Abstract
Previous studies have indicated that 5-Fluoro-2′-deoxyuridine-5′-O-monophosphate 10mer (FdUMP[10]) displays strong antileukemic activity through the dual targeting of thymidylate synthase (TS) and DNA topoisomerase 1 (Top1). The present studies were undertaken to clarify the relationship between the induction of a thymineless state and the formation of Top1 cleavage complexes (Top1CC) for inducing cell death and to clarify the role of DNA replication for induction of lethal DNA double-strand breaks (DSBs) in FdUMP[10]-treated acute myeloid leukemia (AML) cells.
Human promyelocytic (HL60) and AML (KG1a, Molm13, THP-1) cells were synchronized by serum starvation and treated with FdUMP[10] with thymidine (Thy) rescue. Cells were assayed for TS inhibition, DNA DSBs, Top1CC, and apoptosis to clarify the interrelationship of TS inhibition and Top1CC for cell death. FdUMP[10] induced a thymineless state in AML cells and exogenous Thy administered within the first 18 hours of treatment rescued FdUMP[10]-induced Top1CC formation, γH2AX phosphorylation, and apoptosis induction. Exogenous Thy was not effective after cells had committed to mitosis and undergone cell division in the presence of FdUMP[10]. FdUMP[10] treatment resulted in Chk1 activation, and Chk1 inhibition enhanced FdUMP[10]-induced DNA damage and apoptosis. Jnk-signaling was required for FdUMP[10]-induced apoptosis in promyelocytic HL60 cells and in THP1 cells, but was antiapoptotic in Molm13 and to a lesser extent KG1a AML cells. The results are consistent with FdUMP[10] inducing a thymineless state, leading to misincorporation of FdU into genomic DNA of proliferating cells. Top1CC form in cells upon re-entry into S-phase, resulting in DNA double-strand breaks, and initiating apoptotic signaling that can be either muted or enhanced by Jnk-signaling depending on cell type.
Acute myeloid leukemia (AML) is the most common acute leukemia in adults and is highly lethal, resulting in more than 9,000 deaths from 12,000 reported cases in the United States each year [1,2]. Chemotherapy with cytarabine (AraC) and daunorubicin (DNR) frequently induces a remission; however, relapse is common and new chemotherapeutic options are needed, particularly for older patients who are undertreated because of the severe toxicities associated with DNR/AraC chemotherapy [3,4]. We recently demonstrated that the novel polymeric fluoropyrimidine (FP) 5-Fluoro-2′-deoxyuridine-5′-O-monophosphate 10mer (FdUMP[10]) had exceptional activity toward human and murine AML cell lines in vitro and that FdUMP[10] as a single agent had antileukemic activity at least as great as the combination of AraC and doxorubicin (Dox, the preferred anthracycline for mice) in vivo [5]. Furthermore, the strong efficacy observed with FdUMP[10] was achieved with markedly reduced systemic toxicity relative to that associated with AraC/Dox treatment. Importantly, FdUMP [10] had significantly greater efficacy and markedly reduced toxicity in this murine model of AML relative to 5-fluorouracil (5FU), the FP chemotherapeutic that is currently in widespread use [6], indicating that FdUMP[10] is mechanistically distinct from previous FP drugs and may be useful for leukemia treatment.
Studies from the NCI 60 cell line screen revealed that leukemic cells were highly sensitive to FdUMP[10] with GI50 values for several leukemic cell lines in the nM range (<50 nM) [7]. These results were surprising considering the lack of utility for conventional FP drugs, such as 5FU, for leukemia treatment. Our recent studies demonstrated that FdUMP[10] displays nM potency (IC50 < 50 nM) toward a variety of human AML cell lines, including those that express the Flt3-ITD, mutant–deleted p53, and other markers associated with chemoresistance and a poor prognosis [5]. In all AML cell lines tested to date, FdUMP[10] has displayed approximately 1,000-fold greater potency relative to 5FU, which is consistent with fundamental mechanistic differences between these FPs and not merely the result of a stoichiometric advantage for FdUMP[10]. Importantly, FdUMP[10] does not induce appreciable bone marrow toxicity in vivo, and FdUMP [10] displays minimal toxicity toward normal hematopoietic stem cells [5]. In contrast, 5FU is used to eradicate bone marrow in mice [8]; therefore, FdUMP[10] has both the potency and selectivity required to be clinically useful for leukemia treatment.
Our previous studies have demonstrated that FdUMP [10] is an exceptionally potent thymidylate synthase (TS) inhibitor [9] and that TS and topoisomerase 1 (Top1) are dual targets for FdUMP[10] in AML cells [5]. Although previous studies have established that Top1-poisoning is important for FdUMP[10]-mediated cell death [7,10], the relationship between induction of a thymineless state, Top1 cleavage complex (Top1CC) formation, and induction of cell death requires additional clarification. The trapping of Top1 following FdUMP[10] treatment requires FdUTP incorporation into genomic DNA, and this is favored when DNA replication and subsequent DNA repair proceed under thymineless conditions. Under this scenario, exogenous Thy would be expected to rescue cells from Top1CC formation only when administered during the initial replicative cycle. Cells successfully proceeding through mitosis with 5-fluoro-2′-deoxyuridine (FdU) misincorporated into genomic DNA would have the Top1CC-forming lesion fixed in place, and Top1CC would be expected to occur regardless of exogenous Thy levels—provided FdU is not removed from DNA in a postreplicative repair process. The present studies demonstrate that Thy-rescue of Top1CC formation is effective <18 hours after FdUMP [10] treatment, coincident with the timing for DNA replication and cell division in AML cells. The results are consistent with FdUMP[10] efficiently initiating a thymineless state, resulting in stable misincorporation of FdUTP into genomic DNA during replication. Thy rescue of proapoptotic signaling follows the same time dependence as Top1CC formation, which is consistent with apoptosis induction being consequent to Top1CC. FdUMP[10]-induced apoptosis requires Jnk signaling in promyelocytic HL60 cells, but Jnk signaling is antiapoptotic in Molm13 acute monocytic leukemia cells. These results are consistent with FdUMP[10] being a potent general inducer of apoptosis in AML cells by inducing Top1CC in proliferating cells and with cell death signaling subsequent to Chk1 activation being cell-type dependent.
Methods
Materials
All primary and secondary antibodies used for Western blot, flow cytometry, and immunofluorescence [11] were purchased from Cell Signaling unless otherwise noted. AlexaFluor 488 goat anti-rabbit (Invitrogen, Grand Island, NY, USA) was used for flow cytometry, and rhodamine (TRITC)–conjugated donkey anti-rabbit immunoglobulin G for immunofluorescence was purchased from Jackson ImmunoResearch. Z-VAD-FMK caspase inhibitor (Promega, Madison, WI, USA) and ERK inhibitor (U0126, Sigma, St. Louis, MO, USA) were each used at 20 μmol/L. Chk1 inhibitor (SB218078, Calbiochem) and p38 inhibitor (SB203580, Sigma) were each used at 5 μmol/L. JNK inhibitor (SP600125, Sigma) was used at 10 or 50 μmol/L.
Cell culture and ICE bioassay/TOP1 cleavage complex detection
HL60, THP1, KG1a, and Molm13 AML cells (American Type Culture Collection, Manassas, VA, USA) were grown in RPMI 1640 (Gibco, Grand Island, NY, USA) with 10% FBS (Gemini Bio-Products, West Sacramento, CA, USA). HL60 cells were synchronized in G0/G1 by serum starvation for 24 hours followed by serum addition for 12–16 hours before initiating studies. Cells were plated at a density of 1.5 × 106 cells in 100 mm2 plates and grown overnight. ICE bioassays were completed using methods similar to those described previously [10]. AML cells were incubated with FdUMP[10] (1 × 10−8 mol/L) for 0–48 hours. Thymidine rescue was accomplished by adding Thy at 20 μmol/L for the indicated times in combination with FdUMP[10]. Cell samples were counted and equalized for cell number. Primary antibody (mouse anti-human DNA Top I; BD Pharmingen, San Jose, CA, USA) was added at 1:500 dilution. Secondary antibody (Cell Signaling, Danvers, MA, USA) was used at 1:1,000. ECL Lightning Plus (PerkinElmer, Waltham, MA, USA) was used for detection of the Top1CC.
TS catalytic activity assays
TS assays were conducted using procedures similar to those described previously [9]. AML cells were plated at a density of 1.5 × 106 cells in 100-mm2 plates. Cells were grown overnight in RPMI 1640 medium with 10% FBS. Either 5FU (Sigma) (1 × 10−7 mol/L) or FdUMP[10] (1 × 10−9 mol/L) was then added, and the cells were incubated for 8, 16, 24, or 48 hours, harvested, and lysed by freeze-fracturing. Following centrifugation of cell lysates, supernatants were assayed for protein content and TS catalytic activity as described previously [12].
Immunofluorescence
AML cells were washed and spun onto a 35-mm plate coated with poly-L-lysine in serum-free media. Cells were then fixed in 10% buffered formalin, permeabilized with 0.5% Triton-X 100, and consecutively incubated with primary and secondary antibodies and counterstained for DNA with Hoechst dye. Cells were fixed after staining with formalin and visualized using an Olympus-IX70 fluorescence microscope.
Flow cytometry
DNA histograms were obtained for AML cells that were stained with propidium iodide (Sigma) and analyzed using a CANTO flow cytometer. For studies evaluating cell division and cell size, AML cells were incubated with Cell-Trace (Invitrogen) for 20 min and then transferred to fresh media and treated with FdUMP[10] or vehicle only. AML cells were permeabilized with 70% ethanol before incubation with an antibody specific for cleaved caspase 3 (Cell Signaling) and secondary antibody for apoptosis detection.
Caspase activity and viability assays
Caspase 3/7, 8, and 9 activity and cell viability assays were performed using Promega Caspase-Glo Assay reagents for caspase activity and CellTiter-Glo Luminescent Cell Viability Assay reagents for cell viability. Experiments were performed per manufacturer’s instructions. Briefly, 2 × 105 cells were plated in 24-well plates in triplicate, and drug treatments were started 20–24 hours after plating. Cells were resuspended well before 50–100-μL aliquots were taken at the indicated times and mixed with an equal volume of assay kit reagent in a 96-well white plate. The plates were then incubated at room temperature for 30–60 min per instructions before being read using a Tecan Genios luminescence plate reader. Apoptosis data was normalized for cell number using viability data [13].
Comet assays
Comet assays were performed per protocol using Trevigen Comet Assay Reagent Kit (Trevigen, Gaithersburg, MD, USA). Cells were treated with the drug as specified and combined at a concentration of 1 × 105/mL with LMP agarose at a 1:10 ratio and applied to comet slides. Solidified slides underwent lysis overnight at 4°C, were soaked the following day in neutral electrophoresis buffer for 30 min, and were then electrophoresed for 1 hour at 4°C at 1 V/cm. Slides then underwent a DNA precipitation step followed by immersion in 70% ethyl alcohol and drying at 50°C. Slides were stained with SYBR Green Molecular Probes (Invitrogen) and imaged with data collection on a fluorescent microscope using Loats Associates Comet Assay Analysis System.
Statistical analysis
Assay values were expressed as mean ± SE, and comparisons between mean values were made using Student’s t test for paired samples, with p ≤ 0.05 (two-tailed) being considered significant.
Results
FdUMP[10] treatment results in prolonged TS inhibition and induction of apoptosis
Previous studies from our laboratories indicated that FdUMP[10] displayed strong efficacy toward AML cells, including those expressing molecular markers associated with chemoresistance and a poor prognosis such as p53 mutation or deletion and the Flt3-ITD [5]. In contrast to chemotherapeutics widely used for AML treatment, such as anthracycline and AraC [14], that display p53-dependent cytotoxic effects toward AML cells [5], FdUMP[10] displayed similar potency toward AML cells regardless of p53 status. Cell viability studies performed in our laboratory confirmed the potency of FdUMP[10] toward HL60 cells (Fig. 1A), and other AML cells (Supplemental Figure E1, online only, available at www.exphem.org), whereas 5FU at up to 10 μmol/L had a minimal effect on the viability of AML cells (data not shown).
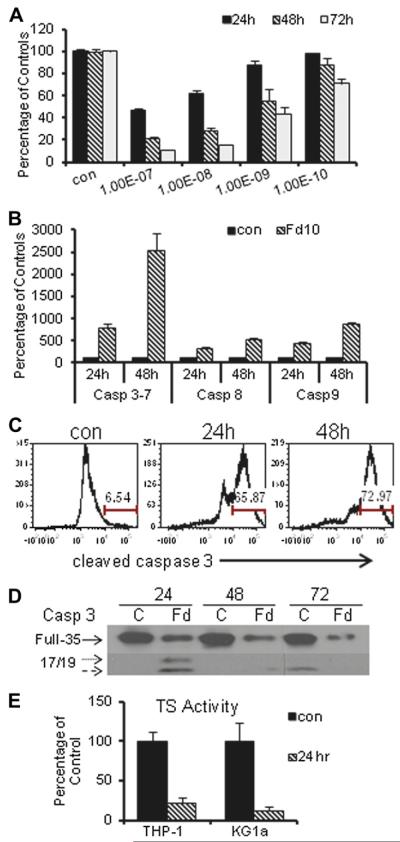
Figure 1.
FdUMP[10] is a potent inducer of thymineless death via induction of apoptosis in HL60 cells. (A) Time and concentration dependence of viability for HL60 cells following FdUMP[10] treatment for the indicated times. Experiments were based on six observations and FdUMP[10] cytotoxicity at 10−10 mol/L was significantly enhanced relative to control at 72 hours (p < 0.001) and at 10−9 mol/L at 24 hours (p < 0.05) and 48 hours (p < 0.009). (B) Luminescent readout of caspase activation indicates FdUMP[10] induces apoptosis. Data were based on at least 12 observations per condition with all pairwise comparisons versus control significant (all p < 0.0001). (C) Flow cytometry histogram for cleaved caspase-3 demonstrating mainly apoptotic cells 24 hours (center) and 48 hours (right) after FdUMP[10] (10−7 mol/L) treatment relative to control (left). (D) Western blot demonstrating cleavage of caspase-3 in HL60 cells treated with FdUMP[10] at 10−8 mol/L for the indicated times. (E) Thymidylate synthase (TS) inhibition in THP-1 (p < 0.008) and KG1a (p < 0.005) cells is significantly reduced relative to control (based on at least five observations) following FdUMP[10] (10−8 mol/L) treatment.
The mode of cell death in AML cells was investigated to determine to what extent these cells undergo apoptosis in response to FdUMP[10] treatment or whether another mode of cell death predominates in these cells. Luminescent assays demonstrated that caspase 3/7 activity was induced upon FdUMP[10] treatment in HL60 cells consistent with induction of p53-independent apoptosis (Fig. 1B). Caspase 3/7 activity was also induced in Molm13, KG1a, and THP-1 cells (Supplemental Figure E2, online only, available at www.exphem.org). Luminescent assays (Fig. 1B) and Western blot (Supplemental Figure E3, online only, available at www.exphem.org) also demonstrated activation of caspases 8 and 9, indicating that both the intrinsic and extrinsic apoptotic pathways [15] were activated. To determine whether apoptosis was the predominant mechanism of cell death, the percentage of cells displaying cleaved caspase 3 was evaluated by flow cytometry (Fig. 1C) and immunofluorescence (Supplemental Figure E4, online only, available at www.exphem.org). Nearly all cells treated with 100 nmol/L FdUMP[10] for 48 hours displayed cleaved caspase 3. Full-length caspase 3 was barely detectable by Western blot following treatment with 10 nmol/L FdUMP [10] (Fig. 1D), whereas cleaved fragments were readily detected, which is also consistent with a majority of FdUMP [10]-treated cells undergoing apoptotic cell death.
Thymidylate synthase is widely recognized as a principal target of FP chemotherapy and FP treatment results in thymineless death [10,16]. Previous studies from our laboratory demonstrated that FdUMP[10] treatment results in more complete TS inhibition in prostate cancer cells [9,17] and AML cells [5] than occurs following a 100-fold greater 5FU treatment, which is consistent with 5FU being mainly an RNA-directed drug [18]. To determine whether apoptotic cell death in AML cells is associated with FdUMP[10]-induced TS inhibition, we evaluated TS activity levels in KG1a, THP-1, and Molm13 cells. TS activity was measured using a 3H-release assay as previously described [9]. The results for KG1a and THP-1 cells are shown in Figure 1E, and results for Molm13 cells are shown in Supplemental Figure E5 (online only, available at www.exphem.org). FdUMP[10] treatment (10 nmol/L) resulted in nearly complete TS inhibition at 24 hours, which is consistent with Top1CC formation and apoptosis induction being associated with induction of a thymineless state in FdUMP[10]-treated AML cells. FdUMP[10] at 10 nmol/L was not effective at inhibiting TS in Molm-13 cells, although TS inhibition was observed at higher concentrations (Supplemental Figure E5, online only, available at www.exphem.org). Interestingly, FdUMP[10]-induced Top1CC and apoptosis induction were detected in Molm13 cells even at FdUMP[10] concentrations that did not cause TS inhibition indicating FdUMP[10] metabolites compete with endogenous Thy for incorporation into genomic DNA (Supplemental Figure E7, online only, available at www.exphem.org).
Thy-rescue of FdUMP[10]-induced apoptosis is ineffective after cells commit to mitosis
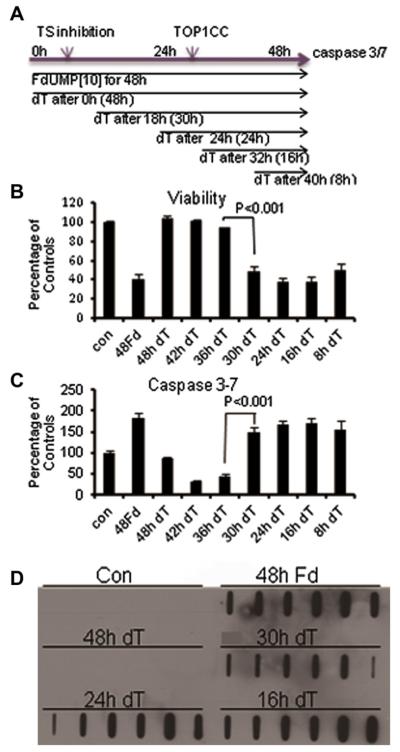
COMPARE analysis evaluating the mechanistic basis for FdUMP[10]-mediated cytotoxicity across the NCI 60 cell line panel indicated that FdUMP[10] mechanistically resembles Top1 poisons such as CPTs [7]. Previous studies in collaboration with the Pommier Laboratory demonstrated that FdUMP[10] treatment caused Top1CC formation and that FdU substitutions in DNA proximal to trapped Top1CC site-specifically inhibit the re-ligation step of Top1 catalysis [10]. To gain further insight into the relationship between TS inhibition and Top1CC formation in AML cells, HL60 cells were synchronized by serum starvation before initiating FdUMP[10] treatment, and the effects of Thy rescue [19,20] on cell viability, apoptosis induction, and Top1CC formation were evaluated (Fig. 2). The time required for a complete cell-cycle was calculated using Cell-Trace (Invitrogen) and found to be <20 h under these conditions (Supplemental Figure E6, online only, available at www.exphem.org). Thy treatment completely rescued FdUMP[10] cytotoxicity and induction of apoptosis when Thy was coadministered with FdUMP[10] during the entire 48-hour drug incubation period or was co-administered during the initial 12 hours of FdUMP[10] treatment (Fig. 2). When Thy was added as a potential rescue agent after cells were treated with FdUMP[10] for longer times (18–40 hours), rescue was not effective. For example, Thy administered for 30 hours starting 18 hours after the initial FdUMP[10] treatment was not effective at rescuing AML cells from the cytotoxic (Fig. 2B) and apoptotic (Fig. 2C) activities of FdUMP[10]. The time dependence of Thy rescue is consistent with exogenous Thy competing with FdU for incorporation into DNA throughout the S phase; however, once FdU is incorporated into DNA and cells have committed to or undergone mitosis, cells can no longer be rescued by exogenous Thy, which is consistent with FdUMP[10]-mediated cytotoxicity being induced at times removed from treatment and with postreplicative repair processes not reducing FdUMP[10]-mediated cytotoxicity.
Figure 2.
The consequences of thymineless death following FdUMP[10] treatment are effectively rescued with exogenous thymidine only when administered during the first replicative cycle (<18 hours). (A) Depiction of the experimental scheme used for Thy rescue experiments and the relative timing of induction of a thymineless state, Top1CC formation, and apoptotic cell death. (B) Cell viability assays showing the time dependence of Thy rescue following FdUMP[10] treatment (10−8 mol/L). (C) Induction of apoptotic cell death and time dependence of rescue by exogenous Thy (10−8 mol/L). Data are a summary of two to six experiments performed in triplicate with significant differences between adjacent time points indicated (time points being farther apart was also significant). (D) In vivo complex of enzyme (ICE) bioassays showing the induction of Top1CC formation following FdUMP[10] treatment (10−8 mol/L) and the time dependence of Thy rescue.
Thy rescue of Top1CC is effective during first replicative cycle
The stable misincorporation of FdU into genomic DNA can cause trapping of Top1CC [10]. ICE bioassays were performed to determine whether Top1CC formed in synchronized FdUMP[10]-treated AML cells; if so, Top1CC formation was rescued by Thy. The results for HL60 cells are shown in Figure 2D. Top1CC were readily detected in genomic DNA isolated from FdUMP[10]-treated cells while coadministration of Thy inhibited Top1CC formation. Thy was effective at rescuing Top1CC formation if present during the entire drug treatment period, but was not effective at preventing Top1CC in cells treated for significant times (e.g., >12 hours) with FdUMP[10]. HL60 cells treated with FdUMP[10] for 18 hours before Thy rescue displayed levels of Top1CC similar to those that were not rescued at all (Fig. 2D). The results were consistent with Top1CC formation occurring only in cells completing one round of replication in the presence of FdUMP[10] and undergoing mitosis after stably misincorporating FdU into genomic DNA. Top1CC were also observed in THP1 and Molm13 cells with similar Thyrescue kinetics (Supplemental Figure E7, online only, available at www.exphem.org). Interestingly, although FdUMP [10] was less efficient at inhibiting TS in Molm13 cells relative to other AML cells examined, it does induce Top1CC formation. Furthermore, Top1CC formation in Molm13 cells was rescued by exogenous Thy, which is consistent with FdU competing with Thy for incorporation into genomic DNA even under Thy-replete conditions.
FdUMP[10]-induced Top1CC results in DNA double-strand breaks and cell-cycle arrest
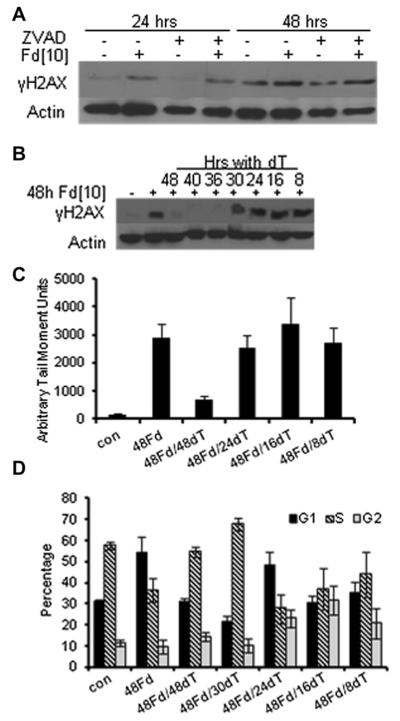
The formation of DNA double-strand breaks (DSBs) following Top1CC formation is the lethal event following treatment with traditional Top1 poisons such as the CPTs [21,22]. DNA DSBs occur upon collision of the advancing replication fork with trapped Top1CC [23]. Because FdUMP[10] induces Top1CC in AML cells (Fig. 2D) and mechanistically resembles CPTs based on COMPARE analysis [7], we examined the formation and timing of DNA DSBs in FdUMP[10]-treated cells and the role of Thy-rescue at preventing DNA damage. γH2AX was evident by Western blot at 24 hours and was enhanced at 48 hours following FdUMP[10] treatment (Fig. 3A). γH2AX was not decreased upon coincubation with Z-VAD at either time point, indicating that DNA DSBs were not a consequence of apoptosis (Fig. 3A). Thy rescue was effective at preventing γH2AX phosphorylation during the initial 12 hours of FdUMP[10] treatment, but Thy rescue was not effective at later time points (Fig. 3B). The formation of DNA DSBs was also evaluated using neutral Comet assays with similar Thy-rescue kinetics (Fig. 3C). Thy rescue affected cell-cycle distribution with the presence of Thy permitting cells to progress further into the S-phase of a second replicative cycle before initiating apoptosis, whereas in the absence of Thy rescue cells were apparently G1-arrested based on DNA histogram fitting (Fig. 3D). Although apparently G1-arrested, FdUMP[10]-treated cells incorporated 5-ethynyl-2′-deoxyuridine (EdU) as readily as untreated cells (Supplemental Figure E8, online only, available at www.exphem.org). In contrast, serum-starved cells were G0/G1-arrested but did not incorporate EdU (Supplemental Figure E8, online only, available at www.exphem.org). The timing and Thy-rescue effects of DNA DSB formation following FdUMP[10] treatment are consistent with DSBs occurring as a consequence of Top1CC formation in cells that have misincorporated FdU into genomic DNA during initial replication, undergone cell division, and subsequently re-entered a second S-phase, only to be arrested early in S-phase because of Top1CC formation. DNA repair processes activated in this second replicative cycle are ineffective regardless of the availability of exogenous Thy.
Figure 3.
FdUMP[10] treatment results in cell-cycle arrest and induces DNA double-strand breaks (DSBs). (A) FdUMP[10]-induced DNA DSBs are not a consequence of apoptotic cell death. γH2AX phosphorylation following FdUMP[10] treatment (10−7 mol/L) is not rescued by Z-VAD (20 μmol/L). (B) FdUMP[10]-induced DNA DSBs (evaluated with γH2AX) can be rescued with exogenous Thy only when administered during the first replicative cycle (<18 hours). (C) Summary of tail moments (arbitrary units) from neutral Comet assays for HL60 cells treated with FdUMP [10] with or without 2′-deoxythymidine (dT) rescue. Values were based on 20 captured images, and mean value differences for 24- and 48-hour dT rescue are significant (p < 0.0006). (D) Summary of DNA histograms from HL60 cells treated with FdUMP[10] with Thy rescue as indicated. FdUMP[10] treatment results in apparent G1-arrest although EdU-incorporation indicates cells have entered S-phase (Supplemental Figure E8, online only, available at www.exphem.org). Thy rescue permits cells to progress further into S-phase before apoptosis induction.
Checkpoint activation, DNA damage, and cell death
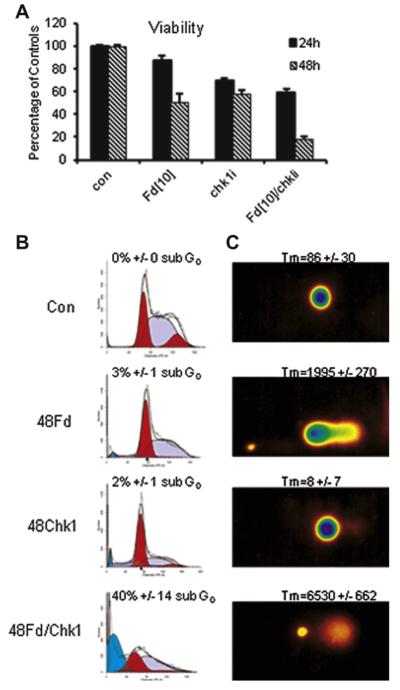
Activation of the checkpoint kinases Chk1 and Chk2 following DNA damage can result in cell-cycle arrest and induction of cell death pathways [24,25]. Top1 poisons, such as CPTs, activate Chk1, which is responsible for mediating cell-cycle arrest consequent to Top1CC formation [26–28]. Chk1 is activated in response to FdUMP[10] treatment (Supplemental Figure E9, online only, available at www.exphem.org). Chk1 is strongly activated at 24 hours, which is consistent with activation occurring consequent to Top1CC formation in FdUMP[10]-treated cells. Chk2 is not strongly activated in response to FdUMP[10] treatment (data not shown). We then evaluated to what extent Chk1 activation was required for FdUMP[10]-mediated cytotoxicity and induction of apoptosis. The Chk1 inhibitor SB218078 significantly enhanced FdUMP[10] cytotoxicity (Fig. 4A), which is consistent with Chk1 involvement in FdUMP[10]-induced cell-cycle arrest by increasing the time for repair of Top1-mediated DNA DSBs. Neither FdUMP[10] nor SB218078 treatment resulted in cells displaying substantial levels of sub-G0 DNA content (Fig. 4B), although FdUMP[10] treatment resulted in Comet tail moments significantly greater than control (Fig. 4C). The FdUMP[10]/SB218078 combination, however, resulted in high levels of sub-G0 DNA content and very high tail moments. These results are consistent with Chk1 activation being required for FdUMP[10]-mediated DNA repair.
Figure 4.
Chk1 inhibition enhances FdUMP[10]-induced DNA damage. DNA histograms and Comet assays for HL60 cells treated with FdUMP [10] (Fd) for the indicated times. (A) Chk1 inhibition (SB218078 5 γmol/L) reduces viability of FUMP[10]-treated cells. The combination (FdUMP[10]/Chk1i) was significantly more cytotoxic than either single agent at both 24 (p < 0.0004) and 48 hours (p < 0.0001) based on six experiments in triplicate. (B) Effects of Chk1 inhibition in combination with FdUMP[10] (10 nmol/L, 48 hours) on cell-cycle progression. FdUMP[10] treatment results in S-phase arrest after one replicative cycle (<18 hours). The combination of FdUMP[10] and the Chk1 inhibitor SB218078 for 48 hours results in fewer arrested cells and greater sub-G0 fraction. (C) Comet assays under neutral conditions indicated that FdUMP[10] induced DNA strand breaks and that Chk1 inhibition substantially enhances FdUMP[10]-induced DNA damage. Tail moments (TM = Tail length × % of DNA in the tail) were calculated from 20 individually imaged cells from parameters measured by computer software (Loats Associates). FdUMP[10] + Chk1i vs. FdUMP[10] p < 0.000004.
FdUMP[10]-induced apoptotic signaling is cell-type dependent
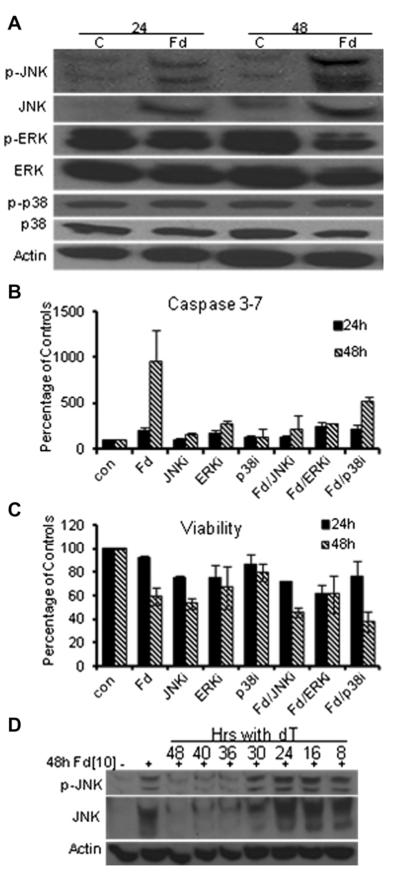
Dual targeting of TS and Top1 with generation of DNA DSBs and Chk1 activation are general features of FdUMP [10]-induced cytotoxicity toward AML cells; however, subsequent cell-death signaling is cell-type dependent. Mitogen-activated protein kinase signaling frequently modulates cell death signaling in response to drug treatment [29], and our studies showed that Erk and p38 are constitutively activated in HL60 cells (Fig. 5A). FdUMP [10] treatment slightly decreased Erk activation and had no effect on p38 activation (Fig. 5A), and inhibiting either decreased FdUMP[10]-induced apoptosis at 48 hours (Fig. 5B). In contrast, FdUMP[10] treatment selectively activated the Jnk pathway in HL60 cells, increasing Jnk protein levels and Jnk phosphorylation at 24 hours with more pronounced effects at 48 hours (Fig. 5A). The relevance of increased Jnk activation for FdUMP[10]-induced apoptosis in HL60 cells was demonstrated by Jnk inhibition (SP200126), reducing apoptosis to control levels at 24 and 48 hours (Fig. 5B). Jnk inhibition did not rescue FdUMP [10]-mediated cytotoxicity in HL60 cells; however, indicating Jnk is important for mediating apoptotic signaling but not overall viability in these cells, with cell death occurring via necrosis or a nonapoptotic process if the apoptotic machinery cannot be activated (Fig. 5C). To gain further insight into the relationship of Top1CC formation, Jnk activation, and apoptosis induction, we evaluated Jnk activation under Thy-rescue conditions. Thy rescue of Jnk activation in HL60 cells followed kinetics similar to those for Thy rescue of Top1CC formation, which is consistent with Jnk activation occurring downstream of Top1-mediated DNA DSBs during a second replicative cycle (Fig. 5D).
Figure 5.
Selective activation of the Jnk pathway is necessary for FdUMP [10]-induced apoptosis, but not cytotoxicity, in HL60 cells. (A) Western blots demonstratingthatFdUMP[10]selectively activatesJnk butnotother mitogen-activated protein kinase pathways such as Erk and p38. (B) Caspase-glo apoptosis assay results demonstrating that Jnk inhibition (SP600125, 50 μmol/L) abrogated FdUMP[10]-induced apoptosis. Mean apoptosis values for FdUMP[10] + Jnki were significantly different from FdUMP[10] at both 24 and 48 hours (p < 0.03). Data were based on six experiments in triplicate. (C) Cell titer-glo assays demonstrating that Jnk inhibition does not significantly rescue FdUMP[10] cytotoxicity. (D) Western blots demonstrating that activation of Jnk following FdUMP[10] treatment may be prevented by exogenous Thy only if administered during the first replicative cycle.
The Jnk pathway significantly modulated cell death and survival following FdUMP[10]-treatment for all AML cell types investigated; however, these effects were cell-type dependent (Supplemental Figure E2, online only, available at www.exphem.org). Jnk inhibition had similar effects on FdUMP[10]-induced apoptosis in THP-1 cells as in HL60 cells; however, Jnk inhibition also reduced FdUMP[10]-mediated cytotoxicity in THP-1 cells. Jnk inhibition was strongly cytotoxic to Molm13 cells and induced apoptosis. Furthermore, Jnk inhibition enhanced FdUMP[10]-mediated cytotoxicity and apoptosis in Molm13 cells. Jnk inhibition had similar cytotoxic and apoptosis-inducing properties toward KG1a cells as for Molm13; however, the effects of combined FdUMP[10] treatment and Jnk inhibition in KG1a were not greater than with either agent singly (Supplemental Figure E2, online only, available at www.exphem.org). The differential effects in cell death signaling observed in these AML cells indicates that Jnk inhibition might be an effective strategy for AML treatment in some instances and could enhance FdUMP[10] therapy in certain cell types.
Discussion
The present studies have established that FdUMP[10]-induced Top1CC and cell death can only be rescued by exogenous Thy administered in the first replicative cycle and before cells have committed to mitosis (Fig. 2). These results are consistent with Top1CC formation in FdUMP [10]-treated cells [30], requiring the stable misincorporation of FdU into genomic DNA with Top1CC generated upon reentry of cells into S-phase. These results indicate that postreplicative repair is not effective at preventing FdUMP[10]-induced cytotoxicity regardless of exogenous Thy levels and that FdUMP[10]-induced cytotoxicity can occur considerably later than drug treatment, providing a sustained therapeutic benefit.
The present studies demonstrated that DNA DSBs generated in FdUMP[10]-treated AML cells are temporally associated with Top1CC formation and are the lethal lesions, as is the case with other Top1 poisons. Our results indicate that the DNA damage response following FdUMP [10] treatment is mediated primarily via Chk1. These results are consistent with previous studies indicating that CPT-induced Top1CC formation resulted in Chk1 activation and that the ATR/Chk1 pathway and not ATM/Chk2 is important for mediating the DNA damage response following CPT treatment [26–28]. These results are consistent with the DNA damage response downstream of Top1CC formation proceeding through similar pathways regardless of whether Top1CC are generated proximal to FdU-substituted DNA following FdUMP[10] treatment or result from ternary complex formation, as occurs with CPTs.
Although Top1 is a common target of CPTs and FdUMP [10], the pharmacologic and biochemical properties of FdUMP[10] are distinct from conventional Top1 poisons and may permit this target to be accessed when systemic toxicities or the development of resistance limit conventional Top1 poisons. Our prior studies demonstrated FdUMP[10] treatment resulted in no bone marrow toxicities [5], whereas treatment with CPTs can cause neutropenia and other systemic toxicities [31–33]. Our laboratory has also shown that FdUMP[10] retains cytotoxicity toward CPT-resistant cells (Gmeiner and Pardee, unpublished data). Thus, FdUMP[10] may be particularly effective for treating malignancies, such as AML, that are not adequately treated with current Top1 poisons.
The present studies have demonstrated that apoptosis is the predominant mode of FdUMP[10]-induced death in human AML cells. Although the general features of induction of a thymineless state and formation of Top1CC in a second replicative cycle appear common to all AML cell lines investigated, the signaling pathways mediating apoptosis appear to be cell-type specific. Jnk-signaling is induced in HL60 cells, and Jnk inhibition blocks FdUMP [10]-induced apoptosis in HL60 and THP1 cells, while in Molm13 and KG1a cells, Jnk inhibition amplified the pro-apoptotic effects of FUMP[10]. Future studies will identify specific Jnk targets that modulate cytotoxicity and apoptosis in AML cells and develop strategies to use this information to maximize the antileukemic activity of FdUMP[10].
Supplementary Material
Supplementary Figure E1. FdUMP[10] (10nM) decreases viability and induces apoptosis in Molm13 (A,B), THP1 (C,D), and KG1a (E,F) AML cells. FdUMP[10] displays strong potency to a variety of AML cells. Assayed at 48hrs with or without addition of thymidine (dT) for the times indicated. Thy rescue for 48 h was significant (p < 1.5 e-7 for viability; p < 3 e-5 for apoptosis).
Supplementary Figure E2. FdUMP[10] (10 nM) induced apoptosis in Molm13 (A,B), THP1 (C,D), and KG1a (E,F) AML cells. Apoptosis is blocked by Jnk inhibition (SP600125 10 μM) in THP-1 cells (significant at 24 (p < 0.0003) and 48 h (p < 0.004) – based on eight observations) as in HL60 cells but is not significantly changed in wt p53 Molm13 cells.
Supplementary Figure E3. WB showing FdUMP[10] treatment (10 nM) for times 24 h or longer results in cleavage of caspase 8 and 9 as well as PARP cleavage, consistent with activation of both the intrinsic and extrinsic apoptotic pathways in HL60 cells.
Supplementary Figure E4. Immunofluorescence images demonstrating that cleaved caspase3 is present in the majority of HL60 cells following treatment with 10 nM FdUMP[10] for 24 h (lower middle) and 48 h (lower right). Few control (vehicle-only) HL60 cells display cleaved caspase 3 (lower left). Bright field images of the same fields across the top.
Supplementary Figure E5. Graph of concentration dependence of TS activity in Molm13 treated with vehicle-only (con) or FdUMP[10] at 1-10 μM for 24 h. FdUMP[10] strongly inhibited TS in THP-1 and KG1a cells at 0.1 μM, but required 1 μM for effective significant inhibition vs control in Molm13 cells (p < 0.005 based on at least five observations).
Supplementary Figure E6. Time-dependence of fluorescence intensity for HL60 cells incubated with Cell-trace (Invitrogen) determined by flow cytometry. The mean fluorescence intensity after 24 h (red) and 48 h (blue) is less than 0.5 and 0.25 times the initial value (black) consistent with HL60 cells replicating and dividing <20 h.
Supplementary Figure E7. ICE bioassays detecting Top1CC formed in THP1 and Molm13 cells following treatment with FdUMP[10] for 48 h or with 32 h thymidine rescue (16dT).
Supplementary Figure E8. Flow cytometry analysis of EdU incorporation into DNA for HL60 cells (A) no EdU in control cell; (B) Control cells with EdU treatment for 3 h - the right-shifted peak is due to newly synthesized DNA in which EdU has been incorporated; (C) Cells treated with FdUMP[10] for 48 h which DNA histogram analysis indicate are predominantly in G1- a similar amount of EdU is incorporated as for the control cells; (D) Cells synchronized in G0/G1 by serum starvation- these cells display very little EdU incorporation.
Supplementary Figure E9. Western blot showing activation of Chk1 (pS345) 24 h following FdUMP[10] treatment.
Acknowledgments
We thank Dr. Mark Willingham for help with immunofluorescence studies and Dr. S. Ghosh for help with cell culture experiments. Funding was provided by National Institutes of Health–National Cancer Institute (NIH-NCI) grant U01CA102532 (to W.G.) and NIH-NCI grant P30CA012197 (to W.G. and T.S.P.), the Doug Coley Leukemia Foundation (to T.S.P.), the Francis P. Tutwiler Fund (to T.S.P.), the McKay Foundation for Cancer Research (to T.S.P.), Wake Forest School of Medicine Translational Sciences Institute (to T.S.P. and W.G.), and Golfers Against Cancer (to T.S.P. and W.G.).
Footnotes
Conflict of interest disclosure W.G. is President and Chief Scientific Officer and T.S.P. is Chief Medical Officer of Salzburg Therapeutics, which holds licenses for FdUMP[10].
Supplementary data related to this article can be found online at http://dx.doi.org/10.1016/j.exphem.2012.10.007.
References
- 1.Roboz GJ. Novel approaches to the treatment of acute myeloid leukemia. Hematology Am Soc Hematol Educ Program. 2011;2011:43–50. doi: 10.1182/asheducation-2011.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Kumar CC. Genetic abnormalities and challenges in the treatment of acute myeloid leukemia. Genes Cancer. 2011;2:95–107. doi: 10.1177/1947601911408076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchner T, Berdel WE, Wormann B, et al. Treatment of older patients with AML. Crit Rev Oncol Hematol. 2005;56:247–259. doi: 10.1016/j.critrevonc.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Robak T, Wierzbowska A. Current and emerging therapies for acute myeloid leukemia. Clin Ther. 2009;31:2349–2370. doi: 10.1016/j.clinthera.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 5.Pardee TS, Gomes E, Jennings-Gee J, et al. Unique dual targeting of thymidylate synthase and topoisomerase 1 by FdUMP[10] results in high efficacy against AML and low toxicity. Blood. 2012;119:3561–3570. doi: 10.1182/blood-2011-06-362442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saif MW, Choma A, Salamone SJ, Chu E. Pharmacokinetically guided dose adjustment of 5-fluorouracil: a rational approach to improving therapeutic outcomes. J Natl Cancer Inst. 2009;101:1543–1552. doi: 10.1093/jnci/djp328. [DOI] [PubMed] [Google Scholar]
- 7.Gmeiner WH, Reinhold WC, Pommier Y. Genome-wide mRNA and microRNA profiling of the NCI 60 cell-line screen and comparison of FdUMP[10] with fluorouracil, floxuridine, and topoisomerase 1 poisons. Mol Cancer Ther. 2010;9:3105–3114. doi: 10.1158/1535-7163.MCT-10-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rich IN. The effect of 5-fluorouracil on erythropoiesis. Blood. 1991;77:1164–1170. [PubMed] [Google Scholar]
- 9.Gmeiner WH, Trump E, Wei C. Enhanced DNA-directed effects of FdUMP[10] compared to 5FU. Nucleosides Nucleotides Nucleic Acids. 2004;23:401–410. doi: 10.1081/ncn-120028336. [DOI] [PubMed] [Google Scholar]
- 10.Liao ZY, Sordet O, Zhang HL, Kohlhagen G, et al. A novel polypyrimidine antitumor agent FdUMP[10] induces thymineless death with topoisomerase I-DNA complexes. Cancer Res. 2005;65:4844–4851. doi: 10.1158/0008-5472.CAN-04-1302. [DOI] [PubMed] [Google Scholar]
- 11.Daniel RA, Rozanska AL, Thomas HD, et al. Inhibition of poly(ADP-ribose) polymerase-1 enhances temozolomide and topotecan activity against childhood neuroblastoma. Clin Cancer Res. 2009;15:1241–1249. doi: 10.1158/1078-0432.CCR-08-1095. [DOI] [PubMed] [Google Scholar]
- 12.Keyomarsi K, Moran RG. Quinazoline folate analogs inhibit the catalytic activity of thymidylate synthase but allow the binding of 5-fluorodeoxyuridylate. J Biol Chem. 1990;265:19163–19169. [PubMed] [Google Scholar]
- 13.Sharpe R, Pearson A, Herrera-Abreu MT, et al. FGFR signaling promotes the growth of triple-negative and basal-like breast cancer cell lines both invitro and invivo. Clin Cancer Res. 2011;17:5275–5286. doi: 10.1158/1078-0432.CCR-10-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feldman EJ. Too much ara-C? Not enough daunorubicin? Blood. 2011;117:2299–2300. doi: 10.1182/blood-2011-01-328633. [DOI] [PubMed] [Google Scholar]
- 15.Kuribayashi K, Finnberg N, Jeffers JR, Zambetti GP, El-Deiry WS. The relative contribution of pro-apoptotic p53-target genes in the triggering of apoptosis following DNA damage in vitro and in vivo. Cell Cycle. 2011;10:2380–2389. doi: 10.4161/cc.10.14.16588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barbour KW, Berger FG. Cell death in response to antimetabolites directed at thymidylate synthase. Cancer Chemother Pharmacol. 2008;61:189–201. doi: 10.1007/s00280-007-0461-4. [DOI] [PubMed] [Google Scholar]
- 17.Gmeiner WH. Novel chemical strategies for thymidylate synthase inhibition. Curr Med Chem. 2005;12:191–202. doi: 10.2174/0929867053363432. [DOI] [PubMed] [Google Scholar]
- 18.Brody JR, Hucl T, Costantino CL, et al. Limits to thymidylate synthase and TP53 genes as predictive determinants for fluoropyrimidine sensitivity and further evidence for RNA-based toxicity as a major influence. Cancer Res. 2009;69:984–991. doi: 10.1158/0008-5472.CAN-08-3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S, Yang SC, Kao CY, Pierce RH, Murthy N. Solid polymeric microparticles enhance the delivery of siRNA to macrophages in vivo. Nucleic Acids Res. 2009;37:e145. doi: 10.1093/nar/gkp758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang W, McLeod HL, Cassidy J, Collie-Duguid ES. Mechanisms of acquired chemoresistance to 5-fluorouracil and tomudex: thymidylate synthase dependent and independent networks. Cancer Chemother Pharmacol. 2007;59:839–845. doi: 10.1007/s00280-006-0384-5. [DOI] [PubMed] [Google Scholar]
- 21.Redon CE, Nakamura AJ, Zhang YW, et al. Histone gammaH2AX and poly(ADP-ribose) as clinical pharmacodynamic biomarkers. Clin Cancer Res. 2010;16:4532–4542. doi: 10.1158/1078-0432.CCR-10-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pommier Y. Topoisomerase I inhibitors: camptothecins and beyond. Nat Rev Cancer. 2006;6:789–802. doi: 10.1038/nrc1977. [DOI] [PubMed] [Google Scholar]
- 23.Lin CP, Ban Y, Lyu YL, Liu LF. Proteasome-dependent processing of topoisomerase I-DNA adducts into DNA double strand breaks at arrested replication forks. J Biol Chem. 2009;284:28084–28092. doi: 10.1074/jbc.M109.030601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dent P, Tang Y, Yacoub A, Dai Y, Fisher PB, Grant S. CHK1 inhibitors in combination chemotherapy: thinking beyond the cell cycle. Mol Interv. 2011;11:133–140. doi: 10.1124/mi.11.2.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garrett MD, Collins I. Anticancer therapy with checkpoint inhibitors: what, where and when? Trends Pharmacol Sci. 2011;32:308–316. doi: 10.1016/j.tips.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 26.Xiao Z, Chen Z, Gunasekera AH, et al. Chk1 mediates S and G2 arrests through Cdc25A degradation in response to DNA-damaging agents. J Biol Chem. 2003;278:21767–21773. doi: 10.1074/jbc.M300229200. [DOI] [PubMed] [Google Scholar]
- 27.Flatten K, Dai NT, Vroman BT, et al. The role of checkpoint kinase 1 in sensitivity to topoisomerase I poisons. J Biol Chem. 2005;280:14349–14355. doi: 10.1074/jbc.M411890200. [DOI] [PubMed] [Google Scholar]
- 28.Cliby WA, Lewis KA, Lilly KK, Kaufmann SH. S phase and G2 arrests induced by topoisomerase I poisons are dependent on ATR kinase function. J Biol Chem. 2002;277:1599–1606. doi: 10.1074/jbc.M106287200. [DOI] [PubMed] [Google Scholar]
- 29.Junttila MR, Li SP, Westermarck J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. Faseb J. 2008;22:954–965. doi: 10.1096/fj.06-7859rev. [DOI] [PubMed] [Google Scholar]
- 30.Gmeiner WH, Salsbury F, Jr, Olsen CM, Marky LA. The stability of a model substrate for topoisomerase 1-mediated DNA religation depends on the presence of mismatched base pairs. J Nucleic Acids. 2011:631372. doi: 10.4061/2011/631372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris EJ, Geller HM. Induction of neuronal apoptosis by camptothecin, an inhibitor of DNA topoisomerase-I: evidence for cell cycle-independent toxicity. J Cell Biol. 1996;134:757–770. doi: 10.1083/jcb.134.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin CP, Ban Y, Lyu YL, Desai SD, Liu LF. A ubiquitin-proteasome pathway for the repair of topoisomerase I-DNA covalent complexes. J Biol Chem. 2008;283:21074–21083. doi: 10.1074/jbc.M803493200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang YW, Regairaz M, Seiler JA, Agama KK, Doroshow JH, Pommier Y. Poly(ADP-ribose) polymerase and XPF-ERCC1 participate in distinct pathways for the repair of topoisomerase I-induced DNA damage in mammalian cells. Nucleic Acids Res. 2011;39:3607–3620. doi: 10.1093/nar/gkq1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pardee TS, Zuber J, Lowe SW. Flt3-ITD alters chemotherapy response in vitro and in vivo in a p53-dependent manner. Exp Hematol. 2011;39:473–485. doi: 10.1016/j.exphem.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hrkach J, Von Hoff D, Ali MM, et al. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci Transl Med. 2012;4:128–139. doi: 10.1126/scitranslmed.3003651. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure E1. FdUMP[10] (10nM) decreases viability and induces apoptosis in Molm13 (A,B), THP1 (C,D), and KG1a (E,F) AML cells. FdUMP[10] displays strong potency to a variety of AML cells. Assayed at 48hrs with or without addition of thymidine (dT) for the times indicated. Thy rescue for 48 h was significant (p < 1.5 e-7 for viability; p < 3 e-5 for apoptosis).
Supplementary Figure E2. FdUMP[10] (10 nM) induced apoptosis in Molm13 (A,B), THP1 (C,D), and KG1a (E,F) AML cells. Apoptosis is blocked by Jnk inhibition (SP600125 10 μM) in THP-1 cells (significant at 24 (p < 0.0003) and 48 h (p < 0.004) – based on eight observations) as in HL60 cells but is not significantly changed in wt p53 Molm13 cells.
Supplementary Figure E3. WB showing FdUMP[10] treatment (10 nM) for times 24 h or longer results in cleavage of caspase 8 and 9 as well as PARP cleavage, consistent with activation of both the intrinsic and extrinsic apoptotic pathways in HL60 cells.
Supplementary Figure E4. Immunofluorescence images demonstrating that cleaved caspase3 is present in the majority of HL60 cells following treatment with 10 nM FdUMP[10] for 24 h (lower middle) and 48 h (lower right). Few control (vehicle-only) HL60 cells display cleaved caspase 3 (lower left). Bright field images of the same fields across the top.
Supplementary Figure E5. Graph of concentration dependence of TS activity in Molm13 treated with vehicle-only (con) or FdUMP[10] at 1-10 μM for 24 h. FdUMP[10] strongly inhibited TS in THP-1 and KG1a cells at 0.1 μM, but required 1 μM for effective significant inhibition vs control in Molm13 cells (p < 0.005 based on at least five observations).
Supplementary Figure E6. Time-dependence of fluorescence intensity for HL60 cells incubated with Cell-trace (Invitrogen) determined by flow cytometry. The mean fluorescence intensity after 24 h (red) and 48 h (blue) is less than 0.5 and 0.25 times the initial value (black) consistent with HL60 cells replicating and dividing <20 h.
Supplementary Figure E7. ICE bioassays detecting Top1CC formed in THP1 and Molm13 cells following treatment with FdUMP[10] for 48 h or with 32 h thymidine rescue (16dT).
Supplementary Figure E8. Flow cytometry analysis of EdU incorporation into DNA for HL60 cells (A) no EdU in control cell; (B) Control cells with EdU treatment for 3 h - the right-shifted peak is due to newly synthesized DNA in which EdU has been incorporated; (C) Cells treated with FdUMP[10] for 48 h which DNA histogram analysis indicate are predominantly in G1- a similar amount of EdU is incorporated as for the control cells; (D) Cells synchronized in G0/G1 by serum starvation- these cells display very little EdU incorporation.
Supplementary Figure E9. Western blot showing activation of Chk1 (pS345) 24 h following FdUMP[10] treatment.