Abstract
Context
The relationship of monocyte/macrophage activation to insulin resistance in obesity is unknown.
Objective
To investigate a marker of macrophage activation, soluble CD163 (sCD163), in relationship to insulin resistance and metabolic parameters in obese and normal-weight subjects.
Design and Participants
95 healthy subjects (65 obese and 30 normal-weight) were studied. Plasma concentrations of sCD163 were assessed, as well as markers of glucose homeostasis, anthropometrics, cytokines, and adipokines. The relationships between sCD163 and these parameters were investigated, and multiple regression modeling assessing the contribution of sCD163 to insulin resistance (HOMA-IR) was performed.
Results
sCD163 was significantly increased in obese subjects compared to normal-weight controls [974 (657, 1272) ng/ml vs. 599 (423, 892) ng/ml, median (IQR); p < 0.0001]. sCD163 was strongly associated with HOMA-IR (Spearman's rho = 0.37, p = 0.0003) and other metabolic parameters. In multiple regression modeling for log HOMA-IR, sCD163 remained significantly associated (p = 0.005) controlling for known mediators of insulin resistance including age, gender, visceral adiposity, and inflammatory markers (model R2 = 0.54, p < 0.0001). Additional nested multiple regression models for log HOMA-IR showed that sCD163 added more than other adipokines and inflammatory markers to the prediction of HOMA-IR.
Conclusions
Monocyte/macrophage activation, as reflected by sCD163 levels, is strongly associated with HOMA-IR in normal-weight and obese subjects after controlling for known mediators of insulin resistance. Moreover, sCD163 adds to standard risk markers for predicting insulin resistance. These data suggest that monocyte/macrophage activation may be an important determinant of insulin resistance in obesity.
Keywords: sCD163, monocyte/macrophage activation, insulin resistance
BACKGROUND
Obesity is a highly prevalent disorder1, which strongly predisposes to the development of type 2 diabetes2, 3 and its attendant cardiovascular co-morbidities4. A deeper understanding of the mechanisms by which obesity contributes to diabetes may facilitate identification and therapeutic targeting of at-risk obese individuals. Recent attention has focused on the hypothesis that tonic subclinical inflammation in obesity5, 6 promotes insulin resistance at the level of the fat, liver, and muscle7. Subclinical inflammation in obesity is thought to be mediated in large part by macrophage infiltration and activation in expanded visceral fat depots7.
Soluble CD163 (sCD163) represents the ectodomain of CD1638, 9, a monocyte/macrophage-specific scavenger receptor known to participate in heme metabolism10 and immunomodulation11. Inflammatory stimuli trigger sCD163 cleavage and subsequent systemic release from monocyte/macrophage cell membranes12-15, suggesting that sCD163 is a marker of monocyte/macrophage activation. The functional role of sCD163, however, remains to be fully characterized11. sCD163 is known to be elevated in obese subjects16 and preliminary data suggest it may be related to the development of diabetes17. However, the relationship of this marker to insulin resistance and other metabolic parameters in obesity is thus far unknown.
In this study, we sought to determine how sCD163 relates to insulin resistance and other metabolic parameters (including specific fat depots, cytokines, and adipokines) in obese and normal-weight subjects.
SUBJECTS AND METHODS
65 obese (BMI ≥ 30 kg/m2) and 30 normal-weight (BMI <25 kg/m2) subjects without significant health problems were recruited from the Boston area between November 2007 and March 2009 as previously described18. Anthropometric, hormonal and carotid IMT (cIMT) data were previously published in this group18, 19, but no data on sCD163 in this cohort have been published. Each subject gave written informed consent. The Massachusetts General Hospital and the Massachusetts Institute of Technology Institutional Review Boards both approved the study. Included subjects were men and women between the ages of 18-55. Exclusion criteria included known diabetes, obesity due to known secondary causes, administration of endocrine hormones (including oral contraceptive pills and glucocorticoids) or anti-hyperglycemic medications, hemoglobin (Hg) < 11 g/dl, creatinine > 1.5 mg/dl, aspartate aminotransferase > 2.5 x the upper limit of normal, and severe chronic illnesses such as the human immunodeficiency virus (HIV) infection.
Methods
All subjects, underwent an overnight fast followed by careful metabolic phenotyping including: a) anthropometric measurements b) fasting blood work for glucose, insulin, lipids, cytokines, adipokines, and sCD163 and c) standard two-hour 75 gram oral glucose tolerance test.
Biochemical Parameters
Fasting lipid levels, glucose levels, and hemoglobin A1c (HbA1c) were determined in the Massachusetts Institute of Technology clinical laboratories using standard techniques. Insulin levels were assessed by a paramagnetic-particle chemiluminescence immunoassay (Beckman Coulter). A 2-hour oral glucose tolerance test was administered using a 75 gram glucose beverage. Insulin sensitivity was quantified by the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR).
sCD163 was determined using an ELISA kit from Trillium Diagnostics as previously described20. All samples were run in duplicate and repeated if there was a >15% difference between duplicates. TNF-alpha (TNF-α), C-reactive protein (CRP), interleukin-6 (IL-6) and adiponectin were measured using commercially available ELISA kits (Invitrogen Corporation Carlsbad, CA (TNF-α); Diagnostic Systems Laboratories Inc, Webster, TX (CRP); and R&D Systems, Inc. Minneapolis, MN (IL-6 and adiponectin)).
Body Composition and Anthropometrics
Height and weight were determined using standard scales. Body mass index (BMI) was calculated. Waist circumference was determined in triplicate at the level of the iliac crest with the patient in an upright position, and average measures were reported21. Cross-sectional abdominal computed tomography (CT) scans (1 cm thickness slice at L4) were performed to determine the distribution of abdominal subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT), as described previously22.
Statistical Analysis
The normality of distribution of continuous variables was assessed via inspection of histogram distribution and application of the Wilk-Shapiro test. Most variables were not normally distributed, and thus comparison between normal-weight and obese subjects were made using the Wilcoxon rank sum test. Univariate association analysis was performed to compare levels of sCD163 with various metabolic parameters using the Spearman's correlation coefficient. Metabolic and inflammatory parameters, including sCD163, were then related to HOMA-IR in univariate association analyses. Multiple regression analyses were first performed using standard least squares modeling with log HOMA-IR as the dependent variable and sCD163 and all other parameters related to HOMA-IR on univariate modeling (in addition to age, gender, and race) as independent variables. The transformation of HOMA-IR normalized its distribution, and when it was used as the dependent variable in multiple regression modeling, normality of residuals was confirmed by the Kolmogirov-Smirnov test. Sensitivity analyses were also performed to determine if sCD163 remained significantly associated with log HOMA-IR when controlling for BMI and for the potential interaction between BMI and sCD163 in this model. Next, a parsimonious multiple regression model for log HOMA-IR was made using forward stepwise regression modeling to identify variables for entry into the model among the larger group of variables related on univariate analysis and to reduce colinearity. Normality of residuals was also confirmed in this model. In addition, a series of nested multiple regression models were constructed sequentially and individually adding sCD163, adiponectin, IL-6, CRP, or TNF-α to a baseline model including only age, sex, and VAT to determine the degree to which sCD163 increased the prediction of HOMA-IR (compared with other adipokines and inflammatory markers). JMP Statistical Database Software (SAS Institute Inc, Cary NC) was used to for statistical analysis, with statistical significance defined as p ≤ 0.05.
RESULTS
Baseline Characteristics of Subjects by Weight-Category
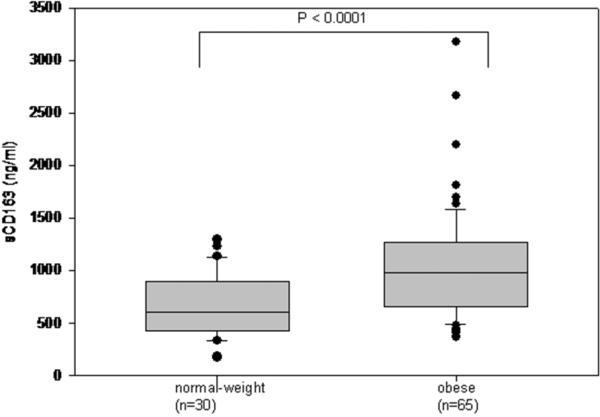
There were no significant differences in age, gender, race, or pack-years of cigarette smoking between obese and normal-weight subjects (Table 1). Compared with normal-weight subjects, obese subjects had higher BMI, waist circumference, SAT, VAT, TNF-α, CRP, triglycerides, LDL cholesterol, HbA1c, fasting glucose, 2-hour glucose, and HOMA-IR, as expected. Obese subjects also had lower levels of HDL cholesterol and adiponectin than did normal-weight subjects (Table 1). sCD163 was significantly higher in obese subjects than in normal-weight subjects [974 (657, 1272) ng/ml vs. 599 (423, 892) ng/ml, median (IQR); p < 0.0001] (Figure 1). There was no significant difference in sCD163 levels between genders.
Table 1.
Baseline Demographics of Subjects by Weight Category
| Normal Weight (n = 30) | Obese (n = 65) | p | |
|---|---|---|---|
| Age (years) | 44.0 (29.8, 48.5) | 45.0 (36.0, 50.5) | 0.69 |
| Gender, no of males (%) | 19 (63) | 39 (60) | 0.76 |
| Race, n (%) | 0.67 | ||
| Caucasian | 18 (60) | 36 (55) | |
| Not Caucasian | 12 (40) | 29 (45) | |
| Cigarette smoking (pack years) | 0 (0, 9.6) | 1.0 (0, 7.8) | 0.33 |
| BMI (kg/m2) | 22.3 (21.3, 23.1) | 35.1 (32.8, 39.3) | <0.0001 |
| Waist circumference (cm) | 78.7 (73.9, 85.7) | 114.6 (106.5, 124.9) | <0.0001 |
| SAT (cm2) | 119 (79, 163) | 468 (396, 582) | <0.0001 |
| VAT (cm2) | 36 (23, 58) | 191 (121, 239) | <0.0001 |
| TNF-α (pg/ml) | 7.8 (5.3, 13.3) | 10.5 (8.1, 15.7) | 0.01 |
| IL-6 (pg/ml) | 6.1 (5.0, 7.9) | 7.2 (5.9, 9.6) | 0.06 |
| CRP (mg/L) | 1.9 (1.5, 7.2) | 19.6 (7.5, 39.2) | <0.0001 |
| Adiponectin (ng/ml) | 6105 (4425, 9336) | 3726 (2188, 5513) | <0.0001 |
| SBP (mm Hg) | 117 (107, 124) | 116 (109, 126) | 1.0 |
| DBP (mm Hg) | 71 (67, 77) | 78 (70, 80) | 0.06 |
| Total cholesterol (mmol/L) | 4.5 (3.6, 5.2) | 4.7 (4.1, 5.4) | 0.13 |
| Triglycerides (mmol/L) | 0.8 (0.5, 1.1) | 1.2 (0.8, 1.7) | 0.0001 |
| HDL cholesterol (mmol/L) | 1.5 (1.3, 1.8) | 1.1 (0.9, 1.4) | <0.0001 |
| LDL cholesterol (mmol/L) | 2.5 (2.1, 2.9) | 3.0 (2.4, 3.6) | 0.01 |
| HbA1c (%) | 5.5 (5.3, 5.6) | 5.6 (5.4, 5.9) | 0.003 |
| Fasting glucose (mmol/L) | 4.7 (4.4, 4.9) | 5.0 (4.7, 5.4) | 0.0004 |
| 2-hour glucose (mmol/L) | 5.3 (3.7, 6.7) | 6.4 (5.1, 8.0) | 0.001 |
| HOMA-IR | 0.5 (0.3, 0.9) | 1.8 (1.1, 3.2) | <0.0001 |
Data are expressed as median (IQR) unless otherwise noted.
Figure 1.
sCD163 levels by weight category (normal versus obese). sCD163 was significantly higher in obese subjects than in normal-weight subjects [974 (657, 1272) ng/ml versus 599 (423, 892) ng/ml, median (IQR); p < 0.0001].
Univariate Associations between sCD163 and Metabolic Parameters Among All Subjects
In univariate analysis, sCD163 was positively and significantly associated with anthropomorphic measurements including BMI (Spearman's rho = 0.43, p < 0.0001), waist circumference (Spearman's rho = 0.46, p < 0.0001), SAT (Spearman's rho = 0.39, p = 0.0001), and VAT (Spearman's rho = 0.41, p < 0.0001), as well as inflammatory markers and adipokines including TNF-α (Spearman's rho = 0.21, p = 0.04), CRP (Spearman's rho = 0.27, p = 0.008), and adiponectin (Spearman's rho = -0.28, p = 0.006) (Table 2). Among glucose homeostatic parameters, sCD163 was significantly associated with fasting glucose (Spearman's rho = 0.22, p = 0.03), 2-hour glucose (Spearman's rho = 0.32, p = 0.002), and HOMA-IR (Spearman's rho = 0.37, p = 0.0003) (Table 2). Figure 2 shows the significant relationship between log HOMA-IR and log sCD163 in obese and normal-weight subjects (r = 0.38, p = 0.0001). In addition, log HOMA-IR remained significantly associated with log sCD163 (r = 0.27, p = 0.03) in an analysis limited to obese subjects.
Table 2.
Univariate associations between Metabolic Parameters and sCD163
| Spearman's rho | p value | |
|---|---|---|
| Age | 0.29 | 0.005 |
| BMI | 0.43 | <0.0001 |
| Waist circumference | 0.46 | <0.0001 |
| SAT | 0.39 | 0.0001 |
| VAT | 0.41 | <0.0001 |
| TNF-a | 0.21 | 0.04 |
| IL-6 | -0.06 | 0.59 |
| CRP | 0.27 | 0.008 |
| Adiponectin | -0.28 | 0.006 |
| SBP | 0.16 | 0.13 |
| DBP | 0.27 | 0.008 |
| Triglycerides | 0.21 | 0.04 |
| HDL | -0.29 | 0.004 |
| HbA1c | 0.18 | 0.09 |
| Fasting glucose | 0.22 | 0.03 |
| 2-hour glucose | 0.32 | 0.002 |
| HOMA-IR | 0.37 | 0.0003 |
Figure 2.
Relationship between log HOMA-IR and log sCD163 by weight category (normal-weight versus obese) (r = 0.38, p = 0.0001).
Multiple Regression Modeling for log HOMA-IR
In univariate modeling, multiple parameters in addition to sCD163 were found to be significantly related to HOMA-IR, including BMI (Supplemental Figure 1), waist circumference, SAT, VAT, TNF-α, IL-6, CRP, and adiponectin (Supplemental Table 1).
In multiple regression modeling, sCD163 remained significantly associated (p = 0.03) with log HOMA-IR after controlling for age, sex, race, and the metabolic parameters found to be associated with HOMA-IR on univariate analysis (model R2 = 0.59, p < 0.0001) (Supplemental Table 2). In this model, sCD163 was the only inflammatory biomarker which remained significant, as known mediators of insulin resistance including TNF-α, CRP, and adiponectin were no longer significant. Moreover, sCD163 remained significantly related to log HOMA-IR controlling simultaneously for BMI and the potential interaction between BMI and sCD163 in this multiple regression model. The interaction term for BMI and sCD163 was not significant in the model.
To make a more parsimonious model, forward stepwise regression modeling for log HOMA-IR was performed. Metabolic parameters related to HOMA-IR on univariate analysis were assessed and the following parameters entered the model on forward stepwise selection: age, sex, VAT, waist circumference, TNF-α, and sCD163. In multiple regression modeling for log HOMA-IR using these parameters as independent variables, waist circumference was no longer significant. To reduce potential colinearity between related anthropometric variables (VAT and waist circumference), a final multiple regression model for log HOMA-IR was constructed using age, sex, VAT, TNF-α, and sCD163 as independent variables. In this final model, R2 was 0.54 (p <0.0001) and sCD163 remained significantly associated (p = 0.005) with log HOMA-IR (Table 3). Adding family history of diabetes and data on diet to the final parsimonious model for log HOMA-IR shown in Table 3 did not diminish the significant independent relationship of sCD163 to log HOMA-IR (p = 0.004).
Table 3.
Final Multiple Regression Model for log HOMA-IR Derived from Forward Stepwise Regression Analysis (overall model R2 = 0.54, P <0.0001)
| β estimate | standard error | p value | |
|---|---|---|---|
| Age (years) | -0.02 | 0.004 | < 0.0001 |
| Sex (male vs. female) | 0.04 | 0.04 | 0.32 |
| VAT (cm2) | 0.003 | 0.0004 | <0.0001 |
| TNF-α (pg/ml) | 0.01 | 0.006 | 0.01 |
| sCD163 (ng/ml) | 0.0002 | <0.0001 | 0.005 |
Additional nested multiple regression models for log HOMA-IR - constructed by sequentially adding sCD163, adiponectin, TNF-α, IL-6, or CRP individually to basal parameters of age, sex and VAT - showed that sCD163 added more than other adipokines and inflammatory markers to the prediction of HOMA-IR (Table 4).
Table 4.
Series of Nested Multiple Regression Models for log HOMA-IR Assessing Predictive Value of Adipokines and Inflammatory Markers above and beyond Age, Sex, and VAT
| Model | Model R2 | p value for inflammatory marker / adipokine in the model |
|---|---|---|
| Model 1: Age, Sex, VAT | 0.45 | N/A |
| Model 2: Age, Sex, VAT, sCD163 | 0.51 | p value for sCD163 = 0.003 |
| Model 3: Age, Sex, VAT, adiponectin | 0.46 | p value for adiponectin = 0.21 |
| Model 4: Age, Sex, VAT, IL-6 | 0.46 | p value for IL-6 = 0.19 |
| Model 5: Age, Sex, VAT, CRP | 0.47 | p value for CRP = 0.07 |
| Model 6: Age, Sex, VAT, TNF-α | 0.50 | p value for TNF-α = 0.006 |
DISCUSSION
In this study, we show for the first time that the monocyte/macrophage activation marker sCD163 strongly relates to insulin resistance, as reflected by HOMA-IR. The relationship holds in a group of healthy subjects, including an obese cohort, and remains highly significant even after controlling for BMI and specific body composition and inflammatory factors known to be associated with insulin resistance. Moreover, sCD163 appears to contribute to the prediction of HOMA-IR (controlling for age, sex, and VAT) to a greater degree than other previously recognized predictive factors such as TNF-α and adiponectin. These novel findings suggest a critical role of monocyte/macrophage activation in the development of insulin resistance.
Our data demonstrate that sCD163 is markedly increased in healthy obese subjects versus normal-weight subjects, and that it correlates strongly with central adiposity. Both SAT and VAT are highly related to sCD163 in our univariate analysis, suggesting that macrophage activation in obesity may be promoted by excess SAT in addition to VAT. Tissue-specific expression of CD163 and local concentrations of sCD163 in specific fat depots will be important to investigate in future studies.
A key aspect of this analysis is the novel demonstration that sCD163 is significantly related to multiple parameters of glucose homeostasis. In particular, sCD163 demonstrates a robust relationship to HOMA-IR, a surrogate of insulin resistance, controlling for numerous factors associated with insulin resistance including BMI and various adipokines and inflammatory markers. Importantly, sCD163 remains highly significantly related to HOMA-IR in a parsimonious final multiple regression model including age, sex, VAT, and TNF-α, as independent variables, in addition to sCD163. This model for HOMA-IR is not only parsimonious but highly physiologically relevant, including well-known predictors of insulin resistance.
Our data support the suggestion that sCD163 may be a more promising biomarker of macrophage activation in adipose tissue and predictor of insulin resistance than other previously established markers, including TNF-α. Elevated systemic levels of TNF-α, a monocyte/macrophage-produced cytokine, have been previously associated with obesity and insulin resistance 23, 24. However, systemic TNF-α levels may underestimate local inflammation in adipose tissue25, potentially due to the relatively rapid clearance of TNF-α from the circulation15. sCD163 is shed from cells of the monocyte/macrophage lineage10, in response to inflammatory stimuli12-14. Macrophages expressing CD163 have the potential to infiltrate fat 26, 27. sCD163 may thus be reflective of systemic monocyte activation and/or fat-specific macrophage activation. Intriguingly, Etzertodt and colleagues recently published that TACE/ADAM 17, a proteinase recognized to promote release of TNF-α from macrophages, also mediates ectodomain shedding of CD16315. This observation potentially explains elevated concentrations of sCD163 in diseases involving macrophage activation (including hemophagocytic syndrome28 and atherosclerosis29).15 In concert with this finding, our data demonstrate that sCD163 levels are indeed associated with TNF-α levels on univariate analysis. Our data further show that sCD163 is more highly related to HOMA-IR in multivariate modeling and increases the predictive value of this modeling more than TNF-α. A potential underlying explanation for the strength of sCD163 as a biomarker of inflammation-mediated insulin resistance is that unlike TNF-α, sCD163 released from stimulated monocytes/macrophages may persist in human plasma for upwards of 24 hours15.
With respect to inflammation-mediated insulin resistance, our data additionally suggest that sCD163 may provide more relevant information than both the acute-phase-reactant CRP and the anti-inflammatory adipocytokine adiponectin. In our study, sCD163 is significantly associated with CRP, a marker of generalized inflammation. Both CRP and sCD163 are highly related to HOMA-IR in univariate analysis. In multiple regression modeling, sCD163 remains a significant contributor to HOMA-IR while CRP does not. sCD163 correlates negatively with adiponectin. In multiple regression modeling, sCD163 remains robustly related to HOMA-IR while adiponectin does not. This study is the first to our knowledge to show that a marker of monocyte/macrophage activation may contribute more to the prediction of insulin resistance than adiponectin. In vitro studies have shown that adiponectin down regulates the expression of CD163 on the cell-surface of monocytes, but not levels of sCD163 released into the supernatant of cultured cells16. The functional relationship between sCD163 and adiponectin in vivo remains unknown.
This study has some limitations. Causality can not be determined based on the study design. The specific mechanisms through which sCD163 increases so dramatically in obesity -e.g. through TNF-α mediated mechanisms, oxidative stress mechanisms, or other pathways -remain to be determined. Finally, the tight relationship between sCD163 and insulin resistance requires further study assessing additional markers of macrophage activation and insulin sensitivity, including the euglycemic clamp.
In sum, our data suggest that sCD163 is a promising marker of monocyte/macrophage activation related to obesity, abdominal fat accumulation, and critical metabolic parameters. Specifically, these data suggest for the first time a robust relationship between sCD163 and insulin resistance that is stronger than that seen with many known diabetes risk factors. Further studies will be needed to elucidate the origins (circulating monocytes versus visceral fat macrophages) and triggers for sCD163 release in obesity, as well as the functional role of sCD163. If sCD163 is not just a marker but also a mediator of insulin resistance, it may prove to be a promising therapeutic target for diabetes prevention efforts in obese individuals with inflamed fat.
Supplementary Material
Acknowledgements
We gratefully acknowledge the MGH bionutrition and nursing staffs and the research volunteers for their participation in the study. We also thank Dr. Hang Lee of the MGH Biostatistics Center for his careful review of the manuscript.
NIH funding was provided through F32 DK085969 to M.Z., K23 DK087857 to H.M, and NS37654 and NS40237 to K.W, and through K24 DK064545 and R01 HL085268 to S.G. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures and Funding: M.Z., T.B., H.M., K.W., and S.G. have nothing to declare.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. Jama. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care. 1994;17:961–969. doi: 10.2337/diacare.17.9.961. [DOI] [PubMed] [Google Scholar]
- 3.Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122:481–486. doi: 10.7326/0003-4819-122-7-199504010-00001. [DOI] [PubMed] [Google Scholar]
- 4.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 5.Festa A, D'Agostino R, Jr., Howard G, Mykkanen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation. 2000;102:42–47. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 6.Brunner EJ, Hemingway H, Walker BR, Page M, Clarke P, Juneja M, Shipley MJ, Kumari M, Andrew R, Seckl JR, Papadopoulos A, Checkley S, Rumley A, Lowe GD, Stansfeld SA, Marmot MG. Adrenocortical, autonomic, and inflammatory causes of the metabolic syndrome: nested case-control study. Circulation. 2002;106:2659–2665. doi: 10.1161/01.cir.0000038364.26310.bd. [DOI] [PubMed] [Google Scholar]
- 7.Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest. 2008;118:2992–3002. doi: 10.1172/JCI34260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moller HJ, Peterslund NA, Graversen JH, Moestrup SK. Identification of the hemoglobin scavenger receptor/CD163 as a natural soluble protein in plasma. Blood. 2002;99:378–380. doi: 10.1182/blood.v99.1.378. [DOI] [PubMed] [Google Scholar]
- 9.Droste A, Sorg C, Hogger P. Shedding of CD163, a novel regulatory mechanism for a member of the scavenger receptor cysteine-rich family. Biochem Biophys Res Commun. 1999;256:110–113. doi: 10.1006/bbrc.1999.0294. [DOI] [PubMed] [Google Scholar]
- 10.Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 11.Van Gorp H, Delputte PL, Nauwynck HJ. Scavenger receptor CD163, a Jack-of-all-trades and potential target for cell-directed therapy. Mol Immunol. 2010;47:1650–1660. doi: 10.1016/j.molimm.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Hintz KA, Rassias AJ, Wardwell K, Moss ML, Morganelli PM, Pioli PA, Givan AL, Wallace PK, Yeager MP, Guyre PM. Endotoxin induces rapid metalloproteinase-mediated shedding followed by up-regulation of the monocyte hemoglobin scavenger receptor CD163. J Leukoc Biol. 2002;72:711–717. [PubMed] [Google Scholar]
- 13.Weaver LK, Hintz-Goldstein KA, Pioli PA, Wardwell K, Qureshi N, Vogel SN, Guyre PM. Pivotal advance: activation of cell surface Toll-like receptors causes shedding of the hemoglobin scavenger receptor CD163. J Leukoc Biol. 2006;80:26–35. doi: 10.1189/jlb.1205756. [DOI] [PubMed] [Google Scholar]
- 14.Timmermann M, Hogger P. Oxidative stress and 8-isoprostaglandin F(2alpha) induce ectodomain shedding of CD163 and release of tumor necrosis factor-alpha from human monocytes. Free Radic Biol Med. 2005;39:98–107. doi: 10.1016/j.freeradbiomed.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 15.Etzerodt A, Maniecki MB, Moller K, Moller HJ, Moestrup SK. Tumor necrosis factor alpha-converting enzyme (TACE/ADAM17) mediates ectodomain shedding of the scavenger receptor CD163. J Leukoc Biol. 2010;88:1201–1205. doi: 10.1189/jlb.0410235. [DOI] [PubMed] [Google Scholar]
- 16.Sporrer D, Weber M, Wanninger J, Weigert J, Neumeier M, Stogbauer F, Lieberer E, Bala M, Kopp A, Schaffler A, Buechler C. Adiponectin downregulates CD163 whose cellular and soluble forms are elevated in obesity. Eur J Clin Invest. 2009;39:671–679. doi: 10.1111/j.1365-2362.2009.02170.x. [DOI] [PubMed] [Google Scholar]
- 17.Moller HJ, Frikke-Schmidt R, Moestrup SK, Nordestgaard BG, Tybjaerg-Hansen A. Serum soluble CD163 predicts risk of type 2 diabetes in the general population. Clin Chem. 2011;57:291–297. doi: 10.1373/clinchem.2010.154724. [DOI] [PubMed] [Google Scholar]
- 18.Makimura H, Stanley T, Mun D, Chen C, Wei J, Connelly JM, Hemphill LC, Grinspoon SK. Reduced growth hormone secretion is associated with increased carotid intima-media thickness in obesity. J Clin Endocrinol Metab. 2009;94:5131–5138. doi: 10.1210/jc.2009-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makimura H, Stanley T, Mun D, You SM, Grinspoon S. The effects of central adiposity on growth hormone (GH) response to GH-releasing hormone-arginine stimulation testing in men. J Clin Endocrinol Metab. 2008;93:4254–4260. doi: 10.1210/jc.2008-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burdo TH, Lentz MR, Autissier P, Krishnan A, Halpern E, Letendre S, Rosenberg ES, Ellis RJ, Williams KC. Soluble CD163 Made by Monocyte/Macrophages Is a Novel Marker of HIV Activity in Early and Chronic Infection Prior to and After Anti-retroviral Therapy. J Infect Dis. 2011;204:154–163. doi: 10.1093/infdis/jir214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lohman TG, Roche AF, Martorell R, editors. Athropometric Standardization Reference Manual. Human Kinetic Books; Champaign, IL: 1988. [Google Scholar]
- 22.Rietschel P, Hadigan C, Corcoran C, Stanley T, Neubauer G, Gertner J, Grinspoon S. Assessment of growth hormone dynamics in human immunodeficiency virus-related lipodystrophy. J Clin Endocrinol Metab. 2001;86:504–510. doi: 10.1210/jcem.86.2.7175. [DOI] [PubMed] [Google Scholar]
- 23.Dandona P, Weinstock R, Thusu K, Abdel-Rahman E, Aljada A, Wadden T. Tumor necrosis factor-alpha in sera of obese patients: fall with weight loss. J Clin Endocrinol Metab. 1998;83:2907–2910. doi: 10.1210/jcem.83.8.5026. [DOI] [PubMed] [Google Scholar]
- 24.Tsigos C, Kyrou I, Chala E, Tsapogas P, Stavridis JC, Raptis SA, Katsilambros N. Circulating tumor necrosis factor alpha concentrations are higher in abdominal versus peripheral obesity. Metabolism. 1999;48:1332–1335. doi: 10.1016/s0026-0495(99)90277-9. [DOI] [PubMed] [Google Scholar]
- 25.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shakeri-Manesch S, Zeyda M, Huber J, Ludvik B, Prager G, Stulnig TM. Diminished upregulation of visceral adipose heme oxygenase-1 correlates with waist-to-hip ratio and insulin resistance. Int J Obes (Lond) 2009;33:1257–1264. doi: 10.1038/ijo.2009.160. [DOI] [PubMed] [Google Scholar]
- 27.Zeyda M, Farmer D, Todoric J, Aszmann O, Speiser M, Gyori G, Zlabinger GJ, Stulnig TM. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes (Lond) 2007;31:1420–1428. doi: 10.1038/sj.ijo.0803632. [DOI] [PubMed] [Google Scholar]
- 28.Schaer DJ, Schleiffenbaum B, Kurrer M, Imhof A, Bachli E, Fehr J, Moller HJ, Moestrup SK, Schaffner A. Soluble hemoglobin-haptoglobin scavenger receptor CD163 as a lineage-specific marker in the reactive hemophagocytic syndrome. Eur J Haematol. 2005;74:6–10. doi: 10.1111/j.1600-0609.2004.00318.x. [DOI] [PubMed] [Google Scholar]
- 29.Aristoteli LP, Moller HJ, Bailey B, Moestrup SK, Kritharides L. The monocytic lineage specific soluble CD163 is a plasma marker of coronary atherosclerosis. Atherosclerosis. 2006;184:342–347. doi: 10.1016/j.atherosclerosis.2005.05.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.