Abstract
Wnt proteins play a critical role in central nervous system development and have been implicated in several neuropathologies, including spinal cord injury (SCI). Ryk, an unconventional Wnt receptor, regulates axonal regeneration after SCI, although its expression pattern in this neuropathology remains unclear. Therefore, we sought to define the spatiotemporal and cellular pattern of Ryk expression after a contusive SCI in adult rats using quantitative reverse transcription polymerase chain reaction (RT-PCR), Western blot, and immunohistochemical analysis. Under physiological conditions, Ryk is expressed in neurons, astrocytes, and blood vessels, but not in oligodendrocytes, microglia, NG2+ glial precursor cells, or axonal projections. Following SCI, we observed an increase in Ryk mRNA expression from 24 h post-injury until 7 days post-injury, whereas its protein levels were significantly augmented at 7 and 14 days post-injury. Moreover, the spatial and cellular Ryk expression pattern was altered in the damaged tissue, where this receptor was observed in reactive astrocytes and microglia/macrophages, NG2+ glial precursors, fibronectin+ cells, oligodendrocytes, and axons. In conclusion, we demonstrate that Ryk is expressed in the unlesioned spinal cord and that, after SCI, its spatiotemporal and cellular expression pattern changed dramatically, being expressed in cells involved in the spinal cord response to damage.
Key words: glia cell response to injury, rat, receptors, traumatic SCI, in vivo studies
Introduction
Spinal cord injury (SCI) is a major central nervous system (CNS) pathology with no currently accepted treatment. SCI affects a significant proportion of the population and causes long-term functional disability.1 From a neuropathological point of view, SCI progression can be separated into two main chronological events, namely primary and secondary injury. Primary injury involves the destruction of the spinal cord parenchyma through direct mechanical trauma. This in turn induces secondary injury, which is characterized by a wide range of complex and interrelated cellular and molecular events that affect uninjured cells as well as circuits that are located in the vicinity of the primary injury core. Furthermore, the secondary injury significantly hinders axonal growth, which represents one of the most significant obstacles to functional recovery after a spinal cord lesion.2 Therefore, to identify new therapeutic targets, a clear understanding of the molecular processes that are characteristically associated with SCI is of the utmost importance.
Wnts are a well-characterized family of glycoproteins that play prominent roles during neural development.3–5 A growing body of evidence suggests that Wnt signalling may be involved in homeostasis and disease progression in adult tissues,6–11 including the spinal cord.10,12–16 Consistent with these findings, we have previously shown that most of Wnt ligands and inhibitors are expressed in the adult spinal cord of rats and, following SCI, are differentially induced with at least Wnt/β-catenin signalling activation in cells that appear to be involved in glial scarring.13 Strategies seeking to modulate Wnt-dependent signaling pathways have been shown to be beneficial in different experimental models of CNS disorders8,17–23 including SCI.10,14,24
Ryk is a well-known unconventional Wnt receptor25 that is composed of a Wnt inhibitory factor 1 (WIF1)-like extracellular domain that enables its interaction with different Wnt ligands. This is in addition to an intracellular domain that is catalytically inactive because of specific amino acid substitutions,26 although Ryk receptor is known to transduce extracellular signals across the plasma membrane through several mechanisms.27–31 Much of what is known about the function of Ryk in the CNS is derived from developmental studies. During this period and among other functions, Ryk acts as a chemorepulsive axon guidance receptor during the establishment of major axon tracts, such as the corpus callosum and the corticospinal tract (CST),32,33 and is vital for the generation of appropriate topographic maps of retinal ganglion cell axons.34
Given the developmental role of Ryk as an essential regulator of axonal growth and the importance of this process in functional recovery following SCI, several studies have investigated the potential of this receptor to mediate axonal regeneration in experimental models of this neuropathological condition.10,14 Blockage of Ryk activity, which is expressed by corticospinal axons, via intrathecal administration of a Ryk-neutralizing antibody, resulted in a significant growth of axons in the CST and enhanced functional recovery following SCI.10,14 This strongly suggests that Ryk influences the progression of SCI, and that modulation of Ryk activity may improve functional recovery.
Despite these intriguing observations, its pattern of expression following SCI remains almost unknown, which limits our understanding of its functions in this neuropathological condition. Therefore, we sought to evaluate the spatiotemporal and cellular pattern of Ryk expression in the unlesioned and lesioned spinal cord.
Methods
Animals, surgical procedures, and experimental design
A total of 51 male Wistar rats (3 months of age, ∼300 g) were used in this study. To analyze Ryk spatiotemporal and cellular expression pattern by quantitative reverse transcription polymerase chain reaction (RT-qPCR), Western blot, and simple or double immunohistochemistry, the animals were divided into an unlesioned control group (n=3 per technique) and lesioned experimental groups that corresponded with the different time points assessed (6 and 24 h post-injury [hpi] and at 3, 7, and 14 days post-injury [dpi]; n=3 per time point and technique).
All of the experimental animal procedures were conducted according to the European Union directives (2010/63/EU) and National Institutes of Health (NIH) guidelines, and were approved by the Bioethics Committee at The National Hospital of Paraplegics (Toledo, Spain) (Permit numbers 51/2009 and 45/2008).
The contusive spinal cord lesions were induced as previously described.13,35 Briefly, using intraperitoneal pentobarbital (40 mg/kg) and xylacine (10 mg/kg) anesthesia and with the rats placed on a thermal pad throughout the surgical process to maintain normothermia, a laminectomy was performed at the level of T8. Subsequently, the injury was induced via a controlled moderate contusion (200 kdynes) using an Infinite Horizon Spinal Cord Impactor (Precision Systems and Instrumentation LLC, Fairfax, VA) and the overlying muscle and skin were sutured. As previously reported,13 the postoperative care included subcutaneous injection of buprenorphine at 24 hpi (Buprex, 0.03 mg/kg) and enrofloxacin (Baytril, 2.5 mg/kg) once daily until 5 dpi. Moreover, the lesioned animals received subcutaneous injections of saline solution for the first 5 dpi in decreasing doses from 5 mL at 24 hpi to 1 mL at 5 dpi. The bladders were emptied twice daily until the lesioned animals completely recovered normal bladder function.
Finally, to establish a homogeneous group in which the variations in the Ryk spatiotemporal and cellular expression pattern were strictly caused by temporal changes after SCI, the 21 point Open-Field Locomotor Basso-Beattie-Bresnahan (BBB) scale was performed as previously described.36 This test was performed by two examiners who were blind to the experimental conditions. Only those animals with a BBB score of 0–3 1 day after surgery and with similar functional progress were included in the study (data not shown).
mRNA isolation and RT-qPCR analysis
The animals used to quantify Ryk mRNA expression were perfused intra-aortically with 150 mL of heparinized saline solution. The total mRNA was isolated using the RNeasy Lipid Tissue Mini Kit (Qiagen, Madrid, Spain) from a 1 cm long spinal cord fragment containing the wound epicenter. The complementary DNA (cDNA) synthesis and amplification, as well as the relative quantification of Ryk mRNA and the endogenous control 18S rRNA, were performed as described.13 The gene-specific primers used for Ryk cDNA amplification were 5′-CGAATAGCCCAGCCAATCA-3′ as forward and 5′-CAACAGGCCATCACAGCAAA-3′ as reverse (GenBank accession number NM_080402.1). As detailed in a previous report,37 the gene-specific primers used for 18S cDNA amplification were 5′-CGGCTACCACATCCAAGGAA-3′ as forward and 5′-GCTGGAATTACCGGGCT-3′ as reverse (Genbank accession number NR_046237.1).
Western blot analysis
The animals used to quantify the amount of Ryk protein were killed as described, and a 1 cm spinal cord fragment containing the wound epicenter was homogenized in ice cold RIPA buffer (Sigma, Steinheim, Germany) containing a protease inhibitor cocktail (Roche, Mannheim, Germany). After the total protein concentration was determined using the Bradford protein method,38 Western blot was performed following the protocol described in previous reports13 using 100 μg of total protein per animal and a rabbit anti-Ryk primary antibody (1:250) (Abgent, San Diego, CA). A mouse anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) primary antibody was used as a loading control as previously reported,13 and densitometrical analysis was performed using the ImageJ software.
Simple and double immunohistochemistry
The animals used for histological analysis were killed by intra-aortic perfusion with 150 mL of heparinized saline solution followed by 1 g/mL of 4% paraformaldehyde (Sigma, Steinheim, Germany). The spinal cord was immediately removed, postfixed for 4 h in the same fixative solution, cryoprotected by immersion in 30% sucrose for 48 h, frozen in Neg-50 frozen medium (Richard-Allan Scientific, Kalamazoo, MI) using dry ice and stored at −20°C. From each spinal cord fragment, parallel cryostat sections (30 μm) were obtained from a 3 cm stretch of spinal cord, 1.5 cm rostral and 1.5 cm caudal of the wound epicenter, and were then mounted onto slides and stored at −20°C.
To determine the histological time course of Ryk expression, a set of parallel sections from each animal was processed for simple immunohistochemistry as previously detailed13 using a polyclonal rabbit anti-Ryk primary antibody (1:50) (Abgent, AP7677a, San Diego, CA) that has been generated against a peptide within amino acids 150–200 of complete human Ryk protein sequence, and has been positively used in previous reports.10,32
To evaluate the cellular Ryk expression, double immunohistochemistry was performed in a set of parallel sections for each animal to visualize Ryk in astrocytes (glial fibrillary acidic protein [GFAP]), neurons (neuronal nuclei [NeuN]), oligodendrocytes (adenomatous polyposis coli [APC]), blood vessels (RECA1), axons (neurofilament 200 [NF200]), microglia/macrophages (ionized calcium binding adaptor molecule 1 [Iba1]), glial progenitors (NG2) and fibronectin+ cells (fibronectin). Briefly, the sections were processed for Ryk immunohistochemistry as described, except that the nonspecific binding was blocked with TSA plus Blocking Reagent (Perkin Elmer, Boston, MA), the rabbit anti-Ryk primary antibody was diluted 1:100, and the TSA plus Tetramethylrhodamine System (Perkin Elmer, Boston, MA) was used to visualize the peroxidase reaction product, according to the manufacturer's instructions. The sections were then processed following the protocol described in a previous report,39 using the following primary antibodies: mouse anti-APC (1:100) (Calbiochem, Darmstadt, Germany), mouse anti-NeuN (1:250) (Chemicon, Temecula, CA), mouse anti-RECA1 (1:500) (AbD Serotec, Oxford, UK), mouse anti-NF200 (1:1000) (Sigma, Steinheim, Germany), rabbit anti-Iba1 (1:500) (Wako, Osaka, Japan), rabbit anti-GFAP (1:1000) (Dako, Glostrup, Denmark), rabbit anti-fibronectin (1:500) (Sigma, Steinheim, Germany) and rabbit anti-NG2 (1:500) (Chemicon, Temecula, CA).
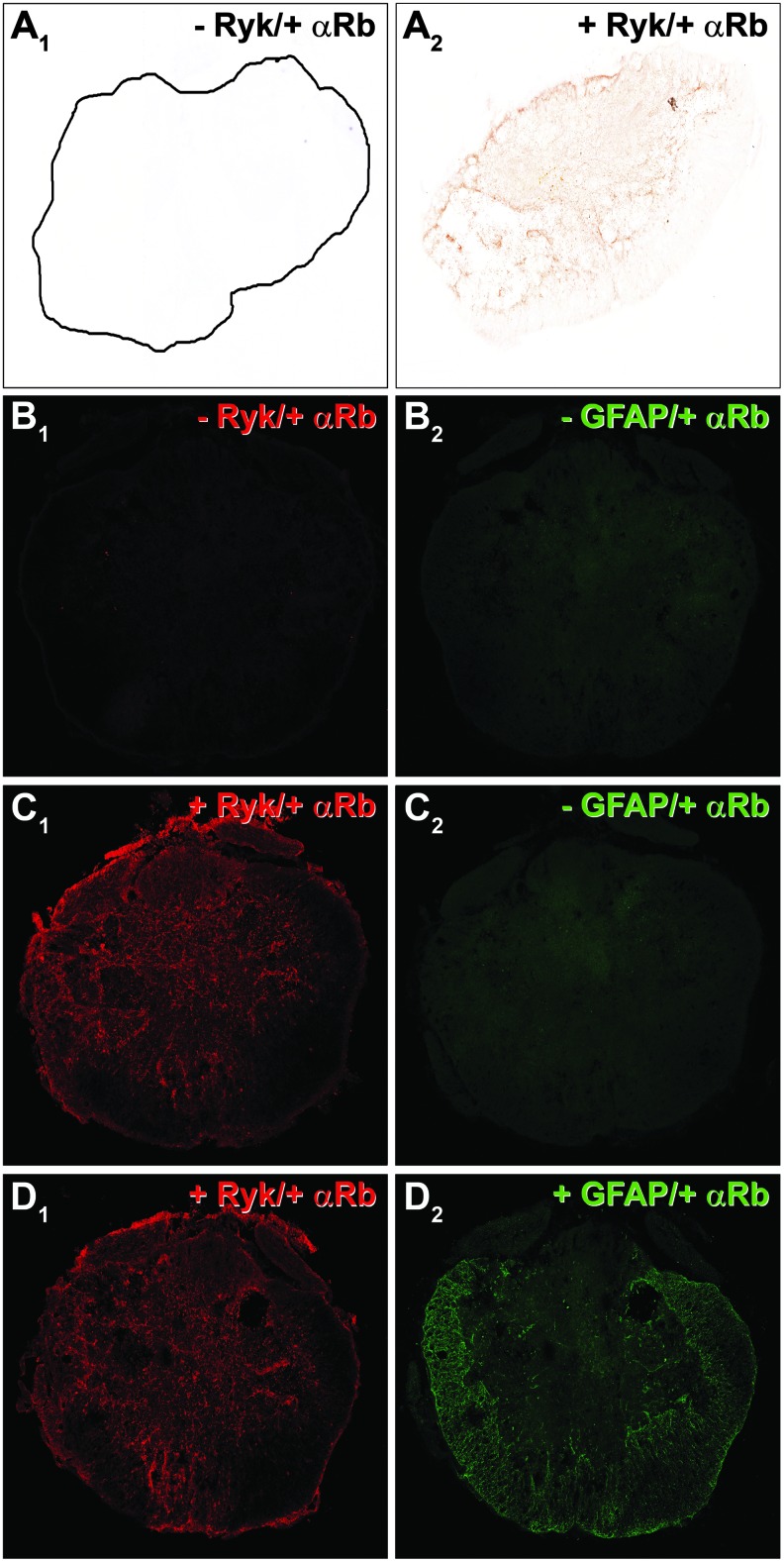
The sections processed for simple immunohistochemistry were examined on a BX61 Motorized Research Microscope (Olympus, Tokyo, Japan), while the double-labelled sections were analyzed using both the BX61 Motorized Research Microscope (Olympus, Tokyo, Japan) and a Leica TCS SP5 confocal microscope (Leica Microsystems, Nussloch, Germany). For simple immunohistochemistry experiments, the sections processed without the primary antibody were used as controls. For double immunohistochemistry and to detect putative cross-reactivity between both simple immunohistochemistries, the sections were processed without the second primary antibody and used as controls. A lack of nonspecific secondary antibody staining was observed in the immunohistochemical controls (Fig. 1). On the other hand, the specificity of the anti-Ryk primary antibody used to perform the present study has been previously proven in the embryonic nervous tissue of Ryk knockout mice.32 To further confirm the specificity of the anti-Ryk antibody used in the present work, we have performed Ryk chromogen-based immunohistochemisty by using a different anti-Ryk primary antibody and following the same experimental protocol detailed previously for simple immunohistochemistry. For this purpose, the antibody used was a monoclonal mouse anti-Ryk that has been generated against a peptide within amino acids 462–475 of human Ryk (Abcam, ab33519, Cambridge, UK) (1:25). In this case, the Ryk protein expression pattern was the same as that observed using the anti-Ryk primary antibody purchased from Abgent, both in the uninjured spinal cords and after SCI at all analyzed times post-injury (Fig. S1) (see online supplementary material at http://www.liebertpub.com).
FIG. 1.
Negative controls for simple and double immunohistochemistry. (A1) A representative section taken from negative controls lacking the primary antibody used for chromogen-based Ryk simple immunohistochemical detection. (A2) A representative section from the same rostro-caudal level processed for the chromogen-based Ryk simple immunohistochemistry. The negative controls performed for the double immunohistochemistry: B1-B2 shows a representative section taken from the negative controls lacking both primary antibodies, C1-C2 shows a representative section taken from the negative controls lacking the second primary antibody, and D1-D2 shows a representative section processed for double immunohistochemistry. Nonspecific staining was not observed in any of the negative controls. Color image is available online at www.liebertpub.com/neu
Statistical analysis
All values are expressed as the mean±SEM. Statistical comparisons of Ryk mRNA and protein expression between the groups were performed using one-way ANOVA followed by the Tukey post-hoc test to determine the individual differences between the means. In all cases, p<0.05 was considered to be statistically significant. All statistical analyses were performed using GraphPad Prism (version 4.0).
Results
Ryk expression in the unlesioned spinal cord
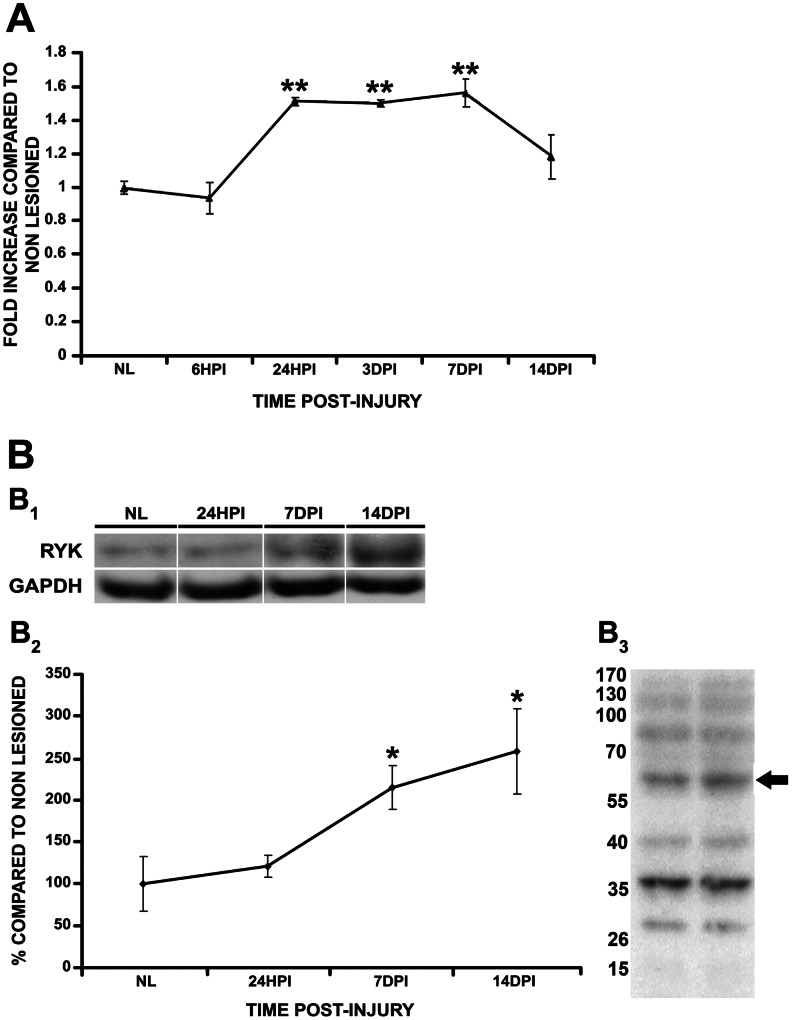
We observed Ryk mRNA (Fig. 2A) and protein (Fig. 2B) expression in unlesioned spinal cords using RT-qPCR and Western blot analysis, respectively.
FIG. 2.
Temporal pattern of Ryk mRNA and protein expression after spinal cord injury (SCI). (A) Ryk mRNA expression significantly increased 24 h post-injury (hpi) and remained elevated until 7 days post-injury (dpi). After 14 dpi, no significant differences were detected when compared with the unlesioned (NL) controls. The data are presented as the mRNA levels from the lesioned animals versus their NL controls. (B) Ryk protein levels significantly increased at 7 dpi and remained elevated at 14 dpi when compared with the NL controls. Data in B2 are presented as the percentage of Ryk protein levels in comparison with the NL controls. B1 shows representative bands of Ryk protein both in the NL spinal cords and at the different analyzed times post-SCI, whereas B3 shows two representative Western blot lanes (the numbers represent the molecular weights and the arrow points to the band corresponding to Ryk protein) In all of the cases, the data represent the mean±SEM. **p<0.01; *p<0.05 versus NL control group.
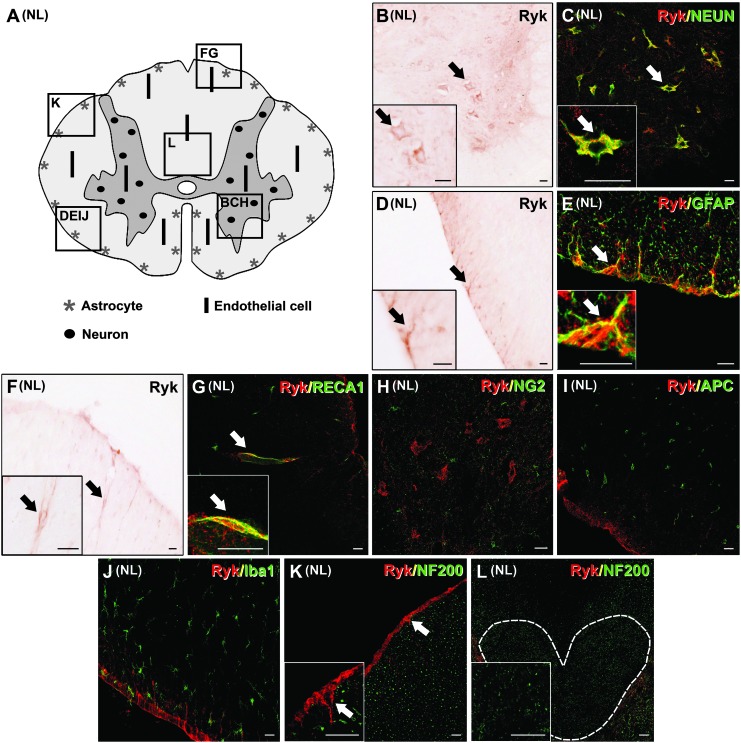
Moreover, qualitative microscopic analysis of unlesioned spinal cord sections processed for simple and double immunohistochemistry revealed Ryk protein expression in a variety of cell types (Fig. 3A–J) (see Fig. 1 for details about the immunohistochemical controls performed). Ryk expression was detected in some neuronal cell bodies, mainly in the ventral horns (Fig. 3B and C). Although neurons at this location were more strongly labeled for Ryk than those in other areas of the spinal cord, neuronal Ryk expression was detected in neurons of all sizes and in all spinal cord laminae. Astroglia also expressed Ryk but only in astrocytes that were located close to the pial surface (Fig. 3D and E). Moreover, Ryk immunofluorescence was detected in a few blood vessels (Fig. 3F and G), although these blood vessels that expressed Ryk were randomly located in different anatomical areas of both the white and gray matter. Finally, in the unlesioned spinal cords, Ryk expression was not observed in NG2+ glial precursors (Fig. 3H), oligodendrocytes (Fig. 3I), quiescent microglial cells (Fig. 3J), or axons (Fig. 3K and L).
FIG. 3.
Immunohistochemical analysis of the spatial and cellular pattern of Ryk expression in unlesioned (NL) spinal cord. (A) A schematic drawing showing Ryk protein expression in a representative transverse section of an NL spinal cord (the squares represent areas where the micrographs were obtained). Ryk immunostaining was observed in neuronal nuclei (NeuN)+ neuronal cell bodies, mainly in the ventral horns (B and C), in glial fibrillary acidic protein (GFAP)+ astrocytes close to the pial surface (D and E), and in some blood vessels in the white (F and G) and gray matter. No Ryk expression was observed in NG2+ glial precursor cells (H), adenomatous polyposis coli (APC)+ oligodendrocytes (I) or Iba1+ microglial cells (J) or neurofilament (NF)200+ axons (K and L; the discontinuous white line in L delimits the corticospinal tract). Scale bars in B–L=20 μm. Color image is available online at www.liebertpub.com/neu
Ryk expression after moderate contusive spinal cord damage
After SCI, we observed a significant increase in Ryk mRNA expression at 24 hpi that remained elevated until 7 dpi when compared with the unlesioned controls. However, at 14 dpi, no significant differences in Ryk mRNA expression were detected in comparison with the unlesioned control animals (Fig. 2A). However, when we assessed whether these variations in the Ryk mRNA expression pattern after SCI were reflected at the protein levels, Ryk protein expression increased significantly at 7 and 14 dpi when compared with the unlesioned controls (Fig. 2B).
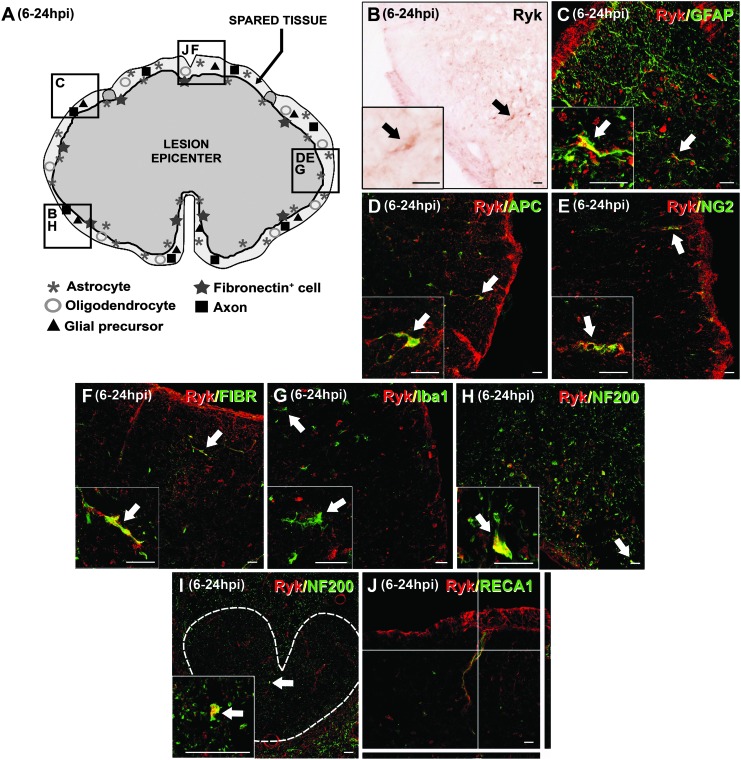
The spatial and cellular distribution of Ryk protein was also qualitatively altered in the lesioned animals in areas that corresponded to the site of impact. In contrast, no changes in the spatial and cellular Ryk expression pattern were observed in regions that were distant from the wound epicenter, in which a normal cytoarchitecture persisted. At 6–24 hpi, Ryk immunolabeling was restricted to the ring of spared tissue that surrounded the lesion epicenter in areas that corresponded to the site of impact (Fig. 4A and B). Although Ryk protein expression was still observed in some astroglia, at these time points the presence of Ryk+ astrocytes was qualitatively increased. Moreover, these Ryk+ astrocytes were not only located close to the external limit of the spinal cord neural parenchyma, as observed in the unlesioned controls, but they were also found in areas that were separated from the pial surface (Fig. 4C). In addition, Ryk protein expression was detected in cell types not observed in unlesioned controls such as few oligodendrocytes (Fig. 4D), NG2+ glial precursors (Fig. 4E), and fibronectin+ cells (Fig. 4F), but not in microglia/macrophages (Fig. 4G). Furthermore, at 6–24 hpi, several axons began to express Ryk in these areas (Fig. 4H), whereas at 24 hpi axonal Ryk expression could be also observed in several CST axons located in preserved spinal cord levels that were located rostral to the site of impact (Fig. 4I). In contrast to the unlesioned animals, no Ryk immunofluorescence was detected in the blood vessels in the lesion area, although in many cases, Ryk+ cells were found to be closely associated with the vasculature (Fig. 4J). No differences in the spatial and cellular Ryk expression pattern were detected between dorsal and ventral regions.
FIG. 4.
Immunohistochemical analysis of the spatial and cellular expression of Ryk at 6–24 h post-injury (hpi). (A) A schematic drawing showing Ryk protein in a representative transverse section corresponding to the level of impact in the lesioned spinal cord at 6–24 hpi (the central dark gray area represents the wound epicenter and the squares represent areas where the micrographs were obtained). Ryk immunolabeling was localized to the ring of spared tissue surrounding the wound epicenter (A and B). Cells that expressed Ryk were identified as glial fibrillary acidic protein (GFAP)+ astrocytes (B and C), adenomatous polyposis coli (APC)+ oligodendrocytes (B and D), NG2+ glial precursors (B and E), fibronectin+ cells (B and F) and neurofilament (NF)200+ axons (B and H). Ryk expression in NF200+ axons was also observed in the corticospinal tract of preserved spinal cord levels that were rostral to the injured tissue (I, the discontinuous white line delimits the corticospinal tract). No Ryk expression was observed in microglia/macrophages (G) or in blood vessels (J). Scale bars in B–J=20 μm. Color image is available online at www.liebertpub.com/neu
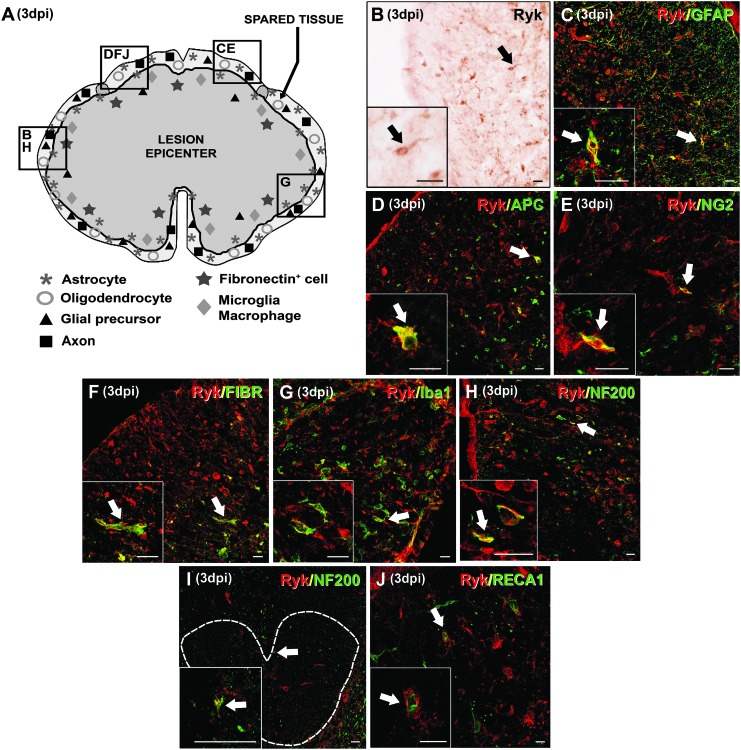
At 3 dpi, the presence of Ryk+ cells was still restricted to the ring of spared tissue that surrounded the wound epicenter, particularly in the areas that corresponded to the site of impact (Fig. 5A and B), although Ryk expression was more evident as compared with the previously evaluated times post-SCI. In these areas, Ryk was similarly expressed by many activated astrocytes (Fig. 5C), and few oligodendrocytes (Fig. 5D), NG2+ glial precursors (Fig. 5E), and fibronectin+ cells (Fig. 5F). In addition, Ryk expression was observed in many activated microglia/macrophages (Fig. 5G) that were characterized by a loss of cellular processes and the acquisition of an amoeboid morphology.40 Again, several Ryk+ axonal projections were also found both in the ring of spared tissue that surrounded the lesion epicenter (Fig. 5H) and in CST axons located in preserved spinal cord levels that were located rostral to the injured tissue (Fig. 5I). Similarly with the 6–24 hpi time point, no Ryk expression was detected in the blood vessels at 3 dpi, although Ryk+ cells were, in most cases, closely associated with them (Fig. 5J).
FIG. 5.
Immunohistochemical analysis of the spatial and cellular expression of Ryk at 3 days post-injury (dpi). (A) A schematic drawing showing Ryk protein in a representative transverse section corresponding to the level of impact in the lesioned spinal cord at 3 dpi (the central dark gray area represents the wound epicenter and the squares represent areas where the micrographs were obtained). Ryk immunostaining was observed at the ring of the spared tissue surrounding the wound epicenter (A and B) in glial fibrillary acidic protein (GFAP)+ astrocytes (B and C), adenomatous polyposis coli (APC)+ oligodendrocytes (B and D), NG2+ glial precursors (B and E), fibronectin+ cells (B and F), Iba1+ microglia/macrophages (B and G) and neurofilament (NF)200+ axons (B and H). Ryk expression in NF200+ axons was also observed in the corticospinal tract of preserved spinal cord levels that were rostral to the injured tissue (I, the discontinuous white line delimits the corticospinal tract). No Ryk expression was observed in blood vessels (J). Scale bars in B–J=20 μm. Color image is available online at www.liebertpub.com/neu
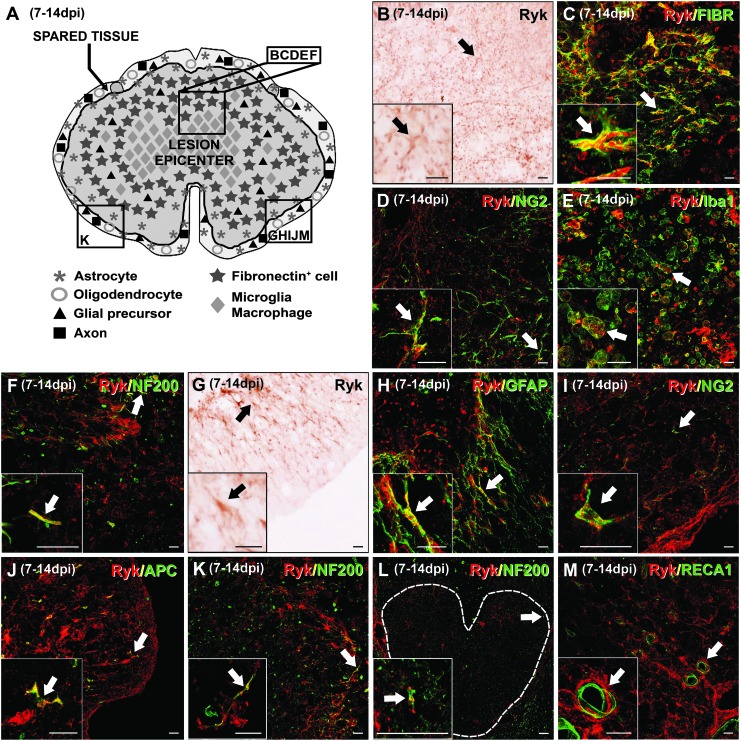
At 7–14 dpi, the maximum presence of Ryk staining of all analyzed times post-injury was observed. Moreover, Ryk+ cells were not only present in the ring of spared tissue that surrounded the lesion epicenter (Fig. 6A and G) but were also found in the epicenter of the lesion, where the most prominent Ryk immunolabeling was observed (Fig. 6A and B). Here, Ryk protein expression was detected in almost all of the fibronectin+ cells (Fig. 6C), in some NG2+ glial precursors (Fig. 6D), and in the majority of activated microglia/macrophages (Fig. 6E). Within the ring of spared tissue that surrounded the wound epicenter, Ryk expression was detected in many astrocytes (Fig. 6H), NG2+ glial precursors (Fig. 6I) and oligodendrocytes (Fig. 6J). Moreover, astroglial Ryk expression in these areas was more prominent in the inner limit of the astroglial scar (Fig. 6H). Interestingly, at these time points (7–14 dpi), Ryk+ astrocytes clearly exhibited morphological signs of activation, demonstrating thicker and longer cellular processes and increased GFAP immunostaining (Figs. 4C, 5C, and 6H).41 Finally, the presence of Ryk+ axons was qualitatively increased as compared with that observed at the previously evaluated time points, as many axons expressing Ryk were detected not only in the ring of spared tissue that surrounded the impact site (Fig. 6K) but also in the lesion epicenter (Fig. 6F). Moreover, Ryk expression was still observed in many CST axons in preserved spinal cord levels that were located rostral to the injured regions (Fig. 6L). As previously described, no Ryk expression was observed in the blood vessels, although there was a close association between Ryk+ cells and the vasculature (Fig. 6M).
FIG. 6.
Immunohistochemical analysis of the spatial and cellular expression of Ryk at 7–14 days post-injury (dpi). (A) A schematic drawing showing Ryk protein in a representative transverse section corresponding to the level of impact in the lesioned spinal cord at 7–14 dpi (the central dark gray area represents the wound epicenter and the squares represent areas where the micrographs were obtained). Although Ryk protein was expressed throughout the entire lesioned area (A), the expression was more prominent in areas corresponding to the center of the lesion (A and B), where Ryk+ cells were identified as fibronectin+ cells (B and C), NG2+ glial precursors (B and D), Iba1+ microglia/macrophages (B and E) and neurofilament (NF)200+ axons (B and F). At the ring of spared tissue surrounding the wound epicenter (A and G), Ryk expression was detected in glial fibrillary acidic protein (GFAP)+ astrocytes (G and H), NG2+ glial precursors (G and I), adenomatous polyposis coli (APC)+ oligodendrocytes (G and J) and axons (G and K). Ryk expression in NF200+ axons was also observed in the corticospinal tract of preserved spinal cord levels that were rostral to the injured tissue (L, the discontinuous white line delimits the corticospinal tract). No Ryk expression was observed in blood vessels (M). Scale bars in B–M=20 μm. Color image is available online at www.liebertpub.com/neu
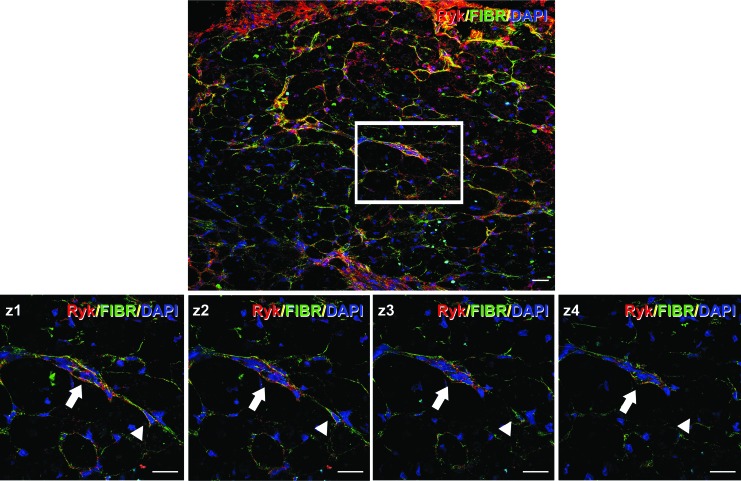
Previous reports have demonstrated extracellular fibronectin deposition in the lesion epicenter after contusive SCI.42–45 For this reason, we sought to evaluate by 14 dpi, when we observed that fibronectin immunolabeling in the injured tissue was clearly evident, whether Ryk was present in the fibronectin+ cells or in the extracellular fibronectin+ scar by performing double immunohistochemistry using antibodies against Ryk and fibronectin as well as DAPI nuclear counterstaining. As is shown in Fig. 7, Ryk immunostaining colocalized with fibronectin+ cells.
FIG. 7.
Ryk is expressed in fibronectin+ cells. This figure shows representative images obtained from sections corresponding to the impact site processed for the visualization of Ryk, fibronectin, and DAPI nuclear counterstaining. The images were captured in lesioned animals at 14 days post-injury (dpi). The white square in the upper image highlights the area selected to obtain the different confocal z planes shown in the lower micrographs. In the lower images, the arrow points to a cluster of cells co-expressing Ryk and fibronectin, while the arrowhead points to a single cell showing Ryk and fibronectin immunostaining that clearly surround its nucleus. Scale bars=20 μm. Color image is available online at www.liebertpub.com/neu
Finally, no neuronal Ryk protein expression was observed in the lesioned areas at any of the time points studied, as no surviving neurons were detected in these regions.
Discussion
This study shows the pattern of Ryk receptor expression in the unlesioned adult spinal cord, in neurons (mainly in the ventral horns), astrocytes close to the pial surface, and blood vessels. Although Ryk expression has been previously described in the developing spinal cord,33,46,47 its expression in the unlesioned adult spinal cord was unclear. In this way, Ryk mRNA expression was detected in the adult spinal cord by in situ hybridization (ISH),48 in agreement with our results that showed Ryk mRNA expression using RT-qPCR. However, previous reports were not able to detect Ryk protein in the unlesioned adult spinal cord by immunohistochemistry.10,14 Likewise, we found no evidence of Ryk immunolabeling in the unlesioned adult spinal cord using the same protocol,10,14 most likely because of the low protein receptor levels. The use of streptavidin or tyramide-based amplification systems revealed Ryk immunopositivity in a wide range of cell types. Consistent with these findings, we have also observed Ryk protein expression in the unlesioned adult spinal cords using Western blot analysis. Interestingly, the spatial and cellular distribution of Ryk protein observed in this study was extremely similar to that reported for its mRNA expression by ISH in adult spinal cord sections, where Ryk mRNA has been found in gray matter cells displaying a neuronal-like morphology as well as in white matter cells displaying a glial-like morphology, predominantly in areas close to the pial surface, and in vascular-like profiles.48 In addition and in agreement with previous reports,10,14 we have found no evidence of axonal Ryk protein expression in the uninjured adult spinal cord.
On the other hand, previous studies have demonstrated widespread Ryk expression under physiological conditions in neuronal and glial-like cells and in specific areas of the mature brain and cerebellum.46 These observations together with our results strongly suggest Ryk involvement in physiological events in adult CNS activity, in addition to its previously described functions during CNS developmental stages.27,31–34 Because the function of Ryk in the mature CNS remains completely unknown, understanding the biological relevance of Ryk expression in neurons, endothelial cells, and astrocytes close to the pial surface in the unlesioned adult spinal cord clearly requires further study, especially when it has been recently demonstrated that most Wnt ligands and its modulators are expressed in the unlesioned spinal cord,13 and that the Wnt family of proteins is involved in different CNS physiological functions during maturity, such as the modulation of blood–brain barrier permeability,49 neurogenesis,9 dendritic arborization, and synaptic function and maintenance.9
We also found that Ryk mRNA and protein expression levels increased significantly after SCI, when compared with unlesioned spinal cords. Specifically, Ryk mRNA expression was upregulated by an ∼1.5-fold change from 24 hpi until 7 dpi, when Ryk mRNA levels began to decrease. Instead, Ryk protein levels did not increase until 7 dpi, and remained elevated until 14 dpi. The delay observed between the Ryk mRNA and protein expression may imply the existence of post-transcriptional regulatory mechanisms or differences between the half-life of Ryk mRNA and protein. Moreover, the spatial and cellular expression pattern of Ryk protein was also dramatically altered following SCI. Although there are little data regarding the expression of Ryk in the lesioned spinal cord, Ryk expression has been previously described in regenerating CST axons after spinal cord hemisection14 or contusion,10 which is consistent with its role in regulating axon regeneration in vitro10,29 and in vivo.10,14 In accordance with these observations, our results demonstrated that from 24 h to 14 days after spinal cord contusion in rats, Ryk was expressed in CST axons that were located in preserved spinal cord levels that were rostral to the injured tissue. Moreover, and at the same times post-injury, we observed that Ryk was expressed in many axonal projections that were located in the ring of spared tissue that surrounded the lesion epicenter, whereas at 7 and 14 dpi, Ryk+ axons were also found in the lesion epicenter. Interestingly, although some conflicting results have been obtained,50 different studies have consistently supported the inability of regenerating CST axons to enter into the injury site in different models of spinal cord injury such as contusion10,51 and dorsal hemisection.52–56 Altogether, these observations raise the possibility that axonal Ryk expression might not be restricted to CST axons and, therefore, that axons belonging to different tracts might be able to express this receptor after SCI. Nevertheless, it should be noted that in the injury model used to perform the present study, few CST axons were observed caudally to the lesion site, clearly indicating that several CST axons were spared after the induction of the damage.
Strikingly, we demonstrated that Ryk is also expressed extensively in fibronectin+ and glial cells after moderate contusive spinal cord damage, including reactive astrocytes, microglia/macrophages, and, to a lesser extent, oligodendrocytes and NG2+ glial precursors, reaching maximum levels of Ryk protein expression at 7–14 dpi.
These observations acquire a higher relevance when the critical involvement of these cell types in the progression of SCI is taken into account. For example, microglia/macrophage reactivity is of the utmost importance, as activated microglia/macrophages are involved in many pivotal processes, such as cellular debris phagocytosis, antigen presentation, cellular trophic support, and the production of a wide range of inflammatory mediators.40,57 Consistent with this idea, modulation of microglia/macrophage reactivity has been shown to significantly improve lesion outcome after SCI.58 Although there are no available data regarding the potential functions of Ryk in microglia/macrophage reactivity, it should be noted that recent reports have suggested the involvement of the Wnt family of proteins in this cellular process. More specifically, Wnt5 and Wnt3a have been found to exert a pro-inflammatory action in activated peripheral macrophages59–62 and cultured microglial cells,63 respectively, by increasing their production of different pro-inflammatory molecules such as cytokines and chemokines. Moreover, different Wnt ligands are able to trigger microglial proliferation in vitro.64 In this context, our observation that, after SCI, only activated microglia/macrophages but not quiescent microglial cells expressed Ryk, strongly points to a possible function of this receptor in microglia/macrophage reactivity.
In addition, astroglial reactivity is another major hallmark of SCI as, among other functions, activated astrocytes are involved in the maintenance of extracellular medium homeostasis, the cellular metabolic and trophic support, the production of many pro- and anti-inflammatory molecules, and the isolation of injured and uninjured nervous tissue by forming the glial scar which, in turn, is one of the most important handicaps in axonal regeneration after SCI.41,65,66 Interestingly, we showed here that most reactive astrocytes expressed Ryk, mainly those located in close relation to the damaged spinal cord parenchyma and at the last evaluated times post-injury (7–14 dpi). These observations suggest that, after SCI, reactive astrocytes are not mere Wnt producers,10 but also are target cells for Wnt ligands, suggesting that the Wnt family of proteins may play a modulatory role in the different processes of spinal cord tissue response to injury that are mediated or modulated by astroglial reactivity.
Another pivotal process in SCI is the migration, proliferation, and differentiation of the slow-dividing NG2+ glial precursors that are present in the nervous parenchyma, which attempt to replace those cells that have been lost because of injury progression.67,68 However, the gliogenic environment of the damaged tissue may favor the differentiation of these cells into astrocytes and not oligodendrocytes, therefore enhancing glial scar formation and possibly exerting a deleterious effect.67 Noticeably, activation of the canonical Wnt-signaling pathway was able to delay the differentiation of oligodendrocyte progenitors during development and remyelination in an experimental model of adult spinal cord demyelination.8,69 Moreover, activation of Ryk in neural precursor cells during CNS development induces their differentiation into neurons, while suppressing their differentiation into oligodendrocytes.70 In this regard, we demonstrate here that NG2+ glial precursors located in the injury site expressed the Wnt receptor Ryk, supporting a role for this receptor in the response to injury of this cell type.
In addition, our results show that the Ryk receptor is also expressed in those oligodendrocytes present in the ring of spared tissue that surrounded the injury epicenter, which are thought to be affected because of secondary injury progression.2 In this way, a major cause of the functional and histopathological deficits associated with SCI is the secondary death of oligodendrocytes.2 Although currently there are no data regarding Ryk functions in oligodendroglial cell death, it should be noted that several reports have shown that, after a CNS injury, the Wnt family of proteins is able to modulate cell survival in other neural cell types, such as neurons.6,20
Finally, an interesting point of discussion derives from the presence of fibronectin+ cells in the lesion epicenter. Several reports have demonstrated the presence of fibronectin in the injury core, even in non-penetrating injuries where meningeal fibroblast invasion and proliferation are minimal.42–45 Different sources have been proposed for this fibronectin+ scar, including vascular extravasation of plasma fibronectin.42,45 However, this intriguing issue has not been completely explored as, for example, there are meningeal-like cells that surround the blood vessels in the CNS, but the extent to which they participate in CNS injuries is not yet clear.71 Moreover, it has been shown that there is an increase in the perivascular cells expressing fibroblast markers after a contusive injury to the rat spinal cord.72 In addition, a recent study demonstrated the existence of perivascular cells, namely pericytes, which, after a spinal cord hemisection in mice, invaded the injury core, expressed fibronectin, and heavily influenced scar formation.73 These findings suggest that fibronectin+ cells invading the lesion epicenter may not only come from the meninges, even in SCI models that do not spare the meningeal layer. Furthermore, the arachnoid and pia mater, also named leptomeninges, are tightly associated with the vessels, and penetrate deeply into the CNS parenchyma.74 Interestingly, we showed here that Ryk was expressed in almost all fibronectin+ cells that were located in the lesion epicenter.
Conclusion
In conclusion, the data presented in this study demonstrate that Ryk is expressed by various cell types and at different locations in the unlesioned spinal cord, suggesting a role for Ryk in the spinal cord under physiological conditions. Moreover, after contusive SCI, we demonstrated that Ryk expression was increased, and that its spatial and cellular expression pattern suffered evident alterations in the lesioned parenchyma, where Ryk expression was observed in axons, reactive astrocytes and microglia/macrophages, NG2+ glial precursors, oligodendrocytes, and fibronectin+ cells. Taken together, these findings strongly suggest that, beyond its well-known role in axonal regeneration after SCI, this receptor may be involved in other pivotal cellular events that characterize the development of spinal cord insults. Further studies are needed to ascertain the function of Ryk in adult neurons and endothelial cells, understand the consequences of switching Ryk expression from these cells to glial meningeal and inflammatory cells during SCI and examine the potential biological relevance of modulating Ryk activity during SCI progression.
Supplementary Material
Acknowledgments
This research was supported by grants obtained from the Fondo de Investigación Sanitaria de Castilla la Mancha (FISCAM) (Grant PI2008-39) and Fondo de Investigación Sanitaria (FIS) (Grant PI08-1475, co-funded by Fondo Europeo de Desarrollo Regional [FEDER]). The funding agencies had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank Virginia Pérez and Sandra Vázquez for their outstanding technical assistance.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Sekhon L.H. Fehlings M.G. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine (Phila Pa 1976) 2001;26:S2–12. doi: 10.1097/00007632-200112151-00002. [DOI] [PubMed] [Google Scholar]
- 2.Profyris C. Cheema S.S. Zang D. Azari M.F. Boyle K. Petratos S. Degenerative and regenerative mechanisms governing spinal cord injury. Neurobiol. Dis. 2004;15:415–436. doi: 10.1016/j.nbd.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Freese J.L. Pino D. Pleasure S.J. Wnt signaling in development and disease. Neurobiol. Dis. 2010;38:148–153. doi: 10.1016/j.nbd.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Logan C.Y. Nusse R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 5.Michaelidis T.M. Lie D.C. Wnt signaling and neural stem cells: caught in the Wnt web. Cell Tissue Res. 2008;331:193–210. doi: 10.1007/s00441-007-0476-5. [DOI] [PubMed] [Google Scholar]
- 6.Caraci F. Busceti C. Biagioni F. Aronica E. Mastroiacovo F. Cappuccio I. Battaglia G. Bruno V. Caricasole A. Copani A. Nicoletti F. The Wnt antagonist, Dickkopf-1, as a target for the treatment of neurodegenerative disorders. Neurochem. Res. 2008;33:2401–2406. doi: 10.1007/s11064-008-9710-0. [DOI] [PubMed] [Google Scholar]
- 7.De Ferrari G.V. Moon R.T. The ups and downs of Wnt signaling in prevalent neurological disorders. Oncogene. 2006;25:7545–7553. doi: 10.1038/sj.onc.1210064. [DOI] [PubMed] [Google Scholar]
- 8.Fancy S.P. Baranzini S.E. Zhao C. Yuk D.I. Irvine K.A. Kaing S. Sanai N. Franklin R.J. Rowitch D.H. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 2009;23:1571–1585. doi: 10.1101/gad.1806309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inestrosa N.C. Arenas E. Emerging roles of Wnts in the adult nervous system. Nat. Rev. Neurosci. 2010;11:77–86. doi: 10.1038/nrn2755. [DOI] [PubMed] [Google Scholar]
- 10.Miyashita T. Koda M. Kitajo K. Yamazaki M. Takahashi K. Kikuchi A. Yamashita T. Wnt-ryk signaling mediates axon growth inhibition and limits functional recovery after spinal cord injury. J. Neurotrauma. 2009;26:955–964. doi: 10.1089/neu.2008.0776. [DOI] [PubMed] [Google Scholar]
- 11.Niu L.J. Xu R.X. Zhang P. Du M.X. Jiang X.D. Suppression of frizzled-2-mediated Wnt/Ca(2+) signaling significantly attenuates intracellular calcium accumulation in vitro and in a rat model of traumatic brain injury. Neuroscience. 2012;213:19–28. doi: 10.1016/j.neuroscience.2012.03.057. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y. Guan Y. Liu H. Wu X. Yu L. Wang S. Zhao C. Du H. Wang X. Activation of the Wnt/beta-catenin signaling pathway is associated with glial proliferation in the adult spinal cord of ALS transgenic mice. Biochem. Biophys. Res. Commun. 2012;420:397–403. doi: 10.1016/j.bbrc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez–Martos C.M. Gonzalez–Fernandez C. Gonzalez P. Maqueda A. Arenas E. Rodriguez F.J. Differential expression of wnts after spinal cord contusion injury in adult rats. PLoS One. 2011;6:e27000. doi: 10.1371/journal.pone.0027000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y. Wang X. Lu C.C. Kerman R. Steward O. Xu X.M. Zou Y. Repulsive Wnt signaling inhibits axon regeneration after CNS injury. J. Neurosci. 2008;28:8376–8382. doi: 10.1523/JNEUROSCI.1939-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi Y. Yuan S. Li B. Wang J. Carlton S.M. Chung K. Chung J.M. Tang S.J. Regulation of Wnt signaling by nociceptive input in animal models. Mol. Pain. 2012;8:47. doi: 10.1186/1744-8069-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan S. Shi Y. Tang S.J. Wnt signaling in the pathogenesis of multiple sclerosis-associated chronic pain. J. Neuroimmune Pharmacol. 2012;7:904–913. doi: 10.1007/s11481-012-9370-3. [DOI] [PubMed] [Google Scholar]
- 17.Cappuccio I. Calderone A. Busceti C.L. Biagioni F. Pontarelli F. Bruno V. Storto M. Terstappen G.T. Gaviraghi G. Fornai F. Battaglia G. Melchiorri D. Zukin R.S. Nicoletti F. Caricasole A. Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is required for the development of ischemic neuronal death. J. Neurosci. 2005;25:2647–2657. doi: 10.1523/JNEUROSCI.5230-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caricasole A. Bakker A. Copani A. Nicoletti F. Gaviraghi G. Terstappen G.C. Two sides of the same coin: Wnt signaling in neurodegeneration and neuro-oncology. Biosci. Rep. 2005;25:309–327. doi: 10.1007/s10540-005-2893-6. [DOI] [PubMed] [Google Scholar]
- 19.Carmichael J. Sugars K.L. Bao Y.P. Rubinsztein D.C. Glycogen synthase kinase-3beta inhibitors prevent cellular polyglutamine toxicity caused by the Huntington's disease mutation. J. Biol. Chem. 2002;277(33):791–33. doi: 10.1074/jbc.M204861200. ,798. [DOI] [PubMed] [Google Scholar]
- 20.Inestrosa N.C. Toledo E.M. The role of Wnt signaling in neuronal dysfunction in Alzheimer's Disease. Mol. Neurodegener. 2008;3:9. doi: 10.1186/1750-1326-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lovestone S. Killick R. Di Forti M. Murray R. Schizophrenia as a GSK-3 dysregulation disorder. Trends Neurosci. 2007;30:142–149. doi: 10.1016/j.tins.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Mastroiacovo F. Busceti C.L. Biagioni F. Moyanova S.G. Meisler M.H. Battaglia G. Caricasole A. Bruno V. Nicoletti F. Induction of the Wnt antagonist, Dickkopf-1, contributes to the development of neuronal death in models of brain focal ischemia. J. Cereb. Blood Flow Metab. 2009;29:264–276. doi: 10.1038/jcbfm.2008.111. [DOI] [PubMed] [Google Scholar]
- 23.Parish C.L. Castelo–Branco G. Rawal N. Tonnesen J. Sorensen A.T. Salto C. Kokaia M. Lindvall O. Arenas E. Wnt5a-treated midbrain neural stem cells improve dopamine cell replacement therapy in parkinsonian mice. J. Clin. Invest. 2008;118:149–160. doi: 10.1172/JCI32273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yin Z.S. Zu B. Chang J. Zhang H. Repair effect of Wnt3a protein on the contused adult rat spinal cord. Neurol. Res. 2008;30:480–486. doi: 10.1179/174313208X284133. [DOI] [PubMed] [Google Scholar]
- 25.Hendrickx M. Leyns L. Non-conventional Frizzled ligands and Wnt receptors. Dev. Growth Differ. 2008;50:229–243. doi: 10.1111/j.1440-169X.2008.01016.x. [DOI] [PubMed] [Google Scholar]
- 26.Fradkin L.G. Dura J.M. Noordermeer J.N. Ryks: new partners for Wnts in the developing and regenerating nervous system. Trends Neurosci. 2009;33:84–92. doi: 10.1016/j.tins.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Kamitori K. Tanaka M. Okuno–Hirasawa T. Kohsaka S. Receptor related to tyrosine kinase RYK regulates cell migration during cortical development. Biochem. Biophys. Res. Commun. 2005;330:446–453. doi: 10.1016/j.bbrc.2005.02.177. [DOI] [PubMed] [Google Scholar]
- 28.Katso R.M. Russell R.B. Ganesan T.S. Functional analysis of H-Ryk, an atypical member of the receptor tyrosine kinase family. Mol. Cell Biol. 1999;19:6427–6440. doi: 10.1128/mcb.19.9.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li L. Hutchins B.I. Kalil K. Wnt5a induces simultaneous cortical axon outgrowth and repulsive axon guidance through distinct signaling mechanisms. J. Neurosci. 2009;29:5873–5883. doi: 10.1523/JNEUROSCI.0183-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu W. Yamamoto V. Ortega B. Baltimore D. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell. 2004;119:97–108. doi: 10.1016/j.cell.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 31.Lyu J. Yamamoto V. Lu W. Cleavage of the Wnt receptor Ryk regulates neuronal differentiation during cortical neurogenesis. Dev. Cell. 2008;15:773–780. doi: 10.1016/j.devcel.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Keeble T.R. Halford M.M. Seaman C. Kee N. Macheda M. Anderson R.B. Stacker S.A. Cooper H.M. The Wnt receptor Ryk is required for Wnt5a-mediated axon guidance on the contralateral side of the corpus callosum. J. Neurosci. 2006;26:5840–5848. doi: 10.1523/JNEUROSCI.1175-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y. Shi J. Lu C.C. Wang Z.B. Lyuksyutova A.I. Song X.J. Zou Y. Ryk-mediated Wnt repulsion regulates posterior-directed growth of corticospinal tract. Nat. Neurosci. 2005;8:1151–1159. doi: 10.1038/nn1520. [DOI] [PubMed] [Google Scholar]
- 34.Schmitt A.M. Shi J. Wolf A.M. Lu C.C. King L.A. Zou Y. Wnt-Ryk signalling mediates medial-lateral retinotectal topographic mapping. Nature. 2006;439:31–37. doi: 10.1038/nature04334. [DOI] [PubMed] [Google Scholar]
- 35.Scheff S.W. Rabchevsky A.G. Fugaccia I. Main J.A. Lumpp J.E., Jr. Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J. Neurotrauma. 2003;20:179–193. doi: 10.1089/08977150360547099. [DOI] [PubMed] [Google Scholar]
- 36.Basso D.M. Beattie M.S. Bresnahan J.C. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 37.Fernandez C.M. Molto E. Gallardo N. del Arco A. Martinez C. Andres A. Ros M. Carrascosa J.M. Arribas C. The expression of rat resistin isoforms is differentially regulated in visceral adipose tissues: effects of aging and food restriction. Metabolism. 2009;58:204–211. doi: 10.1016/j.metabol.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 38.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 39.Fernandez–Martos C.M. Gonzalez P. Rodriguez F.J. Acute leptin treatment enhances functional recovery after spinal cord injury. PLoS One. 2012;7:e35594. doi: 10.1371/journal.pone.0035594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Streit W.J. Walter S.A. Pennell N.A. Reactive microgliosis. Prog. Neurobiol. 1999;57:563–581. doi: 10.1016/s0301-0082(98)00069-0. [DOI] [PubMed] [Google Scholar]
- 41.Ridet J.L. Malhotra S.K. Privat A. Gage F.H. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997;20:570–577. doi: 10.1016/s0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- 42.Farooque M. Zhang Y. Holtz A. Olsson Y. Exudation of fibronectin and albumin after spinal cord injury in rats. Acta Neuropathol. 1992;84:613–620. doi: 10.1007/BF00227738. [DOI] [PubMed] [Google Scholar]
- 43.Galvan M.D. Luchetti S. Burgos A.M. Nguyen H.X. Hooshmand M.J. Hamers F.P. Anderson A.J. Deficiency in complement C1q improves histological and functional locomotor outcome after spinal cord injury. J. Neurosci. 2008;28(13):876–13. doi: 10.1523/JNEUROSCI.2823-08.2008. ,888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma M. Basso D.M. Walters P. Stokes B.T. Jakeman L.B. Behavioral and histological outcomes following graded spinal cord contusion injury in the C57Bl/6 mouse. Exp. Neurol. 2001;169:239–254. doi: 10.1006/exnr.2001.7679. [DOI] [PubMed] [Google Scholar]
- 45.Yu W.R. Westergren H. Farooque M. Holtz A. Olsson Y. Systemic hypothermia following compression injury of rat spinal cord: reduction of plasma protein extravasation demonstrated by immunohistochemistry. Acta Neuropathol. 1999;98:15–21. doi: 10.1007/s004010051046. [DOI] [PubMed] [Google Scholar]
- 46.Kamitori K. Machide M. Osumi N. Kohsaka S. Expression of receptor tyrosine kinase RYK in developing rat central nervous system. Brain Res. Dev. Brain Res. 1999;114:149–160. doi: 10.1016/s0165-3806(99)00033-4. [DOI] [PubMed] [Google Scholar]
- 47.Kamitori K. Machide M. Tomita K. Nakafuku M. Kohsaka S. Cell-type-specific expression of protein tyrosine kinase-related receptor RYK in the central nervous system of the rat. Brain Res. Mol. Brain Res. 2002;104:255–266. doi: 10.1016/s0169-328x(02)00358-3. [DOI] [PubMed] [Google Scholar]
- 48.Lein E.S. Hawrylycz M.J. Ao N. Ayres M. Bensinger A. Bernard A. Boe A.F. Boguski M.S. Brockway K.S. Byrnes E.J. Chen L. Chen T.M. Chin M.C. Chong J. Crook B.E. Czaplinska A. Dang C.N. Datta S. Dee N.R. Desaki A.L. Desta T. Diep E. Dolbeare T.A. Donelan M.J. Dong H.W. Dougherty J.G. Duncan B.J. Ebbert A.J. Eichele G. Estin L.K. Faber C. Facer B.A. Fields R. Fischer S.R. Fliss T.P. Frensley C. Gates S.N. Glattfelder K.J. Halverson K.R. Hart M.R. Hohmann J.G. Howell M.P. Jeung D.P. Johnson R.A. Karr P.T. Kawal R. Kidney J.M. Knapik R.H. Kuan C.L. Lake J.H. Laramee A.R. Larsen K.D. Lau C. Lemon T.A. Liang A.J. Liu Y. Luong L.T. Michaels J. Morgan J.J. Morgan R.J. Mortrud M.T. Mosqueda N.F. Ng L.L. Ng R. Orta G.J. Overly C.C. Pak T.H. Parry S.E. Pathak S.D. Pearson O.C. Puchalski R.B. Riley Z.L. Rockett H.R. Rowland S.A. Royall J.J. Ruiz M.J. Sarno N.R. Schaffnit K. Shapovalova N.V. Sivisay T. Slaughterbeck C.R. Smith S.C. Smith K.A. Smith B.I. Sodt A.J. Stewart N.N. Stumpf K.R. Sunkin S.M. Sutram M. Tam A. Teemer C.D. Thaller C. Thompson C.L. Varnam L.R. Visel A. Whitlock R.M. Wohnoutka P.E. Wolkey C.K. Wong V.Y. Wood M. Yaylaoglu M.B. Young R.C. Youngstrom B.L. Yuan X.F. Zhang B. Zwingman T.A. Jones A.R. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 49.Polakis P. Formation of the blood–brain barrier: Wnt signaling seals the deal. J. Cell Biol. 2008;183:371–373. doi: 10.1083/jcb.200810040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iseda T. Nishio T. Kawaguchi S. Yamanoto M. Kawasaki T. Wakisaka S. Spontaneous regeneration of the corticospinal tract after transection in young rats: a key role of reactive astrocytes in making favorable and unfavorable conditions for regeneration. Neuroscience. 2004;126:365–374. doi: 10.1016/j.neuroscience.2004.03.056. [DOI] [PubMed] [Google Scholar]
- 51.Ferguson I.A. Xian C. Barati E. Rush R.A. Comparison of wheat germ agglutinin-horseradish peroxidase and biotinylated dextran for anterograde tracing of corticospinal tract following spinal cord injury. J. Neurosci. Methods. 2001;109:81–89. doi: 10.1016/s0165-0270(01)00380-6. [DOI] [PubMed] [Google Scholar]
- 52.Brosamle C. Huber A.B. Fiedler M. Skerra A. Schwab M.E. Regeneration of lesioned corticospinal tract fibers in the adult rat induced by a recombinant, humanized IN-1 antibody fragment. J. Neurosci. 2000;20:8061–8068. doi: 10.1523/JNEUROSCI.20-21-08061.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klapka N. Hermanns S. Straten G. Masanneck C. Duis S. Hamers F.P. Muller D. Zuschratter W. Muller H.W. Suppression of fibrous scarring in spinal cord injury of rat promotes long-distance regeneration of corticospinal tract axons, rescue of primary motoneurons in somatosensory cortex and significant functional recovery. Eur. J. Neurosci. 2005;22:3047–3058. doi: 10.1111/j.1460-9568.2005.04495.x. [DOI] [PubMed] [Google Scholar]
- 54.Lee J.K. Chan A.F. Luu S.M. Zhu Y. Ho C. Tessier–Lavigne M. Zheng B. Reassessment of corticospinal tract regeneration in Nogo-deficient mice. J. Neurosci. 2009;29:8649–8654. doi: 10.1523/JNEUROSCI.1864-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seif G.I. Nomura H. Tator C.H. Retrograde axonal degeneration "dieback" in the corticospinal tract after transection injury of the rat spinal cord: a confocal microscopy study. J. Neurotrauma. 2007;24:1513–1528. doi: 10.1089/neu.2007.0323. [DOI] [PubMed] [Google Scholar]
- 56.Steward O. Zheng B. Tessier–Lavigne M. Hofstadter M. Sharp K. Yee K.M. Regenerative growth of corticospinal tract axons via the ventral column after spinal cord injury in mice. J. Neurosci. 2008;28:6836–6847. doi: 10.1523/JNEUROSCI.5372-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.David S. Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat. Rev. Neurosci. 2011;12:388–399. doi: 10.1038/nrn3053. [DOI] [PubMed] [Google Scholar]
- 58.Loane D.J. Byrnes K.R. Role of microglia in neurotrauma. Neurotherapeutics. 2010;7:366–377. doi: 10.1016/j.nurt.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blumenthal A. Ehlers S. Lauber J. Buer J. Lange C. Goldmann T. Heine H. Brandt E. Reiling N. The wingless homolog WNT5A and its receptor Frizzled-5 regulate inflammatory responses of human mononuclear cells induced by microbial stimulation. Blood. 2006;108:965–973. doi: 10.1182/blood-2005-12-5046. [DOI] [PubMed] [Google Scholar]
- 60.George S.J. Wnt pathway: a new role in regulation of inflammation. Arterioscler. Thromb. Vasc. Biol. 2008;28:400–402. doi: 10.1161/ATVBAHA.107.160952. [DOI] [PubMed] [Google Scholar]
- 61.Pereira C. Schaer D.J. Bachli E.B. Kurrer M.O. Schoedon G. Wnt5A/CaMKII signaling contributes to the inflammatory response of macrophages and is a target for the antiinflammatory action of activated protein C and interleukin-10. Arterioscler. Thromb. Vasc. Biol. 2008;28:504–510. doi: 10.1161/ATVBAHA.107.157438. [DOI] [PubMed] [Google Scholar]
- 62.Pereira C.P. Bachli E.B. Schoedon G. The wnt pathway: a macrophage effector molecule that triggers inflammation. Curr. Atheroscler. Rep. 2009;11:236–242. doi: 10.1007/s11883-009-0036-4. [DOI] [PubMed] [Google Scholar]
- 63.Halleskog C. Mulder J. Dahlstrom J. Mackie K. Hortobagyi T. Tanila H. Kumar Puli L. Farber K. Harkany T. Schulte G. WNT signaling in activated microglia is proinflammatory. Glia. 2011;59:119–131. doi: 10.1002/glia.21081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kilander M.B. Halleskog C. Schulte G. Recombinant WNTs differentially activate beta-catenin-dependent and -independent signalling in mouse microglia-like cells. Acta Physiol. (Oxf) 2011;203:363–372. doi: 10.1111/j.1748-1716.2011.02324.x. [DOI] [PubMed] [Google Scholar]
- 65.Norenberg M.D. Astrocyte responses to CNS injury. J. Neuropathol. Exp. Neurol. 1994;53:213–220. doi: 10.1097/00005072-199405000-00001. [DOI] [PubMed] [Google Scholar]
- 66.Silver J. Miller J.H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 67.Barnabe–Heider F. Frisen J. Stem cells for spinal cord repair Cell. Stem Cell. 2008;3:16–24. doi: 10.1016/j.stem.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 68.Horner P.J. Thallmair M. Gage F.H. Defining the NG2–expressing cell of the adult CNS. J. Neurocytol. 2002;31:469–480. doi: 10.1023/a:1025739630398. [DOI] [PubMed] [Google Scholar]
- 69.Feigenson K. Reid M. See J. Crenshaw E.B., 3rd Grinspan J.B. Wnt signaling is sufficient to perturb oligodendrocyte maturation. Mol. Cell Neurosci. 2009;42:255–265. doi: 10.1016/j.mcn.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 70.Zhong J. Kim H.T. Lyu J. Yoshikawa K. Nakafuku M. Lu W. The Wnt receptor Ryk controls specification of GABAergic neurons versus oligodendrocytes during telencephalon development. Development. 2011;138:409–419. doi: 10.1242/dev.061051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fawcett J.W. Asher R.A. The glial scar and central nervous system repair. Brain Res. Bull. 1999;49:377–391. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- 72.Okada M. Miyamoto O. Shibuya S. Zhang X. Yamamoto T. Itano T. Expression and role of type I collagen in a rat spinal cord contusion injury model. Neurosci. Res. 2007;58:371–377. doi: 10.1016/j.neures.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 73.Goritz C. Dias D.O. Tomilin N. Barbacid M. Shupliakov O. Frisen J. A pericyte origin of spinal cord scar tissue. Science. 2011;333:238–242. doi: 10.1126/science.1203165. [DOI] [PubMed] [Google Scholar]
- 74.Mercier F. Kitasako J.T. Hatton G.I. Fractones and other basal laminae in the hypothalamus. J. Comp. Neurol. 2003;455:324–340. doi: 10.1002/cne.10496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.