP discharge during depolarizing current injection; the proportions of tonic firing, initial bursting, single spiking, and delayed firing neurons were similar in trained and untrained mice. The properties of spontaneous excitatory synaptic currents (sEPSCs) did not differ in trained and untrained animals. In contrast, evoked excitatory synaptic currents recorded after dorsal column stimulation were markedly increased in trained animals (peak amplitude 78.9±17.5 vs. 42.2±6.8 pA; charge 1054±376 vs. 348±75 pA·ms). These data suggest that 3 weeks of treadmill exercise does not affect the intrinsic properties of spinal neurons after SCI; however, excitatory synaptic drive from dorsal column pathways, such as the corticospinal tract, is enhanced.
Key words: action potential, intrinsic properties, patch clamp electrophysiology, synaptic input
Introduction
Incomplete lesions to the spinal cord are accompanied by anatomical changes, such as axonal sprouting in the vicinity of the lesion within weeks following spinal cord injury (SCI).1–3 This post-SCI axonal sprouting can form new intraspinal circuits that allow descending pathways to bypass the site of the lesion.1 Importantly, recent studies have shown that exercise training can enhance sprouting following SCI.4,5 Although these studies have documented anatomical changes in the vicinity of a lesion post-injury, functional changes in the synaptic or intrinsic electrical properties of neurons likely to be involved in forming new intraspinal circuits after SCI have not been extensively explored, especially in adult animals.
Here, we use in vitro patch-clamp electrophysiology to compare the synaptic and intrinsic properties of neurons in the immediate vicinity of an incomplete spinal lesion in untrained and trained adult mice. By utilizing a small animal such as the mouse, we are able to obtain horizontal spinal cord slices that maintain longitudinal fiber pathways and significant spinal cord circuitry.6 Importantly, axons in the corticospinal tract (CST), which lie in the deep dorsal columns of rodents,7,8 can be preserved and subsequently activated rostral to the lesion site with an appropriately placed stimulating electrode. Together these features of our slice preparation allow detailed examination of the effect of various interventions, in this instance exercise training, on neuron properties and connectivity following SCI.
Methods
All procedures were approved by the University of Newcastle Animal Care and Ethics Committee. Animals (C57BL/6 male mice, 9–10 weeks of age) received a left spinal cord hemisection beneath the T10 vertebra (i.e., between T10 and T11 spinal nerves) while under isoflurane (5% induction and 1.5–2.5% maintenance) and medetomidine (0.03 mg/kg s.c.) anaesthesia. Postsurgical analgesia was provided by buprenorphine (0.1 mg/kg s.c. every 8 h for 48 h). After 1 week of recovery, mice exhibiting left hindlimb paralysis were randomly allocated to untrained or trained groups. Over the next 3 weeks, the trained group received enforced treadmill exercise (two 10 min sessions, 5 days/week) at speeds that matched their ability (ranging from 6 to 12 m/min). The untrained group remained in their cages during this period. To acclimate mice to the treadmill, all animals completed 2 weeks of treadmill training prior to their surgery.
In order to control for lesion variability, the same surgeon performed all hemisections, and we quantified the lesion in slices used for recording (see dashed rectangle in Fig. 1A). The measured area of the injury including cavitation (i.e., missing tissue) as well as glial/scar tissue was similar in untrained and trained animals (0.53±0.08 mm2 vs. 0.40±0.04 mm2). We also measured lesion “extent” as the distance between the medial apex of the lesion and the midline of the spinal cord. A value of 0 mm indicated that the lesion extended all the way to the midline of the cord. The average extent of the lesion (±SE) was 0.15±0.03 mm2 in untrained and trained groups. Together, these data suggest that our SCI hemisections were similar in the two groups.
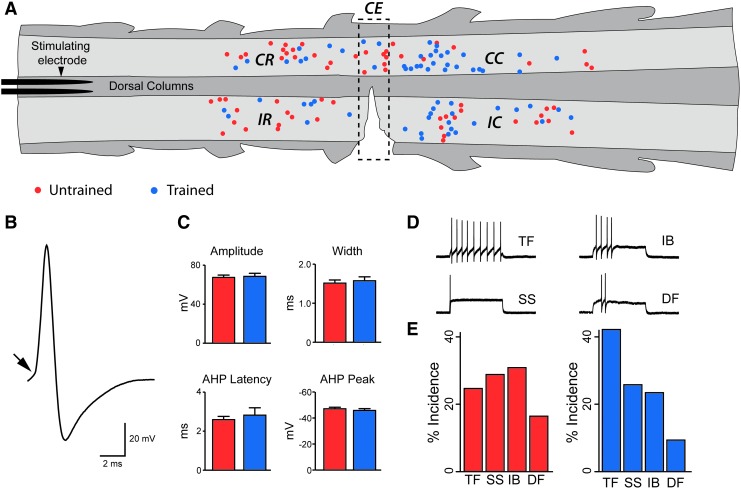
FIG. 1.
Location of recorded neurons and their intrinsic properties in SCI mice. (A) Schematic showing the location of recorded neurons on a horizontal slice. Light and dark gray shading represent gray and white matter, respectively, which are clearly visible under infrared differential interference contrast optics. Neurons from untrained (red circles) and trained (blue circles) mice were recorded one to two segments rostral and caudal to the hemisection (dashed rectangle: made between T10 and T11 spinal nerves). Recording location, relative to the lesion, was divided into five regions: ipsilateral-rostral (IR), ipsilateral-caudal (IC), contralateral-rostral (CR), contralateral-caudal (CC), and contralateral-epicenter (CE; inside dashed rectangle). A bipolar stimulating electrode was placed in the dorsal columns at the rostral end of the slice (left) for stimulation of descending inputs. (B) Representative action potential (AP) showing inflection point (arrow, where dV/dt is >15–20 mV/ms) from which various measurements were made (see text). (C) Group comparisons for AP properties in untrained (red) and trained (blue) mice. These properties were not different in untrained and trained mice. Error bars=SEM on all bar charts. (D) Typical AP discharge patterns observed in response to square step depolarizing current injection (800 ms duration). Discharge patterns fall into four categories: tonic firing (TF), single spiking (SS), initial bursting (IB), and delayed firing (DF). (E) The incidence of each firing pattern did not differ in untrained (red) and trained (blue) mice. Color image is available online at www.liebertpub.com/neu
Following the 3 week training period after SCI, both trained and untrained mice (now ∼13 weeks of age) were killed for in vitro patch-clamp electrophysiology. Investigators were blinded to the training status of the animal had been. Horizontal spinal cord slices were prepared as previously described.6 Briefly, animals were deeply anaesthetized with ketamine (100 mg/kg i.p.) and decapitated. The torso was immersed in ice-cold, oxygenated, sucrose substituted, low calcium/high magnesium artificial cerebrospinal fluid (S-ACSF; containing [in mM]: 250 sucrose, 25 NaHCO3, 11 glucose, 2.5 KCl, 1 NaH2PO4, 6 MgCl2, and 1 CaCl2; pH 7.3). The spinal cord was then excised using a ventral approach. A length of spinal cord (T6–L1) was fixed to an agar cutting stage (ventral side down) with cyanoacrylate glue, and submerged in a bath containing ice-cold S-ACSF. Horizontal slices (250 μm thick) were then cut using a vibrating microtome (HM 650V; Microm, Walldorf, Germany). A single slice containing the dorsal horn and/or intermediate zone and a continuous strip of the dorsal columns was obtained for recording. This slice was chosen for analysis, as it permitted stimulation of dorsal column pathways. The slice was immediately transferred to a recording bath with a constant flow (6 bath volumes/min) of oxygenated ACSF (250 mM sucrose substituted with 118 mM NaCl and MgCl2 and CaCl2 adjusted to 1 mM and 2.5 mM, respectively). The slice was positioned in the bath, secured under a custom-made net, and left for 1 h to equilibrate (22–24°C) before experimental procedures commenced.
After the equilibration period, a bipolar tungsten electrode was placed in the dorsal columns rostral to the lesion for stimulation (Fig. 1A and as described by Flynn et al.).6 Patch pipettes were pulled from borosilicate glass (1.5 mm OD×1.16 mm ID; Harvard Apparatus, Kent, UK) and had tip resistances of 3–5 MΩ. They were filled with a K+-based internal solution containing (in mM): 135 KCH3SO4, 6 NaCl, 2 MgCl2, 10 HEPES, 0.1 ethylene glycol tetraacetic acid (EGTA), 2 Mg-adenosine triphosphate (ATP), and 0.3 NaGTP; pH 7.3 using KOH. Neurons in the vicinity of the lesion were targeted for whole-cell patch clamp electrophysiology using infrared differential interference contrast optics,9 and photographed for subsequent mapping of the location of recorded neurons (see Fig. 1A). The whole-cell recording configuration was first established in voltage-clamp mode (holding potential −60 mV). All reported membrane potential values were corrected for a 10 mV junction potential.10 Data were acquired with a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, USA), sampled at 50 kHz, filtered between 2 and 10 kHz, and stored on a computer using AxoGraph X software (AxoGraph Scientific, Sydney, Australia). All recordings were made at room temperature (22–24°C).
A 5 mV hyperpolarizing step (10 ms duration, 10 repetitions) was used to measure series and input resistance. Only data from neurons in which series resistance was <20 MΩ were included in our analysis. Action potentials (APs) were elicited by injecting depolarizing, square current pulses (800 ms duration, 20 pA increments) from resting membrane potential. AP properties such as peak amplitude (measured from point of inflection to peak), width (measured at 50% of peak), after-hyperpolarization (AHP) latency and peak (measured from point of inflection to negative peak), were quantified from rheobase APs (Fig. 1B). AP discharge patterns in response to depolarising current steps were categorized as tonic firing (TF), single spiking (SS), initial bursting (IB), and delayed firing (DF) (as previously described in Flynn et al.).6
Spontaneous excitatory synaptic currents (sEPSCs) were recorded for at least 3 min in voltage-clamp mode (holding potential −60 mV) and analyzed offline. Captured sEPSCs were averaged, and peak amplitude (baseline to peak negative current), rise-time (calculated over 10–90% of peak amplitude), half-width (measured at 50% of peak amplitude), and decay time constant (calculated over 20–80% of the decay phase) were obtained. sEPSC frequency was calculated from raw traces over a period of 3 min. Dorsal column evoked synaptic responses, also recorded in voltage-clamp mode (holding potential −60 mV), were recorded after stimulation (0.1 ms duration current pulse at 0.2 Hz, 1.2×threshold). Dorsal column evoked responses were averaged over 14–15 trials, and peak amplitude (baseline to peak negative current), charge (area under the current trace baseline), and response latency (stimulation to current onset) were measured from the averaged response. Such responses were abolished by the addition of CNQX (10 μM) to the bath (not shown) as previously demonstrated.6
Statistical analysis
Comparisons of intrinsic and synaptic properties based on cell location or average training distance were made using a one-way ANOVA with a Tukey post-hoc test of all values, or a Kruskal–Wallis test with a Dunn post-hoc test, depending upon the distribution of the data set. Comparisons between data from untrained and trained groups were made using Student's t tests or Mann–Whitney U tests, depending upon the normality of the data set. The prevalence of AP discharge patterns was compared in untrained and trained groups using χ2 tests. Correlation analysis was applied to data from trained animals to test for relationships between distance travelled and electrophysiological parameters. In all comparisons, whether data fit a normal distribution was determined using a Kolmogorov–Smirnov test. Significance was set at p<0.05.
Results
In total, 137 neurons (70 untrained vs. 67 trained) were recorded from 28 adult mice (15 untrained vs. 12 trained): yield per animal ∼5 neurons. Neurons were located in segments adjacent to the lesion. Recorded cells were divided into five regions based on their location relative to the lesion: ipsilateral-rostral (IR), ipsilateral-caudal (IC), contralateral-rostral (CR), contralateral-caudal (CC), and contralateral-epicenter (CE - immediately contralateral to the lesion) (Fig 1A). Comparisons between untrained and trained groups showed no significant differences between passive and active intrinsic membrane properties analyzed (i.e., resting membrane potential, input resistance, AP amplitude, AP width, AHP latency, or AHP peak) based on cell location. Comparisons of sEPSC properties within untrained and trained groups also demonstrated no significant differences in rise time, peak amplitude, decay time constant, or frequency between cells from different regions. The latency of evoked responses was, however, greater in CE-located cells compared with IR- and CR-located cells in untrained animals. We believe this difference is related to the limited sampling across some of the location categories for this parameter (number of cells in each location category: IR=6, CR=9, IC=2, CC=2, CE=2). Overall, our analysis suggests the location our recorded neurons, relative to the lesion, was similar in both groups. Accordingly, subsequent comparisons were made between pooled neurons in untrained and trained groups.
Passive membrane properties for neurons in untrained versus trained groups were comparable in terms of resting membrane potential (−60.9±1.2 vs.−62.3±1.4 mV) and input resistance (809±84 vs. 881±78 MΩ). AP properties were also similar in untrained versus trained animals: AP amplitude (67.2±2.4 vs. 68.2±3.2 mV), AP width (1.5±0.1 vs. 1.6±0.1 ms), AHP latency (2.6±0.2 vs. 2.8±0.4 ms), or AHP peak (46.9±1.2 vs. 45.6±1.4 mV) (Fig. 1C). Four AP discharge patterns were observed in response to square step depolarizing current injection (Fig. 1D). The proportions of each discharge pattern did not differ in the untrained and trained mice (Fig. 1E).
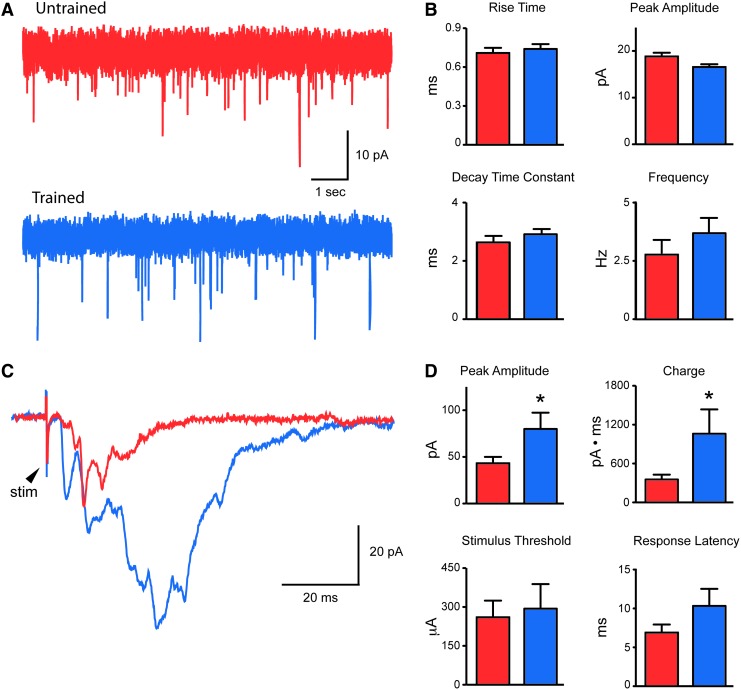
The properties of sEPSCs for neurons in the untrained and trained groups were similar, with no significant differences in rise time (0.71±0.04 vs. 0.74±0.04 ms), peak amplitude (18.7±0.8 vs. 16.7±0.3 pA), decay time constant (2.6±0.2 vs. 2.9±0.2 ms), or frequency (2.7±0.5 vs. 3.7±0.6 Hz) (Fig. 2B). In contrast, dorsal column evoked responses from trained mice had significantly larger peak amplitudes (78.9±17.5 vs. 42.2±6.8 pA) and greater charge (1054±376 vs. 348±75 pA·ms) compared with neurons from untrained mice (Fig. 2C and D). Stimulus threshold (262±64 vs. 295±95 μA) and response latency (6.8±1.0 vs. 10.3±2.2 ms) were similar in both groups, suggesting that stimulus parameters did not account for this observation. The clearly polysynaptic nature of the evoked EPSCs suggests the involvement of multiple synapses and neurons between the stimulation site in the dorsal columns and the recorded neuron.
FIG. 2.
Properties of spontaneous and evoked excitatory postsynaptic currents (EPSCs) in SCI mice. (A) Spontaneous EPSCs (sEPSCs) recorded in untrained (red trace) and trained (blue trace) mice (holding potential −60mV). (B) Group comparisons for selected sEPSC properties. These properties did not differ in untrained and trained mice. Error bars=SEM on all bar charts. (C) Overlaid dorsal column evoked synaptic responses from untrained (red) and trained mice (blue). (D) Group comparisons for dorsal column evoked synaptic responses. Peak amplitude and charge for evoked responses were significantly larger in the trained mice (*p<0.05). Color image is available online at www.liebertpub.com/neu
Animals in the trained group exercised at treadmill speeds commensurate with their ability, and therefore travelled varying distances over 3 weeks of exercise training. As the amount or “dose” of exercise administered to each animal during training may have had a differential effect on intrinsic or synaptic properties, comparisons were made based on the average distance travelled by each mouse. Animals were divided into tertiles (n=4 animals in each group) based on total distance travelled over 3 weeks of treadmill exercise (15 sessions): shorter distance (4190–4800 m), mid-distance (4930–5320 m) and longer distance (6050–6870 m). No significant differences were detected in the intrinsic properties (resting membrane potential, input resistance, AP amplitude, AP width, AHP latency, or AHP peak) of cells from trained animals based on total distance traveled. Likewise, there were no significant differences in the sEPSC properties (frequency, rise time, peak amplitude, or decay time constant) of cells based on the total distance traveled during the 3 week training period. We also examined the correlations for distance traveled relative to each (n=17) of the continuous electrophysiological variables measured (i.e., intrinsic, synaptic, and evoked properties). This analysis showed poor and nonsignificant correlations for all variables, bar one. Correlation coefficients (r values) averaged 0.017±0.23 (range −0.47 to +0.49). Only one variable (sEPSC frequency) was significantly correlated with running distance (r=−0.28) and p=0.05. Overall this analysis suggests that any differences in the capacity of animals to run (i.e., distance traveled or exercise dose) within the trained group did not affect the results of our study.
Discussion
This study expands on previous work in which we showed that it was possible to obtain a horizontal spinal cord slice that allowed high-resolution study of both intrinsic and synaptic properties of spinal neurons in adult mice using patch-clamp electrophysiology.6 Here, we have used our horizontal slice preparation to investigate the effects of exercise training on the function of neurons in the vicinity of an SCI. The major findings of our study are that 3 weeks of treadmill training following SCI: 1) do not alter the intrinsic membrane properties of neurons; 2) do not alter local excitatory synaptic inputs based on the properties of sEPSCs; but 3) markedly increase excitatory synaptic drive from axons located in the dorsal columns.
Our study shows that the intrinsic properties of neurons in the vicinity of an SCI are not altered by a short period (3 weeks) of exercise training (Fig. 1B–D). This finding differs from studies on rat motor neurons after SCI. For example, passive exercise training (4 weeks) attenuates the changes in motor neuron intrinsic properties that normally accompany complete SCI.11 The precise identity of our recorded neurons is unknown; however, based on previous work12,13 and mouse spinal cord anatomy,14 our recordings would have included neurons in the dorsal horn and intermediate zone of the spinal cord, which are in the terminal field of corticospinal tract (CST) axons in rodents.15 Neurons in these locations include those involved in processing tactile information, commissural neurons, and short and long propriospinal neurons.3 Importantly, the axons of these neurons do not exit the spinal cord. Therefore, unlike motor neurons, our recorded neurons are not deprived of the important afferent input and peripheral trophic factors that come from exercising paralyzed muscle.16,17
Dorsal column evoked responses exhibited markedly larger peak amplitude and charge in mice that underwent 3 weeks of exercise training after SCI (Fig 2D). This finding is consistent with anatomical studies showing that dorsal column pathways exhibit significant axonal sprouting following SCI both above and below the lesion.2,18,19 Moreover, exercise training after SCI is known to enhance sprouting, and increase the number of contacts on spinal neurons.4,5,20 To our knowledge, our study is the first demonstration using whole-cell patch clamp electrophysiology techniques, that exercise may induce such sprouting and form new synaptic connections whose functional properties can be measured electrically, around a lesion site in the spinal cord. The precise details of these new connections await further study; however, CST axons likely contribute to this plasticity, as they lie in ventral regions of the dorsal columns in rodents.21,22
Although dorsal column evoked responses were altered by exercise training after SCI, the properties (amplitude, kinetics, or frequency) of sEPSCs were unchanged. The simplest interpretation of this finding is that the axon terminals involved in spontaneous neurotransmitter release differ from those recruited by dorsal column stimulation. Together, these data on excitatory synaptic connections suggest that plasticity caused by exercise training preferentially targets connections between dorsal column pathways, such as the CST, and spinal neurons around the site of an SCI.
Conclusion
In summary, our study represents a necessary first step in providing a critical functional addition to current anatomical and molecular techniques for examining plasticity in spinal cord circuits after injury and exercise therapy. Achieving this in a mouse model of SCI is also important, given the increasing availability of genetic and molecular tools to identify and alter discrete neuronal populations within the spinal cord.23–25 In future studies, patch clamp electrophysiology can be used to examine how the two major determinates of neuron output, intrinsic properties and synaptic inputs,26 are altered by various forms of exercise therapy and other interventions in animal models of SCI.27
Acknowledgments
This study was supported by National Health and Medical Research Council Project grant 628765 and The Hunter Medical Research Institute.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bareyre F.M. Kerschensteiner M. Raineteau O. Mettenleiter T.C. Weinmann O. Schwab M.E. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat. Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- 2.Fouad K. Pedersen V. Schwab M.E. Brosamle C. Cervical sprouting of corticospinal fibers after thoracic spinal cord injury accompanies shifts in evoked motor responses. Curr. Biol. 2001;11:1766–1770. doi: 10.1016/s0960-9822(01)00535-8. [DOI] [PubMed] [Google Scholar]
- 3.Flynn J.R. Graham B.A. Galea M.P. Callister R.J. The role of propriospinal interneurons in recovery from spinal cord injury. Neuropharmacology. 2011;60:809–822. doi: 10.1016/j.neuropharm.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 4.Engesser–Cesar C. Ichiyama R.M. Nefas A.L. Hill M.A. Edgerton V.R. Cotman C.W. Anderson A.J. Wheel running following spinal cord injury improves locomotor recovery and stimulates serotonergic fiber growth. Eur. J. Neurosci. 2007;25:1931–1939. doi: 10.1111/j.1460-9568.2007.05469.x. [DOI] [PubMed] [Google Scholar]
- 5.Goldshmit Y. Lythgo N. Galea M.P. Turnley A.M. Treadmill training after spinal cord hemisection in mice promotes axonal sprouting and synapse formation and improves motor recovery. J. Neurotrauma. 2008;25:449–465. doi: 10.1089/neu.2007.0392. [DOI] [PubMed] [Google Scholar]
- 6.Flynn J.R. Brichta A.M. Galea M.P. Callister R.J. Graham B.A. A horizontal slice preparation for examining the functional connectivity of dorsal column fibres in mouse spinal cord. J. Neurosci. Methods. 2011;200:113–120. doi: 10.1016/j.jneumeth.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 7.Hsu J.Y. Stein S.A. Xu X.M. Development of the corticospinal tract in the mouse spinal cord: a quantitative ultrastructural analysis. Brain Res. 2006;1084:16–27. doi: 10.1016/j.brainres.2006.02.036. [DOI] [PubMed] [Google Scholar]
- 8.Steward O. Zheng B. Tessier–Lavigne M. Hofstadter M. Sharp K. Yee K.M. Regenerative growth of corticospinal tract axons via the ventral column after spinal cord injury in mice. J. Neurosci. 2008;28:6836–6847. doi: 10.1523/JNEUROSCI.5372-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dodt H.U. Zieglgänsberger W. Visualizing unstained neurones in living brain slices by infrared dic-videomicroscopy. Brain Res. 1990;537:333–336. doi: 10.1016/0006-8993(90)90380-t. [DOI] [PubMed] [Google Scholar]
- 10.Barry P.H. Lynch J.W. Liquid junction potentials and small cell effects in patch-clamp analysis. J. Membr. Biol. 1991;121:101–117. doi: 10.1007/BF01870526. [DOI] [PubMed] [Google Scholar]
- 11.Beaumont E. Houle J.D. Peterson C.A. Gardiner P.F. Passive exercise and fetal spinal cord transplant both help to restore motoneuronal properties after spinal cord transection in rats. Muscle Nerve. 2004;29:234–242. doi: 10.1002/mus.10539. [DOI] [PubMed] [Google Scholar]
- 12.Szucs P. Odeh F. Szokol K. Antal M. Neurons with distinctive firing patterns, morphology and distribution in laminae v-vii of the neonatal rat lumbar spinal cord. Eur. J. Neurosci. 2003;17:537–544. doi: 10.1046/j.1460-9568.2003.02484.x. [DOI] [PubMed] [Google Scholar]
- 13.Willis W.D. Coggeshall R.E. Sensory Mechanisms of the Spinal Cord. 3rd. Vol. 1. NewYork: Kluwer; 2004. [Google Scholar]
- 14.Watson C. Paxinos G. Kayalioglu G. Academic Press; London: 2009. The Spinal Cord. [Google Scholar]
- 15.Tracey D.J. Ascending and descending pathways in the spinal cord, in: The Rat Nervous System. In: Paxinos G., editor. Academic Press; San Diego: 2004. pp. 149–164. [Google Scholar]
- 16.Harkema S.J. Hurley S.L. Patel U.K. Requejo P.S. Dobkin B.H. Edgerton V.R. Human lumbosacral spinal cord interprets loading during stepping. J. Neurophysiol. 1997;77:797–811. doi: 10.1152/jn.1997.77.2.797. [DOI] [PubMed] [Google Scholar]
- 17.Fouad K. Rank M.M. Vavrek R. Murray K.C. Sanelli L. Bennett D.J. Locomotion after spinal cord injury depends on constitutive activity in serotonin receptors. J. Neurophysiol. 2010;104:2975–2984. doi: 10.1152/jn.00499.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ballermann M. Fouad K. Spontaneous locomotor recovery in spinal cord injured rats is accompanied by anatomical plasticity of reticulospinal fibers. Eur. J. Neurosci. 2006;23:1988–1996. doi: 10.1111/j.1460-9568.2006.04726.x. [DOI] [PubMed] [Google Scholar]
- 19.Weidner N. Ner A. Salimi N. Tuszynski M.H. Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proc. Natl. Acad. Sci. U. S. A. 2001;98:3513–3518. doi: 10.1073/pnas.051626798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Courtine G. Song B. Roy R.R. Zhong H. Herrmann J.E. Ao Y. Qi J. Edgerton V.R. Sofroniew M.V. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat. Med. 2008;14:69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hantman A.W. Jessell T.M. Clarke's column neurons as the focus of a corticospinal corollary circuit. Nat. Neurosci. 2010;13:1233–1239. doi: 10.1038/nn.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown L.T., Jr. Projections and termination of the corticospinal tract in rodents. Exp. Brain Res. 1971;13:432–450. doi: 10.1007/BF00234340. [DOI] [PubMed] [Google Scholar]
- 23.Brownstone R.M. Wilson J.M. Strategies for delineating spinal locomotor rhythm-generating networks and the possible role of hb9 interneurones in rhythmogenesis. Brain Res. Rev. 2008;57:64–76. doi: 10.1016/j.brainresrev.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goulding M. Circuits controlling vertebrate locomotion: moving in a new direction. Nat. Rev. Neurosci. 2009;10:507–518. doi: 10.1038/nrn2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng B. Lee J.K. Xie F. Genetic mouse models for studying inhibitors of spinal axon regeneration. Trends Neurosci. 2006;29:640–646. doi: 10.1016/j.tins.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Turrigiano G. Abbott L.F. Marder E. Activity-dependent changes in the intrinsic properties of cultured neurons. Science. 1994;264:974–977. doi: 10.1126/science.8178157. [DOI] [PubMed] [Google Scholar]
- 27.Battistuzzo C.R. Callister R.J. Callister R. Galea M.P. A systematic review of exercise training to promote locomotor recovery in animal models of spinal cord injury. J. Neurotrauma. 2012;29:1600–1613. doi: 10.1089/neu.2011.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]