Abstract
Chronic neck pain affects up to 70% of persons, with the facet joint being the most common source. Intra-articular injection of the non-steroidal anti-inflammatory drug ketorolac reduces post-operative joint-mediated pain; however, the mechanism of its attenuation of facet-mediated pain has not been evaluated. Protease-activated receptor-1 (PAR1) has differential roles in pain maintenance depending on the type and location of painful injury. This study investigated if the timing of intra-articular ketorolac injection after painful cervical facet injury affects behavioral hypersensitivity by modulating spinal astrocyte activation and/or PAR1 expression. Rats underwent a painful joint distraction and received an injection of ketorolac either immediately or 1 day later. Separate control groups included injured rats with a vehicle injection at day 1 and sham operated rats. Forepaw mechanical allodynia was measured for 7 days, and spinal cord tissue was immunolabeled for glial fibrillary acidic protein (GFAP) and PAR1 expression in the dorsal horn on day 7. Ketorolac administered on day 1 after injury significantly reduced allodynia (p=0.0006) to sham levels, whereas injection immediately after the injury had no effect compared with vehicle. Spinal astrocytic activation followed behavioral responses and was significantly decreased (p=0.009) only for ketorolac given at day 1. Spinal PAR1 (p=0.0025) and astrocytic PAR1 (p=0.012) were significantly increased after injury. Paralleling behavioral data, astrocytic PAR1 was returned to levels in sham only when ketorolac was administered on day 1. Yet, spinal PAR1 was significantly reduced (p<0.0001) by ketorolac independent of timing. Spinal astrocyte expression of PAR1 appears to be associated with the maintenance of facet-mediated pain.
Key words: astrocyte, facet joint, ketorolac, pain, PAR1
Introduction
Persistent neck pain is a major cause of disability, affecting between 10% and 70% of persons worldwide.1,2 The cervical facet joint is a common source of neck pain identified in clinical studies,3,4 with mechanical reports also documenting nociceptor innervation of the capsule and its mechanical vulnerability for injury from non-physiologic joint and spine motions.5–7 Despite reports suggesting that injury to the cervical facets is a major contributor to persistent neck pain,8 the cellular mechanisms by which pain is initiated and maintained after joint injury have yet to be defined.
One source of joint-mediated pain is joint inflammation.9 Painful joint inflammation can be caused by a variety of pathologies including arthritis and is often associated with increased concentrations of prostaglandins within the fluid of the inflamed joint.9 Inflammatory factors within a painful joint can cause direct activation of nociceptive fibers, which can lead to a release of peptides at their synaptic terminals within the superficial laminae of the spinal cord.9 Specifically, painful injury to the cervical facet joints in the rat causes increased production of the peptide, substance P, in the spinal cord.10 There is also evidence of glial activation after facet injury with an increase in astrocyte activation in the dorsal horn as early as 1 week11 and maintained for at least 2 weeks after injury12 following the trends in produced behavioral sensitivity. Although these studies point to a link between peripheral inflammation, spinal glial activation, and facet-mediated pain, no study directly investigates the effect of inflammation in the facet joint on centrally mediated pain.
Protease-activated receptor-1 (PAR1) has been implicated as a potential regulator of inflammatory and neuropathic pain.13,14 PAR1 is a member of a family of four G-protein coupled receptors that are activated by serine proteases and are typically found in high concentrations at an injury site.15,16 Expression of PAR1 has been confirmed on neurons, astrocytes, and microglia, all of which are cells known to contribute to pain.17–21 Yet the role of PAR1 in the initiation and maintenance of pain is still not clear. For example, PAR1 activation via intrathecal injection of exogenous thrombin induces sustained thermal hyperalgesia and tactile allodynia in the mouse,22 whereas plantar injection of thrombin causes an immediate increase in the nociceptive threshold for mechanical stimulation in the rat.23
In addition to PAR1 activation directly affecting pain outcomes, PAR1 expression is modulated after neural tissue trauma; PAR1 mRNA is increased in the sciatic nerve after partial nerve ligation in the rat as early as day 1 after injury,24 and spinal PAR1 protein is increased 1 week after painful sciatic nerve ligation in the mouse.24 Although many studies implicate PAR1 activation and its role in certain types of peripheral neuropathic and inflammatory pain states, no study has determined if PAR1 has a role in joint-mediated pain and whether it can be modulated in the presence or absence of pain. PAR1 is expressed by astrocytes and activated microglia,18,19,21 both of which have been shown to contribute to the initiation and maintenance of pain.25–27
Ketorolac, a non-steroidal anti-inflammatory drug (NSAID) with strong analgesic activity, has been used clinically to treat post-operative, inflammatory, and neuropathic pain.28–32 The primary mechanism of action of ketorolac is by local non-selective inhibition of the activity of cyclooxygenase (COX)-1 and COX-2, which diminishes prostaglandin production, and is associated with a reduction in behavioral sensitivity.33–35 Intra-articular ketorolac injection reduces joint inflammation and postoperative pain in the knee in both clinical and animal models.30,36,37 Although the local anti-inflammatory action of ketorolac in the periphery may contribute to its analgesic effects, other studies report that ketorolac reduces excitatory peptides in the dorsal horn after partial sciatic nerve ligation, which has been correlated with a reduction in pain.38 Our laboratory has shown that a single joint injection of ketorolac given after a painful facet joint injury can attenuate pain only when it is given after pain has developed, although the specific mechanism of action was not determined.39
The current study aims to determine if, and how, local ketorolac treatment after a painful facet joint injury can reduce pain. Because of its speculated role in pain and its expression by glial cells, PAR1 was also evaluated in the spinal cord to understand if it has a role in joint-mediated pain. As such, a model of painful mechanical facet joint loading was used,10,12,40,41 and a single bilateral intra-articular injection of ketorolac was given immediately or at day 1 after injury. Behavioral sensitivity was monitored for 7 days after injury, and astrocytic activation, spinal PAR1 expression, and astrocytic expression of PAR1 were evaluated at day 7 in the spinal dorsal horn. Glial fibrillary acidic protein (GFAP) was used as an indicator of astrocytic activation and has been shown to be increased in association with behavioral sensitivity in this model of facet joint injury.11,12,25
Methods
Male Holtzman rats weighing 375–425 g were housed under United States Department of Agriculture- and Association for Assessment and Accreditation of Laboratory Animal Care-compliant conditions, with a 12–12 h light-dark cycle and free access to food and water. All procedures were performed according to the guidelines of the University of Pennsylvania Institutional Animal Care and Use Committee, the National Research Council for the care and use of laboratory animals, and the Committee for Research and Ethical Issues of the International Association for the Study of Pain.42 Surgical procedures were performed under inhalation isoflurane anesthesia (4% for induction, 2.5% for maintenance). According to previously described methods,41,43 the bilateral cervical facet joints and their capsules were exposed at C6/C7 by clearing the surrounding musculature and transecting the interspinous ligaments. A controlled bilateral facet joint distraction was imposed across the facet capsule using a customized loading device that distracted the C6 joint rostrally in a controlled fashion that has been shown to reliably produce persistent pain symptoms.10,41,43 The severity of each joint injury was measured using imaging to track the motions of markers affixed to the laminar bones of the joint during distraction.12,40 Sham procedures were also performed in separate rats (sham, n=5) undergoing surgery but with no joint distraction to serve as controls. After surgery, wounds were closed with 3-0 polyester suture and surgical staples. Rats were allowed to recover in room air with controlled temperature and humidity and were monitored continuously throughout the study period.
Rats that underwent the painful facet joint distraction injury were randomly selected to receive treatment in the joint either on day 0 or day 1 after injury. The group undergoing ketorolac (Sigma-Aldrich; St. Louis, MO) treatment on day 0 received a joint injection of 12 μg of ketorolac in 10 μL sterile H2O (ketorolac d0; n=5); two groups received treatment at 1 day after the initial injury: either a joint injection of 10 μL sterile H2O (vehicle; n=4) or a joint injection of 12 μg of ketorolac in 10 μL sterile H2O (ketorolac d1; n=6). Under inhalation anesthesia (2.5% isoflurane), ketorolac was administered via intra-articular injection in the bilateral C6/C7 facet joints using a 10 μL syringe with a 33-gauge needle (Hamilton; Reno, NV). This volume and needle size were optimized based on pilot studies indicating that 10 μL is the maximum volume of fluid that can be contained in the cervical facet joint space and that a 33-gauge needle is sufficiently small not to induce ligament damage or fluid leakage from the injection. During an injection, the needle was gently inserted into the facet joint by piercing through its capsule in the dorsal medial region of the capsular ligament. After injection, the needle was withdrawn and the incision was cleaned with betadine (Purdue Pharma; Stamford, CT) before the wound was closed with polyester suture and surgical staples.
Mechanical allodynia was measured in the bilateral forepaws of all rats to characterize the patterns of behavioral sensitivity after injury and treatment, on days 1, 3, 5, and 7 after injury. Baseline measurements were also recorded on day 0 before the surgical procedure for each rat as a matched un-operated control for its responses after injury. For groups receiving a joint injection with either vehicle or ketorolac treatment at day 1 (ketorolac d1), additional testing was performed at day 2 to measure allodynia on the day immediately after treatment. Methods for quantifying forepaw allodynia used in this study have been previously validated.44–46
Briefly, rats were acclimated to the environment and tester before undergoing any behavioral assessment. Each testing session consisted of three rounds of 10 tactile stimulations to the plantar surface of each forepaw using a 4 g von Frey filament (Stoelting; Wood Dale, IL). A positive response was counted when the rat immediately withdrew its paw on stimulation, usually accompanied by licking or tightening of the paw. For each testing session, the total number of paw withdrawals for each paw was counted. Responses for the left and right paws were averaged for each rat on every testing day and further averaged within groups. Data are expressed as the average total number of paw withdrawals with the standard deviation. Repeated measures analysis of variance (ANOVA) with the Tukey honestly significant difference test was used to compare allodynia responses across all groups for days 1, 3, 5, and 7. An additional comparison between vehicle and ketorolac d1 groups was performed using repeated measures ANOVA with the Tukey correction for days 1, 2, 3, 5, and 7.
After behavioral assessment on day 7, spinal cord tissue at the C5 level was harvested to evaluate astrocytic activation by assessing GFAP immunolabeling and PAR1 expression. After transcardiac perfusion, tissue was post-fixed for 1 h followed by cryopreservation in 30% sucrose/phosphate buffered saline and stored for 3 days at 4°C. Spinal cord tissue was then freeze-mounted with Histoprep (Fisher Diagnostic; Fair Lawn, NJ). Thin cryosections (16 μm, n=4 sections per animal) were mounted onto APES-slides for staining. Slides were incubated in mouse anti-GFAP (1:1000; Wako; Richmond, VA) or rabbit anti-PAR1 (1:250; Abcam, Cambridge, MA) overnight at 4°C, followed by incubation with either goat anti-mouse Alexa 546 (1:150; Invitrogen, Carlsbad, CA) or goat anti-rabbit Alexa 488 (1:250; Invitrogen, Carlsbad, CA) before cover-slipping. Co-labeling of GFAP and PAR1 was performed by incubating the slides simultaneously in mouse anti-GFAP (1:1000) and rabbit anti-PAR1 (1:250) overnight at 4°C, followed by incubation in both goat anti-mouse Alexa 546 (1:150) and goat anti-rabbit Alexa 488 (1:250) before cover-slipping.
Spinal cord sections were imaged using a Carl Zeiss LSM 510 microscope (Carl Zeiss LLS; Thronwood, NY). Images were cropped to include a region of interest in the superficial dorsal horn before GFAP or PAR1 expression was quantified using a customized densitometry program created in MATLAB (matrix laboratory), as previously reported.12,44,47,48 Briefly, spinal cord sections were imaged at 20X magnification, and images were cropped to a uniform region of interest including only the dorsal horn tissue. Cropped images were then inverted, and the percentage of pixels above a pre-defined threshold was measured. That threshold was set based on levels of GFAP and PAR1 in normal un-operated tissue. The number of positive pixels for GFAP and PAR1 was divided by the number of pixels defining the total area of tissue for each image. Data were expressed as the mean percentage of positive pixels normalized to percentages in un-operated tissue for each group with the standard deviation. For all immunohistochemistry assays, negative controls with no primary antibody were included for verification of staining technique and analyses. Normalized group averages (sham, vehicle, ketorolac d0, ketorolac d1) were compared using one-way ANOVA with post-hoc Bonferroni correction.
To quantify the astrocytic expression of PAR1, a customized MATLAB program was used to measure the co-labeling of PAR1 with GFAP in spinal cord tissue.39 The percent co-localization was defined by the percentage of pixels that were positively labeled for both PAR1 and GFAP within a given section. Data were represented as the mean percent co-localization for each group normalized to the percent of PAR1 and GFAP co-localization in normal un-operated tissue with the standard deviation. Differences between normalized group averages were determined using one-way ANOVA with a post-hoc Tukey test.
Results
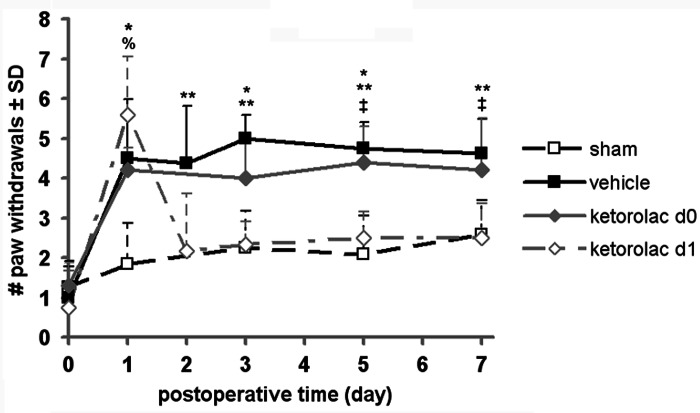
The injury severity that was imposed was similar for all rats undergoing any joint distraction. Mean applied joint distraction was 0.64±0.12 mm for vehicle, 0.64±0.05 mm for ketorolac d0, and 0.75±0.11 mm for ketorolac d1, with the ketorolac d1 group undergoing significantly greater (p=0.032) joint distractions than either of the two other groups. Despite this difference in applied injury magnitude between the two treatment groups, allodynia for all injury groups (vehicle, ketorolac d0, and ketorolac d1) at day 1 was significantly (p<0.006) higher than sham and not different between any injury group (Fig. 1). Mechanical allodynia after facet distraction for the vehicle treated group remained significantly elevated above sham on each day of testing (p<0.013) and was significantly different from sham overall (p=0.0003) (Fig. 1). Further, injection of ketorolac in the injured joint on day 1 (ketorolac d1) produced a significant (p<0.008) immediate reduction in allodynia at day 2 that was sustained for 7 days compared with vehicle treatment; this attenuation of sensitivity was significant (p=0.0006) (Fig. 1). Forepaw sensitivity for the group receiving treatment immediately after injury (ketorolac d0) remained unchanged from vehicle and was significantly greater than sham (p=0.0014). Ketorolac d0 also exhibited more mechanical allodynia than the group that received treatment on day 1 (ketorolac d1; p=0.005) and on days 5 and 7 (p<0.047).
FIG. 1.
Average forepaw mechanical allodynia after facet joint injury with ketorolac or vehicle treatment or sham procedures. Allodynia is significantly elevated over sham on day 1 for all injury groups and remains elevated for vehicle (p<0.013) and ketorolac d0 (*p<0.0356). Allodynia for ketorolac d1 is significantly (%p<0.0001) elevated over sham on day 1 but is significantly reduced by day 2 compared with vehicle (**p<0.008) and by day 5 compared with ketorolac d0 (‡p<0.0474). Data are plotted as mean±standard deviation (SD).
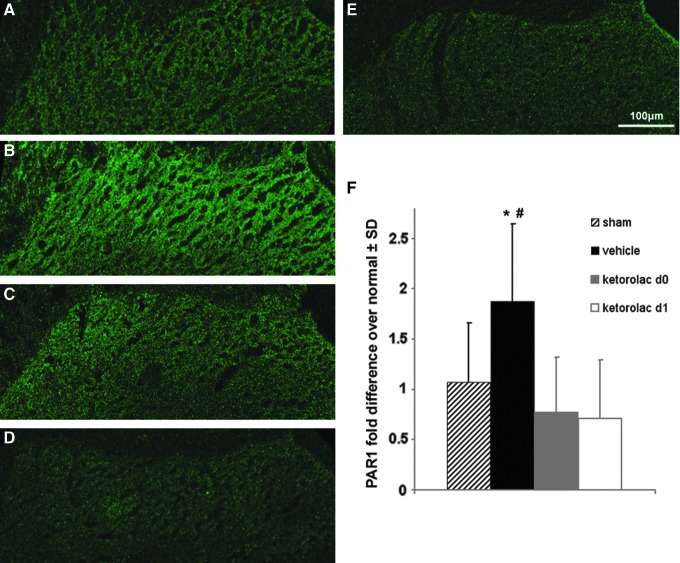
GFAP expression on day 7 in the superficial dorsal horn of the spinal cord paralleled the behavioral response patterns observed between groups (Fig. 2). Spinal GFAP expression in the vehicle treated group was significantly (p=0.037) increased over sham at day 7 (Fig. 2). Rats receiving ketorolac treatment on day 1 (ketorolac d1) also exhibited GFAP expression levels that were significantly (p=0.009) lower than those levels in vehicle treated rats; however, this same reduction was not evident in the rats treated on day 0 (ketorolac d0; Fig. 2). Further, GFAP expression was not different between ketorolac d1 and sham groups at day 7. Although GFAP in the ketorolac d0 group was elevated above ketorolac d1, this was not significant (Fig. 2).
FIG. 2.
Immunolabeling of glial fibrillary acidic protein (GFAP) in the spinal dorsal horn at day 7 after vehicle, ketorolac treatments, and sham. GFAP immunolabeling is less in sham (A) compared with vehicle (B), and ketorolac d1 (C) levels are similar to sham. GFAP levels for ketorolac d0 (D) are similar to vehicle (B) levels. The GFAP labeling in both sham (A) and ketorolac d1 (C) is not different from levels in un-operated naïve control tissue (E). (F) Normalized quantification of the extent of GFAP expression indicates that expression in vehicle is elevated over sham (*p=0.037) and is reduced after ketorolac d1 compared with vehicle (**p=0.009). Data are shown as normalized mean±standard deviation (SD); the scale bar (100 μm) applies to all images. Color image is available online at www.liebertpub.com/neu
Similar to the increase in spinal GFAP for vehicle relative to sham, the PAR1 expression in the superficial dorsal horn in the vehicle group was significantly (p=0.0025) elevated over sham on day 7 after injury (Fig. 3). In contrast, spinal PAR1 levels were significantly (p<0.0001) reduced for both groups treated with ketorolac regardless of whether it was on day 1 or on day 0 (Fig. 3). In addition, PAR1 expression was not different from sham for either of those treated groups at that time point, with all groups exhibiting variability that is evident by the quantification (Fig. 3F).
FIG. 3.
Immunolabeling of protease-activated receptor (PAR)1 in the spinal dorsal horn at day 7 after vehicle, ketorolac treatments, and sham. On day 7, PAR1 in the dorsal horn is less in sham (A) compared with vehicle (B). Groups treated with ketorolac at the time of injury (ketorolac d0) (C) and at day 1 (ketorolac d1) (D) exhibit PAR1 staining similar to sham (A). Sham (A) expression levels are similar to tissue from un-operated naïve controls (E). (F) Quantification of the normalized percent positive pixels of PAR1 is significantly (*p=0.0025) increased for vehicle over sham at day 7. Treatment with ketorolac at either time point (ketorolac d0; ketorolac d1) significantly (#p<0.0001) decreases PAR1 levels compared with vehicle. Data are represented as normalized mean±standard deviation (SD). Color image is available online at www.liebertpub.com/neu
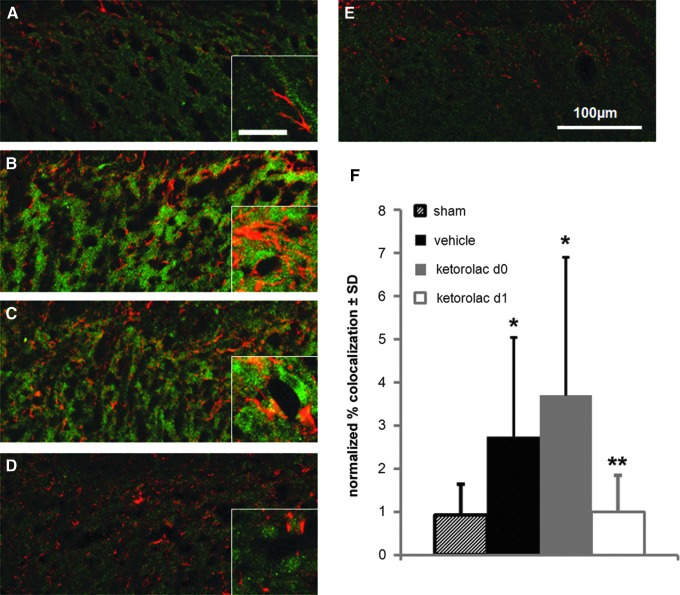
Astrocytic PAR1, taken as the co-localization of GFAP and PAR1, in sham operated rats was unchanged from levels in normal un-operated rats (Fig. 4). Rats that received facet injury with a vehicle injection on day 1 exhibited significantly (p=0.012) more astrocytic PAR1 compared with sham (Fig. 4). Similarly, rats treated with ketorolac immediately after injury (ketorolac d0) had significantly (p<0.0001) more co-localization of PAR1 and GFAP compared with sham (Fig. 4). Of note, the degree of PAR1 and GFAP co-localization in both the ketorolac d0 and vehicle groups exhibited variability across rats (Fig. 4F). Astrocytic PAR1 was reduced to sham levels only after ketorolac administration on day 1 after injury (ketorolac d1) and was significantly (p<0.0077) reduced for ketorolac d1 compared with both vehicle and ketorolac d0.
FIG. 4.
Quantification of astrocytic PAR1 in the spinal dorsal horn at day 7. Astrocytic PAR1 (yellow), denoted by the co-localization of PAR1 (green) and GFAP (red), is lower in sham (A) compared with vehicle (B). Levels of PAR1 and GFAP co-localization are similar between vehicle (B) and ketorolac d0 (C) and ketorolac d1 (D) appears to be less than both of these groups. Levels of co-localization in normal (E) tissue are similar to both sham (A) and ketorolac d1 (D). The scale bar (100 μm) for the full-size images applies to all, and the scale bar for the inset images is 20 μm. (F) Quantification of normalized percent co-localization of PAR1 and glial fibrillary acidic protein is significantly increased for vehicle (*p=0.012) and ketorolac d0 (*p<0.0001) compared with sham. Treatment with ketorolac at day 1 after injury (ketorolac d1) significantly (**p<0.0077) reduces co-localization compared with vehicle and ketorolac d0. Data represented as mean±standard deviation (SD). Color image is available online at www.liebertpub.com/neu
Discussion
This study demonstrates that a delayed intra-articular injection of the NSAID ketorolac produces immediate and sustained attenuation of allodynia after painful facet injury that can be at least partially attributed to a decrease in spinal astrocyte activation and a decrease in astrocytic expression of PAR1 after injury (Figs. 1 and 4). Spinal PAR1 expression, not localized to a specific cell type, is increased at day 7 after painful facet joint injury and is at least partially attributable to an increase in astrocyte expression of the receptor (Figs. 3 and 4), which is consistent with increased spinal PAR1 reported after a painful peripheral nerve injury.22 Peripheral ketorolac administration at either the immediate or delayed time point (day 1) after facet injury was able to reverse this increase in spinal PAR1 to levels observed in normal un-operated rats (Fig. 3); however, this was the case regardless of whether there was any change in allodynia or astrocytic response (Figs. 1–3).
Co-labeling of PAR1 and GFAP indicates that an increase in PAR1 expression localized to astrocytes is increased at day 7 after a painful injury and is only reduced to levels in sham operated rats when that injury is treated with ketorolac after the development of pain (Fig. 4). This attenuated spinal astrocytic PAR1 expression parallels both the behavioral and GFAP expression data (Figs. 1, 2, and 4). Although the modulation of total spinal PAR1 may not be directly related to pain after facet joint injury, the co-localization studies suggest that spinal astrocytic expression of PAR1 may be specifically related to, or responsible for, pain from the facet joint.
The timing of administration of ketorolac affects the attenuation of allodynia (Fig. 1). When ketorolac treatment is delayed until after sensitivity is produced (at day 1), allodynia is reduced immediately and remains at sham levels for up to 7 days; when it is injected before the development of pain (at the time of injury; day 0), allodynia is not reduced even transiently (Fig. 1). These behavioral findings suggest that the analgesic effects of this anti-inflammatory agent are time-dependent relative to the onset of pain and/or the injury event and are supported by a study that demonstrated that ketorolac given after partial sciatic nerve ligation was effective at immediately attenuating tactile allodynia only when administered after the development of pain and inflammation.49 Ketorolac has been shown to reduce tactile allodynia within 3 hours and for at least 5 days if it is administered at the peak of inflammation in an injured nerve.38,49,50
Although the early inflammatory responses have not been defined for the facet injury model used here, substance P is modulated in the spinal cord by day 1,10 and glial activation develops in this model associated with the presence of behavioral sensitivity.11,12 In fact, these time points coincide with the onset and modification of painful behaviors in the current study (Fig. 1). Because ketorolac given at day 1 was sufficient to attenuate pain in this model and in neuropathic pain,49 it may be possible that this NSAID might be more effective in alleviating joint-mediated pain once inflammatory symptoms have developed. This study, however, did not evaluate the time course of inflammation or PAR1 expression in the joint or dorsal root ganglion (DRG). As such, it is not known whether the primary action of ketorolac was not in the joint. Nonetheless the strong behavioral findings suggest it to be effective at attenuating traumatically induced joint pain when administered early on after trauma.
Future studies defining the longer term outcomes and more widespread tissue responses, as well as the potential clinical efficacy of intra-articular delivery at later time points after injury and the feasibility of systemic administration of ketorolac on facet-induced pain would help determine the mechanism(s) by which this treatment may attenuate pain in this model.
Ketorolac is postulated to attenuate pain by decreasing COX-mediated inflammation locally at the administration site, which reduces the sensitivity of injured afferents and can lead to biochemical changes in the spinal cord where these afferents synapse.9,38,51,52 The facet capsule that encloses the facet joint is innervated by nociceptors, and non-physiologic stretch of the facet capsule has been shown to activate those receptors and to induce neuronal hyperexcitability in the dorsal horn at day 7 after injury.5–7,43 Injured afferents release excitatory neuropeptides, such as substance P, glutamate, and calcitonin gene-related peptide, at their terminals that directly activate astrocytes and other glial cells in the spinal cord that can amplify neuronal excitability via the release of pro-inflammatory cytokines and prostaglandins.25–27
Glial activation was observed here at 7 days after painful facet joint distraction (Fig. 2), which has been reported previously for this model.11 Further, delayed intra-articular ketorolac injection reduced spinal astrocyte activation on day 7 after injury in association with a decrease in sensitivity, whereas immediate ketorolac treatment did not (Fig. 2). Because ketorolac injection on day 1 reduced astrocytic activation in the dorsal horn and attenuated allodynia, these findings suggest that it might also reduce other central mechanisms associated with persistent pain, such as neuronal hyperexcitability.
Painful facet joint injury increases PAR1 expression in the spinal dorsal horn that is abolished by a single intra-articular injection of ketorolac at either time point after injury (Fig. 3). Because a decrease in total spinal PAR1 expression is observed despite behavioral sensitivity and spinal glial activation only being reduced for the delayed treatment (Figs. 1–3), this suggests that PAR1 may not specifically contribute to facet-induced pain. Although PAR1 has not been studied explicitly in the context of joint pain, PAR1 protein and mRNA have been shown to increase in the spinal cord after neuropathic pain caused by sciatic nerve ligation.22,24 Although this study did not measure PAR1 expression in any cell type in the injured joint or in the DRG, astrocytic PAR1 is increased in the spinal cord after injury (Fig. 4).
Although PAR1 expression has not been measured previously in activated astrocytes, the activation of PAR1 on primary rat astrocytes in vitro has been shown to lead to the release of intracellular calcium stores, phosphorylation of extracellular signal-regulated kinase53, and production of matrix metalloproteinases,21,53 which suggests a potentially strong role of astrocytic PAR1 in pain. Considering the findings that spinal astrocytic PAR1 increases in the dorsal horn by day 7 after painful facet injury and is reduced only when ketorolac is administered on day 1 after injury and in association with attenuation of behavioral sensitivity (Figs. 1 and 4), astrocytic PAR1 may be more closely related to facet-mediated pain than general spinal PAR1. Further studies are needed to define the other cellular responses and their relationship to PAR1 in the spinal cord.
Acknowledgments
This work was funded in part by grants from the National Institutes of Health/National Institute of Arthritis, Musculoskeletal and Skin Diseases (#AR056288), the Department of Defense (#W81XWH-10-1-1002), and the Catharine D. Sharpe Foundation.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Haldeman S. Carroll L. Cassidy J.D. Schubert J. Nygren A. The Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders: Executive summary. Spine. 2008;33(Suppl):S5–S7. doi: 10.1097/BRS.0b013e3181643f40. [DOI] [PubMed] [Google Scholar]
- 2.Lidgren L. Preface: Neck pain and the decade of the bone and joint 2000–2010. Spine. 2008;33(Suppl):S1–S2. doi: 10.1016/j.jmpt.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Barnsley L. Lord S.M. Wallis B.J. Bogduk N. The prevalence of chronic cervical zygapophysial joint pain after whiplash. Spine. 1995;20:20–25. doi: 10.1097/00007632-199501000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Lord S.M. Barnsley L. Wallis B.J. Bogduk N. Chronic cervical zygapophysial joint pain after whiplash. A placebo-controlled prevalence study. Spine. 1996;21:1737–1745. doi: 10.1097/00007632-199608010-00005. [DOI] [PubMed] [Google Scholar]
- 5.Inami S. Shiga T. Tsujino A. Yabuki T. Okado N. Ochiai N. Immunohistochemical demonstration of nerve fibers in the synovial fold of the human cervical facet joint. J. Orthop. Res. 2001;19:593–596. doi: 10.1016/S0736-0266(00)00048-6. [DOI] [PubMed] [Google Scholar]
- 6.Kallakuri S. Singh A. Lu Y. Chen C. Patwardhan A. Cavanaugh J.M. Tensile stretching of cervical facet joint capsule and related axonal changes. Eur. Spine J. 2008;17:556–563. doi: 10.1007/s00586-007-0562-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLain R.F. Mechanoreceptor endings in human cervical facet joints. Spine. 1994;19:495–501. doi: 10.1097/00007632-199403000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Cavanaugh J.M. Ozaktay A.C. Yamashita H.T. King A.L. Lumbar facet pain: biomechanics, neuroanatomy and neurophysiology. J. Biomech. 1996;29:1117–1129. doi: 10.1016/0021-9290(96)00023-1. [DOI] [PubMed] [Google Scholar]
- 9.Kidd B.L. Morris V.H. Urban L. Pathophysiology of joint pain. Ann. Rheum. Dis. 1996;55:276–283. doi: 10.1136/ard.55.5.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee K.E. Winkelstein B.A. Joint distraction magnitude is associated with different behavioral outcomes and substance P levels for cervical facet joint loading in the rat. J. Pain. 2009;4:436–445. doi: 10.1016/j.jpain.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Winkelstein B.A. Santos D.G. An intact facet capsular ligament modulates behavioral sensitivity and spinal glial activation produced by cervical facet joint tension. Spine. 2008;33:856–862. doi: 10.1097/BRS.0b013e31816b4710. [DOI] [PubMed] [Google Scholar]
- 12.Lee K.E. Davis M.B. Mejilla R.M. Winkelstein B.A. In vivo cervical facet capsule distraction: mechanical implications for whiplash and neck pain. Stapp Car Crash J. 2004a;48:373–395. doi: 10.4271/2004-22-0016. [DOI] [PubMed] [Google Scholar]
- 13.Garcia P.S. Gulati A. Levy J.H. The role of thrombin and protease activated receptors in pain mechanisms. Thromb. Haemost. 2010;103:1145–1151. doi: 10.1160/TH09-12-0848. [DOI] [PubMed] [Google Scholar]
- 14.Suo Z. Citron B.A. Festoff B.W. Thrombin: a potential proinflammatory mediator in neurotrauama and neurodegenerative disorders. Curr. Drug Targets Inflamm. Allergy. 2004;3:105–114. doi: 10.2174/1568010043483953. [DOI] [PubMed] [Google Scholar]
- 15.Coughlin S.R. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 16.Déry O. Corvera C.U. Steinhoff M. Bunnett N.W. Proteinase-activated receptors: novel mechanisms of signaling by proteases. Am. J. Physiol. 1998;274:C1429–C1452. doi: 10.1152/ajpcell.1998.274.6.C1429. [DOI] [PubMed] [Google Scholar]
- 17.Junge C.E. Lee C.J. Hubbard K.B. Zhang Z. Olson J.J. Hepler J.R. Brat D.J. Traynelis S.F. Protease-activated receptor-1 in human brain: localization and functional expression in astrocytes. Exp. Neurol. 2004;188:94–103. doi: 10.1016/j.expneurol.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 18.Pompili E. Fabrizi C. Nori S.L. Panetta B. Geloso M.C. Corvino V. Michetti F. Fumagalli L. Protease-activated receptor-1 expression in rat microglia after trimethyltin treatment. J. Histochem. Cytochem. 2011;59:302–311. doi: 10.1369/0022155410397996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shavit E. Michaelson D.M. Chapman J. Anatomical localization of protease-activated receptor-1 and protease-mediated neuroglial crosstalk on peri-synaptic astrocytic endfeet. J. Neurochem. 2011;119:460–473. doi: 10.1111/j.1471-4159.2011.07436.x. [DOI] [PubMed] [Google Scholar]
- 20.Vellani V. Kinsey A.M. Prandini M. Hechtfischer S.C. Reeh P. Magherini P.C. Giacomoni C. McNaughton P.A. Protease activated receptors 1 and 4 sensitize TRPV1 in nociceptive neurones. Mol. Pain. 2010;6:61. doi: 10.1186/1744-8069-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H. Ubl J.J. Reiser G. Four subtypes of protease-activated receptors, co-expressed in rat astrocytes, evoke different physiological signaling. Glia. 2002;37:53–63. doi: 10.1002/glia.10012. [DOI] [PubMed] [Google Scholar]
- 22.Narita M. Usui A. Narita M. Niikura K. Nozaki H. Khotib J. Nagumo Y. Yajima Y. Suzuki T. Protease-activated receptor-1 and platelet-derived growth factor in spinal cord neurons are implicated in neuropathic pain after nerve injury. J. Neurosci. 2005;25:10000–10009. doi: 10.1523/JNEUROSCI.2507-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asfaha S. Brussee V. Chapman K. Zochodne D.W. Vergnolle N. Proteinase-activated receptor-1 agonists attenuate nociception in response to noxious stimuli. Br. J. Pharmacol. 2002;135:1101–1106. doi: 10.1038/sj.bjp.0704568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niclou S.P. Suidan H.S. Pavlik A. Vejsada R. Monard D. Changes in the expression of protease-activated receptor 1 and protease nexin-1 mRNA during rat nervous system development and after nerve lesion. Eur. J. Neurosci. 1998;10:1509–1607. doi: 10.1046/j.1460-9568.1998.00183.x. [DOI] [PubMed] [Google Scholar]
- 25.McMahon S.B. Cafferty W.B. Marchand F. Immune and glial cell factors as pain mediators and modulators. Exp. Neurol. 2005;192:444–462. doi: 10.1016/j.expneurol.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Watkins L.R. Milligan E.D. Maier S.F. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001;24:450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- 27.Watkins L.R. Milligan E.D. Maier S.F. Spinal cord glia: new players in pain. Pain. 2001;93:201–205. doi: 10.1016/S0304-3959(01)00359-1. [DOI] [PubMed] [Google Scholar]
- 28.Cassinelli E.H. Dean C.L. Garcia R.M. Furey C.G. Bohlman H.H. Ketorolac use for postoperative pain management following lumbar decompression surgery: a prospective, randomized, double-blinded, placebo-controlled trial. Spine. 2008;33:1313–1317. doi: 10.1097/BRS.0b013e31817329bd. [DOI] [PubMed] [Google Scholar]
- 29.Kim S.J. Lo W.R. Hubbard G.B., 3rd Srivastava S.K. Denny J.P. Martin D.F. Yan J. Bergstrom C.S. Cribbs B.E. Schwent B.J. Aaberg T.M., Sr. Topical ketorolac in vitreoretinal surgery: a prospective, randomized, placebo-controlled, double-masked trial. Arch. Ophthalmol. 2008;126:1203–1208. doi: 10.1001/archopht.126.9.1203. [DOI] [PubMed] [Google Scholar]
- 30.Ng H.P. Nordström U. Axelsson K. Perniola A.D. Gustav E. Ryttberg L. Gupta A. Efficacy of intra-articular bupivacaine, ropivacaine, or a combination of ropivacaine, morphine, and ketorolac on postoperative pain relief after ambulatory arthroscopic knee surgery: A randomized double-blind study. Reg. Anesth. Pain. Med. 2006;31:26–33. doi: 10.1016/j.rapm.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 31.Turner C.L. Eggleston G.W. Lunos S. Johnson N. Wiedmann T.S. Bowles W.R. Sniffing out endodontic pain: use of an intranasal analgesic in a randomized clinical trial. J. Endod. 2011;37:439–444. doi: 10.1016/j.joen.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 32.Vintar N. Rawal N. Pohar M. Veselko M. Intra-articular patient-controlled analgesia improves early rehabilitation after knee surgery. Coll. Antropol. 2010;34:941–945. [PubMed] [Google Scholar]
- 33.Dogan N. Erdem A.F. Gundogdu C. Kursad H. Kizilkaya M. The effects of ketorolac and morphine on articular cartilage and synovium in the rabbit knee joint. Can. J. Physiol. Pharmacol. 2004;82:502–505. doi: 10.1139/y04-066. [DOI] [PubMed] [Google Scholar]
- 34.Gerstenfeld L.C. Thiede M. Seibert K. Mielke C. Phippard D. Svagr B. Cullinane D. Einhorn T.A. Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. J. Orthop. Res. 2003;21:670–675. doi: 10.1016/S0736-0266(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 35.Irwin M.G. Cheung K.M. Nicholls J.M. Thompson N. Intra-articular injection of ketorolac in the rat knee joint: effect on articular cartilage and synovium. Br. J. Anaesth. 1998;80:837–839. doi: 10.1093/bja/80.6.837. [DOI] [PubMed] [Google Scholar]
- 36.Buvanendran A. Kroin J.S. Kari M.R. Tuman K.J. A new knee surgery model in rats to evaluate functional measures of postoperative pain. Anesth. Analg. 2008;107:300–308. doi: 10.1213/ane.0b013e3181732f21. [DOI] [PubMed] [Google Scholar]
- 37.Convery P.N. Milligan K.R. Quinn P. Scott K. Clarke R.C. Low-dose intra-articular ketorolac for pain relief following arthroscopy of the knee joint. Anesthesia. 1998;53:1125–1129. doi: 10.1046/j.1365-2044.1998.00582.x. [DOI] [PubMed] [Google Scholar]
- 38.Ma W. Eisenach J.C. Intraplantar injection of a cyclooxygenase inhibitor ketorolac reduces immunoreactivities of substance P, calcitonin gene-regulated peptide, and dynorphin in the dorsal horn of rats with nerve injury or inflammation. Neuroscience. 2003;121:681–690. doi: 10.1016/s0306-4522(03)00497-4. [DOI] [PubMed] [Google Scholar]
- 39.Dong L. Guarino B.B. Jordan-Sciutto K.L. Winkelstein B.A. Activating transcription factor 4, a mediator of the integrated stress response, is increased in the dorsal root ganglia following painful facet joint distraction. Neuroscience. 2011;193:377–386. doi: 10.1016/j.neuroscience.2011.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong L. Winkelstein B.A. Simulated whiplash modulates expression of the glutamatergic system in the spinal cord suggesting spinal plasticity is associated with painful dynamic cervical facet loading. J. Neurotrauma. 2010;27:163–174. doi: 10.1089/neu.2009.0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee K.E. Davis M.B. Winkelstein B.A. Capsular ligament involvement in the development of mechanical hyperalgesia after facet joint loading: behavioral and inflammatory outcomes in a rodent model of pain. J. Neurotrauma. 2008;25:1383–1393. doi: 10.1089/neu.2008.0700. [DOI] [PubMed] [Google Scholar]
- 42.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 43.Quinn K.P. Dong L. Golder F.J. Winkelstein B.A. Neuronal hyperexcitability in the dorsal horn after painful facet joint injury. Pain. 2010;151:414–421. doi: 10.1016/j.pain.2010.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hubbard R.D. Winkelstein B.A. Transient cervical nerve root compression in the rat induces bilateral forepaw allodynia and spinal glial activation: Mechanical factors in painful neck injuries. Spine. 2005;30:1924–1932. doi: 10.1097/01.brs.0000176239.72928.00. [DOI] [PubMed] [Google Scholar]
- 45.Lee K.E. Thinnes J.H. Gokhin D.S. Winkelstein B.A. A novel rodent neck pain model of facet-mediated behavioral hypersensitivity: Implications for persistent pain and whiplash injury. J. Neurosci. Methods. 2004;137:151–159. doi: 10.1016/j.jneumeth.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 46.Rothman S.M. Kreider R.A. Winkelstein B.A. Spinal neuropeptide responses in persistent and transient pain following cervical nerve root injury. Spine. 2005;30:2491–2496. doi: 10.1097/01.brs.0000186316.38111.4b. [DOI] [PubMed] [Google Scholar]
- 47.Dong L. Odeleye A.O. Jordan-Sciutto K.L. Winkelstein B.A. Painful facet joint injury induces neuronal stress activation in the DRG: Implications for cellular mechanisms of pain. Neurosci. Lett. 2008;443:90–94. doi: 10.1016/j.neulet.2008.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rothman S.M. Winkelstein B.A. Cytokine antagonism reduces pain and modulates spinal astrocytic reactivity after cervical nerve root compression. Ann. Biomed. Eng. 2010;38:2563–2576. doi: 10.1007/s10439-010-0012-8. [DOI] [PubMed] [Google Scholar]
- 49.Ma W. Eisenach J.C. Morphological and pharmacological evidence for the role of peripheral prostaglandins in the pathogenesis of neuropathic pain. Eur. J. Neurosci. 2002;15:1037–1047. doi: 10.1046/j.1460-9568.2002.01940.x. [DOI] [PubMed] [Google Scholar]
- 50.Ma W. Du W. Eisenach J.C. Role for both spinal cord COX-1 and COX-2 in maintenance of mechanical hypersensitivity following peripheral nerve injury. Brain Res. 2002;937:94–99. doi: 10.1016/s0006-8993(02)02593-3. [DOI] [PubMed] [Google Scholar]
- 51.Bustamante D. Paeile C. Ketorolac tromethamine: an experimental study of its analgesic effects in the rat. Gen. Pharmacol. 1993;24:693–698. doi: 10.1016/0306-3623(93)90233-n. [DOI] [PubMed] [Google Scholar]
- 52.Sandrini M. Central effects of non-opiod analgesics review of actions and their clinical implications. CNS Drugs. 1999;12:337–345. [Google Scholar]
- 53.Choi M.S. Kim Y.E. Lee W.J. Choi J.W. Park G.H. Kim S.D. Jeon S.J. Go H.S. Shin S.M. Kim W.K. Shin C.Y. Ko K.H. Activation of protease-activated receptor1 mediates induction of matrix metalloproteinase-9 by thrombin in rat primary astrocytes. Brain Res. Bull. 2008;76:368–375. doi: 10.1016/j.brainresbull.2008.02.031. [DOI] [PubMed] [Google Scholar]