Abstract
Background
Central nervous system (CNS) disease as the site of first relapse after exposure to adjuvant trastuzumab has been reported. We carried out comprehensive meta-analysis to determine the risk of CNS metastases as the first site of recurrence in patients with HER2-positive breast cancer who received adjuvant trastuzumab.
Methods
Eligible studies include randomized trials of adjuvant trastuzumab administered for 1 year to patients with HER2-positive breast cancer who reported CNS metastases as first site of disease recurrence. Statistical analyses were conducted to calculate the incidence, relative risk (RR), and 95% confidence intervals (CIs) using fixed-effects inverse variance and random-effects models.
Results
A total of 9020 patients were included. The incidence of CNS metastases as first site of disease recurrence in HER2-positive patients receiving adjuvant trastuzumab was 2.56% (95% CI 2.07% to 3.01%) compared with 1.94% (95% CI 1.54% to 2.38%) in HER2-positive patients who did not receive adjuvant trastuzumab. The RR of the CNS as first site of relapse in trastuzumab-treated patients was 1.35 (95% CI 1.02–1.78, P = 0.038) compared with control arms without trastuzumab therapy. The ratio of CNS metastases to total number of recurrence events was 16.94% (95% CI 10.85% to 24.07%) and 8.33% (95% CI 6.49% to 10.86%) for the trastuzumab-treated and control groups, respectively. No statistically significant differences were found based on trastuzumab schedule or median follow-up time. No evidence of publication bias was observed.
Conclusions
Adjuvant trastuzumab is associated with a significant increased risk of CNS metastases as the site of first recurrence in HER2-positive breast cancer patients.
Keywords: breast cancer, central nervous system, hER2, meta-analysis, metastases, trastuzumab
introduction
Trastuzumab, the monoclonal antibody to the extracellular domain of HER2, has significantly improved both disease-free survival (DFS) and overall survival (OS) in patients with locally advanced HER2-positive breast cancer [1–3]. Since its US Food and Drug Administration approval in 2006, trastuzumab has become the cornerstone of adjuvant therapy in this patient population. Despite these advances in patient outcome, 13%–28% of patients will develop recurrent disease [1–6].
In the post-trastuzumab era, it is well known that approximately one-third of patients with metastatic HER2-positive breast cancer will develop central nervous system (CNS) metastases, but these events tend to be a relatively late occurrence [7]. With the continuation of HER2-targeted therapy throughout the majority of a patient's metastatic disease course, CNS progression is normally preceded by metastases to other distant organs such as a liver, lung, and bone [8]. Proposed hypotheses suggest that the CNS may be a sanctuary site for micro-metastatic disease by failure of trastuzumab to penetrate the blood–brain barrier or by the loss of HER2 overexpression in breast cancer cells migrating to the brain [9]. Importantly, HER2-positive breast cancer that has spread to the CNS has been primarily characterized in patients who were never exposed to adjuvant trastuzumab and who only received trastuzumab-based treatment in the metastatic setting.
The CNS as a site of first relapse has been sporadically reported in the large adjuvant trials using trastuzumab. This topic is of particular importance because it suggests that the natural history of HER2-positive breast cancer has changed in the era of adjuvant trastuzumab therapy, as the incidence of CNS disease as the first site of recurrence before adjuvant trastuzumab was relatively uncommon. In this report, we investigate the incidence and risk of CNS metastases detected at the time of first recurrence in an up-to-date, comprehensive meta-analysis of randomized, controlled trials of adjuvant trastuzumab administered for 1 year in patients with HER2-amplified breast cancer.
methods
selection of studies
PubMed citations were reviewed from January 1966 to December 2011. The search criteria were randomized trials published in English, and the key words were trastuzumab (as well as the trade name Herceptin) and breast cancer or adenocarcinoma of the breast. Abstracts from the American Society of Clinical Oncology (ASCO; www.asco.org) and the San Antonio Breast Cancer Symposium (SABCS; www.sabcs.org) were also queried. When more than one publication or presentation was identified from the same clinical trial, the most recent report was included for analysis. The most recent package insert of trastuzumab was also accessed to identify relevant information.
data extraction and clinical end points
Data abstraction was conducted independently by three investigators (EMO, JM, and CSW) according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement [10], any discrepancies were resolved by consensus. For each study, we extracted the following information: first author's name, year of publication, trial phase, number of enrolled patients, treatment arms, duration and schedule of trastuzumab therapy, median follow-up, number of recurrence events and CNS metastases as a site of first relapse events in experimental and control arms, and hazard rate (HR) of OS and DFS.
Trials that met the following criteria were chosen for analysis: randomized adjuvant or neoadjuvant phase II or phase III trials that included 1 year of trastuzumab, where patients could be assigned to a control arm without trastuzumab, and contain adequate reporting of CNS disease as a site of first relapse.
statistical analysis
For the calculation of incidence, the number of patients with CNS disease as a site of first relapse and the number of patients treated with trastuzumab were extracted from the selected clinical trials. The proportion of patients with CNS disease and the 95% confidence intervals (CI) were derived from each trial. We also calculated the relative risk (RR) and CI of CNS disease as a site of first relapse in patients assigned to trastuzumab versus controls in the same trial.
We assessed statistical heterogeneity among trials included in the meta-analysis using the Cochran Q statistic, and inconsistency was quantified with the I2 statistic that estimates the percentage of total variation across studies due to heterogeneity ration that chance [11]. We considered an I2-value of >50% as indicative of substantial heterogeneity. When substantial heterogeneity was not observed, the pooled estimate calculated on the basis of the fixed-effects model was reported using inverse variance method. When substantial heterogeneity was observed, the pooled estimate calculated on the basis of the random-effects model was reported using the DerSimonian and Laird method that considers both within-study and between-study variations [12].
We examined a possible dose–administration relationship between trastuzumab and CNS disease as the site of first relapse by dividing trials into those in which trastuzumab was given with chemotherapy (concurrent) or after chemotherapy (sequential), and stratified trials by trastuzumab administered once a week versus every 3 weeks. To test the variation in risk estimates by trastuzumab administration, we conducted a meta-regression analysis. We also tested variation in risk estimates by the median follow-up time by meta-regression analysis. Finally, publication bias was evaluated through funnel plots and with Begg's and Egger's tests [13, 14]. A two-tailed P-value of <0.05 was considered statistically significant. All statistical analyses were carried out using Stata/SE version 11.0 software (Stata Corp., College Station, TX).
results
population characteristics
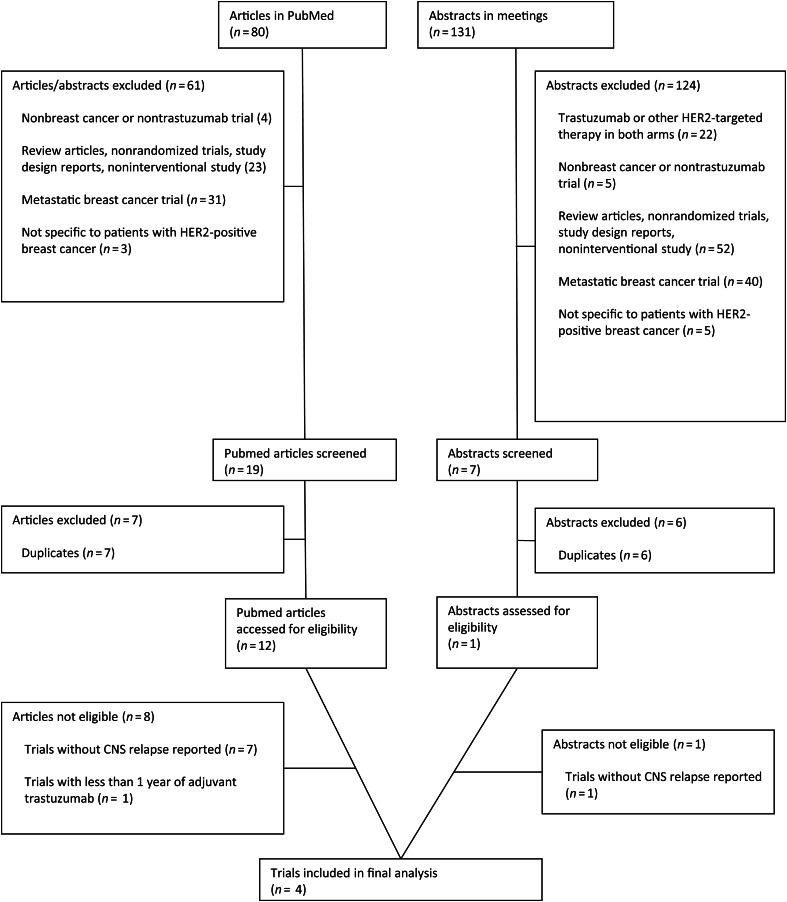
The original search yielded a total of 211 potentially relevant trastuzumab studies: 80 abstracts from PubMed and 131 from ASCO or SABCS meetings. A detailed selection process is presented in Figure 1. After evaluating each study, 185 were initially excluded. The remaining 26 studies were carefully screened, and an additional 13 trials were excluded for being duplicates. Thirteen of the remaining studies were thoroughly evaluated, and seven trials were excluded because of lack of CNS relapse reporting [15–22]. One trial was excluded because trastuzumab was only given for <1 year [6]. Four trials were selected for inclusion in the meta-analysis [1–4].
Figure 1.
Selection process for the randomized, controlled trials included in the meta-analysis.
A total of 9020 patients were available for the meta-analysis. Baseline characteristics of each trial are presented in Table 1. All selected trials included patients with localized HER2-positive breast cancer as defined by an immunohistochemistry of 3+ or by fluorescence in situ hybridization with at least criteria of a HER2:CEP17 ratio of ≥2 [1–4]. Two trials included node-positive patients only, [2, 3] and the remaining included patients with both node-positive and high-risk, node-negative diseases [1, 4]. Patients were required to have adequate baseline organ and hematologic function. All trials excluded patients with an abnormal left ventricular ejection fraction. Chemotherapeutic agents given before or during trastuzumab therapy included anthracyclines, cyclophosphamide, 5-fluorouracil, and taxanes. Trastuzumab was administered for a total of 1 year in all studies. All trials separated recurrence events by local and distant recurrence. Terminology for relapsed disease was defined as in the brain in two studies [1, 2], and reported as in the CNS in the remaining two trials [3, 4].
Table 1.
Baseline characteristics of the patients on the trials included in the meta-analysis
| Study and treatment arm | Phase | Crossover allowed | Total number of patients enrolled on study regardless of HER2 status | Median follow-up (months) | HR of OS (95% CI) | HR of DFS (95% CI) | No. of patients for analysis | No. of CNS eventsa | No. recurrence eventsb |
|---|---|---|---|---|---|---|---|---|---|
| NSABP B31 [1]c | III | Yes | 2101 | 46.8d | 0.59 (0.48–0.73)e | 0.51 (0.44–0.59)e | |||
| AC followed by paclitaxel | 1046 | 17 | 243 | ||||||
| AC followed by paclitaxel and concurrent trastuzumab | 1055 | 32 | 137 | ||||||
| NCCTG N9831 [2]c | III | Yes | 3505 | 72 | 0.88 (0.67–1.15)f | 0.67 (0.54–0.81)f | |||
| AC followed by paclitaxel | 1087 | 21 | 225 | ||||||
| AC followed by paclitaxel and sequential trastuzumab | 954 | 19 | 174 | ||||||
| AC followed by paclitaxel and concurrent trastuzumab | 949 | 26 | 139 | ||||||
| HERA [3]g | III | Yes | 5102 | 48.4 | 0.85 (0.701–1.04) | 0.76 (0.66–0.87) | |||
| Observation | 1698 | 32 | 458 | ||||||
| One year of trastuzumab | 1703 | 37 | 369 | ||||||
| Two years of trastuzumab | NR | NR | NR | ||||||
| PACS 04 [4]h | III | No | 3010 | 47 | 1.27 (0.68–2.38) | 0.86 (0.61–1.22) | |||
| FEC versus ED followed by observation | 268 | 8 | 52 | ||||||
| FEC versus ED followed by 1 year of trastuzumab | 260 | 11 | 44 |
All trials evaluated patients with localized HER2-positive breast cancer. Crossover indicates that patients were allowed to receive trastuzumab after the initial efficacy results were made public.
aNumber of CNS events reported as first site of recurrent disease.
bTotal number of patients with a recurrence event.
dMedian follow-up for combined analysis of NSABP B31 and NCCTG N9831. Follow-up for NSABP B31 alone NR.
cDosing for NSABP B31 [1] and NCCTG N9831 [2]: doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 every 3 weeks for four cycles followed by paclitaxel 175 mg/m2 every 3 weeks for four cycles or paclitaxel 80 mg/m2 every week for 12 weeks. Patients randomized to trastuzumab received an initial loading dose of 4 mg/kg followed by 2 mg/kg given once a week for a total of 52 weeks.
eHR reported in the combined analysis of NSABP B31 and the concurrent arm of NCCTG N9831.
fHR reported for sequential trastuzumab compared with chemotherapy alone. The concurrent trastuzumab outcome data are combined with the NSABP B31 data above.
gDosing for HERA [3] Patients must have received at least four cycles of chemotherapy, choice of agent was at the discretion of the treating physician. Patients randomized to trastuzumab was administered at a loading dose of 8 mg/kg (day 1 of first cycle) with subsequent doses administered at 6 mg/kg every 3 weeks for a total course of 1 or 2 years. Outcome data for the 2-year arm have not yet been reported.
hDosing for PACS04 [4] Patients were randomized initially to either six courses of FEC or ED regimen. FEC regimen included fluorouracil 500 mg/m2, epirubicin 100 mg/m2, and cyclophosphamide 500 mg/m2 every 3weeks. ED regimen included epirubicin 75 mg/m2 and docetaxel 75 mg/m2. Patients were subsequently randomized to trastuzumab was administered at a loading dose of 8 mg/kg (day 1 of first cycle) with subsequent doses administered at 6 mg/kg every 3 weeks for a total course of 1 year.
NSABP, National Surgical Adjuvant Breast and Bowel Project (NSABP); AC, doxorubicin and cyclophosphamide; NCCTG, North Central Cancer Treatment Group; HERA, herceptin adjuvant; HER2, human epidermal growth factor receptor 2; FEC, fluorouracil, epirubicin, and cyclophosphamide; ED, epirubicin and docetaxel; HR, hazard ratio; DFS, disease-free survival; OS, overall survival; NR, not recorded.
incidence of CNS metastases
Among the 4921 patients that received adjuvant trastuzumab, 125 developed CNS metastases as the site of first recurrence. By using fixed-effects inverse variance models for this analysis (heterogeneity test: Q = 4.90; P = 0.179; I2 = 38.8%) the overall incidence of CNS metastases as the first site of relapse was 2.56% (95% CI 2.07% to 3.01%). For the control group, there were 78 CNS events among 4099 patients, which conferred an incidence of 1.94% (95% CI 1.54% to 2.38%) (heterogeneity test: Q = 2.07; P = 0.558; I2 = 0.0% Table 2). The ratio of CNS metastases to total number of recurrence events was 16.94% (95% CI 10.85% to 24.07%) (heterogeneity test: Q = 17.47; P = 0.001; I2 = 82.8%) and 8.33% (95% CI 6.49% to 10.86%) (heterogeneity test: Q = 4.76; P = 0.190; I2 = 37.0%) for the trastuzumab-treated and control groups, respectively, using random-effects models.
Table 2.
Incidence and relative risk (RR) of central nervous system (CNS) metastases as site of first recurrence in HER2-positive breast cancer patients treated with adjuvant trastuzumab, stratified by concurrent or sequential chemotherapy
| Treatment | Number of trials | Trastuzumab arm |
Control arm |
Trastuzumab-related CNS metastases as a site of first recurrence |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients |
Incidence (%) | 95% CI | Number of patients |
Incidence (%) | 95% CI | ||||||
| CNS as a site of first recurrence | Total | CNS as a site of first recurrence | Total | RR | 95% CI | ||||||
| Overall | 4 | 125 | 4921 | 2.56 | 2.07–3.01 | 78 | 4099 | 1.94 | 1.54–2.38 | 1.35a | 1.02–1.78 |
| Timing of Chemotherapy | |||||||||||
| Concurrent | 1b | 58 | 2004 | 2.94 | 2.25–3.72 | 38 | 2133 | 1.82 | 1.30–2.43 | 1.62 | 1.08–2.44 |
| Sequential | 2b,c | 67 | 2917 | 2.31 | 1.80–2.89 | 61 | 3053 | 2.03 | 1.56–2.56 | 1.15 | 0.81–1.62 |
| Weekly | 2 | 77 | 2958 | 2.63 | 2.08–3.23 | 38 | 2133 | 1.82 | 1.30–2.43 | 1.47 | 1.00–2.16 |
| Every 3 weeks | 2 | 48 | 1963 | 2.45 | 1.81–3.18 | 40 | 1966 | 2.07 | 1.48–2.74 | 1.21 | 0.80–1.83 |
P-value for the difference in RR of concurrent versus sequential adjuvant trastuzumab for CNS as a site of first recurrence = 0.29. P-value for difference in RR of weekly versus every 3 weeks adjuvant trastuzumab for CNS as a site of first recurrence = 0.56.
aP = 0.038; heterogeneity test: Q = 1.78, P = 0.620, I2 = 0.0%.
bNSABP B31 [1] randomized to concurrent trastuzumab therapy. HERA [3] and PACS04 [4] randomized to sequential trastuzumab therapy. NCCTG 9831 [2] randomized to concurrent or sequential trastuzumab therapy and therefore the control patients from this study were used in both the concurrent and sequential analyses.
CNS, central nervous system; RR, relative risk; CI, confidence interval.
relative risk of CNS metastases
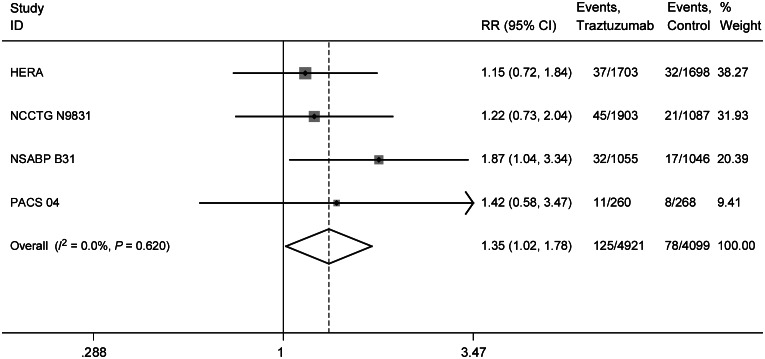
We determined the overall RR of CNS metastases as the first site of recurrence from the four randomized control trials. The overall RR of developing CNS metastases as the first site of recurrence with 1 year of adjuvant trastuzumab was 1.35 (95% CI 1.02–1.78, P = 0.038) compared with patient who did not receive trastuzumab therapy. No significant heterogeneity among the included trials was observed. (Q = 1.78; P = 0.620; I2 = 0.0%; Figure 2). Among patients who developed recurrent disease, the RR of CNS involvement after receiving trastuzumab was 1.85 (95% CI 1.241–2.765, P = 0.0026) (heterogeneity test: Q = 6.26; P = 0.0996; I2 = 52.1%) compared with relapsed patients who were not administered trastuzumab therapy using a random-effects model to account for potential heterogeneity.
Figure 2.
Relative risk (RR) of central nervous system (CNS) recurrence events as a site of first relapse associated with adjuvant trastuzumab versus control among patients with HER2-positive breast cancer.
influence of concurrent versus sequential trastuzumab
Adjuvant trastuzumab may be administered concurrently with chemotherapy or treatment can begin after chemotherapy is complete. Therefore, we attempted to examine the incidence and RR stratified by timing of trastuzumab administration. One trial [1] randomized patients to adjuvant trastuzumab given concurrently with chemotherapy (n = 2101) and two studies [3, 4] to sequential trastuzumab (n = 3929; Table 2.) In one trial, randomization to either a concurrent or sequential arm was possible (n = 2990) [2]. The incidence of CNS metastases as a site of first recurrence was 2.94% (95% CI 2.26% to 3.72%) and 2.31% (95% CI 1.80% to 2.89%) for concurrent and sequential trastuzumab, respectively. The overall RRs for concurrent and sequential trastuzumab dosing were 1.62 (95% CI 1.080–2.44) and RR 1.15 (95% CI 0.81–1.62). Temporality of trastuzumab treatment was not significantly related to risk of CNS metastases as a site of first relapse in a meta-regression model (P = 0.29).
influence of weekly versus every 3-week trastuzumab
Adjuvant trastuzumab therapy may be administered at an initial loading dose of 4 mg/kg followed by 2 mg/kg given once a week or alternatively at a loading dose of 8 mg/kg (day 1 of first cycle) with subsequent doses administered at 6 mg/kg every 3 weeks. Regardless of dose, patients in each trial were prescribed trastuzumab for 1 year. The incidence of CNS metastases as a site of first recurrence were 2.63% (95% CI 2.08% to 3.23%) and 2.45% (95% CI 1.81% to 3.18%) in the weekly versus every 3-week dosing, respectively. The RR for patients treated with weekly trastuzumab was 1.47 (95% CI 1.00–2.16) compared with 1.21 (95% CI 0.80–1.82) when administered on an every 3-week schedule. Administration time of trastuzumab treatment was not significantly related to risk of CNS metastases as a site of first recurrence in a meta-regression model (P = 0.561).
influence of the duration of follow-up
To determine whether the amount of follow-up time for a study impacted on the occurrence of CNS disease as a site of first recurrence, median duration of follow-up was included as a continuous covariate in a meta-regression model. No difference was seen in the trastuzumab groups due to median follow-up (P = 0.677).
quality of the study and publication bias
All trials included in the meta-analysis were randomized, multicenter, open-label, phase III trials published in peer-reviewed journals [1–4]. Each trial contained a control arm where patients were not administered trastuzumab. No evidence of publication bias was detected for the RR of CNS metastases as the first site of recurrence in trastuzumab-treated patients with HER2-positive breast cancer by Egger (P = 0.5774) or Begg's test (P = 0.3337).
discussion
To our knowledge, this is the largest and most current report to show a significant increase in the risk of CNS metastases as the first site of recurrence for HER2-positive breast cancer patients who received 1 year of adjuvant trastuzumab. The incidence of CNS metastases as the first site of distant relapse was 2.56% (95% CI 2.07% to 3.01%). Despite the reasonably low absolute incidence, there is an overall increase of 76% in risk of the detection of a CNS lesion at diagnosis of first relapse in trastuzumab-treated patients compared with controls. Previous meta-analyses report similar findings with a 1.5- to 1.8-fold increased risk of the CNS as the first site of recurrent disease [9, 23, 24]. Importantly, data from these studies did not include CNS relapse information from the PACS04 trial. Unlike prior analyses, we report the ratio of CNS metastases to total number of recurrence events at 16.94% (95% CI 10.85% to 24.07%) for patients receiving adjuvant trastuzumab therapy, which is equivalent to double the ratio noted in the control arms. These data suggest that while trastuzumab is excellent at controlling extracranial relapse, the monoclonal antibody appears to have limited value in preventing CNS recurrence.
Stratified analyses were carried out to evaluate subgroups with the potentially higher incidence of CNS disease as first site of relapse. No differences were found between concurrent versus sequential trastuzumab administration, and similarly, no differences were noted between weekly versus every 3-week administration of trastuzumab. These findings suggest that concurrent chemotherapy, dose or frequency of trastuzumab administration may not change the limited ability of trastuzumab to be delivered to known sanctuaries of microscopic HER2-positive cancer cells. Additionally, risk of CNS recurrence is independent of the duration of follow-up in each study. This remains a hypothesis-generating analysis, and caution should be used when interpreting these subgroups due to limited sample size.
HER2-positive breast cancer that has spread to the brain is a well-known occurrence in women receiving a metastatic trastuzumab-based regimen. A CNS lesion identified at time of metastatic diagnosis is relatively uncommon and occurs in 8% of patients with advanced disease who were never exposed to adjuvant trastuzumab [8]. These reported numbers are similar to the 8.33% (95% CI 6.49% to 10.86%) calculated for control arms in this analysis. However, after prolonged use of anti-HER2 therapy, the number of patients with CNS involvement dramatically increases with approximately one-third to one-half demonstrating radiographically detected CNS lesions by the time of death [7, 25]. In our analysis, we show a doubling in the percentage of patients presenting with CNS disease as a first site of recurrence; suggesting that adjuvant trastuzumab—similar to sequential HER2-directed regimens for metastatic disease—controls extracranial disease but may expose the brain as both a milieu primed for HER2-positive cancer growth and as an organ relatively protected from trastuzumab's anticancer properties by its inability to cross the blood–brain barrier.
Despite the size of our analysis, there are several limitations to this study. Data were abstracted from published clinical trial results, and therefore, individual patient information was not available. We are unable to learn about the timing and subsequent outcomes of these events. Establishment of clinical risk factors associated with development of CNS metastases as the first site of recurrence after adjuvant trastuzumab including stage, nodal status, or hormonal status is not possible in this analysis. It is unknown if the prognosis or clinical course of patients with a CNS first recurrence differ significantly from patients who only develop an extracranial relapse after adjuvant trastuzumab. Importantly, patients recruited to these adjuvant studies did not undergo screening CNS imaging after administration of adjuvant therapy, and CNS imaging at time of first diagnosis of extracranial recurrence is not routinely carried out in patients without CNS symptomatology. Therefore, the rates of CNS involvement at time of first relapse may be underestimated in this meta-analysis.
While not available in our dataset, it would be important to also understand the number of patients with a subsequent CNS progression and compare their outcomes to patients with a brain lesion on first presentation. The publications used in this study report an overall decrease in breast cancer recurrence in patients that received trastuzumab, which may translate into a progressive disparity between the numbers of patients at risk for a first CNS relapse event in the trastuzumab-treated versus the control groups. A time to first CNS event analysis may help to understand this potential bias; however, this information is not available within the confines of our study. An examination of the HERA trial by Pestalozzi et al. [26] reports that trastuzumab decreases the cumulative incidence of extracranial recurrence, while CNS relapses (first or subsequent) occur with equal frequency in both trastuzumab-treated and nontrastuzumab arms. These data, along with our analysis, may imply that adjuvant trastuzumab does not directly cause CNS disease; instead, trastuzumab fails to prevent recurrence in the brain resulting in an increase in the ratio of CNS metastases to total number of first relapse events.
Our findings may suggest that trastuzumab therapy has changed the pattern of recurrence in the era of adjuvant therapy and these data prompt re-evaluating our clinical management of this patient population. Confirmatory prospective studies are needed to further characterize the timing of these events and the impact on patient care. For example, in the metastatic setting, radiographic screening for CNS disease is not a routine clinical practice. Similarly, while CNS disease can cause significant morbidity, early interventions such as prophylactic cranial irradiation can have considerable toxicity [27]. The incidence of CNS relapse is small and until new methods of identifying patients at increased risk of CNS recurrence are validated, pursuing interventions to decrease this risk are currently unappealing in the HER2-positive breast cancer population. Similarly, the benefit of routine screening to diagnose asymptomatic CNS metastases remains unknown.
Dual HER2-inihibiton targeting both the extracellular and intracellular components of the tyrosine kinase receptor have led to improved outcomes in patients with metastatic HER2-amplified breast cancer [28]. Clinical trial development utilizing anti-HER2 therapy continues to add targeted agents to improve ‘crippling’ of the HER2 oncogenic pathway [28]. Small molecules that target the HER pathway, including lapatinib and afatinib, are hypothesized to have improved CNS penetration and may have some activity in HER2-positive patients with brain metastases. As these newer anti-HER2 agents move to the adjuvant setting, it is important to assess if they will impact on the incidence of CNS disease as a first site of relapse.
In conclusion, the use of adjuvant trastuzumab may fail to prevent CNS metastases at time of first recurrence in patients with HER2-amplified breast cancer. Although the overall incidence remains low, the RR is significant with a doubling in the proportion of relapsed patients with brain metastases after trastuzumab compared with control arms. While adjuvant trastuzumab has dramatically lowered the risk of recurrence, clinicians should be cognizant of these occurrences and monitor survivors closely for worrisome neurologic symptoms. Additionally, investigators need to insure that CNS disease events are appropriately captured on any future adjuvant HER2-positive trials utilizing trastuzumab and next-generation HER2-targeted therapy to appropriately understand this phenomenon in the context of dual anti-HER2 regimens. Novel agents with enhanced CNS penetration and the ability to target microscopic disease in the brain are needed, as CNS metastases will continue to cause significant morbidity and mortality in patients with HER2-amplifed breast cancer. This study highlights the continued need for post-approval investigation into the outcomes of patients treated with targeted agents to accurately describe the evolving natural history of different breast cancer subtypes.
funding
EMO is supported by Translational Grant No. K12 CA 133250 in experimental therapeutics from the National Cancer Institute.
disclosure
NUL receives research support from Genentech, Inc.
acknowledgements
Data from this study were reported in part at the American Society of Clinical Oncology Annual Meeting (2012 Chicago, IL, USA).
references
- 1.Perez EA, Romond EH, Suman VJ, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;29:3366–3373. doi: 10.1200/JCO.2011.35.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perez EA, Suman VJ, Davidson NE, et al. Sequential versus concurrent trastuzumab in adjuvant chemotherapy for breast cancer. J Clin Oncol. 2011;29:4491–4497. doi: 10.1200/JCO.2011.36.7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gianni L, Dafni U, Gelber RD, et al. Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: a 4-year follow-up of a randomised controlled trial. Lancet Oncol. 2011;12:236–244. doi: 10.1016/S1470-2045(11)70033-X. [DOI] [PubMed] [Google Scholar]
- 4.Spielmann M, Roché H, Delozier T, et al. Trastuzumab for patients with axillary-node–positive breast cancer: results of the FNCLCC-PACS 04 Trial. J Clin Oncol. 2009;27:6129–6134. doi: 10.1200/JCO.2009.23.0946. [DOI] [PubMed] [Google Scholar]
- 5.Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Eng J Med. 2011;6:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joensuu H, Bono P, Kataja V, et al. Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: final results of the FinHer Trial. J Clin Oncol. 2009;27:5685–5692. doi: 10.1200/JCO.2008.21.4577. [DOI] [PubMed] [Google Scholar]
- 7.Lin NU, Winer EP. Brain metastases: the HER2 paradigm. Clin Cancer Res. 2007;13:1648–1655. doi: 10.1158/1078-0432.CCR-06-2478. [DOI] [PubMed] [Google Scholar]
- 8.Olson EM, Najita JS, Sohl J, et al. Clinical outcomes and treatment practice patterns of patients with HER2-positive metastatic breast cancer in the post-trastuzumab era. Breast. 2013 doi: 10.1016/j.breast.2012.12.006. January 23 [Epub ahead of print], doi: 10.1016/j.breast.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bria E, Cuppone F, Fornier M, et al. Cardiotoxicity and incidence of brain metastases after adjuvant trastuzumab for early breast cancer: the dark side of the moon? A meta-analysis of the randomized trials. Breast Cancer Res Treat. 2008;109:231–239. doi: 10.1007/s10549-007-9663-z. [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 13.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 14.Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–3685. doi: 10.1200/JCO.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 16.Untch M, Rezai M, Loibl S, et al. Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: results from the GeparQuattro Study. J Clin Oncol. 2010;28:2024–2031. doi: 10.1200/JCO.2009.23.8451. [DOI] [PubMed] [Google Scholar]
- 17.von Minckwitz G, Rezai M, Loibl S, et al. Capecitabine in addition to anthracycline- and taxane-based neoadjuvant treatment in patients with primary breast cancer: phase III GeparQuattro Study. J Clin Oncol. 2010;28:2015–2023. doi: 10.1200/JCO.2009.23.8303. [DOI] [PubMed] [Google Scholar]
- 18.Pierga J-Y, Delaloge S, Espié M, et al. A multicenter randomized phase II study of sequential epirubicin/cyclophosphamide followed by docetaxel with or without celecoxib or trastuzumab according to HER2 status, as primary chemotherapy for localized invasive breast cancer patients. Breast Cancer Res Treat. 2010;122:429–437. doi: 10.1007/s10549-010-0939-3. [DOI] [PubMed] [Google Scholar]
- 19.Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375:377–384. doi: 10.1016/S0140-6736(09)61964-4. [DOI] [PubMed] [Google Scholar]
- 20.Buzdar AU, Valero V, Ibrahim NK, et al. Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2–positive operable breast cancer: an update of the initial randomized study population and data of additional patients treated with the same regimen. Clin Cancer Res. 2007;13:228–233. doi: 10.1158/1078-0432.CCR-06-1345. [DOI] [PubMed] [Google Scholar]
- 21.Chang HR, Glaspy J, Allison MA, et al. Differential response of triple-negative breast cancer to a docetaxel and carboplatin-based neoadjuvant treatment. Cancer. 2010;116:4227–4237. doi: 10.1002/cncr.25309. [DOI] [PubMed] [Google Scholar]
- 22.Paz I, Lau S, Garberoglio C, et al. Nab-paclitaxel and carboplatin with or without trastuzumab (trast) as part of neoadjuvant chemotherapy (NCT) in patients (pts) with stage II-III breast cancer (BC). ASCO Annual Meeting, Int J Clin Oncol. 2008;26:(Abstr. 567). [Google Scholar]
- 23.Viani G, Afonso S, Stefano E, et al. Adjuvant trastuzumab in the treatment of her-2-positive early breast cancer: a meta-analysis of published randomized trials. BMC cancer. 2007;7:153. doi: 10.1186/1471-2407-7-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dahabreh IJ, Linardou H, Siannis F, et al. Trastuzumab in the adjuvant treatment of early-stage breast cancer: a systematic review and meta-analysis of randomized controlled trials. Oncologist. 2008;13:620–630. doi: 10.1634/theoncologist.2008-0001. [DOI] [PubMed] [Google Scholar]
- 25.Lin NU, Diéras V, Paul D, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res. 2009;15:1452–1459. doi: 10.1158/1078-0432.CCR-08-1080. [DOI] [PubMed] [Google Scholar]
- 26.Pestalozzi B, Homes E, Metzger O, et al. Trastuzumab does not increase the incidence of central nervous system (CNS) relapses in HER2-positive early breast cancer: the HERA trial experience. San Antonio Breast Conference Symposium; San Antonio, TX. 2012. [Google Scholar]
- 27.Slotman B, Faivre-Finn C, Kramer G, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Eng J Med. 2007;357:664–672. doi: 10.1056/NEJMoa071780. [DOI] [PubMed] [Google Scholar]
- 28.Olson EM. Maximizing human epidermal growth factor receptor 2 inhibition: a new oncologic paradigm in the era of targeted therapy. J Clin Oncol. 2012;30:1712–1714. doi: 10.1200/JCO.2011.40.2545. [DOI] [PubMed] [Google Scholar]