Abstract
The extent to which changes in ultradian and circadian rhythms (URs and CRs) reflect seasonal variations in pineal melatonin secretion was assessed in male Siberian hamsters transferred from long to short day lengths. The period of the locomotor activity UR increased from 2.5 h in long days to 4.5 h in short day lengths, but this and most other features of the short-day ultradian phenotype were unaffected by pinealectomy; only the short-day increase in UR amplitude was counteracted by pineal extirpation. Virtually all UR components were unaffected by gonadectomy or replacement testosterone or estradiol treatment; changes in testicular hormone secretion appear insufficient to account for seasonal fluctuation in URs. Pinealectomy did not affect activity onsets and offsets or phase angles of CR entrainment in short and long day lengths; the duration of nocturnal activity was equivalently longer in short than long days in both pinealectomized and pineal-intact hamsters. CR robustness of pinealectomized hamsters in short days was intermediate between values of long-day and short-day sham-pinealectomized males. Hourly nocturnal locomotor activity was markedly reduced in SD, and this effect was completely reversed by PINx. We conclude that seasonal transitions in UR and CR waveforms controlled by day length are mediated primarily by melatonin-independent mechanisms, with lesser contributions from melatonin-dependent processes. Most seasonal changes in ultradian and circadian rhythms in males of this species are not influenced by gonadal hormones. URs may allow animals to respond appropriately to changing environmental contingencies. In winter reduced activity combined with temporal restructuring of activity to include longer intervals of rest may be adaptive in maintaining body temperature at lower values and down-regulating energy expenditure when above ground temperatures are extremely low.
Keywords: Pinealectomy, Melatonin, Castration, Testosterone, Estradiol, Circadian rhythms
Introduction
Ultradian rhythms (URs) impose temporal order at multiple levels of biological organization (Veldhuis, 2008; Yates and Yates, 2008), yet little is known about the neuroendocrine substrates that generate and entrain URs in behavior (reviewed in Prendergast and Zucker, 2012; Prendergast et al., 2012a,b).
The influence of pinealectomy and pineal hormones on mammalian circadian locomotor activity is well documented (reviewed in Armstrong, 1989; Cassone, 1998). Pinealectomized (PINx) female Syrian hamsters and rats manifest more rapid re-entrainment of circadian wheel-running activity after a phase shift of the light–dark cycle (Finkelstein et al., 1978; Quay, 1970). In male Syrian hamsters, however, the circadian period of wheel-running is not influenced by pinealectomy during testing in bright or dim light or in constant darkness (Aschoff et al., 1982; Morin, 1993; Morin and Cummings, 1981); nor does pinealectomy affect phase angles of circadian entrainment to light (Morin, 1993) or circadian phase response curves to light in this species (Aschoff et al., 1982). The effects of pineal extirpation on free-running or entrained circadian rhythms of Siberian hamsters have not been reported previously.
Striking seasonal differences in UR components of locomotor activity are under proximate control of day length in male Siberian hamsters; e.g., the period (τ′), amplitude, and complexity of the UR waveform increases in day lengths shorter than 13 h light/day (Prendergast and Zucker, 2012). Because virtually all effects of short photoperiods on neuroendocrine traits are mediated by the pineal gland (Bartness and Wade, 1985; Prendergast et al., 2009), we anticipated that modulation of hamster URs by day length might be eliminated by pinealectomy. Alternatively, the seasonal change in URs could be transduced by pineal-independent effects of light on ultradian pacemakers. The first experiment evaluated these hypotheses.
The testes of Siberian hamsters undergo profound regression in short day lengths, but this response is eliminated by pinealectomy (Hoffmann, 1979). Consequently, pinealectomy may influence photoperiodic control of URs by eliminating melatonin-dependent decreases in gonadal hormone secretion in short day lengths. Gonadal steroids have long been implicated in the control of circadian rhythms (CRs) of locomotor activity of male (Daan et al., 1975; Eskes and Zucker, 1978) and female (Morin et al., 1977) rodents. Castration lengthens the period of the wheel-running CR of male mice housed in constant dim light, but not in darkness; increasing intensities of constant light potentiate the effect of gonadectomy on the period of the CR (Butler et al., 2012). Castration and hormone replacement do not, however, significantly affect the period of circadian wheel-running behavior of male Syrian hamsters (Morin and Cummings, 1981), but some sex differences in circadian function are organized early in development by gonadal hormones (Albers, 1981; Zucker et al., 1980).
Gonadal influences on rodent behavioral URs are presently unspecified, except for a single study that monitored URs in locomotor activity of 7 male LEW rats, 5 of them orchidectomized after baseline testing. URs with periods of 4–4.8 h persisted in all 5 males with 3 of the castrates generating more complex UR waveforms, with additional periods of 3 h (Wollnik and Döhler, 1986).
The second experiment assessed the impact of gonadectomy on ultradian and circadian rhythms of Siberian hamsters. It is the first study to address gonadal modulation of URs in a seasonal photoperiodic rodent and tests the hypothesis that gonadal steroids contribute to seasonal differences in ultradian behavior.
Methods
Animals
Siberian hamsters were from a colony maintained at the University of Chicago. Pairs were housed in polypropylene cages in a room illuminated 15 h/day (long day; LD) with incandescent light (400–700 lx at cage level, light onset at 02:00 h, light offset 17:00 h). Food (Teklad, Harlan, Indianapolis, IN), filtered tap water and cotton nesting material were available ad libitum. Ambient temperature and relative humidity were held constant at 19±2 °C and 53±10%, respectively. Pups were weaned at 18–20 days of age and housed in same-sex groups of siblings until experimental manipulations began (described below), after which they were housed singly for the duration of the experiment. All experimental procedures were approved by the institutional animal care and use committee at the University of Chicago (Protocol # 71443).
Experiment 1. Does the pineal gland mediate effects of day length on URs?
Animals and surgical procedures
Adult male hamsters (4–6 months of age, n=63) from the LD colony were pinealectomized (PINx; n=27) or subjected to a sham PINx (n=36) under anesthesia (pentobarbital, 50 mg/kg, ip) according to procedures described by Carter and Goldman (1983). Briefly, anesthetized hamsters were immobilized in a stereotaxic apparatus and a midline skin incision exposed the skull from bregma to approximately 3 mm caudal to lambda. A trephine bit centered on lambda was used to remove a portion of the skull, and the pineal gland removed with microdissecting forceps. Pi-nealectomy was verified via histological examination of the excised tissue under a dissecting microscope. Gelfoam was inserted into the wound site, the skin closed with wound clips, and topical antibiotic ointment applied externally. Hamsters received buprenorphine analgesic (0.01 mg/kg, sc) 2×/day for 2 days after surgery. The sham-PINx and PINx procedures were similar, except that the pineal gland was not removed; in addition, in PINx hamsters the absence of the pineal gland was confirmed at autopsy.
Photoperiod treatments
One week after surgery (week 0), hamsters were either transferred to a short-day photoperiod (SD; 9 h light/day; n=31) or remained in the LD photoperiod (n=32). Light offset (17:00 h) remained the same for both photoperiods. Final sample sizes are indicated in Fig. 1.
Fig. 1.

Mean±SEM changes in (A) body mass and (B) estimated testis volume of hamsters maintained to adulthood in a long day (LD; 15L:9D) photoperiod, surgically pinealectomized (PINx) or sham-PINx (Sham) on week 0, then transferred to short days (SD; 9L:15D) or retained in LD. Changes in body and testis mass obtained on week 12 are expressed as a percentage of week 0 values. ***P≤0.001 vs. PINx, within photoperiod; ###P≤0.001 vs. LD value, within surgical condition.
Somatic and reproductive measurements
On week 8, hamsters were weighed (±0.1 g) during the light phase and the length and width of the left testis measured (±0.1 mm), under methoxyflurane anesthesia. The product of (testis width)2×(testis length) provides a measure of estimated testis volume (ETV) that is positively correlated (R>0.9) with testis weight (Gorman and Zucker, 1995). Stage in the pelage color cycle was assessed using an integer scale of 1–4 (1 = dark ‘summer’ fur, 4 = white ‘winter’ fur, Duncan and Goldman, 1984) without knowledge of the hamster’s treatment condition.
Data from sham-PINx hamsters that failed to exhibit gonadal regression (ETV>400) in SD (photoperiod nonresponders, n=3) were omitted from all subsequent analyses.
Locomotor activity
Activity was recorded in the home cage with passive infrared motion detectors on week 12 (see “Activity measurements”).
Experiment 2. Do gonadal hormones affect ultradian rhythms?
Animals and surgical procedures
Adult male Siberian hamsters (5–8 month of age, n=57) from the LD breeding colony (15L:9D) were bilaterally gonadectomized (GonadX; n=44) or sham gonadectomized (sham-GonadX; n=13) under pentobarbital anesthesia (50 mg/kg) via a single midline incision. Testicular blood vessels were ligated and cauterized, and the testes removed. The incision was closed with non-resorbable vinyl sutures and stainless steel wound clips. Topical antibiotic ointment was applied externally to the wound site, and hamsters treated with buprenorphine 2×/day for 2 days after surgery. The Sham-GonadX procedure replicated the GonadX procedure, except that the blood vessels were not ligated and the testes remained in situ.
Hormone implants were inserted sc at the time of GonadX/sham surgery. Implants were constructed of Silastic tubing (Dow Corning, Midland, MI; ID: 1.47 mm; OD: 1.96 mm) closed at both ends with silicone sealant. Testosterone-filled (TP) implants 2 mm in length were packed with crystalline testosterone propionate (T1875; Sigma, St. Louis, MO, USA). 2 mm long estradiol (E2) implants were filled with β-estradiol (E8875; Sigma). Cholesterol-filled implants (C) were 2 mm in length (C8667; Sigma). Prior to implantation, implants were soaked in 37 °C sterile saline in separate containers for 24–36 h and rinsed prior to implantation. Silastic implants were placed sc in the dorsal interscapular region, and the skin closed with stainless steel wound clips. GonadX hamsters received TP (n=15), E2 (n=14) or C (n=15) implants; Sham-GonadX hamsters were treated with C (n=13).
Locomotor activity
Activity was recorded in the home cage during a 10 day interval immediately prior to surgical treatments (baseline), and on weeks 2, 6 and 10 after surgery/implant (see “Activity measurements”).
Activity measurements
URs and CRs of spontaneous general locomotor activity—a behavior that correlates highly with daily rhythms of sleep-wakefulness, body temperature, and drinking behavior (Rusak and Zucker, 1979) was recorded as previously described (Prendergast et al., 2012a,b) to assess properties of the underlying circadian and ultradian timing systems. In the present context, “URs” and “CRs” specifically reference home-cage locomotor behavior rhythms.
In experiment 1, activity data were collected continuously in the home cage between weeks 10–13 (designated week 12). In experiment 2, locomotor data were collected prior to surgical procedures (baseline), between weeks 1–3 (week 2), weeks 5–7 (week 6), and weeks 9–11 (week 10). In both experiments, data were collected for 10 consecutive days with passive infrared motion detectors (Coral Plus, Visonic, Bloomfield, CT) positioned above the wire cage top, 22 cm above the cage floor. Motion detectors registered activity when 3 of 27 zones were crossed. Activity triggered closure of an electronic relay, recorded by a computer running ClockLab software (Actimetrics, Evanston, IL). Cumulative activity counts were binned at 6 min intervals.
Activity analyses
Ultradian rhythms (URs)
Activity data collected at 6 min intervals were parsed into light-phase only activity (150 or 90 data points/24 h, depending on photoperiod) and dark-phase only activity (90 or 150 data points/ 24 h) files. The number of photophases and scotophases sampled was adjusted to equalize the number of data points to 900 for each hamster, thereby ensuring equivalent statistical power in periodogram analyses from different photoperiods. For LD hamsters (experiments 1 and 2), 10 consecutive nights and 6 consecutive days generated dark-phase and light-phase activity files, each with 900 data points. For SD hamsters (experiment 2), 6 nights and 10 days were used. Successive days of scotophase- and photophase-specific activity data concatenated into separate files were subjected to Lomb–Scargle periodogram (LSP) and cosinor periodogram analyses, as described below.
Circadian rhythms
Unparsed files (240 data points/24 h) 10 days in length were subjected to LSP and Cosinor periodogram analyses to extract quantitative CR parameters.
Total and hourly activity calculations
Total dark phase and light phase activity was calculated as the mean number of activity counts in each photophase, without correction for photophase duration. Mean hourly activity counts were calculated as the total activity counts per photophase, divided by the number of hours in the photophase.
Statistical analyses
Lomb–Scargle periodogram analyses (Lomb, 1976) identified the presence/absence of URs and CRs, and the number of significant peaks in the UR spectrum (complexity; range: 0.1–7.9 h). The level of statistical significance (α) was set to 0.01. Cosinor analyses determined several quantitative measures of URs (range: 0.1–7.9 h) and CRs (range: 22–26 h): robustness (or ‘prominence’, the percent of variance accounted for by the best-fit cosine model, which corresponds to the coefficient of determination R2 in regression analyses; Refinetti et al., 2007); mesor (rhythm-adjusted mean value around which the waveform oscillates); amplitude (the difference between the peak or trough value and the mesor), expressed as absolute values (activity counts) and relative values referenced to the photophase-specific mesor value; the latter measure incorporates baseline activity levels during each photophase in determining rhythm amplitude. Acrophase was computed as the mean time (relative to onset or offset of light) at which the waveform peaks. The level of statistical significance was set to 0.05.
The LSP detects ultradian periodicities from incomplete evenly-sampled time series and optimizes detection of URs by not displaying peaks at multiples of all rhythms detected (Ruf, 1999; van Dongen, et al., 1999). Supplemental analyses after completion of LSP analysis (van Dongen et al., 2001) were adopted as recommended by Refinetti et al. (2007). The cosinor periodogram (Bingham et al., 1982) is a reliable, preferred curve-fitting tool to quantify rhythm parameters (Refinetti et al., 2007).
LSP and cosinor analyses were performed with software written by R. Refinetti (available at http://www.circadian.org/softwar.html; Refinetti et al., 2007). Analyses of variance (ANOVAs) and post-hoc pairwise comparisons were performed with Statview 5.0 (SAS Institute, Cary, NC, USA). Effects of day length on reproductive and somatic measures were assessed by ANOVA and effects of day length on fur color with Kruskal–Wallis tests (H scores), followed by Mann–Whitney U tests. The proportion of hamsters displaying URs and CRs was evaluated with chi-square tests. Quantitative aspects of URs and CRs were examined with ANOVA. Pairwise comparisons were performed with t tests. Differences were considered significant only if P≤0.05.
Results
Experiment 1. Does the pineal gland mediate effects of day length on URs?
Somatic and gonadal responses
Photoperiod and surgical manipulations interacted to affect body mass (F1,59=13.02, P<0.001) and reproductive condition (F1,59= 84.49, P<0.001; Fig. 1). Sham-operated hamsters lost body mass and exhibited gonadal regression in SD (P<0.001 vs. LD-sham, both comparisons). Pinealectomy counteracted the short-day induced body mass loss and decreases in testis size; values for SD-PINx hamsters did not differ from those of LD-sham or LD-PINx hamsters (P>0.20, all comparisons), but were significantly attenuated relative to values for SD-sham-PINx hamsters (P<0.001, both comparisons, Fig. 1).
Ultradian rhythms
UR Prevalence
Photoperiod and PINx treatments did not affect UR prevalence. Dark-phase URs were evident in 63% of LD-sham and 70% of SD-sham hamsters (χ2=0.23, P>0.60), and 50% of LD-PINx and 82% of SD-PINx hamsters (χ2=2.83, P=0.09; Fig. 2A). Light-phase URs were detected in 19% of LD-sham and 45% of SD-sham hamsters (χ2=2.86, P=0.09) and in 25% of LD-PINx and 18% of SD-PINx hamsters (χ2=0.18, P>0.60; Fig. 2A).
Fig. 2.
(A) Percent hamsters exhibiting significant URs during the dark phase and light phase and mean±SEM (B) period, (C) robustness, (D) mesor, and (E) amplitude of ultradian waveforms of males pinealectomized (PINx) or sham-PINx (Sham) and either maintained in long (LD) or transferred to short days (SD) for 12 weeks. *P≤0.05 vs. Sham, within photoperiod; ##P≤0.01, ###P≤0.001 vs. LD value, within surgical condition.
UR complexity
Neither photoperiod (F1,59=0.17, P>0.60) nor surgical treatments (F1,59=0.47, P>0.40) affected dark-phase UR complexity. Among LD and SD groups, the median number of significant UR peaks was 1 (range: 0–3 and 0–2, respectively). Effects of photoperiod (F1,59=0.09, P>0.70) and surgical treatments (F1,59=1.03, P>0.30) on light-phase UR complexity were similarly absent. In LD and SD, the median number of peaks was 0 (range: 0–6 and 0–7, respectively).
UR τ′
Dark-phase τ′ was significantly affected by photoperiod (F1,57=18.5, P<0.001) but not by PINx (F1,57=0.20, P>0.60; Fig. 2B) and was significantly longer in SD-sham than LD-sham hamsters (P=0.01); the τ′-lengthening effect of SD was also significant among PINx hamsters (P<0.005, SD PINx>LD PINx and LD sham). Light-phase τ′ was not affected by photoperiod (F1,59=0.85, P>0.30) or surgical treatments (F1,59=0.41, P>0.50; Fig. 2B).
UR robustness
Dark-phase and light-phase robustness were not affected by photoperiod (F1,59=0.57, P>0.40) or surgical treatment (F1,59=1.28, P>0.20 all comparisons; Fig. 2C). There was a trend effect of surgical treatment (F1,59=3.55, P=0.06; Fig. 2C).
UR mesor
Photoperiod (F1,59=8.80, P<0.005), but not surgical treatment (F1,59<0.01, P>0.90), significantly affected UR dark-phase mesor values (Fig. 2D) which were greater in LD-sham than SD-sham hamsters (P<0.01). In contrast, UR mesor values did not differ between LD PINx and SD PINx hamsters (P>0.10).
UR amplitude
In the dark phase amplitude was significantly enhanced by SD (F1,59=21.47, P<0.001), but surgical treatment (F1,59=3.00, P=0.08) and the interaction of photoperiod with surgery (F1,59= 3.56, P=0.06) fell short of statistical significance (Fig. 2E). UR amplitude was significantly greater in SD sham-PINx than LD sham-PINx hamsters, (P<0.001). The effect of photoperiod was not statistically significant in PINx hamsters (P=0.08). Pinealectomy did not affect UR amplitude in LD hamsters (P>0.80), but reduced amplitude in SD hamsters (P<0.05).
Neither photoperiod nor surgical treatment affected light-phase UR mesor (Fig. 2D), or amplitude (Fig. 2E) values.
UR acrophase
Dark-phase acrophases occurred significantly earlier in LD than SD groups (F1,59=8.71, P<0.005), but PINx was without effect on this measure (F=0.01, P>0.90). In LD, acrophases occurred 1.2±0.3 h and 1.1±0.3 h after light offset in sham-PINx and PINx hamsters, respectively (P>0.80); in SD, acrophases occurred 2.4± 0.5 h and 2.4±0.6 h after light offset in sham-PINx and PINx hamsters, respectively (P>0.80). In contrast, light-phase acrophases were not affected by photoperiod (F1,59=1.71, P>0.10) or surgical treatment (F1,59=2.92, P>0.05).
Circadian rhythms
CR prevalence
CRs were present in 100% of LD hamsters, 100% of SD-PINx hamsters and 85% of SD-sham hamsters on week 12, with no significant differences between the groups.
CR robustness
Photoperiod (F1,59=18.9, P<0.001) significantly affected robustness, but no main effect of surgery was evident (F1,59=1.86, P>0.10; Fig. 3A). Among sham-PINx hamsters, CRs were more robust in LD than SD (P<0.001); this effect of photoperiod persisted in PINx hamsters (P<0.05), although reduced in magnitude. Pinealectomy did not affect CR robustness in LD hamsters (P>0.70), but did increase robustness in SD hamsters (P<0.05).
Fig. 3.
Mean±SEM (A) robustness and (B) amplitude of circadian waveforms of hamsters pinealectomized (PINx) or sham-PINx (Sham) and either maintained in to long (LD) or transferred to short days (SD) for 12 weeks. *P≤0.05 vs. Sham, within photoperiod; #P≤0.05, ##P≤0.01, ###P≤0.001 vs. LD value, within surgical condition.
CR mesor
Neither photoperiod (F1,58=0.88, P>0.30) nor surgical treatment (F<0.01, P>0.90) significantly altered CR mesor values.
CR amplitude
Amplitude was diminished in SD (F1.58=36.4, P<0.001), but the effect of surgical treatments was not significant (F1,58=3.29, P=0.07; Fig. 3B). In SD amplitude was lower in both sham-PINx (P<0.001) and PINx (p<0.005) groups than in corresponding LD groups.
CR acrophase
Timing of the CR acrophase was significantly altered by photoperiod (F1,58=79.3, P<0.001), but not by surgical treatments (F1,58=0.03, P>0.80). In LD, acrophases occurred 4.0±0.2 h and 4.3± 0.3 h after light offset in sham-PINx and PINx hamsters, respectively (P>0.40); in SD acrophases occurred 8.2±0.4 h and 8.1±0.9 h after light offset in sham-PINx and PINx hamsters, respectively (P>0.80).
CR entrainment
Day length affected the timing of circadian locomotor activity onset (F1,59=22.1, P<0.001) and activity offset (F1,59= 20.59, P<0.001). Activity onset occurred later in SD than LD hamsters (Fig. 4, P<0.001, all comparisons). Pinealectomy did not affect activity onset (F1,59=1.20, P>0.20) or offset (F1,59=0.29, P>0.50) in either photoperiod. Activity onsets were more variable in PINx than Sham-PINx hamsters in SD, but onset times did not differ between these groups (P>0.30). The duration of nocturnal locomotor activity (α) was significantly longer in SD (12.2±5.6 h) than LD (8.3±0.6 h) hamsters (F1,59=71.6, P<0.001), but PINx was without effect on α (F1,59=1.11, P>0.40, Fig. 4).
Fig. 4.
Mean±SEM onset and offset of circadian locomotor activity rhythms of hamsters pinealectomized (PINx) or sham-PINx (Sham) and kept in long (LD) or short days (SD) for 12 weeks. Background shading depicts the duration of the photoperiod dark phase. ###P≤0.001 vs. LD, within surgical condition.
Total locomotor activity
Phase-specific total locomotor activity
Across all treatment conditions total locomotor activity was greater in the dark than the light phase (F1,118=52.8, P<0.001; Fig. 5A). Among pineal-intact hamsters, total activity was greater in LD than SD in both the dark and light phases (P<0.05 in each case; Fig. 5A). Pinealectomy counteracted the short-day decreases in activity in both the dark phase and light phase (SD-PINx versus LD sham-PINx: dark phase: P>0.80; light phase: P>0.30).
Fig. 5.
Mean±SEM (A) total locomotor activity and (B) hourly locomotor activity during the dark (left) and light (right) phases of hamsters pinealectomized (PINx) or sham-PINx (Sham) and kept in long (LD) or short days (SD) for 12 weeks. (C, D) Mean±SEM hourly locomotor activity of sham-PINx (panel C) and PINx (panel D) hamsters across the 24 h cycle. The duration of the light and dark phases of the LD and SD photoperiods is depicted at the top of panels C and D with white and black bars, respectively. In panels A and B, †P<0.05, ††P<0.01 vs. LD value within photophase and surgical condition. In panels C and D, *P<0.05 vs. SD value within surgical condition; #P<0.05 vs. SD-sham PINx value at corresponding time point.
Phase-specific activity per hour
Locomotor activity was assessed on a per hour basis to account for different light and dark phase durations in the two photoperiods. Counts/hour were higher in LD than SD (F1,118=47.0, P<0.001) and in the dark than the light phase (F1,118=7.09, P<0.01; Fig. 5B). There was no main effect of pinealectomy (F1,118=0.03, P>0.80), but photoperiod (LD/SD) and photophase (light/dark phase) interacted to affect hourly activity counts (F1,118=9.39, P<0.005), which were greater in LD than SD in pineal-intact hamsters in the dark phase (P<0.01) but not among pinealectomized hamsters (P>0.10; Fig. 5B).
In pineal-intact hamsters, hourly activity was lower in SD than LD for the first 5 (17:00–22:00) and the last 3 h (23:00–02:00) of the dark phase (P<0.05, all comparisons; Fig. 5C). Between 02:00 h and 08:00 h, when illumination was present in LD but not in SD, hourly activity was higher in SD between 03:00–04:00 and 05:00–8:00 (P<0.05, all comparisons).
In SD, pinealectomy altered the waveform of hourly activity. During 5 consecutive one-hour sampling epochs, from 02:00 through 07:00 h, activity was greater in SD-PINx than SD sham-PINx hamsters (P<0.05, all comparisons; Figs. 5C, D); hourly activity was comparable in these groups at all other times (P>0.05, all comparisons).
Experiment 2. Gonadal hormone effects on ultradian rhythms
Body mass and accessory sex tissue
During the 10 weeks after surgery, the pattern of body mass change differed across treatment groups (F30, 530=6.61, P<0.001, Fig. 6). In the first two weeks after surgery body mass decreased in all groups. Sham-GonadX hamsters treated with cholesterol (C) steadily gained body mass during the ensuing 9 weeks, whereas GonadX+C hamsters did not (P<0.001). GonadX+TP and GonadX+E2 groups both regained body mass postoperatively with recovery more rapid in TP-treated than E2-treated hamsters (P<0.05). The pattern of body mass accretion in GonadX+TP hamsters did not differ from that of Sham+C hamsters (P>0.10), whereas mass gain was reduced in GonadX+E2 compared to Sham+C hamsters (P<0.001; Fig. 6). On week 10, body mass values were higher in Sham+C and GonadX+TP hamsters than in all other groups (P<0.05, all comparisons). Body mass values of GonadX+E2 hamsters were significantly greater than those of GonadX+C hamsters (P<0.05; Fig. 6).
Fig. 6.
Mean±SEM change in body mass relative to baseline values. Males housed in LD were gonadectomized (GonadX) or sham-GonadX (Sham) on week 0 (baseline, BL) and treated with sc implants of cholesterol (C), testosterone propionate (TP), or β-estradiol (E2) that remained in situ throughout postoperative testing. **P≤0.01, ***P≤0.001 vs. Sham+C. +P≤0.01 vs. GonadX+C.
Surgical/implant treatments affected vas deferens mass (F3,56= 26.9, P<0.001), which was comparable in Sham+C (mean+SD: 100.2+23 mg) and GonadX+TP (96.8+25 mg) hamsters (P>0.60), both values exceeding those of GonadX+E2 (59.1+23) and GonadX+C (38.7+16.4 mg) groups (P<0.01, all comparisons).
Ultradian rhythms
Dark-phase URs
URs were detected in >60% of all groups during post-surgical monitoring; surgery and hormone treatments did not affect UR incidence (χ2<1.52, P>0.20, all comparisons; Fig. 7A). Surgical/hormone treatments did not alter UR τ′, UR robustness, UR mesor, or UR amplitude during any of the three post-surgical sampling intervals (F3,53<1.77, P>0.10, all comparisons; Figs. 7A–E).
Fig. 7.
(A) Percent hamsters exhibiting significant URs during the dark and light phases and mean±SEM (B) period, (C) robustness, (D) mesor, and (E) amplitude of ultradian waveforms of hamsters housed in LD that were gonadectomized (GonadX) or sham-GonadX (Sham) on week 0 (baseline, BL) and received sc implants of cholesterol (C), testosterone propionate (TP), or β-estradiol (E2). *P≤0.05 vs. GonadX+TP value and GonadX+E2 value.
Light-phase URs
Expression of URs was more variable in the light than the dark-phase (Fig. 7A). On week 2, URs were more prevalent in GonadX+C than GonadX+TP hamsters (χ2=5.07, P<0.05), but not relative to other groups (χ2<1.68, P>0.10). Surgical and hormone treatments did not affect the incidence of URs at any other time point (χ2<2.39, P>0.10, all comparisons).
With one exception (see below), surgical and hormone treatments did not affect τ′, robustness, mesor, or amplitude through week 10, (F<2.50, P>0.05, all comparisons; Fig. 7). On week 6, there was a significant main effect of surgical and hormone treatment on robustness (F3,53=3.52, P<0.05; Fig. 7C) with significantly more robust light-phase URs in GonadX+C than GonadX+TP (P<0.005) and GonadX+E2 (P<0.05) hamsters.
Circadian rhythms
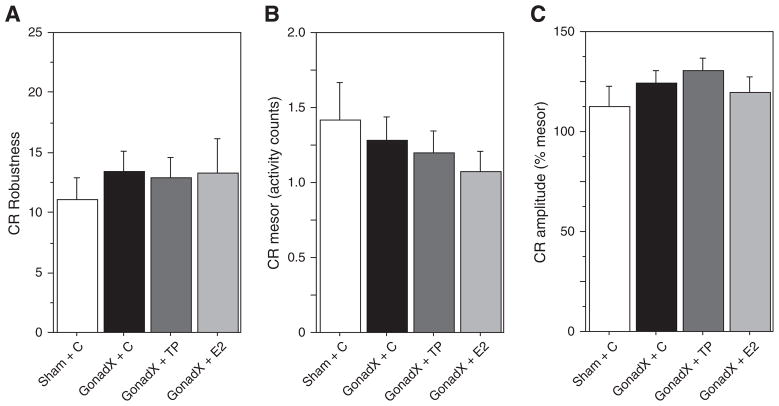
Surgical/hormone treatments did not affect CR robustness, mesor, or amplitude at any of the post-surgical sampling intervals (F3,53<0.89, P>0.40, all comparisons; Fig. 8).
Fig. 8.
Mean±SEM (A) robustness, (B) mesor and (C) amplitude of week 10 circadian waveforms of LD hamsters that were gonadectomized (GonadX) or sham-GonadX (Sham) and treated with sc implants of cholesterol (C), testosterone propionate (TP), or β-estradiol (E2).
There also was no effect of surgical/hormone treatment on the acrophase of the CR (F3,53=0.53, P>0.60). Timing of activity onset (F3,53=3.31, P<0.05) and activity offset (F3,53=5.16, P<0.005; Fig. 9) was affected by the treatments. Locomotor activity of Sham+C hamsters began shortly before the onset of darkness. Castration with cholesterol replacement caused a significant delay in the onset of activity (P=0.01 GonadX+C vs. Sham+C)—an effect that was mitigated, in part, by TP and E2 treatments, which resulted in activity onsets that did not differ from those of GonadX+C hamsters (P>0.10, both comparisons) or Sham+C hamsters (P>0.05, both comparisons). Activity offset occurred significantly later in GonadX+C than Sham+C hamsters (P<0.05). The castration-induced delay in activity offset was counteracted by TP (P<0.05) and E2 (P<0.005) treatment (Fig. 9).
Fig. 9.

Mean±SEM onset and offset of week 10 circadian locomotor activity rhythms (horizontal bars) of hamsters housed in LD, gonadectomized (GonadX) or sham-gonadectomized (Sham-GonadX) and treated sc with implants of cholesterol (C), testosterone propionate (TP), or β-estradiol (E2). Vertical dashed lines indicate the onset (17:00 h) and offset (02:00 h) of the dark phase. #P≤0.05, ##P≤0.05 vs. GonadX+C; *P≤0.05, **P≤0.01 vs. Sham+C.
Discussion
Pineal influences on ultradian rhythms
The period of the locomotor activity UR was ~60% longer, rhythm amplitude was substantially greater, and mesor values much lower in SD than LD (cf. Prendergast and Zucker, 2012). The increase of the dark phase UR period from 2.5 to 4.5 h and changes in rhythm robustness and complexity were indistinguishable in PINx and sham-PINx hamsters transferred from long to short day lengths. Pineal melatonin secretion, which normally transduces effects of day length on the neuroendocrine axis (Bartness et al., 1993), evidently is not an essential mediator of several short photoperiod effects on behavioral URs. This contrasts with the complete elimination or marked reduction in PINx Siberian hamsters of short-day seasonal differences of body and testicular mass (Fig. 1; Bartness and Wade, 1985), prolactin and LH secretion (Imundo et al., 2001), pelage color (Badura and Goldman, 1992), daily torpor (Vitale et al., 1985) and estrous cycles (Schlatt et al., 1993), but is similar to the failure of pinealectomy to prevent short-day expansion of the duration of circadian locomotor activity in Siberian hamsters (Prendergast and Freeman, 1999). Many seasonal changes in this species are mediated by the pineal gland, but others appear controlled by day length, perhaps via circadian mechanisms, without requiring pineal participation.
In a preliminary study, the ultradian body temperature rhythm of a SD-PINx hamster was comparable to that of a LD-intact male and dissimilar to that of a SD-intact male (Steinlechner et al., 1986). Pending replication in a full scale study, this raises the possibility that the pattern of short-day melatonin secretion may influence some but not other URs. Several exceptions to the general pattern in the present study are the counteraction by PINx of short-day increases in locomotor UR amplitude and short-day decreases in total locomotor activity. This suggests that in intact males the expansion of nocturnal melatonin secretion in short days (reviewed in Bartness et al., 1993) enhances these rhythm components. The hypothesis that elevated gonadal hormone secretion in pinealectomized short-day hamsters diminishes UR amplitude tentatively can be discounted based on results of the second experiment. We conclude that seasonal variation in locomotor UR characteristics other than rhythm amplitude is controlled by melatonin-independent mechanisms, either via direct effects of light on ultradian oscillators, or lighting information transmitted from circadian clockworks to UR substrates (cf. Gerkema et al., 1990, 1993; Prendergast et al., 2012b).
As anticipated, removal of the pineal gland was without effect on URs of long-day hamsters, congruent with numerous observations that pineal hormones do not affect the seasonal phenotype in long summer day lengths (e.g., Hoffmann, 1979).
Gonadal influences on ultradian rhythms
Because elimination of gonadal hormone secretion was not sufficient to induce the short-day UR phenotype, and replacement hormone treatments were completely without impact on URs in long day lengths, we tentatively conclude that the effect of day length on male URs is mediated via gonadal steroid-independent mechanisms.
Pineal influences on circadian rhythms
The duration of nocturnal pineal melatonin secretion has little or no impact on the circadian system of male Siberian (this study) or male Syrian hamsters (Aschoff et al., 1982; Morin, 1993). Although supraphysiological melatonin treatments exert modest effects on CRs (Armstrong, 1989; Cassone, 1998; Prendergast, 2010), there is no evidence in any rodent model that endogenous fluctuations in melatonin secretion influence circadian rhythms (reviewed in Armstrong, 1989; Butler et al., 2008).
Gonadal influences on circadian rhythms
Both activity onset and offset occurred significantly later in castrated males treated with cholesterol implants than similarly-treated intact males, suggesting that androgens affect some aspects of the circadian waveform, as previously documented for male mice (Butler et al., 2012; Daan et al., 1975) and Syrian hamsters (Eskes and Zucker, 1978; Morin and Cummings, 1981). These effects of castration were mitigated in part (activity onset) or completely (activity offset) by chronic treatment with TP or E2. The present experiment does not permit conclusions about sexual differentiation of the Siberian hamster circadian system, but it is noteworthy that, in contrast to the circadian system of unmanipulated male Syrian hamsters, which is largely unresponsive to estradiol treatment (Morin et al., 1977; Zucker et al., 1980), male Siberian hamsters in the present study exhibited comparable responses to E2 and TP treatments.
Adaptive significance of URs
URs may allow animals to respond appropriately to changing environmental contingencies (Yates and Yates, 2008), particularly in short day lengths when robustness and the presence of nocturnal circadian rhythms are greatly diminished (Warner et al., 2010). URs generally are more prominent when circadian rhythms are partly disintegrated or entirely absent (reviewed in Prendergast et al., 2012b).
URs of body temperature generally coincide with URs of locomotor activity, with lower values during rest periods (Heldmaier et al., 1989). Mean body temperature of Siberian hamsters is 0.7 °C lower in short than long days; “… the lower level of mean body temperature and the extended periods with minimum body temperature will reduce energy requirements for maintenance” (Heldmaier et al., 1989), a view supported by prominent changes in the duration and number of vigilance state episodes in SD, a lowering of cortical temperature by 0.7 °C, and a 50% reduction in EEG power density, all of which may contribute to energy conservation in SD (Deboer and Tobler, 1996). In the present study, the lengthening of UR period in SD was accompanied by a decrease in overall locomotor activity, in both the light and the dark phases, corroborating a recent report of a reduction in general activity measured 2–4 months after transfer from LD to SD (Warner et al., 2010, Table 1). The combination of reduced overall activity and temporal restructuring of that activity (i.e., imposition of longer rest periods) may maintain body temperature at lower values and down-regulate energy expenditure during foraging bouts in winter, when above ground temperatures are extremely low (Ross, 1998; Weiner, 1987). Aschoff and Gerkema (1985, p.330) noted that it is an “… economic principle not to spend energy continuously at a relatively high level (as demanded at times) but to alternate between expenditure and restoration of energy.”
Lastly, the ultradian pattern of thermogenesis that accompanies URs in locomotor activity in Siberian hamsters may reflect UR rhythms of neural activity that maintain neuronal integrity and alertness and support cognitive function (Meyer et al., 2012). Many authors (e.g., Meyer et al., 2012) have speculated that the ultradian basic rest activity cycle (BRAC) positions animals to respond to both salient environmental events and internal physiological challenges.
General discussion
Short day lengths induced widespread changes to the ultradian timing system of male Siberian hamsters. The present work establishes that the robust seasonal changes in pineal and gonadal hormone secretion likely are not causally related to seasonal modulation of the locomotor UR period. UR amplitude is similarly unaffected by gonadal hormones, but pineal modulation cannot be discounted in the control of several aspects of the UR waveform. Short days also were associated with marked reductions in the robustness and amplitude of circadian rhythms—responses evidently not contingent on photoperiodic changes in melatonin secretion or feedback effects of gonadal hormones. Similar loss of circadian coherence of locomotor activity in short-day Siberian hamsters was documented recently by Warner et al. (2010). Together the data point to the pre-eminence of pineal- and gonadal hormone-independent control of seasonal changes in ultradian and circadian timing.
Acknowledgments
The authors are grateful to Ken Onishi, Ela Sehic and Dr. Betty Theriault for expert assistance and to anonymous reviewers for their helpful criticism. This work was supported by Grant AI-67406 from the National Institute of Allergy and Infectious Diseases.
References
- Albers HE. Gonadal hormones organize and modulate the circadian system of the rat. Am J Physiol. 1981;241:R62–R66. doi: 10.1152/ajpregu.1981.241.1.R62. [DOI] [PubMed] [Google Scholar]
- Armstrong SM. Melatonin and circadian control in mammals. Experientia. 1989;45:932–938. doi: 10.1007/BF01953050. [DOI] [PubMed] [Google Scholar]
- Aschoff J, Gerkema M. On diversity and uniformity of ultradian rhythms. Exp Brain Res. 1985;(Suppl 12):321–334. [Google Scholar]
- Aschoff J, Gerecke U, von Goetz C, Groos GA. Phase responses and characteristics of free-running activity rhythms in golden hamsters: independence of the pineal gland. In: Aschoff J, Daan S, Groos G, editors. Vertebrate Circadian Systems. Springer Verlag; Berlin: 1982. pp. 129–140. [Google Scholar]
- Badura LL, Goldman BD. Prolactin-dependent seasonal changes in pelage: role of the pineal gland and dopamine. J Exp Zool. 1992;261:27–33. doi: 10.1002/jez.1402610105. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Wade GN. Photoperiodic control of seasonal body weight cycles in hamsters. Neurosci Biobehav Rev. 1985;9:599–612. doi: 10.1016/0149-7634(85)90006-5. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Powers JB, Hastings MH, Bittman EL, Goldman BD. The timed infusion paradigm for melatonin delivery: what has it taught us about the melatonin signal, its reception, and the photoperiodic control of seasonal responses? J Pineal Res. 1993;15:161–190. doi: 10.1111/j.1600-079x.1993.tb00903.x. [DOI] [PubMed] [Google Scholar]
- Bingham C, Arbogast B, Guillaume GC, Lee JK, Halberg F. Inferential statistical methods for estimating and comparing cosinor parameters. Chronobiologia. 1982;9:397–439. [PubMed] [Google Scholar]
- Butler MP, Paul MJ, Turner KW, Park JH, Driscoll JR, Kriegsfeld LJ, Zucker I. Circadian rhythms of photorefractory Siberian hamsters remain responsive to melatonin. J Biol Rhythms. 2008;23:160–169. doi: 10.1177/0748730407312949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MP, Karatsoreos IN, LeSauter J, Silver R. Dose-dependent effects of androgens on the circadian timing system and its response to light. Endocrinology. 2012;153:2344–2352. doi: 10.1210/en.2011-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter DS, Goldman BD. Antigonadal effects of timed melatonin infusion in pinealectomized male Djungarian hamsters (Phodopus sungorus sungorus): duration is the critical parameter. Endocrinology. 1983;113:1261–1267. doi: 10.1210/endo-113-4-1261. [DOI] [PubMed] [Google Scholar]
- Cassone VM. Melatonin’s role in vertebrate circadian rhythms. Chronobiol Int. 1998;15:457–473. doi: 10.3109/07420529808998702. [DOI] [PubMed] [Google Scholar]
- Daan S, Damassa D, Pittendrigh CS, Smith ER. An effect of castration and testosterone replacement on a circadian pacemaker in mice (Mus musculus) Proc Natl Acad Sci U S A. 1975;72:3744–3747. doi: 10.1073/pnas.72.9.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deboer T, Tobler I. Shortening of the photoperiod affects sleep distribution, EEG and cortical temperature in the Djungarian hamster. J Comp Physiol A. 1996;179:483–492. doi: 10.1007/BF00192315. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Goldman BD. Hormonal regulation of the annual pelage color cycle in the Djungarian hamster, Phodopus sungorus I Role of the gonads and pituitary. J Exp Zool. 1984;230:89–95. doi: 10.1002/jez.1402300112. [DOI] [PubMed] [Google Scholar]
- Eskes GA, Zucker I. Photoperiodic regulation of the hamster testis: dependence on circadian rhythms. Proc Natl Acad Sci U S A. 1978;75:1034–1038. doi: 10.1073/pnas.75.2.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein JS, Baum FR, Campbell CS. Entrainment of the female hamster to reversed photoperiod: role of the pineal. Physiol Behav. 1978;21:105–111. doi: 10.1016/0031-9384(78)90283-4. [DOI] [PubMed] [Google Scholar]
- Gerkema MP, Groos GA, Daan S. Differential elimination of circadian and ultradian rhythmicity by hypothalamic lesions in the common vole, Microtus arvalis. J Biol Rhythms. 1990;5:81–95. doi: 10.1177/074873049000500201. [DOI] [PubMed] [Google Scholar]
- Gerkema MP, Daan S, Wilbrink M, Hop MW, van der Leest F. Phase control of ultradian feeding rhythms in the common vole (Microtus arvalis): the roles of light and the circadian system. J Biol Rhythms. 1993;8:151–171. doi: 10.1177/074873049300800205. [DOI] [PubMed] [Google Scholar]
- Gorman MR, Zucker I. Seasonal adaptations of Siberian hamsters. II Pattern of change in day length controls annual testicular and body weight rhythms. Biol Reprod. 1995;53:116–125. doi: 10.1095/biolreprod53.1.116. [DOI] [PubMed] [Google Scholar]
- Heldmaier G, Steinlechner S, Ruf T, Wiesenger H, Klingenspor M. Photoperiod and thermoregulation in vertebrates: body temperature rhythms and thermogenic acclimation. J Biol Rhythms. 1989;4:251–265. [PubMed] [Google Scholar]
- Hoffmann K. Photoperiod, pineal, melatonin and reproduction in hamsters. Prog Brain Res. 1979;52:397–415. doi: 10.1016/S0079-6123(08)62946-5. [DOI] [PubMed] [Google Scholar]
- Imundo J, Bielefeld E, Dodge J, Badura LL. Relationship between norepinephrine release in the hypothalamic paraventricular nucleus and circulating prolactin levels in the Siberian hamster: role of photoperiod and the pineal gland. J Biol Rhythms. 2001;16:173–182. doi: 10.1177/074873001129001755. [DOI] [PubMed] [Google Scholar]
- Lomb N. Least-squares frequency analysis of unequally spaced data. Astrophys Space Sci. 1976;39:447–462. [Google Scholar]
- Meyer CW, Blessing W, Heldmaier G. Ultradian episodes of thermogenesis in mammals: implications for the timing of torpor entry and arousal. In: Ruf T, Bieber C, Arnold W, Millesi E, editors. Living in a Seasonal World. Springer Verlag; Berlin: 2012. pp. 219–229. [Google Scholar]
- Morin LP. Age, but not pineal status, modulates circadian periodicity of golden hamsters. J Biol Rhythms. 1993;8:189–197. doi: 10.1177/074873049300800302. [DOI] [PubMed] [Google Scholar]
- Morin LP, Cummings LA. Effect of surgical or photoperiodic castration, testosterone replacement or pinealectomy on male hamster running rhythmicity. Physiol Behav. 1981;26:825–838. doi: 10.1016/0031-9384(81)90106-2. [DOI] [PubMed] [Google Scholar]
- Morin LP, Fitzgerald KM, Zucker I. Estradiol shortens the period of hamster circadian rhythms. Science. 1977;196:305–307. doi: 10.1126/science.557840. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ. MT1 melatonin receptors mediate somatic, behavioral, and reproductive neuroendocrine responses to photoperiod and melatonin in Siberian hamsters (Phodopus sungorus) Endocrinology. 2010;151:714–721. doi: 10.1210/en.2009-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast BJ, Freeman DA. Pineal-independent regulation of photo-nonresponsiveness in the Siberian hamster (Phodopus sungorus) J Biol Rhythms. 1999;14:62–71. doi: 10.1177/074873099129000452. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Zucker I. Photoperiodic influences on ultradian rhythms of male Siberian hamsters. PLoS One. 2012;7:e41723. doi: 10.1371/journal.pone.0041723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast BJ, Nelson RJ, Zucker I. Seasonal rhythms of mammalian behavioral neuroendocrinology. In: Pfaff D, Arnold A, Etgen A, Fahrbach S, Moss R, Rubin R, editors. Hormones, Brain, and Behavior. 2. Vol. 1. Academic Press; San Diego: 2009. pp. 507–538. [Google Scholar]
- Prendergast BJ, Beery AK, Paul MJ, Zucker I. Enhancement and suppression of ultradian and circadian rhythms across the female hamster reproductive cycle. J Biol Rhythms. 2012a;27:246–256. doi: 10.1177/0748730412441315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast BJ, Cisse YM, Cable EJ, Zucker I. Dissociation of ultradian and circadian phenotypes in female and male Siberian hamsters. J Biol Rhythms. 2012b;27:287–298. doi: 10.1177/0748730412448618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quay WB. Precocious entrainment and associated characteristics of activity patterns following pinealectomy and reversal of photoperiod. Physiol Behav. 1970;5:1281–1290. doi: 10.1016/0031-9384(70)90041-7. [DOI] [PubMed] [Google Scholar]
- Refinetti R, Cornélissen G, Halberg F. Procedures for numerical analysis of circadian rhythms. Biol Rhythm Res. 2007;38:275–325. doi: 10.1080/09291010600903692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross PD. Mammalian Species No 595. 1998. Phodopus sungorus; pp. 1–9. [Google Scholar]
- Ruf T. The Lomb–Scargle periodogram in biological rhythm research: analysis of incomplete and unequally spaced time-series. Biol Rhythm Res. 1999;30:178–201. [Google Scholar]
- Rusak B, Zucker I. Neural regulation of circadian rhythms. Physiol Rev. 1979;59:449–526. doi: 10.1152/physrev.1979.59.3.449. [DOI] [PubMed] [Google Scholar]
- Schlatt S, Niklowitz P, Hoffmann K, Nieschlag E. Influence of short photoperiods on reproductive organs and estrous cycles of normal and pinealectomized female Djungarian hamsters, Phodopus sungorus. Biol Reprod. 1993;49:243–250. doi: 10.1095/biolreprod49.2.243. [DOI] [PubMed] [Google Scholar]
- Steinlechner S, Heldmaier G, Weber C, Ruf T. Role of photoperiod: pineal gland interaction in torpor control. In: Heller HC, Musacchia XJ, Wang LCH, editors. Living in the Cold. Elsevier; New York: 1986. pp. 301–307. [Google Scholar]
- van Dongen HPA, Olofsen E, Van Hartevelt JH, Kruyt EW. A procedure of multiple period searching in unequally spaced time series with the Lomb–Scargle method. Biol Rhythm Res. 1999;30:149–177. doi: 10.1076/brhm.30.2.149.1424. [DOI] [PubMed] [Google Scholar]
- van Dongen HPA, Ruf T, Olofsen E, van Hartevelt JH, Kruyt EW. Analysis of problematic time series with the Lomb–Scargle method, a reply to ‘Emphasizing difficulties in the detection of rhythms with Lomb–Scargle periodograms’. Biol Rhythm Res. 2001;32:347–354. doi: 10.1076/brhm.32.3.347.1348. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD. Pulsatile hormone secretion: mechanisms, significance and evaluation. In: Lloyd D, Rossi E, editors. Ultradian Rhythms from Molecules to Mind. Springer Science and Business; Berlin: 2008. pp. 229–247. [Google Scholar]
- Vitale PM, Darrow JM, Duncan MJ, Shustak CA, Goldman BD. Effects of photoperiod, pinealectomy and castration on body weight and daily torpor in Djungarian hamsters (Phodopus sungorus) J Endocrinol. 1985;106:367–375. doi: 10.1677/joe.0.1060367. [DOI] [PubMed] [Google Scholar]
- Warner A, Jethwa PH, Wyse CA, I’Anson H, Brameld JM, Ebling FJP. Effects of photoperiod on daily locomotor activity, energy expenditure, and feeding behavior in a seasonal mammal. Am J Physiol. 2010;298:R1409–1416. doi: 10.1152/ajpregu.00279.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner J. Limits to energy budget and tactics in energy investments during reproduction in the Djungarian hamster (Phodopus sungorus sungorus Pallas 1770) Symp Zool Soc Lond. 1987;57:167–187. Suppl. [Google Scholar]
- Wollnik F, Döhler KD. Effects of adult or perinatal hormonal environment on ultradian rhythms in locomotor activity of laboratory LEW/Ztm rats. Physiol Behav. 1986;38:229–240. doi: 10.1016/0031-9384(86)90158-7. [DOI] [PubMed] [Google Scholar]
- Yates FE, Yates LB. Ultradian rhythms as the dynamic signature of life. In: Lloyd D, Rossi E, editors. Ultradian Rhythms from Molecules to Mind. Springer Science and Business; Berlin: 2008. pp. 249–260. [Google Scholar]
- Zucker I, Fitzgerald KM, Morin LP. Sex differentiation of the circadian system in the golden hamster. Am J Physiol. 1980;238:R97–R101. doi: 10.1152/ajpregu.1980.238.1.R97. [DOI] [PubMed] [Google Scholar]