Abstract
Studies examining the association between menstrual cycle phases and smoking behavior in women have yielded mixed results. The purpose of this study was to elucidate the associations between ovarian hormones and smoking by directly measuring ovarian hormone levels and obtaining a laboratory assessment of smoking behaviors. Four hypotheses were tested: increased smoking will be associated with 1) low absolute levels of estradiol and progesterone; 2) decreasing (i.e., dynamic changes in) estradiol and progesterone; 3) lower ratios of progesterone to estradiol, and 4) higher ratios of estradiol to progesterone. Female smokers (≥10 cigarettes/day) with regular menstrual cycles were recruited as part of a larger, ongoing study examining the influence of ovarian hormones on smoking cessation treatment. Participants completed two study visits, including a one-hour adlib smoking topography session, which provided a detailed assessment of smoking behavior. Both the change in hormone levels over time and the relative ratios of ovarian hormones were associated with smoking behavior, but each to a limited extent. Decreases in estradiol (r=−.21, p=.048), and decreases in progesterone (r=−.23, p=.03) were associated with increased puff intensity. Lower ratios of progesterone to estradiol were associated with a greater number of puffs (r=−.26, p=.01) and weight of cigarettes smoked (r=−.29, p=.005). The best predictors of smoking behavior were the ratio of progesterone to estradiol (z=−2.7, p=.004) and the change in estradiol and progesterone over time (z=−2.1, p=.02). This pattern of results may help to explain inconsistent findings in previous studies and suggest potential mechanisms by which hormones influence nicotine addiction.
Keywords: Estrogen, progesterone, nicotine, tobacco, women
Smoking is the leading cause of preventable death in the United States (Danaei et al., 2009). Although men are more likely to smoke than women (USDHS, 2010), women may be more sensitive to certain aspects of nicotine addition. For example, women are more sensitive to the subjective effects of nicotine (Myers, Taylor, Moolchan, & Heishman, 2008; Sofuoglu, Mitchell, & Mooney, 2009) and more likely to cite tension reduction, stimulation, social dynamics (Berlin et al., 2003), and appetite suppression as reasons for smoking (Reid, Pipe, Riley, & Sorensen, 2009). Smoking is associated with significant health risks in women (Perkins, 2001), and more women than men report having tried nicotine replacement therapy (NRT) to quit smoking (Reid, et al., 2009). However, women are less likely than men to achieve sustained abstinence following NRT (Bohadana, Nilsson, Rasmussen, & Martinet, 2003; Japuntich et al., 2011; Perkins, 2001).
Ovarian hormones may contribute to gender differences in nicotine addiction. Estradiol is thought to enhance women’s sensitivity to nicotine, whereas progesterone is thought to be protective (Lynch & Sofuoglu, 2010). Tonic progesterone administration in the context of relatively low estradiol levels increases the subjective negative effects of nicotine, decreases the subjective positive effects of nicotine, and reduces the urge to smoke in women (Sofuoglu & Mooney, 2009). These findings are supported by experimental animal studies, which have demonstrated decreased motivation for nicotine when progesterone levels are high (Lynch, 2009). However, the effects of progesterone on smoking behavior in women are less clear (Sofuoglu, Babb, & Hatsukami, 2001; Sofuoglu, Mouratidis, & Mooney, 2011).
The majority of research examining the effects of ovarian hormones on smoking has used menstrual cycle phase as a proxy for ovarian hormone function. These studies typically compare women in different phases of the menstrual cycle (e.g., follicular vs. luteal), and the results have been quite mixed. Some studies show increased smoking during menses (DeBon, Klesges, & Klesges, 1995; Marks, Hair, Klock, Ginsburg, & Pomerleau, 1994), others show increased smoking during the late-luteal phase (DeBon, et al., 1995; Snively, Ahijevych, Bernhard, & Wewers, 2000), and others show no relationship between cigarette smoking and menstrual phase (Allen, Hatsukami, Christianson, & Nelson, 1996; Allen, Hatsukami, Christianson, & Nelson, 1999; Pomerleau, Cole, Lumley, Marks, & Pomerleau, 1994).
Certain methodological issues may have contributed to the inconsistent findings across studies. Previous studies used arbitrary phase classifications to denote differences in hormonal milieu. Comparisons of one phase vs. another (follicular vs. luteal) can be problematic to the extent that such phases are imperfect proxies of actual hormone levels because estradiol and progesterone levels fluctuate significantly within cycle phases (see Figure 1). Thus, the low absolute levels of estradiol and progesterone experienced during menses {DeBon, 1995 #116}{Marks, 1994 #149}, the decreasing levels of estradiol and progesterone experienced during the luteal-phase {DeBon, 1995 #116}{Snively, 2000 #113}, or the relative ratio of estradiol to progesterone (or vice versa) may be associated with increased smoking. The ratios of ovarian hormones (estradiol/progesterone or progesterone/estradiol) are associated with the exacerbation of a number of neurological and psychiatric conditions, including the frequency of seizures in women with epilepsy (Herzog, 1999), the number and volume of brain lesions associated with multiple sclerosis (Pozzilli et al., 1999), and menstrual distress in women with migraines (Beckham et al., 1992). Previous studies of smoking cessation have used the ratio of estradiol to progesterone rather than the reverse (Allen, Bade, Center, Finstad, & Hatsukami, 2008). Given that the two ratios (i.e., estradiol/progesterone and progesterone/estradiol) are not equivalent or linearly related, either or both ratios may be of importance. Thus, a more rigorous examination of hormonal influences on smoking behavior would focus on absolute and changing levels of both progesterone and estradiol, and the relationship between hormones (i.e., the ratio of progesterone to estradiol or vice versa).
Figure 1.
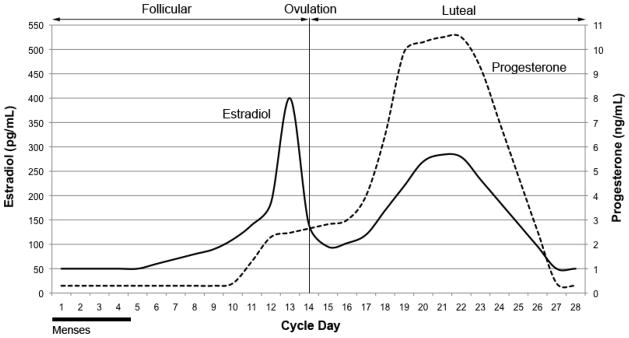
Approximate ovarian hormone levels during the menstrual cycle.
A second methodological issue that may have contributed to previous discrepant findings is related to the assessment of smoking behaviors. Many previous studies have relied on self-reported smoking behavior occurring hours or days following the assessment of menstrual cycle phase or hormone levels, and this varying lag in time between predictor and outcome likely accounts for different conclusions. It is critical that assessments, hormones and smoking behaviors are temporally congruent in order to detect meaningful associations. Thus, laboratory assessment is advantageous because smoking behaviors can be examined in relation to hormones measured at the same time.
The current study examines data from an ongoing smoking cessation trial to elucidate the relationship between ovarian hormones and smoking by directly measuring ovarian hormone levels and obtaining a detailed laboratory assessment of smoking behaviors. While it is difficult to derive a single, definitive hypothesis from the inconsistent findings noted above, we tentatively propose the following four hypotheses: increased smoking will be associated with 1) low levels of estradiol and progesterone; 2) decreasing estradiol and decreasing progesterone; 3) lower ratios of progesterone to estradiol; and 4) higher ratios of estradiol to progesterone.
Methods
Participants
Treatment-seeking female smokers (≥10 cigarettes/day) ages 18–45 years, having regular menstrual cycles between 25 and 35 days and not taking hormonal contraceptives or replacement were recruited from the community as part of a larger, ongoing study examining the influence of ovarian hormones on smoking cessation treatment. Participants were allowed to enter the study at any time during their menstrual cycle phase. Exclusion criteria were current unstable major psychiatric or medical disorders, current substance use disorders, and pregnancy or breastfeeding within the last three months. Women with premenstrual symptoms were not excluded from participating in this study.
Procedures
The Medical University of South Carolina Institutional Review Board approved this study. Participants were recruited from the community through local media advertising for a smoking cessation study. At the first study visit (T1), participants provided written informed consent and completed self-report questionnaires, including a demographic questionnaire and the Fagerström Test for Nicotine Dependence (FTND; Heatherton et al., 1991). FTND scores range from zero to 10, with higher scores indicating a greater degree of dependence. Psychiatric interview using the Mini International Neuropsychiatric Interview (MINI; Sheehan et al., 1998) was conducted to assess for exclusionary Axis I psychiatric diagnoses. Five milliliters of blood were collected in order to conduct estradiol and progesterone assays.
The second study visit (T2) was conducted approximately one to two weeks later (M=10.8 days, SD=8.0). Participants were instructed to abstain from smoking for 12 hours prior to attending the second study visit, which was verified via carbon monoxide (CO) breathalyzer. Women CO levels higher than 10ppm (n=5) were excluded from this study. A blood draw was performed to allow determination of serum estradiol and progesterone levels. Participants completed a one-hour ad libitum smoking topography session following a cue reactivity session described elsewhere (see Saladin et al., in submission). Importantly, both study visits and all data included in the current analyses occurred prior to the beginning of the smoking cessation treatment phase. As such, none of the women were receiving treatment.
Smoking Topography
At the beginning of T2, the participant’s CO level was measured and her entire supply of cigarettes was weighed. The participant was provided with an ashtray and permitted to smoke her preferred brand of cigarettes, indoors ad libitum for 60 minutes using smoking topography equipment (CReSSmicro System, Borgwadlt KC, Inc). During this time, the participant had access to reading material provided by study personnel and was told to deposit all cigarette butts in the ashtray provided by study personnel. At the end of the 60 minute period, the participant’s CO level was measured again to calculate the absolute change in CO during the session. The number of cigarette butts was counted and the smoked and unsmoked cigarettes were weighed.
The smoking topography device measured and recorded various aspects of the participant’s smoking behavior, including number of puffs on the first cigarette and all cigarettes, total number of cigarettes smoked, mean puff volume (mL) for the first cigarette and all cigarettes, total puff volume (mL) for the first cigarette and all cigarettes, mean puff duration (seconds) for the first cigarette and all cigarettes, mean flow rate (mL/second) for the first cigarette and all cigarettes, and peak flow rate (mL/second) for the first cigarette and all cigarettes. Flow rate represents the rate at which a volume of air is pulled in through the smoking topography device and has been shown to affect CO delivery independently of puff volume or duration (Robinson & Forbes, 1975).
Ovarian Hormone Measurement
Hormone assays were performed at the MUSC Clinical Core Laboratory using chemilluminescent immunoassay. The unit of measurement was pg/mL for estradiol and ng/mL for progesterone. Three separate ovarian hormone measurements served as independent variables: 1) absolute hormone levels; 2) the absolute change in hormone levels between study visits: and 3) the relative ratios of progesterone and estradiol. Absolute hormone levels reflect estradiol and progesterone levels assessed at T2. The hormone change index represents the absolute change in hormone levels between T1 and T2 (i.e., hormone change = T2 estradiol level – T1 estradiol level). The ratio of progesterone to estradiol represents the level of progesterone at T2 divided by the level of estradiol at T2. The ratio of progesterone to estradiol is highest during the luteal phase and lowest during the follicular phase of the menstrual cycle. Several studies examining the association between hormones and craving have used the opposite ratio: estradiol divided by progesterone, and since the E:P ratio is not linearly equivalent to the P:E ratio, we included both.
Statistical Analyses and Data Reduction
The smoking topography variables were subjected to data reduction techniques, including factor analysis, in order to identify broader, underlying behavioral constructs. The rationale for using factor analysis was threefold. First, the smoking topography device yielded a large number of behavioral outcome measures, many of which were highly correlated. Analyzing all of the outcomes separately would have required a large number of statistical tests, which would have increased the family wise error rate. Moreover, running several analyses with essentially the same dependent variable would have provided little incremental validity. Second, data reduction would yield a more comprehensive representation of the underlying behavioral constructs; this would be preferable to simply choosing a reduced set of variables based on a theoretical-rational approach (Clark & Watson, 1995). Third, reducing the variables to more robust constructs may have enhanced power and improved the likelihood of finding meaningful associations with the ovarian hormone measures.
As a preliminary step, bivariate correlations between topography variables were examined. When two variables were highly correlated (r > 0.80), one of the variables was eliminated. Determination of which variable to retain when two were correlated was based on the strength of their associations with the remaining variables. Remaining variables were subjected to a principal components analysis with varimax rotation. Estimated factor score coefficients were used to create standardized composite variables, which have a mean of zero and a standard deviation of one.
Of the 13 topography variables measured, five were eliminated to reduce redundancy in the item pool. The remaining eight variables were subjected to a principal components analysis. One variable, total puff volume, loaded highly on both factors 2 and 3, and was therefore eliminated from the item pool. As shown in Table 1, the final principal components analysis with varimax rotation yielded a three-factor solution. The three factors and the resulting standardized variables were labeled “number of puffs,” “flow rate,” and “puff intensity,” respectively.
Table 1.
Average values and rotated factor loadings of smoking topography variables.
| Smoking Topography Variables | M | SD | Factor Loadings
|
||
|---|---|---|---|---|---|
| Factor 1: Number of Puffs | Factor 2: Flow Rate | Factor 3: Puff Intensity | |||
| Number of Puffs (on All Cigarettes) | 35.68 | 21.02 | .969 | −.043 | −.126 |
| Number of Puffs on First Cigarette | 12.85 | 4.64 | .791 | .010 | .124 |
| Number of Cigarettes Smoked During Entire Session | 2.97 | 1.03 | .740 | −.141 | −.078 |
| Mean Flow Rate (mL/s) of First Cigarette | 33.01 | 9.19 | −.034 | .943 | −.083 |
| Peak Flow Rate (mL/s) of First Cigarette | 46.55 | 14.21 | −.113 | .938 | .092 |
| Mean Puff Duration (s) of First Cigarette | 1.97 | 2.20 | .042 | −.250 | .939 |
| Mean Puff Volume (mL) for Entire Session | 52.17 | 23.54 | −.102 | .264 | .919 |
Note: The smoking topography variables entered into the principal components analysis are listed along with the unit of measure, mean (M), and standard deviation (SD) for the entire sample. The principal components analysis yielded three factors, which were entitled, “number of puffs,” “flow rate,” and “puff intensity.” Mean and standard deviation scores represent raw data prior to factor analysis and standardization, which yielded three standardized factor scores with a mean of zero and a standard deviation of one.
Partial correlations were used to examine associations between ovarian hormone measures and the smoking variables, controlling for age and FTND scores because both age and FTND were significantly associated with the smoking variables (p’s<.05). The hormone variables included: 1) absolute hormone levels at T2; 2) the ratio of progesterone to estradiol (P:E) and the ratio of estradiol to progesterone (E:P); and 3) the change in hormone levels between study visits T1 and T2 (as outlined in section 2.5). In order to examine which hormone variable best predicts smoking behavior, the difference between effect sizes was directly tested using methods described by Meng, Rosenthal, & Rubin (1992). In addition, hierarchical linear regression analyses were used to determine the amount of variance in smoking behavior accounted for by ovarian hormones. Age and FTND were entered into the first block, and the ovarian hormone variable of interest was entered into the second block.
Results
Participant Characteristics
On average, participants (N=98) were 31.2 (SD=7.6) years old. Almost all (97%) were non-Hispanic, and the racial composition of the participants was 79% White, 16% African-American, 2% Native Hawaiian or Pacific Islander, and 3% other. Most participants had completed high school (60%), and an additional 32% had completed college. The average FTND score was 4.8 (SD=2.3). The mean (SD) estradiol level was 128.5 pg/mL (72.7) at T1 and 95.9 pg/mL (69.9) at T2. The mean (SD) progesterone level was 6.4 ng/mL (7.1) at T1 and 4.7 ng/mL (6.2) at T2. Reference ranges adapted for the Siemens Advia Centaur Immunoassay System used in this study suggest that progesterone levels less than 1.5 ng/mL are indicative of the follicular phase in premenopausal women, whereas levels greater than 1.6 ng/mL are indicative of the luteal phase. Based on these ranges, we estimate that at T1, 63% of participants were in the luteal phase and 37% were in the follicular phase; and at T2, 51% were in the luteal phase and 49% were in the follicular phase.
Ovarian Hormones and Smoking Behaviors
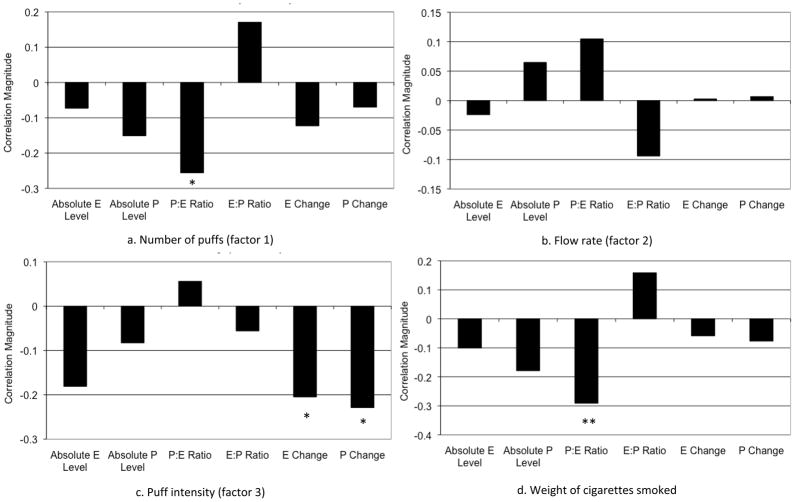
Average values of the smoking topography measures prior to standardization are shown in Table 1. Partial correlations between the hormone variables, the three standardized smoking topography composite variables, and the boost in CO levels, controlling for age and FTND, are shown in Figure 2. The boost in CO levels during the topography session was not significantly associated with any of the hormone variables and is not included in the figure. In addition, absolute levels of estradiol and progesterone were not significantly associated with any of the smoking variables.
Figure 2.
Correlations between ovarian hormone measures and smoking behaviors. E = estradiol. P = progesterone. Absolute P Level = absolute level at T2. Absolute E level = absolute level at T2. P:E Ratio = ratio of progesterone to estradiol at T2. E:P Ratio = ratio of estradiol to progesterone at T2. E Change = absolute change in estradiol level between T1 and T2. P Change = absolute change in progesterone between T1 and T2.
*p<.05, **p<.01.
Partial correlations between the ratio of progesterone to estradiol and each of the smoking variables, controlling for age and FTND, are shown in Figure 2. The ratio of progesterone to estradiol was negatively correlated with the number of puffs variable (r=−.26, p=.01) and the weight of cigarettes smoked (r=−.29, p=.005). Thus, participants with lower levels of progesterone relative to estradiol took more puffs during the topography session and smoked a greater mass of cigarettes. Hierarchical linear regression demonstrated that the ratio of progesterone to estradiol accounted for 5% of the variance in the weight of cigarettes smoked and 6% of the variance in the number of puffs. In contrast, the ratio of estradiol to progesterone was not significantly correlated with any of the smoking variables.
Correlations between the hormone change indexes and each of the smoking variables are also shown in Figure 2. There was a significant negative association between puff intensity and absolute changes in both estradiol (r=−.21, p=.048) and progesterone (r=−.23, p=.03). Thus, during the time between T1 and study T2, larger decreases in estradiol and progesterone were associated with greater puff intensities during the adlib smoking session. Hierarchical linear regression demonstrated that the change in estradiol and progesterone each accounted for 4% and 5% of the variance in puff intensity, respectively.
Compared to the other hormone variables, the ratio of progesterone to estradiol was the best predictor of the number of puffs (z=−2.3, p=.01) and weight of cigarettes smoked (z=−2.7, p=.004) during the topography session. The estradiol and progesterone change indexes were the best predictors of puff intensity (z=−2.1, p=.02).
Discussion
This study aimed to elucidate the relations between ovarian hormones and smoking behavior. Our study is strengthened by the direct and multivariate assessment of hormone levels at a time when they were temporally congruent with the assessment of smoking behavior, which was also defined in multiple ways. Topography variables were reduced to three meaningful factors: number of puffs, flow rate, and puff intensity. The three factors appear to assess different aspects of smoking behavior, and they were differentially related to the ovarian hormone variables assessed.
The results of this study suggest that both the change in hormone levels over time and the relative ratio of progesterone to estradiol appear to influence smoking behavior, but each to a limited extent. Our results may help to explain inconsistent findings in previous studies and suggest potential mechanisms by which hormones influence nicotine addiction. Decreasing estradiol and decreasing progesterone were also associated with greater puff intensities in the current study. These results are consistent with a previous study by Snively and colleagues that found increased smoking during the late luteal phase (Snively, et al., 2000), which is characterized by both decreasing estradiol and progesterone.
Although variance in smoking behaviors accounted for by ovarian hormone measures seen in this study was small (4–6%), gender differences in nicotine dependence and ovarian hormone function remain an important area of investigation. According to Cohen (1992), r=.10 represents a small effect size, whereas .3 and .5 represent medium and large effect sizes, respectively. Rosenthal (1990) has asserted that even small associations between variables are often meaningful in a practical context. Examples include the “small” associations between smoking and mortality, aspirin use and myocardial infarction, and gender and chronic heart disease (Rutledge & Loh, 2004).
Consistent with the notion that estradiol may increase sensitivity to nicotine, whereas progesterone is protective (Lynch & Sofuoglu, 2010), high levels of progesterone relative to estradiol (i.e., P:E ratio) were associated with a lower number of puffs and a smaller mass of cigarettes smoked. Conversely, low levels of progesterone relative to estradiol were associated with a higher number of puffs and a greater mass of cigarettes smoked. Notably, this effect cannot be explained by the effect of progesterone alone on smoking because the absolute level of progesterone was not significantly associated with smoking. Instead, progesterone appears to reduce smoking behavior only when it is not opposed by estradiol. These findings are consistent with animal and human studies demonstrating diminished interest in nicotine following progesterone administration in the context of low levels of estradiol (Lynch, 2009; Sofuoglu & Mooney, 2009) and diminished relapse rates among female smokers who quit during the luteal phase compared to the follicular phase (Allen, et al., 2008). In this study, the P:E ratio was more strongly correlated with smoking behavior than the E:P ratio, which suggests that the level of progesterone relative to estradiol rather than the reverse should be examined in future studies of smoking behavior.
Results of this study suggest that the best predictors of smoking behavior are the ratio of progesterone to estradiol and the change in ovarian hormones over time. However, the mechanism by which decreasing ovarian hormones influence the volume and duration of cigarette puffs is unclear, as is how a low ratio of progesterone to estradiol influences smoking behavior. Future studies should replicate these findings and explore potential mechanisms by which changes in hormone levels and the ratio of progesterone to estradiol differentially influence smoking behavior.
In the current study, the smoking measures were collected during an adlib smoking session without any alternative reinforcers. The addition of an alternative reinforcer to smoking (e.g., money) may increase the effect size of steroid hormones on smoking behavior in future studies. Moreover, ovarian hormones may affect smoking behaviors in some women more than others, which could explain the small effect sizes detected in the current study. For example, it is well established that some women are differentially sensitive to the effects of ovarian hormones (Rubinow, Schmidt, & Roca, 1998). Moreover, previous research suggests an increased desire to smoke and to relieve negative affect during the late luteal phase only among women with premenstrual symptoms (Allen, et al., 1999). The effects of hormones on smoking may therefore be greater in women who experience premenstrual symptoms. Future studies may identify subpopulations of female smokers acutely sensitive to the effects of ovarian hormones.
This study had certain weaknesses. First, the controlled laboratory environment in which the study was conducted and the inclusion of treatment-seeking women limits the generalizability of the results. Second, estradiol and progesterone were measured relatively infrequently. The hormone change indexes created in this study represent the change in hormone levels between the first and second study visits, which were approximately 11 days apart. Hormones change significantly over the course of 11 days in normally cycling women, and thus, the hormone change index was a very rough indicator of change over time. More refined (e.g., daily) hormonal assessment is preferred, but feasibility may be diminished. Recent advances in hormone assay techniques allow for ovarian hormone detection in saliva rather than plasma. Salivary sampling methods are not only easier and more acceptable to women, but they also detect the unbound or “biologically available” portion of ovarian hormones, allowing for more powerful analyses of associations between hormones and behavioral symptoms (Edler, Lipson, & Keel, 2007; Shirtcliff et al., 2000). Fourth, the effects of the cue reactivity assessment that immediately preceded the adlib smoking procedure are unknown. However, all subjects received the same cue reactivity procedure, regardless of menstrual phase, and thus, it is unlikely that the cue reactivity session had a meaningful effect on the associations between hormones and smoking. Finally, a more direct test of the hypothesis that ovarian hormones influence smoking behavior in women could come from future experimental studies wherein exogenous estradiol and progesterone are administered to female smokers and the subsequent effects on smoking are examined. Future studies could also examine whether nicotine consumption per se or smoking behavior more generally are affected by changing hormone levels by examining differences in behavior when subjects are given denicotinized versus regular nicotine-containing cigarettes.
Advances in this line of research may lead to novel pharmacological treatments for nicotine dependence. For example, women who are less likely to achieve abstinence may benefit from a combination treatment such as varenicline plus transdermal progesterone. Alternatively, reducing the cyclic fluctuations in ovarian hormones with hormonal contraceptives may attenuate the exacerbation of smoking behavior seen in the late luteal phase. An improved understanding of the association between ovarian hormone levels and smoking could be used to enhance behavioral smoking interventions. By helping women to identify predictable biological triggers for smoking, behavioral strategies could be implemented to prepare women for periods of increased urges to smoke.
In conclusion, the ratio of progesterone to estradiol and the change in hormone levels over time were the strongest hormonal predictors of smoking behavior. Ovarian hormone function remains a primary candidate for explaining gender differences in nicotine addiction. Future studies are needed to elucidate the neurobiological mechanisms by which hormones influence smoking and to determine the utility of ovarian hormone manipulation in augmenting women’s response to smoking cessation treatment.
Acknowledgments
This research was supported by grants from NIDA (P50DA016511 Component 4 awarded to Dr. Saladin and Dr. Gray and K23DA020482 awarded to Dr. Carpenter), NICHD (K12HD055885 awarded to Dr. Hartwell), and NCRR (UL1RR029882), which supports the MUSC Clinical and Translational Research Center. NIDA, NICHD, and NCRR had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Dr. Hartwell received research funding for a separate project from Global Research Awards for Nicotine Dependence (GRAND), an independent competitive grants program supported by Pfizer, Inc.
The authors would like to acknowledge Dr. Kathleen Brady for her mentorship and direction of the MUSC Specialized Center for Research (SCOR) on Sex and Gender Factors Affecting Women’s Health. They would also like to thank S. Ashley McCullough, Erin Klintworth, and Jessica Olsen for their assistance with data collection and management.
Footnotes
Drs. Saladin, Gray, Carpenter, and Hartwell designed the study and wrote the protocol. Dr. Schiller conducted literature searches, provided summaries of previous research studies, and conducted the statistical analysis. Dr. Schiller wrote the first draft of the manuscript, and all authors contributed to and have approved the final manuscript.
The authors declare that they have no additional conflicts of interest.
References
- Allen SS, Bade T, Center B, Finstad D, Hatsukami D. Menstrual phase effects on smoking relapse. [Randomized Controlled Trial; Research Support, N.I.H., Extramural] Addiction. 2008;103(5):809–821. doi: 10.1111/j.1360-0443.2008.02146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SS, Hatsukami D, Christianson D, Nelson D. Symptomatology and energy intake during the menstrual cycle in smoking women. [Research Support, U.S. Gov’t, P.H.S.] Journal of substance abuse. 1996;8(3):303–319. doi: 10.1016/s0899-3289(96)90170-4. [DOI] [PubMed] [Google Scholar]
- Allen SS, Hatsukami DK, Christianson D, Nelson D. Withdrawal and pre-menstrual symptomatology during the menstrual cycle in short-term smoking abstinence: effects of menstrual cycle on smoking abstinence. [Clinical Trial, Randomized Controlled Trial, Research Support, U.S. Gov’t, P.H.S.] Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 1999;1(2):129–142. doi: 10.1080/14622299050011241. [DOI] [PubMed] [Google Scholar]
- Beckham JC, Krug LM, Penzien DB, Johnson CA, Mosley TH, Meeks GR, Prather RC. The relationship of ovarian steroids, headache activity and menstrual distress: a pilot study with female migraineurs. [Research Support, U.S. Gov’t, P.H.S.] Headache. 1992;32(6):292–297. doi: 10.1111/j.1526-4610.1992.hed3206292.x. [DOI] [PubMed] [Google Scholar]
- Berlin I, Singleton EG, Pedarriosse AM, Lancrenon S, Rames A, Aubin HJ, Niaura R. The Modified Reasons for Smoking Scale: factorial structure, gender effects and relationship with nicotine dependence and smoking cessation in French smokers. [Clinical Trial, Randomized Controlled Trial, Research Support, Non-U.S. Gov’t] Addiction. 2003;98(11):1575–1583. doi: 10.1046/j.1360-0443.2003.00523.x. [DOI] [PubMed] [Google Scholar]
- Bohadana A, Nilsson F, Rasmussen T, Martinet Y. Gender differences in quit rates following smoking cessation with combination nicotine therapy: influence of baseline smoking behavior. [Clinical Trial Randomized Controlled Trial Research Support, Non-U.S. Gov’t] Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2003;5(1):111–116. doi: 10.1080/1462220021000060482. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D. Constructing validity: Basic issues in objective scale development. Psychological Assessment. 1995;7(3):309–319. doi: 10.1037/1040-3590.7.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112(1):155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJ, Ezzati M. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. [Research Support, U.S. Gov’t, P.H.S.] PLoS medicine. 2009;6(4):e1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBon M, Klesges RC, Klesges LM. Symptomatology across the menstrual cycle in smoking and nonsmoking women. [Comparative Study, Research Support, Non-U.S. Gov’t, Research Support, U.S. Gov’t, P.H.S.] Addictive behaviors. 1995;20(3):335–343. doi: 10.1016/0306-4603(94)00070-f. [DOI] [PubMed] [Google Scholar]
- Edler C, Lipson SF, Keel PK. Ovarian hormones and binge eating in bulimia nervosa. [Research Support, N.I.H., Extramural] Psychological medicine. 2007;37(1):131–141. doi: 10.1017/S0033291706008956. [DOI] [PubMed] [Google Scholar]
- Herzog AG. Psychoneuroendocrine aspects of temporolimbic epilepsy. Part II: Epilepsy and reproductive steroids. Psychosomatics. 1999;40(2):102–108. doi: 10.1016/S0033-3182(99)71255-7. [DOI] [PubMed] [Google Scholar]
- Japuntich SJ, Leventhal AM, Piper ME, Bolt DM, Roberts LJ, Fiore MC, Baker TB. Smoker characteristics and smoking-cessation milestones. [Randomized Controlled Trial Research Support, N.I.H., Extramural] American journal of preventive medicine. 2011;40(3):286–294. doi: 10.1016/j.amepre.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ. Sex and ovarian hormones influence vulnerability and motivation for nicotine during adolescence in rats. [Research Support, Non-U.S. Gov’t] Pharmacology, biochemistry, and behavior. 2009;94(1):43–50. doi: 10.1016/j.pbb.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Sofuoglu M. Role of progesterone in nicotine addiction: evidence from initiation to relapse. [Research Support, N.I.H., Extramural, Research Support, Non-U.S. Gov’t, Research Support, U.S. Gov’t, Non-P.H.S., Review] Experimental and clinical psychopharmacology. 2010;18(6):451–461. doi: 10.1037/a0021265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks JL, Hair CS, Klock SC, Ginsburg BE, Pomerleau CS. Effects of menstrual phase on intake of nicotine, caffeine, and alcohol and nonprescribed drugs in women with late luteal phase dysphoric disorder. [Research Support, Non-U.S. Gov’t, Research Support, U.S. Gov’t, P.H.S.] Journal of substance abuse. 1994;6(2):235–243. doi: 10.1016/s0899-3289(94)90265-8. [DOI] [PubMed] [Google Scholar]
- Meng X-l, Rosenthal R, Rubin DB. Comparing correlated correlation coefficients. Psychological Bulletin. 1992;111(1):172–175. doi: 10.1037/0033-2909.111.1.172. [DOI] [Google Scholar]
- Myers CS, Taylor RC, Moolchan ET, Heishman SJ. Dose-related enhancement of mood and cognition in smokers administered nicotine nasal spray. [Randomized Controlled Trial; Research Support, N.I.H., Intramural] Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33(3):588–598. doi: 10.1038/sj.npp.1301425. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Smoking cessation in women. Special considerations. [Research Support, U.S. Gov’t, P.H.S., Review] CNS drugs. 2001;15(5):391–411. doi: 10.2165/00023210-200115050-00005. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Cole PA, Lumley MA, Marks JL, Pomerleau OF. Effects of menstrual phase on nicotine, alcohol, and caffeine intake in smokers. [Research Support, U.S. Gov’t, P.H.S.] Journal of substance abuse. 1994;6(2):227–234. doi: 10.1016/s0899-3289(94)90253-4. [DOI] [PubMed] [Google Scholar]
- Pozzilli C, Falaschi P, Mainero C, Martocchia A, D’Urso R, Proietti A, Filippi M. MRI in multiple sclerosis during the menstrual cycle: relationship with sex hormone patterns. [Research Support, Non-U.S. Gov’t] Neurology. 1999;53(3):622–624. doi: 10.1212/wnl.53.3.622. [DOI] [PubMed] [Google Scholar]
- Reid RD, Pipe AL, Riley DL, Sorensen M. Sex differences in attitudes and experiences concerning smoking and cessation: results from an international survey. [Research Support, Non-U.S. Gov’t] Patient education and counseling. 2009;76(1):99–105. doi: 10.1016/j.pec.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Robinson JC, Forbes WF. The role of carbon monoxide in cigarette smoking. I. Carbon monoxide yield from cigarettes. Archives of environmental health. 1975;30(9):425–434. doi: 10.1080/00039896.1975.10666743. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. How are we doing in soft psychology? American Psychologist. 1990;45(6):775–777. doi: 10.1037/0003-066X.45.6.775. [DOI] [Google Scholar]
- Rubinow DR, Schmidt PJ, Roca CA. Estrogen-serotonin interactions: implications for affective regulation. [Research Support, Non-U.S. Gov’t, Review] Biological psychiatry. 1998;44(9):839–850. doi: 10.1016/s0006-3223(98)00162-0. [DOI] [PubMed] [Google Scholar]
- Rutledge T, Loh C. Effect sizes and statistical testing in the determination of clinical significance in behavioral medicine research. [Review] Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 2004;27(2):138–145. doi: 10.1207/s15324796abm2702_9. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Schwartz EB, Curran MJ, Booth A, Overman WH. Assessing estradiol in biobehavioral studies using saliva and blood spots: simple radioimmunoassay protocols, reliability, and comparative validity. [Comparative Study, Research Support, Non-U.S. Gov’t, Research Support, U.S. Gov’t, P.H.S.] Hormones and behavior. 2000;38(2):137–147. doi: 10.1006/hbeh.2000.1614. [DOI] [PubMed] [Google Scholar]
- Snively TA, Ahijevych KL, Bernhard LA, Wewers ME. Smoking behavior, dysphoric states and the menstrual cycle: results from single smoking sessions and the natural environment. [Clinical Trial, Randomized Controlled Trial, Research Support, Non-U.S. Gov’t, Research Support, U.S. Gov’t, P.H.S.] Psychoneuroendocrinology. 2000;25(7):677–691. doi: 10.1016/s0306-4530(00)00018-4. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK. Progesterone treatment during the early follicular phase of the menstrual cycle: effects on smoking behavior in women. [Clinical Trial, Randomized Controlled Trial, Research Support, U.S. Gov’t, P.H.S.] Pharmacology, biochemistry, and behavior. 2001;69(1–2):299–304. doi: 10.1016/s0091-3057(01)00527-5. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mitchell E, Mooney M. Progesterone effects on subjective and physiological responses to intravenous nicotine in male and female smokers. [Clinical Trial; Research Support, N.I.H., Extramural; Research Support, U.S. Gov’t, Non-P.H.S.] Human psychopharmacology. 2009;24(7):559–564. doi: 10.1002/hup.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Mooney M. Subjective responses to intravenous nicotine: greater sensitivity in women than in men. [Research Support, N.I.H., Extramural, Research Support, U.S. Gov’t, Non-P.H.S.] Experimental and clinical psychopharmacology. 2009;17(2):63–69. doi: 10.1037/a0015297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Mouratidis M, Mooney M. Progesterone improves cognitive performance and attenuates smoking urges in abstinent smokers. [Randomized Controlled Trial, Research Support, N.I.H., Extramural, Research Support, U.S. Gov’t, Non-P.H.S.] Psychoneuroendocrinology. 2011;36(1):123–132. doi: 10.1016/j.psyneuen.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDHS. Vital signs: current cigarette smoking among adults aged >or=18 years ---United States, 2009. MMWR Morbidity and mortality weekly report. 2010;59(35):1135–1140. [PubMed] [Google Scholar]