Abstract
Omega-3 polyunsaturated fatty acids (ω-3 PUFAs) confer multiple health benefits and decrease the risk of neurological disorders. Studies are needed, however, to identify promising cellular targets and to assess their prophylactic value against neurodegeneration. The present study (1) examined the efficacy of a preventive diet enriched with ω-3 PUFAs to reduce dysfunction in a well-established spinal cord injury (SCI) animal model and (2) used a novel metabolomics data analysis to identify potential neurolipidomic targets. Rats were fed with either control chow or chow enriched with ω-3 PUFAs (750 mg/kg/day) for 8 weeks before being subjected to a sham or a contusion SCI operation. We report new evidence showing that rats subjected to SCI after being pre-treated with a diet enriched with ω-3 PUFAs exhibit significantly better functional outcomes. Pre-treated animals exhibited lower sensory deficits, autonomic bladder recovery, and early improvements in locomotion that persisted for at least 8 weeks after trauma. We found that SCI triggers a robust alteration in the cord PUFA neurolipidome, which was characterized by a marked docosahexaenoic acid (DHA) deficiency. This DHA deficiency was associated with dysfunction and corrected with the ω-3 PUFA-enriched diet. Multivariate data analyses revealed that the spinal cord of animals consuming the ω-3 PUFA-enriched diet had a fundamentally distinct neurolipidome, particularly increasing the levels of essential and long chain ω-3 fatty acids and lysolipids at the expense of ω-6 fatty acids and its metabolites. Altogether, dietary ω-3 PUFAs prophylaxis confers resiliency to SCI mediated, at least in part, by generating a neuroprotective and restorative neurolipidome.
Key words: DHA, functional recovery, prevention, lipidomics
Introduction
Spinal cord injury (SCI) leads to debilitating and long-lasting motor, sensory, and bladder dysfunction. Primary mechanical injury to the spinal cord initiates a complex cascade of secondary damaging events, including prominent metabolic alterations, axonal damage, inflammation, cell death, and demyelination.1 Although current therapeutic strategies aimed at alleviating the secondary injury are promising,2,3 studies identifying and addressing potential determinants of vulnerability to neurological dysfunction in experimental models of injury are very limited. Accumulating evidence suggests that the susceptibility to damage that follows SCI depends on the intrinsic balance between neurorestorative and neurodestructive signals.4
Injury to the spinal cord results in a robust deregulation of membrane polyunsaturated fatty acid (PUFA) homeostasis, leading to membrane derangements and preferential release of arachidonic acid (AA; 20:4, ω-6 PUFA) and peroxidation of docosahexaenoic acid (DHA; 22:6, ω-3 PUFA).5–11 These derangements can lead to ω-6 fatty acid metabolism and marked ω-3 PUFA deficiencies, altering PUFA nutritional requirements. Further, the essential role of these lipids in physiology and cell signaling is believed to be important in the neural responses to injury and could predispose nerve cells to dysfunction. For instance, AA and its potent pro-inflammatory mediators are implicated in secondary damage cascades and dysfunction after SCI.12–16 In contrast, ω-3 PUFAs, such as DHA, are anti-inflammatory, confer neuroprotection, and play significant roles in facilitating functional recovery in various SCI animal models.12,17–22
One important hypothesis arising from our work and from other published epidemiological evidence is that dietary ω-3 PUFAs can confer resiliency and facilitate recovery, even when administered before SCI. We recently reported that acute parenteral administration of DHA before injury leads to early improvements in conduction and locomotor function after SCI.22 This finding is consistent with studies showing the efficacy of ω-3 PUFAs as a post-treatment modality to ameliorate secondary damage and dysfunction.12,17–24 There is still a significant gap in our knowledge, however, concerning the therapeutic effects of prophylactic ω-3 PUFAs against neurotrama.
We assessed whether SCI significantly alters ω-3 PUFA requirements in the cord, and whether pre-treatment with dietary ω-3 PUFAs, without further supplementation, is sufficient to reduce dysfunction after SCI. Addressing this question is particularly important for populations where neurological injury is an unavoidable high risk (i.e., neurodegenerative conditions, contact sports, and military conflicts) and can have major implications for the identification of biomarkers of neural vulnerability to dysfunction.
Methods
Animals
Experimental procedures were performed in compliance with the Loma Linda University School of Medicine regulations and institutional guidelines consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. A total of 54 female Sprague-Dawley rats weighing 182–212 g were received from Charles River Laboratories (Portage, MI). Animals were housed in individual cages with food and water ad libitum and exposed to alternate light and dark periods of 12 h.
Study design
The animals were allowed to acclimatize for 1 week after arriving at the animal facility and were randomly divided into two main groups: (Group A) animals received the control diet (n=27) and (group B) animals received the ω-3 PUFA-enriched diet (n=27) (Fig. 1A). After 8 weeks on these diets, the rats were further divided into four groups based on their dietary and surgical interventions: Group 1 was the control diet, sham operated group (n=8); group 2 was the control diet, spinal cord injured group (n=19); group 3 was the ω-3 PUFA-enriched diet, sham operated group (n=8); and group 4 was the ω-3 PUFA-enriched diet, spinal cord injured group (n=19). The respective dietary interventions were continued after surgery. Autonomic bladder, sensory and motor functions were assessed after surgery. The best attempts were made to perform behavioral tests in a blinded manner. Spinal cord tissue was collected for analyses at 8 weeks post-operation (wpo).
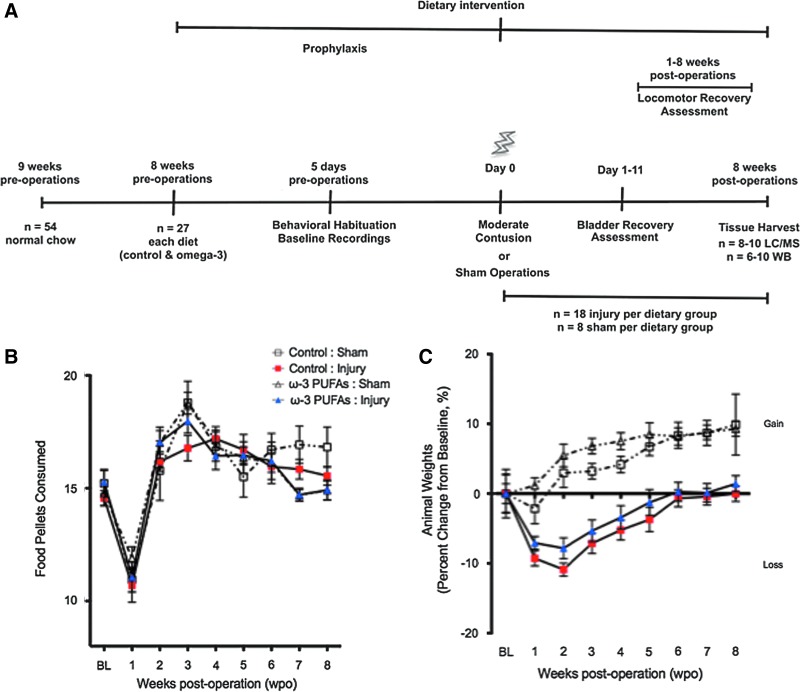
FIG. 1.
Dietary and weight monitoring. (A) Timeline outlining experimental design. (B) Baseline food resulted in an average intake of 15 pellets per day (500 mg docosahexaenoic acid and 250 mg eicosapentaenoic acid per kg of animal body weight per day). Surgery resulted in a significant reduction in food intake that returned to baseline levels at 2 weeks post-operation (wpo). No significant differences were found between groups (p>0.05, n=8–18 animals per group). (C) Dietary ω-3 polyunsaturated fatty acids (PUFAs) pre-treatment prevented significant weight losses when compared with injured rats receiving control diets at 1 week post-injury (wpi). Although dietary intake was similar, sham animals gained more weight than injured counterparts. Color image is available online at www.liebertpub.com/neu
Diet composition
Custom isocaloric AIN-93G-based diet formulations were designed with modifications to the fat composition (Bio-Serv, Frenchtown, NJ). The level of dietary fat was approximately 6% of dry weight supplied as either soybean oil (control chow) or menhaden fish oil (ω-3 PUFA-enriched chow). Both of the diets were stored refrigerated. Laboratory analyses using gas chromatography coupled with mass spectrometry demonstrated that the level of DHA and eicosapentaenoic acid (EPA) in the menhaden fish oil was 12.82-g and 6.91-g, respectively, per 100 g of diet. The level of cholesterol was 0.582-g/100 g. This amount was added to control diets to make the levels consistent with that of the fish oil diet. The selection of soybean oil as the control fat in our diets was based on the following observations: (1) Soybean oil is the principal source of ω-3 fatty acids in the United States, and (2) mammals have a limited capacity to convert DHA from linolenic acid (LA: 18:3, ω-3).
Spinal cord injury surgical and post-operative procedures
After 8 weeks of receiving either dietary intervention, the animals were deeply anesthetized with a mixture of ketamine (80 mg/kg) and xylazine (10 mg/kg). The spinal cord was injured at thoracic level 10 (T10) using the well-characterized NYU Impactor.25 Briefly, the skin and muscles overlying the spinal column were incised. A laminectomy was performed at T9–T10, leaving the dura intact, and the T8 and T12 spinal processes were clamped to the Impactor. A 10-g rod was released from 12.5-mm onto the exposed spinal cord to induce mild to moderate trauma. Analysis of the biomechanical impact parameters revealed no significant differences between control and treated groups (control diet group: 1.799±0.051-mm, 0.478±0.005 m/s, and 0.382±0.007 m/s vs. ω-3 PUFA-enriched diet group: 1.748±0.058-mm, 0.478±0.008 m/s, and 0.385±0.013 m/s values for compression, impact velocity, and compression rate, respectively; average±standard error of the mean [SEM]). The sham animals received only a laminectomy to expose the spinal cord.
During surgery, the body temperature of the animals was maintained at 37°C using a thermostatically controlled heating pad with a rectal thermometer (Physitemp TCAT-2LV; Physitemp, Clifton, NJ). After surgery, the muscle layers were sutured, and the skin layers were closed with wound clips. The bladders of injured rats were expressed using the Crede maneuver three times a day until voiding reflexes were restored. Cefazolin (Bristol Myers Squibb, New York, NY; 25 mg/kg, s.q.) and Buprenex® (buprenorphine; Reckett and Colman Pharmaceuticals, Inc. Richmond, VA; 0.05 mg/kg, s.c.) were given to all rats for 5 and 3 consecutive days, respectively. Two animals died because of surgical complications during this study. All other animals were allowed to survive for 8 wpo.
Autonomic function testing
Autonomic bladder control recovery
For all injured animals, the residual volume of urine expressed each morning was measured during the first 11 days post-injury (dpi; period during which full recovery of bladder function occurs after mild to moderate contusive injury in the rat). We also recorded the day on which autonomic bladder control was restored (e.g., when the maximal volume of urine collected in one session is consistently below 500 μL).
Motor function testing
Behavioral evaluation of spontaneous locomotion
Rats' spontaneous open-field locomotion was evaluated using the 22-point (0–21) Basso-Beattie-Bresnahan (BBB) scale.26,27 Briefly, animals were acclimatized to the open field environment during five daily sessions 1 week before SCI. After the SCI, the open field locomotion test was videotaped weekly. Subsequently, two observers assessed the locomotive function, joint movement, paw placement and rotation, coordination, and tail and trunk position and stability. For each hind paw, the averaged consensus score was used for analyses. In this scale, a completely paralyzed rat scores 0, a rat with increasing joint movements but without weight support scores between 1 and 8 (recovery stage 1), a rat with abnormal locomotion but with weight supported steps scores between 9 and 13 (recovery stage 2), graded coordination patterns, paw position, and trunk stability scores between 14 and 20 (recovery stage 3), and a normal (and sham) rat scores 21.
Locomotor scores were transformed using a simple post-hoc method that reduces error variance and improves the metric properties of the BBB, effect size, and power.22,28 This transformation produces a continuous distribution by pooling together BBB scores from 2–4 and 14–21 and thus avoids potentially suspect measures of performance from unlikely observations in the lower/higher end of the scale.
Sensory function testing
Habituation
Animals were habituated to sensory behavioral tests during five daily sessions held 1 week before baseline recordings.
Electronic von Frey test
Sensory function was assessed by measuring the withdrawal threshold of the hind paws in response to a mechanical stimulus using an electronic von Frey aesthesiometer (model 2391C; IITC Life Science, Woodland Hills, CA). Each animal was placed in a Plexiglas chamber positioned on an elevated metallic grid floor, which provided access to the plantar surface of the hind paw. Animals were allowed to acclimate to the environment for 30 min before testing. A rigid blunt tip attached to the meter was applied to the plantar surface from under the floor. The withdrawal threshold was defined as the average force (g) required for paw removal in five trials separated by a 1-min interval. The maximum and minimum threshold values were excluded from each paw after each testing session. The data were normalized to the percent change from baseline and sham animals. The normalized % change from baseline represents [(withdrawal threshold injury @ baseline – withdrawal thresholdinjury @ time point X) / withdrawal thresholdinjury @ baseline] X 100 – [(withdrawal thresholdshams @ baseline – withdrawal thresholdshams @ time point X) / withdrawal thresholdshams @ baseline] X 100.
Metabolomic analyses
Metabolon's sample preparation and metabolic profiling
The unbiased metabolic profiling platform used for this analysis combined three independent platforms: ultrahigh performance liquid chromatography/tandem mass spectrometry (UHPLC/MS/MS2) optimized for basic species, UHPLC/MS/MS2 optimized for acidic species, and gas chromatography/mass spectrometry (GC/MS). The spinal cord samples were processed essentially as described previously.29,30 For each sample, 100 μL was used for analyses. Using an automated liquid handler (Hamilton LabStar, Salt Lake City, UT), protein was precipitated from the plasma with methanol that contained four standards to report on extraction efficiency. The resulting supernatant was split into equal aliquots for analysis on the three platforms. Aliquots, dried under nitrogen and vacuum-desiccated, were subsequently either reconstituted in 50 μL 0.1% formic acid in water (acidic conditions) or in 50 μL 6.5 mM ammonium bicarbonate in water, pH 8 (basic conditions) for the two UHPLC/MS/MS2 analyses or derivatized to a final volume of 50 μL for GC/MS analysis using equal parts bistrimethyl-silyl-trifluoroacetamide and solvent mixture acetonitrile:dichloromethane:cyclohexane (5:4:1) with 5% triethylamine at 60°C for 1 h.
In addition, three types of controls were analyzed in concert with the experimental samples: aliquots of a well-characterized human plasma pool served as technical replicates throughout the data set, extracted water samples served as process blanks, and a cocktail of standards spiked into every analyzed sample allowed instrument performance monitoring. The experimental samples and controls were randomized across the platform run days.
For UHLC/MS/MS2 analysis, aliquots were separated using a Waters Acquity UPLC (Waters, Millford, MA) and analyzed using an LTQ mass spectrometer (Thermo Fisher Scientific, Inc., Waltham, MA), which consisted of an electrospray ionization source and linear ion-trap mass analyzer. The MS instrument scanned 99–1000 m/z and alternated between MS and MS2 scans using dynamic exclusion with approximately 6 scans per sec. Derivatized samples for GC/MS were separated on a 5% phenyldimethyl silicone column with helium as the carrier gas and a temperature ramp from 60°C to 340°C and then analyzed on a Thermo-Finnigan Trace DSQ MS (Thermo Fisher Scientific, Inc.) operated at unit mass resolving power with electron impact ionization and a 50–750 atomic mass unit scan range.
Metabolites were identified by automated comparison of the ion features in the experimental samples compared to a reference library of chemical standard entries that included retention time, molecular weight (m/z), preferred adducts, and in-source fragments as well as associated MS spectra. The metabolites were curated by visual inspection for quality control using software developed at Metabolon.31
RNA isolation and reverse transcription
Animals were submitted to humane euthanasia with Fatal-Plus, and perfused transcardially with ice-cold 0.01 M phosphate buffered saline, pH 7.4 (Sigma-Aldrich). Spinal cord segments containing the injury areas adjacent to the injury site (5 mm each) were collected at 8 wpo. (wpo). Total RNA was extracted using the Trizol reagent (Invitrogen) according to the manufacturer's instructions, and the RNA concentration was determined on a NanoDrop spectrophotometer (Thermo Scientific). cDNA was prepared with 800 ng of RNA by reverse transcription using SuperScript II and random primers (Invitrogen).
Real-time polymerase chain reaction (PCR)
PCR amplification and analyses were performed on a CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA). The cDNA was amplified by PCR using pairs of primers specific for protein kinase B (Akt1; FWD: 5'-TAC CAT GAA CGA CGT AGC CA-3' and REV: 5'-AGG TGC CAT CAT TCT TGA GG-3'), and the cyclic AMP responsive element binding protein (CREB; FWD: 5'-CAT GGA CTC TGG AGC AGA CA-3' and REV: 5'-GGG CTA ATG TGG CAA TCT GT 3'). Each well contained 25 μL as the final PCR reaction volume containing cDNA, primers and the SYBR green PCR master mix (Applied Biosystems, Foster City, CA). All samples were run in triplicates. Thermal cycling conditions were as follows: 10 sec at 95°C for denaturation, and 30 sec at 60°C for annealing/extension.
Relative mRNA quantities were compared between groups using the crossing threshold values. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH; FWD: 5'-TGC CAC GAA GAC TGT GG-3' and REV: 5'-TTC AGC TCT GGG ATG ACC TT-3') was used as the internal control gene, and amplification specificity was checked using melting curve analyses. Mock reactions were performed as negative controls.
Immunodetection
Western blot
Eight weeks after the operations, the spinal cord segments were processed for Western blot analysis as described previously.32 Briefly, 5 mm spinal cord segments were dissected and immediately frozen. The tissue containing injured epicenter-to-caudal regions was then homogenized manually with a Dounce tissue grinder in 200 μL of 1% SDS in Tris–EDTA buffer with proteinase inhibitors (10 μg/mL aprotinin, 1 μg/mL leupeptin, and 1 mM PMSF) and then sonicated. After centrifugation at 14,000 rpm, the supernatant was assayed for protein concentration using the method of Lowry33 and bovine serum albumin as a standard. Thirty micrograms of protein were diluted in Laemmli buffer, loaded and run in 4–12% SDS-polyacrylamide gels, and transferred to nitrocellulose membranes. Membranes were blocked using Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE). The relative amounts of Akt and CREB proteins were detected using rabbit anti-Akt and anti-CREB antibodies (1:1000 each; Cell Signaling, Danvers, MA).
After a 2 h incubation in primary antibodies, the membranes were washed three times for 10 min in Tris-buffered saline with 0.1% Tween-20 and incubated in donkey anti-rabbit IgG conjugated with IRDye® 800CW for 1 h (1:15,000; LI-COR Biosciences). Membranes were washed as above, and the images were captured using an Odyssey infrared imaging system (LI-COR Biosciences). Protein relative expression was standardized to β-actin levels, which were detected using mouse anti-β-actin antibodies (1:2000; Abcam, Cambridge, MA) and goat anti-mouse IRDye® 680RD secondary antibodies (1:20000, LI-COR Biosciences). Quantitative analyses were performed using ImageJ 1.45I software (http://rsb.info.nih.gov/ij/index.html).
Statistical analysis
Statistical analyses were performed using SPSS 20.0 (IBM: SPSS, Armonk, NY), Prism 5 software v5d (GraphPad Software Inc., San Diego, CA), MetaboAnalyst (www.metaboanalyst.ca), and the “R” program (http://cran.r-project.org/). Two-way analysis of variance (ANOVA) followed by Bonferroni post-hoc comparisons were used to determine the effect of our dietary/surgical intervention and time on rat pellet consumption, weights, residual urine volume, open-field locomotion scores, and sensory threshold changes. Analysis of covariance was also used to test multi-interactions between weight, pellet consumption, and functional behavior at baseline and at different time points within and between the groups. All other data were assessed by one-way ANOVA, t tests, or the Mann-Whitney U test.
The Kolmogorov-Smirnov and Shapiro-Wilk normality tests were used to investigate outliers and spread. Outliers were also identified using the Grubbs method, also known as extreme studentized deviate; www.graphpad.com. Only two animals were excluded from the study after using these exclusion methods in our behavioral data. To analyze neurometabolic alterations, multivariate data analysis—partial least squares projections to latent structures discriminant analysis (PLS-DA)—was performed using MetaboAnalyst software. Spearman rank correlation tests were used to explore associations between the top metabolic ratios contributing to model variability and the behavioral phenotypes. Data are presented as mean±SEM. Statistical differences were considered significant at p<0.05.
Results
The rationale behind the chosen ω-3 PUFAs dose, pre-treatment schedule, and end-point measures was based on (1) studies that indicate that animals that are fed diets enriched with 6% fish oil, such as the one used in this study, can take up to 8 weeks to significantly alter the lipid profile of the rat brain,34 (2) studies that demonstrate that fatty acid incorporation occurs in a manner that is highly correlated with the amount consumed and with brain needs,35,36 (3) studies revealing that the beneficial effects of ω-3 PUFAs in the central nervous system (CNS) could be readily observed with dietary interventions lasting 6–8 weeks and at doses ranging from 10 mg/day to 400 mg/kg/day,19,37–39 and (4) evidence that supports that damage/repair processes take place after chronic SCI.40–43 The study design is summarized in Figure 1A.
Animals were housed in individual cages and the baseline food consumption was monitored daily. Food intake resulted in an average intake of 15 pellets per day (Fig. 1B). This amount resulted in approximately 500 mg DHA and 250 mg EPA per kg BW. It is known that rats and humans have similar dietary requirements for PUFAs (% of total energy). The relationship between body size and the rate of metabolic processes (and thus time), however, needs to be taken into account when dosing. For instance, the relationship between basal metabolic rate (BMR) and BW in different size mammals is described by the function Y=aX0.75, where Y is BMR (kJ/d), X is BW (kg) and a is BMR per kg0.75 per day, which is 300 kJ/(kg0.75 · d)
Thus, the BMR in different size species is proportional to the BW raised to the 0.75 power, also known as the metabolic weight. Assuming that the BMR comprises 75% of the total metabolic rate, then the total metabolic rate can be described by the function Y=400 X0.75. From this relationship, the total metabolic rate of a 70-kg human is 400×700.75=9680 kJ or 138 kJ per kg BW. Similarly, the total metabolic rate of a 200 g rat is 400×0.2000.75=120 or 600 kJ per kg BW. Thus, the absolute energy expenditure expressed in kJ per kg body weight is greater in rats than in humans. Therefore, although the DHA dose used in this study (500 mg/kg) would be equivalent to approximately 35 mg of DHA/day in a 70-kg human, this amount would be far less when corrected for metabolic weight. Also, when using this correction, the 8-week feeding period used herein is analogous to approximately 1 year in humans.44
Two-way ANOVA analyses identified time as the only significant source of variation in food intake between groups (F[8,1082]=29.33; p<0.0001, n=8 control groups, n=18 experimental groups). Post-hoc analysis revealed a significant reduction in the amount of pellets ingested at 1 wpo when compared with baseline consumption (∼30 % reduction; p<0.05). Normal feeding behavior was re-established by week 2 after surgery when compared with baseline values (p>0.05).
Two-way ANOVA revealed that diet/surgical intervention [F(3,7044)=62.97] and time (F[8,3439]=11.53) had significant effects on animal weights (p≤0.0001 for both) (Fig. 1B). Post-hoc analysis showed no significant differences in animal BW at baseline (p>0.05). Although in both groups, the injury resulted in an 8–10% reduction in BW, post-hoc analysis demonstrated no significant differences in BW when comparing 1 week post-injury (wpi) with baseline values in animals fed with the ω-3 PUFA-enriched diet (p>0.05). In contrast, significant BW loss was found in the control-diet fed animals at 1 wpi (p<0.01). In both diet groups, baseline BW was re-established at 3 wpi. It is noteworthy that although sham animals showed similar pellet consumption when compared with injured rats, no differences in weight were observed in the first few weeks after operations. In contrast, injured rats showed a marked metabolic disconnect, as evidenced by weight loss despite similar caloric intake to sham counterparts.
Dietary ω-3 PUFA prophylaxis accelerates bladder recovery, improves locomotor function, and ameliorates sensory dysfunction
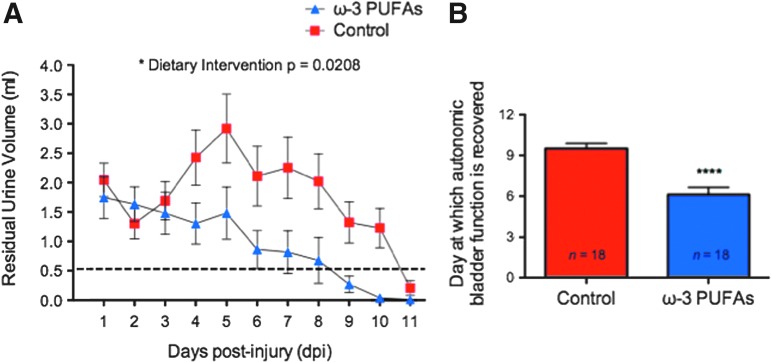
SCI results in a period of marked bladder dysfunction.45 Because animals need assistance with bladder voiding during the first few days after SCI, we collected and quantified the residual urine volume to assess whether a dietary ω-3 PUFA intervention show efficacy in accelerating autonomic bladder recovery. Of special significance, ANOVA identified the type of diet as a significant source of variance between groups in the course of 11 dpi (F[1,69.42]=5.88; p=0.0208, n=18 per group) (Fig. 2A). Further, full autonomic recovery of bladder control (two or more days with a residual volume equal or less than 0.5 mL) occurred significantly earlier in animals receiving ω-3 PUFA-enriched diets (6.1±0.6 dpi) when compared with controls (9.5±0.4 dpi) (unpaired t test t=4.97 df=34; p<0.0001) (Fig. 2B).
FIG. 2.
Beneficial effects of dietary ω-3 PUFAs prophylaxis on autonomic function after contusion injury. (A) Residual urine volumes (mL) differed significantly between control and ω-3 polyunsaturated fatty acids (PUFAs) pre-treated groups (analysis of variance, p=0.0208, n=18). Dashed line represents cutoff volume used to determine complete autonomic recovery. (B) For each animal, the number of days needed to attain full autonomic recovery was defined as residual volume of 0.5 mL for 2 or more consecutive days. Dietary ω-3 PUFAs prophylaxis resulted in fewer days to attain full bladder recovery (Mann-Whitney U test, p<0.0001, n=18). Color image is available online at www.liebertpub.com/neu
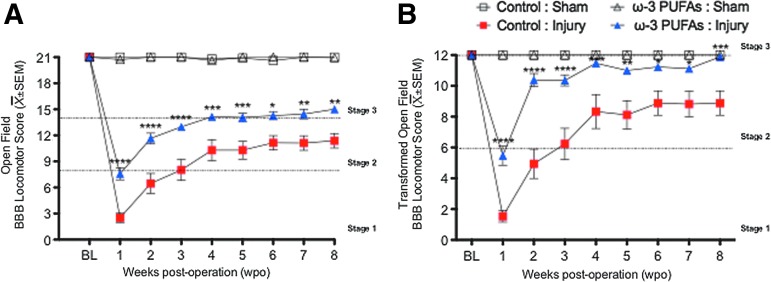
To characterize the effects of dietary ω-3 PUFA prophylaxis in motor recovery after SCI, we used the BBB locomotor behavior scale followed by score transformations.28 Two-way ANOVA followed by Bonferroni post-hoc testing demonstrated significant differences in BBB locomotor scores between animals receiving ω-3 PUFA-enriched diets and control diets (untransformed data: F[3,8636]=460.1; transformed data: (F[3,1542]=117.7; for both p<0.0001) (Figs. 3A and B).
FIG. 3.
Dietary ω-3 polyunsaturated fatty acids (PUFAs) prophylaxis improves somatic function after contusion injury. (A) Dietary ω-3 PUFAs prophylaxis results in significant early and long-lasting functional milestones after SCI. (B) These results remained significant after pooling unusual observations and improving the metric properties using the Basso-Beattie-Bresnahan (BBB) score transformations. Interestingly, control animals reached a recovery plateau at 4 weeks (occasional weight supported plantar steps with no coordination: BBB score=10; transformed BBB score=8), whereas dietary prophylaxis resulted in prolonged recovery when compared with controls (displayed consistent weight-supported plantar steps and coordination at 8 weeks post-injury: BBB score=14; transformed BBB score: 12). Data are presented as mean±standard error of the mean (SEM); asterisks indicate significance level: Two-way analysis sof variance followed by Bonferroni post hoc *p<0.05, **p<0.01, ***p<= 0.001, n=8–18. Color image is available online at www.liebertpub.com/neu
A striking finding of this study is that locomotor recovery was significantly accelerated in the group of animals receiving ω-3 PUFA-enriched diets when compared with controls. This was evidenced by their ability to produce extensive joint movements and occasional weight supported steps at 1 wpi (transformed BBB scores, mean±SEM: control diet=1.53±0.38, n=17 vs. ω-3 PUFA-enriched diet=5.47±0.62, n=17; p<0.0001) (Fig. 3B). Interestingly, post-hoc analysis showed that these differences in locomotor behavior persisted at least until 8 wpi (p<0.05). End-point outcome measures showed that animals receiving control diets were able to step occasionally or frequently but with no signs of locomotive coordination at 8 wpi (transformed score: 8.2±0.86). In contrast, rats receiving ω-3 PUFA-enriched diets displayed consistent weight-supported plantar steps (or frequent plantar stepping and occasional dorsal stepping), consistent coordination, and paw rotation during locomotion at 8 wpi (transformed score: 11.88±0.08).
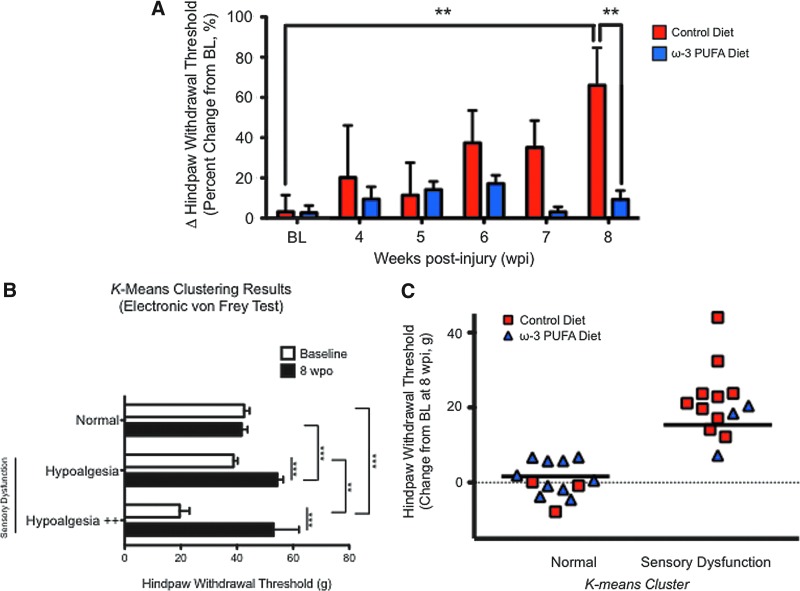
To examine the effect of dietary ω-3 PUFA on sensory function after SCI, the rats were subjected to electronic von Frey testing using a nonpunctate blunt probe. A remarkable finding of this study is that the ω-3 PUFA diet prevented major sensory deficits (mechanical hypoalgesia or loss of sensitivity) associated with chronic SCI in adult rats43 (F[1,14919]=8.592; p=0.0039) (Fig. 4A). Post-hoc analyses showed significant differences in normalized hind paw withdrawal thresholds between animals fed with control diets (≈66% change from baseline) and ω-3 PUFA-fed groups (≈9% change from baseline) at 8 wpi (p<0.01) (Fig. 4A).
FIG. 4.
Dietary ω-3 polyunsasturated fatty acids (PUFA)s prevents sensory dysfunction after chronic spinal cord injury (SCI). (A) Electronic von Frey test shows that SCI results in increased withdrawal threshold (hypoalgesia). Remarkably, ω-3 PUFAs prophylaxis prevented alterations in paw sensitivity when compared with baseline (BL) (p>0.01). Post hoc revealed significant effects were observed between dietary groups at 8 wpi (p<0.05). Data are expressed as %Δ paw withdrawal threshold from BL (and normalized to %Δ in sham animals; mean±standard error of the mean [SEM], n=13). (B) K-means clustering divided mechanical sensory thresholds (g of force required to elicit hind paw withdrawal) into three groups: (1) normal (no differences between baseline and 8 weeks post-operation (wpo); two-way analysis of variance followed by Bonferroni's post-hoc test, p>0.05), (2) hypoalgesia (significant difference between baseline and 8 wpo; p<0.05), and (3) hypoalgesia++ (extremely significant difference between baseline and 8 wpo; p<0.05). Data are presented as mean±SEM. (C) Dietary ω-3 PUFAs prophylaxis significantly reduced the number of animals in which sensory dysfunction developed. K-means clustering was followed by the Fisher exact test to determine whether diet altered the predisposition of animals for development of sensory deficits. Analyses revealed that dietary ω-3 PUFAs reduced the probability of development of major sensory dysfunction (hypoalgesia) by 54% when compared with controls (Fisher exact test p=0.02, n=26). Color image is available online at www.liebertpub.com/neu
To determine whether a diet enriched in ω-3 PUFA reduces the likelihood of developing sensory dysfunction, we used the K-means clustering method to assign all rats to three groups according to their mechanical sensory thresholds changes at 8 wpi. Clustering resulted in a group of animals with statistically significant increases in hind paw withdrawal thresholds at 8 wpi when compared with their respective baseline values (hypoalgesia cluster). The second cluster of animals did not show significant threshold changes between baseline and 8 wpo (normal cluster). The third cluster (hypoalgesia ++) also revealed hypoalgesic behaviors when compared with baseline (this cluster contained three animals fed with control diets). It is noteworthy that the average baseline value from this third cluster was also significantly lower when compared with the other two groups. Clustering validation results are summarized in Fig. 4B (hypoalgesic behavior [cluster]: F[2,634.2]=4.03, p=0.022; time: F[1,2543]=32.33, p<0.0001).
Remarkably, we found that only 23% of the animals receiving ω-3 PUFA diets were clustered in the group of animals with significant sensory dysfunction (hypoalgesic clusters combined; Fig. 4C). In marked contrast, more than 75% of the control animals showed significant sensory deficits. The Fisher exact test revealed that this difference was significant (p=0.02; strength of association analysis demonstrated an odds ratio of 0.09 [95% confidence interval (CI)=0.01 to 0.60], with a sensitivity of 0.23 [95% CI=0.05 to 0.54] and a specificity of 0.23 [95% CI=0.05 to 0.54]).
Distinctive neurolipidomic signatures are associated with injury operations and dietary interventions
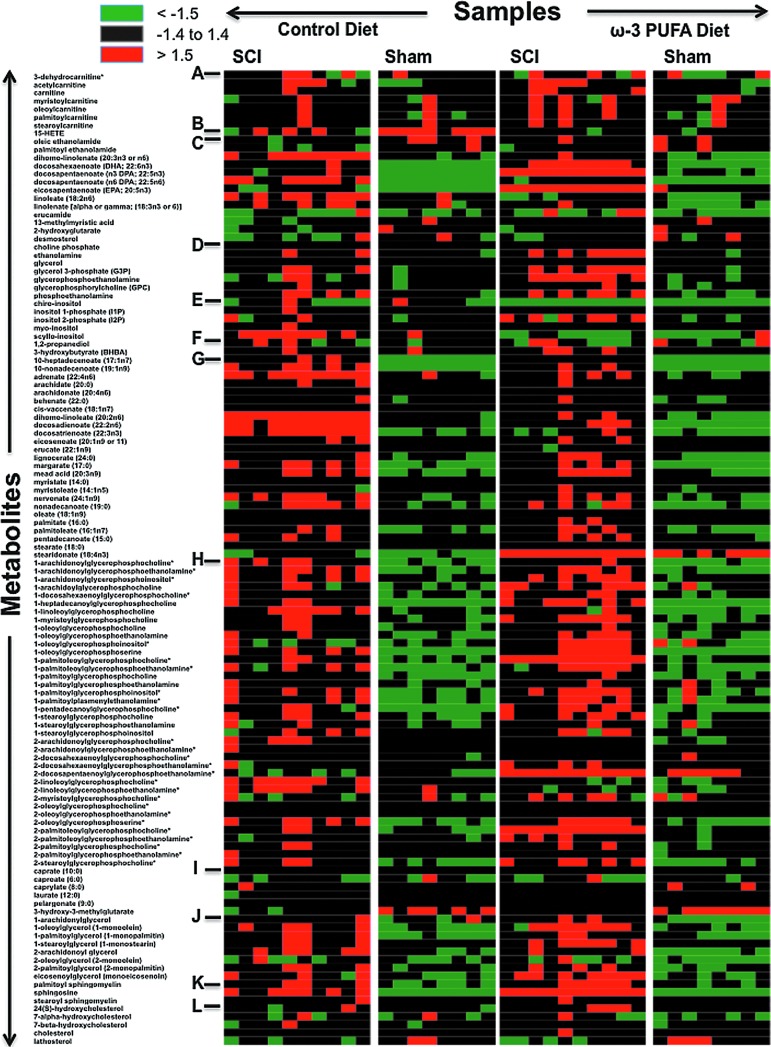
To investigate the neurolipidome, we interrogated the levels of lipid metabolic features across 36 spinal cord epicenters using both LC/MS and GC/MS. Unsupervised hierarchical clustering was used to arrange the lipid metabolites on the basis of their relative levels across tissue samples and generate heat maps of the spinal cord neurolipidome (Fig. 5). Remarkably, we found distinctive metabolic lipid signatures in each studied group.
FIG. 5.
Heat map representation of unsupervised hierarchical clustering. Data are arranged in metabolites (rows) by sample type (columns). Metabolite family description: A, carnitine metabolism; B, eicosanoids; C, essential fatty acids and free fatty acids; D, glycerolipids; E, inositol metabolism; F, ketone bodies; G, long chain fatty acids; H, lysolipids; I, medium chain fatty acids; J, monoacylglycerols; K, sphingolipids; L, sterols and steroids. Green and red colors represent metabolite decreases and increases, respectively, relative to the median metabolite levels. See color scale. PUFA=polyunsaturated fatty acids; SCI=spinal cord injury. Color image is available online at www.liebertpub.com/neu
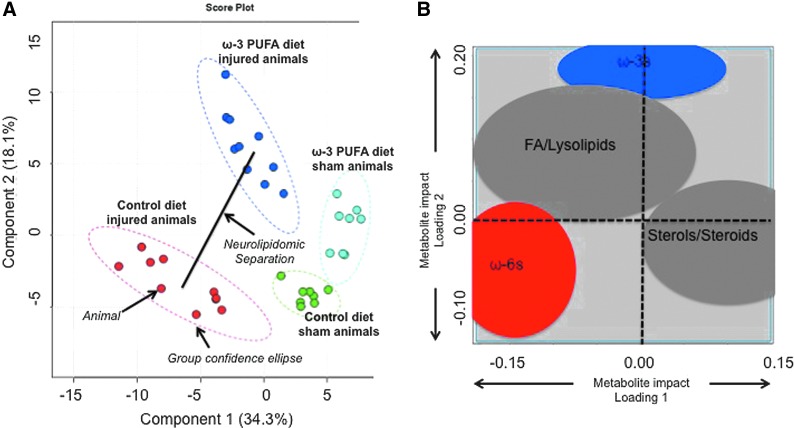
Partial least squares PLS-DA was performed to determine if the lipid features were capable of distinguishing group separations using a supervised projection technique (Fig. 6B). Of significance, our analyses revealed a projection in which the groups were significantly separated (p<0.01 by permutation test). PLS-DA score plot was obtained using the variation scores of the first two principal components, PC1 (34.3%) and PC2 (18.1%) (Fig. 6A). Each plot mark corresponds to an animal subject and the variability in relative lipid metabolite levels detected for that animal. The first component, PC1, separated the sham animal spectra from injured animals. The second component, PC2, separated the spectra from control diets and animals receiving ω-3 PUFA-enriched diets. Hotelling's T2 confidence ellipse, at a significance level of 0.05, revealed no outliers. Because score plots and loading plots are complementary, superimposing them depicts the impact of metabolites in the score plot model and show how the metabolites are correlated (most influential variables are further from zero on each loading; correlated variables are grouped together).
FIG. 6.
Multivariate data analyses. (A) PLS-DA score plot containing two first components. Each plot mark corresponds to an observation (individual rat spinal cord sample). The confidence ellipses are based on the Hotelling T2 and illustrate the 95% confidence regions. Principal component 1 (PC1; x-axis) describes differences in the spectra between sham and injury operations. Principal component 2 (PC2; y-axis) shows a marked separation between control and ω-3 diets. Score plot analyses revealed statistically significant separations between groups (p<0.01 by permutation test). (B) Loading plot between PC1 and PC2. Loading 1 explains differences between sham and injured animals, whereas features in loading 2 explain differences between dietary interventions. Groups with stronger impact are further away from the plot origin (represented by dashed lines). Data demonstrate that ω-3 polyunsaturated fatty acids (PUFAs) contributed significantly to lipidomic differences between injured animals (location in loading 2 is far from zero). In contrast, analyses revealed that ω-6 PUFAs present a major contribution to both model loadings (loading 1: surgery effects and loading 2: diet effects), validating their role in chronic spinal cord injury and modulation by dietary ω-3 PUFAs. Correlated metabolites were grouped together to facilitate data presentation. Color image is available online at www.liebertpub.com/neu
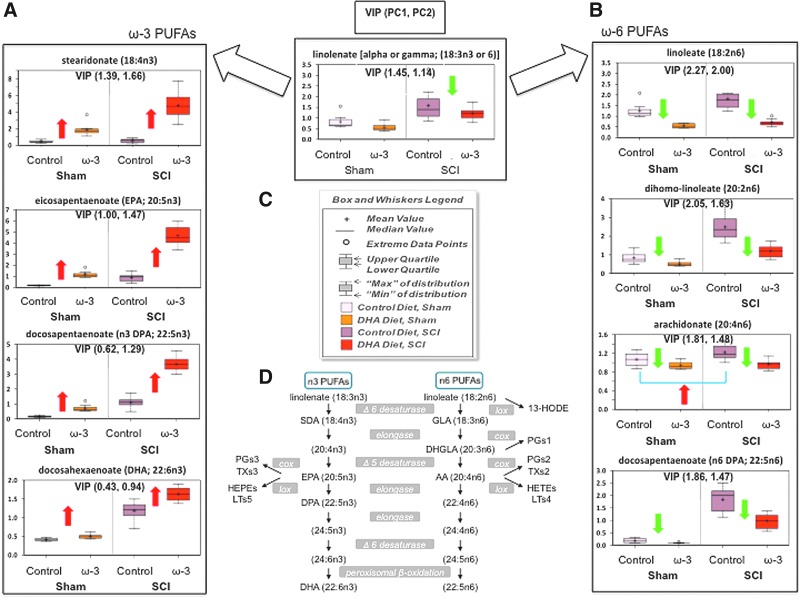
As expected, the PLS-DA loading plot showed that the levels of ω-3 and ω-6 PUFAs had the strongest influence on the separation observed in our score plot model (Fig. 6B). Variable influence on projection (VIP) analyses, which reflect the impact of metabolites in the PLS-DA model, confirmed these findings by showing that PUFAs had a robust impact in both components of our PLS-DA model (VIP values higher than 1). Interestingly, DHA levels had only a slight effect in the projection model (low VIP values or low influence), which supports its fast metabolism and avid incorporation in neural membrane phospholipids. Figure 7 summarizes the main effects of chronic SCI and diet on the biosynthetic pathway of ω-3 and ω-6 PUFAs. We found that the ω-3 PUFA-enriched diet significantly skewed the PUFA metabolism toward increased ω-3 at the expense of reduced ω-6 PUFA levels in both sham and injured animals.
FIG. 7.
Chronic spinal cord injury (SCI) results in marked deregulation of ω-3 (A) and ω-6 (B) Polyunsaturted fatty acid (PUFA) metabolic pathways corrected by ω-3 PUFA-enriched diet. Green (decrease) and red (increase) arrows represent statistically significant alterations when compared with controls (two-way analysis of variance followed by post-hoc testing was performed. Differences were significance at p<0.05). (C) Box and whiskers legend depict metabolite relative levels relative to the median metabolite levels in each group. For each ω-3 and ω-6 PUFA metabolite, variable influence on projection (VIP) values were included for projections to latent structures discriminant analysis principal component (PC) 1 and 2. For each component, VIP scores higher than 1 are considered significant contributors to the group separations. (D) Summary of PUFA biosynthetic pathways. AA, arachidonic acid; DGLA, dihomo-γ-linolenic acid; GLA, γ-linolenic acid; DPA, docosapentaenoic acid; SDA, stearidonic acid; PG, prostaglandins; LT, leukotrienes; TX, thromboxanes; HEPE, hydroxyeicosapentaenoic acid; HETE, hydroxyeicosatetraenoic acid; HODE, hydroxyoctadecadienoic acid. Color image is available online at www.liebertpub.com/neu
Chronic spinal cord injury dysfunction is linked to DHA deficiency and corrected by a preventive ω-3 PUFA dietary intervention
A number of studies have shown that feeding animals increased amounts of fish oil ω-3 PUFAs has a profound effect on PUFA biosynthetic pathways, particularly increasing tissue levels of DHA (22:6, ω-3), docosapentaenoic acid (DPA, 22:5, ω-3) and EPA (20:5, ω-3) at the expense of AA (20:4, ω-6).46 Our results validate these findings and demonstrate that chronic SCI results in dramatic alterations in PUFA metabolism that can be corrected and prevented by dietary prophylaxis with ω-3 PUFAs.
Previous evidence supports that dietary supplementation with ω-3 PUFAs results in improved CNS function.47 On the other hand, deficiencies in ω-3 PUFAs have been associated with numerous neurological impairments.48–52 DHA deficiency is accompanied by concomitant increases in DPA (22:5, ω-6) levels; thus, the ratio of ω-6 DPA to DHA has been suggested as a biochemical marker of low ω-3 PUFA status.53,54
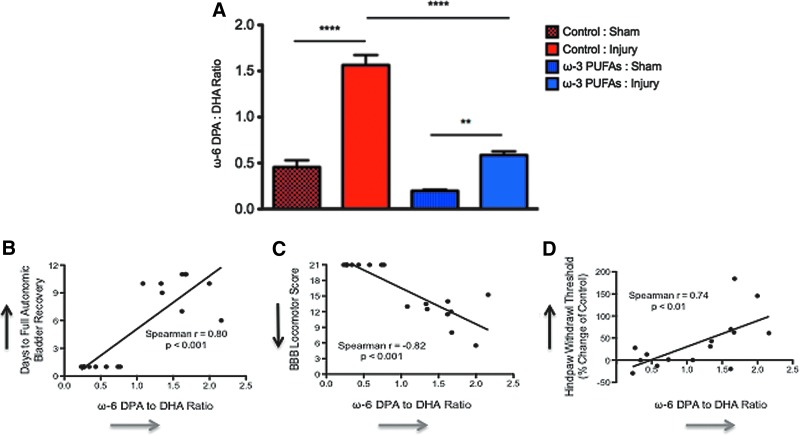
Using liquid and gas chromatography coupled with mass spectrometry (LC/MS and GC/MS), we measured the relative levels of ω-6 DPA and DHA in the spinal cord lesion/laminectomy epicenter area. We used the ω-6 DPA to DHA ratio to determine: (1) whether chronic SCI results in a DHA deficiency, (2) whether our dietary intervention altered this ratio, and (3) whether this deficiency has functional consequences in SCI. One-way ANOVA showed very significant differences in ω-6 DPA to DHA ratios between groups (F[3,10.66]=67.53, p<0.0001).
Post-hoc analyses demonstrated that SCI resulted in a significant ω-6 DPA to DHA ratio increase (DHA deficiency) when compared with sham animals at 8 wpo (p<0.0001 and p<0.01, respectively) (Fig. 8A), showing that DHA nutritional requirements may be enhanced after SCI. Remarkably, the dietary ω-3 PUFA diet significantly reduced this DHA deficiency when compared with the animals fed with control chow during chronic SCI (p<0.0001; n=10). Although DHA levels were increased in the animals receiving diets enriched with ω-3 PUFAs, no significant differences were observed when comparing the ω-6 DPA to DHA ratio in sham animals from both dietary groups (p>0.05, n=8 rats per sham group). This supports the proposed idea that PUFAs are tightly regulated under physiological conditions and shows that the control diet itself did not generate the DHA deficiency. It is also noteworthy that the observed effects are not likely from a deficiency in ω-6 fatty acids in the animals fed with ω-3 PUFA. This is supported by the finding that diet type had no significant effect on the levels of mead acid (20:3, ω-9), a biomarker of linoleic acid (18:2, ω-6) deficiency (data not shown).
FIG. 8.
Chronic spinal cord injury results in significant dicosahexaenoic acid (DHA) deficiency that is corrected by a ω-3 polyunsaturated fatty acid (PUFA)-enriched diet. (A) Deficiency of the dietary-essential DHA (22:6, ω-3) was measured by assessing the levels of docosapentaenoic acid (DPA) (22:5, ω-6). After injury, both dietary groups presented significant increases in the ω-6 DPA to DHA ratio. Notably, injured animals consuming ω-3 PUFAs showed a significant reduction in the ω-6 DPA to DHA ratio when compared with injured animals receiving control diets at 8 weeks post-injury (wpi). This reduction was comparable to the baseline levels observed in the sham-operated animals receiving the control diet. The ω-6 DPA to DHA ratio was similar in both sham groups. Spearman rank correlation showed that the ω-6 DPA to DHA ratio is strongly associated with bladder (B), locomotor (C), and sensory function (D). Data were generated by correlating the levels of the ω-6 DPA to DHA with the number of days needed for full bladder recovery (1–11), the Basso-Beattie-Bresnahan (BBB) locomotor scores (0–21), and mechanical threshold changes from baseline at 8 wpi (% change from baseline). This was repeated for each animal receiving control diets (n=15–17 pairs for each behavioral test). R and p values are included for each graph. Line depicts the regression line. Black arrows in Y-axes represent direction of increased dysfunction in each behavioral test. Grey arrows in X-axes represent direction of increased DHA deficiency. Color image is available online at www.liebertpub.com/neu
Dietary ω-3 PUFAs increase protein kinase B/Akt and CREB protein mRNA levels, even in sham-operated animals
To validate our findings and investigate the potential consequences of DHA deficiency in functional recovery after SCI, we performed Spearman correlation analyses to determine the relationship between the ω-6 DPA to DHA ratio and functional behavior in the animals receiving control diets. Because it has been proposed that molecular determinants of early autonomic recovery may also influence locomotor function (and vice versa),55 we also investigated the correlation between chronic DHA deficiency and bladder recovery. Notably, increases in the ω-6 DPA to DHA ratio were positively associated with the number of days needed to full bladder recovery in animals receiving control diets (r value=0.80, p<0.001; n=17 pairs) (Fig. 8B). The ω-6 DPA to DHA ratio showed a robust negative correlation with BBB locomotor scores (r=−0.82, p<0.0001) (Fig. 8C). Further, this ratio was positively correlated with mechanical hypoalgesia (r=0.74, p<0.01) (Fig. 8D). Together, these findings show for the first time that DHA deficiency after SCI may increase susceptibility for dysfunction and/or hinder functional recovery. However, the mechanisms underlying these beneficial roles in recovery remain largely unknown.
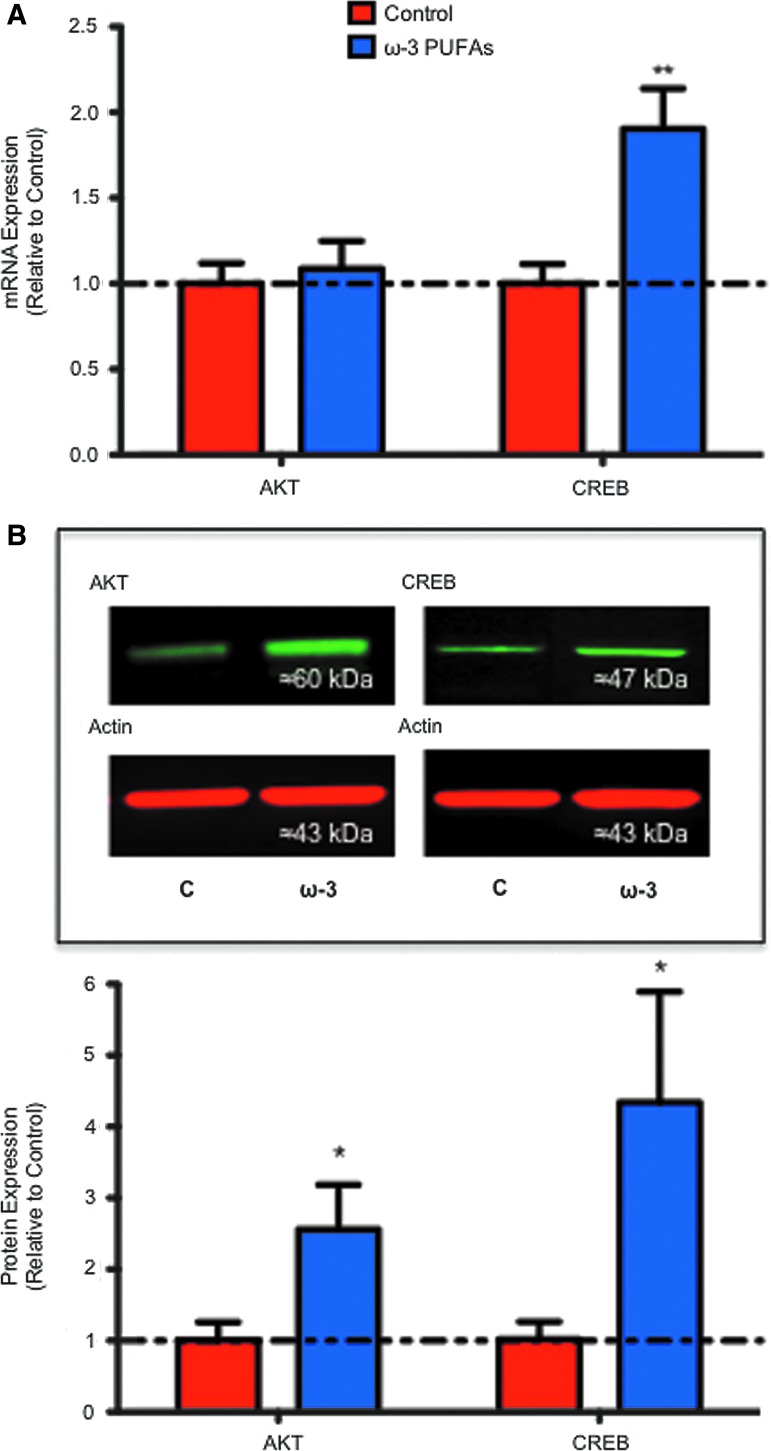
Protein kinase B/Akt (Akt) and the CREB protein have been implicated in regulating neural plasticity56–58 and pro-survival and regenerative responses in SCI.22,59–61 Previous studies from our laboratory and others have shown that DHA-mediated beneficial effects may be mediated, at least partially, through activation of Akt and/or CREB signaling pathways.22,62 To determine whether a diet rich in ω-3 PUFAs results in modulation of these neuroreparative molecules after chronic SCI, we examined Akt and CREB mRNA levels at 8 wpi. ANOVA followed by Bonferroni multiple comparison test revealed no significant changes in Akt mRNA levels when comparing both injury groups (F[3,0.26]=0.91, t=0.40, CI [−0.76 to 0.56], p>0.05) (Fig. 9A). In contrast, our analyses revealed a 1.9-fold increase in CREB mRNA levels in animals that were fed with ω-3 PUFAs when compared with controls at 8 wpi (F[3,3.73]=9.03, t=3.44, CI [−1.72 to −0.09], p<0.05, n≥4) (Fig. 9A). Western blot analyses demonstrated that n ω-3 PUFA-enriched diet resulted in a 2.6-fold increase in Akt protein levels when compared with controls at 8 wpi (Mann Whitney U (4.0), two-tailed, sum [25,53], p=0.026, n=6) (Fig. 9B). Further, when compared with controls, dietary ω-3 PUFAs induced a significant 4.3-fold increase in the protein levels of CREB at 8 wpi (Mann Whitney U [2.0], two-tailed, sum [17,49], p=0.017, n=6) (Fig. 9B).
FIG. 9.
Dietary ω-3 polyunsaturated fatty acids (PUFAs) prophylaxis results in increased expression of pro-restorative signaling molecules. (A) Quantitative analyses of real-time polymerase chain reaction crossing thresholds showed a significant increase in cyclic AMP responsive element binding (CREB) protein mRNA levels in the ω-3 PUFA-pre-treated group when compared with control animals at 8 weeks post-injury (analysis of variance followed by Bonferroni post hoc, p<0.05, n≥4–5). (B) Immunoblot of spinal cord samples showing Akt (60 kDa) and CREB (approximately 47 kDa) protein upregulation after dietary pre-treatment with ω-3 PUFAs. Analyses show that dietary ω-3 PUFA prophylaxis resulted in increased Akt and CREB protein levels when normalized to actin levels (Mann-Whitney U test, p<0.05, n=6). Error bars represent standard error of the mean. Color image is available online at www.liebertpub.com/neu
Discussion
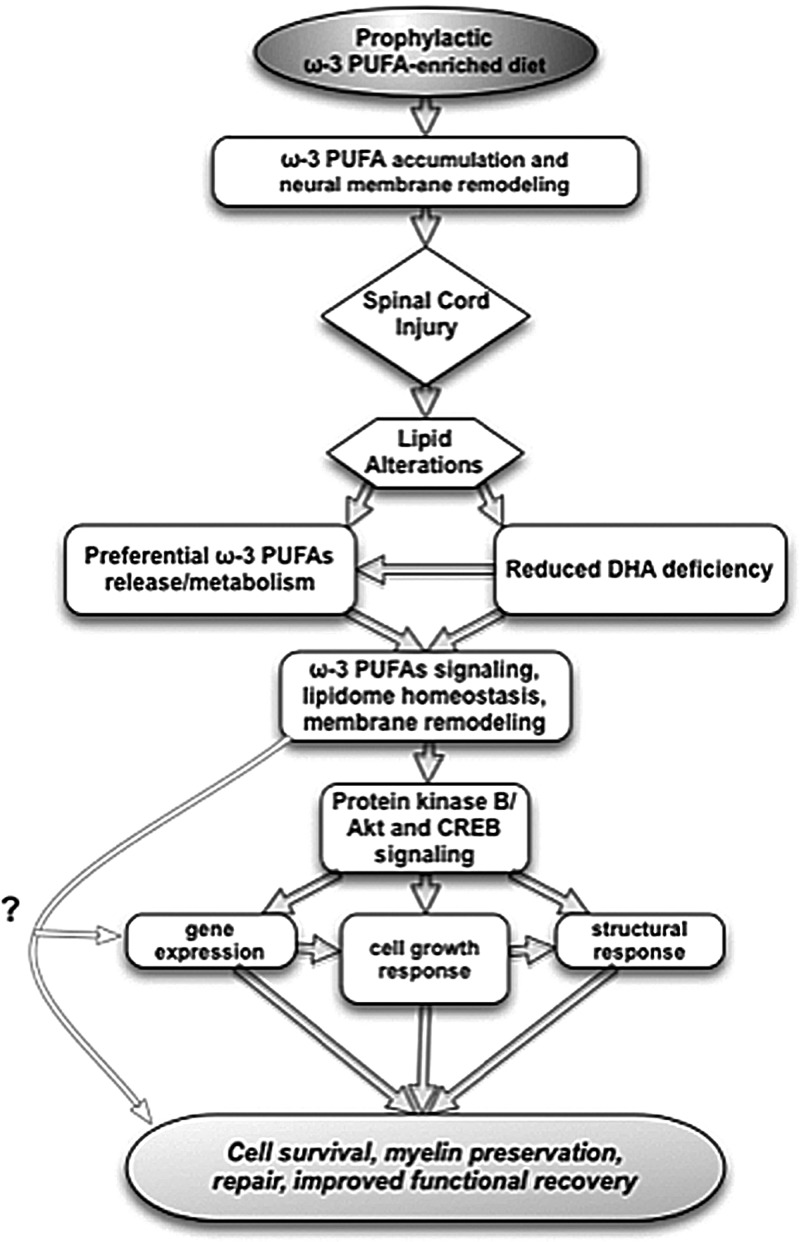
The present study shows that a diet enriched in ω-3 PUFAs prophylaxis is sufficient to ameliorate autonomic, motor, and sensory function after experimental contusion SCI in rats. These improvements showed a significant association with the cord lipidome, particularly a ω-3 PUFAs accumulation at the expense of ω-6 PUFAs. Chronic SCI resulted in distinct lipidomic signatures, including a marked DHA deficiency, which was accompanied by dysfunction and a limited capacity to support functional recovery. Notably, a diet enriched with ω-3 PUFAs was effective in reducing this deficiency and resulted in the up-regulation of the pro-survival and repair proteins, Akt and CREB. A proposed mechanism for ω-3 PUFA-mediated prophylaxis against SCI is presented in Fig. 10.
FIG. 10.
Putative mechanisms underlying the beneficial effects of dietary ω-3 polyunsaturated fatty acids (PUFAs) prophylaxis in spinal cord injury (SCI). A preventive nutritional therapy with ω-3 PUFAs results in accumulation of ω-3 PUFAs in the spinal cord neural membranes, which may provide extended neuroprotection and repair substrates after SCI. Lipid metabolism alterations under this dietary regime leads to reduced ω-3 deficiencies, particularly dicosahexaenoic acid (DHA), and preferentially activates ω-3 PUFA metabolism. This response may result in regaining PUFA homeostasis and enhanced pro-restorative signaling, such as protein kinase B/Akt and cyclic AMP responsive element binding (CREB) protein and/or additional uncharacterized mechanisms. Omega-3 PUFAs may provide important substrates implicated in stress resistance, such as induction of antioxidant gene expression, cell growth, and membrane homeostasis and remodeling. Together, this response should result in improved tissue sparing, plasticity, and repair.
Pre-treatment with a ω-3 PUFA-enriched diet improves somatic and autonomic recovery after contusion SCI
The spinal cord is at high risk of injury during surgeries, high-impact sports, military conflicts, and in several congenital and degenerative CNS disorders.63–65 Of significance, for patients undergoing SCI, the recovery of autonomic bladder function is a high priority for improving their quality of life.66 After incomplete SCI, there is initial disruption of bladder control followed by partial recovery, which correlates negatively with injury severity and damage.67 Full autonomic bladder function recovery is a consequence of adaptive plasticity, particularly sprouting and reconnection of spinospinal pathways.68 Here, we found that a ω-3 PUFA-enriched diet not only results in amelioration of autonomic bladder function but also accelerates its complete recovery, suggesting reduced damage and/or activation of repair responses.
Recent published reports have shown that supplementation with ω-3 PUFAs or with interventions that increase DHA levels promote locomotor recovery when administered after SCI.12,17–24 These findings led us to examine the efficacy of a preventive diet enriched with ω-3 PUFAs to reduce dysfunction after injury. Our results validate the role of fatty acids in functional recovery and show for the first time that dietary enrichment with ω-3 PUFA alone, without additional DHA supplementation or AA blockade, is sufficient to significantly improve hind limb function in a clinically relevant contusion SCI. This was evidenced by weight-supported steps during the first week after injury in the group of animals receiving ω-3 PUFA prophylaxis, which represents an important finding, because voluntary locomotion with full weight support requires supraspinal control.69,70 More importantly, our results show that these beneficial effects are long lasting, because BBB scores continued to improve in relation to controls for at least 8 weeks. These rapid and prolonged beneficial effects suggest that dietary ω-3 PUFA prophylaxis may be attributable to a combination of early (i.e., neuroprotection, plasticity, and remyelination) and late (i.e., sprouting and regeneration) protective/repair mechanisms.
Although previous findings demonstrate that a combined intravenous and dietary DHA regime has the ability to sustain functional recovery, the efficacy of dietary DHA had limited positive effects when administered alone after injury.18 Our findings suggest that the lack of dietary DHA efficacy reported by these investigators may have resulted as a consequence of reduced food intake and altered lipid metabolism during the first wpi, which may lead to suboptimal DHA levels. Likewise, administration of fenretinide (a retinoic acid analog shown to reduce AA and increase DHA), after 24 h post-injury failed to sustain locomotor recovery in mice,23 suggesting the relevance of the PUFA balance in the acute stages of SCI and supporting a role for endogenous ω-3 PUFAs in prophylaxis against neurotrauma. The novel experimental paradigm used in this study, which fed the animals with a ω-3 PUFA-enriched diet before SCI, underscores the value of the pre-treatment. Further, these data clearly demonstrate a potential therapeutic window for ω-3 PUFAs, which may be dependent on the ω-3 source and dosage, and warrants further investigation.
Sensory deficits and pain are common clinical problems after chronic SCI.71 We show that a diet enriched with ω-3 PUFA prevents the development of major sensory dysfunction (hypoalgesia), as evidenced by no significant changes in the force needed to elicit hind limb withdrawal when compared with controls. Although the pathophysiological mechanisms responsible for the loss or presence of sensory function after injury are only partly understood, evidence demonstrates a paradoxical combination of sensory loss within the area where chronic pain is felt, suggesting that both neurodegenerative and pro-inflammatory responses may play a role.72,73 Because we used the spinal cords for lipidomic profiling and RNA/protein extractions, we could not morphologically determine the extent of injury in this study. Sensory function, however, has been correlated with the amount of white matter spared.74 Further, assessment of recovery using the BBB locomotor scale and bladder function provide an indirect measure of injury magnitude and spared tissue.27,67 Along with the results of others, we have shown that this ω-3 PUFA-mediated positive functional outcome could be explained, at least in part, by the ability of DHA and EPA to dampen various secondary damage events, activate neuroprotective mechanisms, increase white matter sparing, and reduce axonal pathology in SCI.12,17–22
ω-3 PUFA-enriched diet reduces DHA deficiency and results in distinctive lipid signatures associated with functional recovery after SCI
The precise mechanisms underlying the beneficial effects of dietary ω-3 PUFAs prophylaxis in the CNS are only partly understood, and our findings showing the important nutritional contributions and implications in functional behavior after trauma reveal various novel features. First, although the CNS is highly resistant to dietary PUFAs deficiencies,75,76 our data show that SCI itself leads to DHA nutritional deficiencies. These data are supported by rodent studies showing that SCI results in an acute reduction in DHA plasma levels23 and accelerates DHA metabolism.77 More importantly, we show that this SCI-induced DHA deficiency is associated with autonomic, motor, and sensory functional impairments in rats. Several studies support this observation by showing that ω-3 PUFA deficiencies result in neurological deficits and disease, including Alzheimer's disease.48–52
Second, both chronic SCI and dietary supplementation with ω-3 PUFAs had a profound impact on the spinal cord neurolipidome. Notably, dietary enrichment with ω-3 PUFAs prevented a chronic DHA deficiency and led to across-the-board increases in short and long chain ω-3 PUFAs, which were accompanied by similar decreases in ω-6 species. Retroconversion of DHA to shorter chain PUFAs is known to occur and could account, in part, for the increased levels of other ω-3 PUFAs.78,79
On the other hand, the corresponding decrease in ω-6 PUFAs supports an ω-3 PUFA role in modulating genes and enzymes involved in the transport, synthesis, esterification, storage, and degradation of fatty acids.80 Moreover, the PUFA balance can impair the interconversion of EPA and DHA by modulating the activity of Δ6-desaturase in vivo.81 Although little is known about the particular mechanisms involved in the regulation of lipid metabolism after SCI, it is now clear that an ω-3 PUFA-enriched diet causes a global shift toward ω-3 lipids, which may have an important impact on resiliency to damage.
Despite its therapeutic and prognostic potential, the literature is scarce with regard to reports discussing endogenous determinants of vulnerability to neurological dysfunction in experimental models of injury. A very likely post-traumatic endogenous site is the cell membrane, which undergoes marked structural and functional alterations. After SCI, membrane phospholipids are converted into potent signaling molecules through the action of multiple phospholipases, including the calcium-dependent phospholipase A2 that preferentially hydrolyzes AA from the sn-2 position of phospholipids.82 Therefore, altering the membrane phospholipid acyl-chain composition by administration of ω-3 PUFAs has the potential to change phospholipid-mediated cell signaling by three important processes: (1) altering the availability of AA, (2) changing the suitability of the phospholipids to serve as substrates for an array of phospholipases, and (3) mediating broad scale changes to membrane lipid composition that, in turn, may affect fluidity, signaling, and function. Although the involvement of membrane remodeling in the response to SCI has not been studied extensively, abnormal remodeling after injury has been implicated in membrane protein and channel dysfunction.83,84
A third and final notable feature of this study is that dietary supplementation with ω-3 PUFAs results in increased levels of protein kinase B/Akt and CREB. Interestingly, several studies, including ours, have shown that Akt and CREB may play a role in DHA-mediated neural resiliency, neuroprotection, and plasticity.22,52,62,85 Our finding supports the idea that ω-3 PUFAs may modulate cellular switches involved in controlling vulnerability to damage after SCI. Future studies incorporating inhibitors of Akt/CREB could provide further insight on whether this mechanism is essential for the prophylactic efficacy of dietary ω-3 PUFAs in SCI.
Conclusion
We show that SCI leads to chronic DHA deficiency associated with dysfunction. We present the first evidence that demonstrates that dietary ω-3 PUFA prophylaxis results in distinctive neurolipidomic alterations that may reduce cellular vulnerability and facilitate functional recovery after SCI. Our findings have significant implications for the current Western diet. For instance, recovery after neurotrauma may be hindered by our modern diet. Thus, based on clinical and epidemiological evidence for the beneficial effects of ω-3 PUFAs,86 together with evidence of safety and tolerability,87,88 dietary ω-3 PUFAs prophylaxis against neurotrauma deserves consideration.
Acknowledgments
The authors wish to thank Dr. Pappan from Metabolon for his technical assistance with the lipidomic data sets. We are grateful for Dr. Montero's support with animal care. We also thank Lorena Salto for her editorial assistance. This work was supported in part by NIH grants 5R25GM060507 and 1P20MD001632.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Hulsebosch C.E. Recent advances in pathophysiology and treatment of spinal cord injury. Adv. Physiol. Educ. 2002;26:238–255. doi: 10.1152/advan.00039.2002. [DOI] [PubMed] [Google Scholar]
- 2.Kwon B.K. Okon E. Hillyer J. Mann C. Baptiste D. Weaver L.C. Fehlings M.G. Tetzlaff W. A systematic review of non-invasive pharmacologic neuroprotective treatments for acute spinal cord injury. J. Neurotrauma. 2011;28:1545–1588. doi: 10.1089/neu.2009.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwon B.K. Okon E.B. Plunet W. Baptiste D. Fouad K. Hillyer J. Weaver L.C. Fehlings M.G. Tetzlaff W. A systematic review of directly applied biologic therapies for acute spinal cord injury. J. Neurotrauma. 2011;28:1589–1610. doi: 10.1089/neu.2009.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yip P.K. Malaspina A. Spinal cord trauma and the molecular point of no return. Mol. Neurodegener. 2012;7:6. doi: 10.1186/1750-1326-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demediuk P. Saunders R.D. Clendenon N.R. Means E.D. Anderson D.K. Horrocks L.A. Changes in lipid metabolism in traumatized spinal cord. Prog. Brain Res. 1985;63:211–226. doi: 10.1016/S0079-6123(08)61985-8. [DOI] [PubMed] [Google Scholar]
- 6.Demediuk P. Saunders R.D. Anderson D.K. Means E.D. Horrocks L.A. Membrane lipid changes in laminectomized and traumatized cat spinal cord. Proc. Natl. Acad. Sci. U. S. A. 1985;82:7071–7075. doi: 10.1073/pnas.82.20.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu C.Y. Halushka P.V. Hogan E.L. Cox R.D. Increased thromboxane level in experimental spinal cord injury. J. Neurol. Sci. 1986;74:289–296. doi: 10.1016/0022-510x(86)90114-0. [DOI] [PubMed] [Google Scholar]
- 8.Saunders R. Horrocks L.A. Eicosanoids, plasma membranes, and molecular mechanisms of spinal cord injury. Neurochem. Pathol. 1987;7:1–22. doi: 10.1007/BF02834288. [DOI] [PubMed] [Google Scholar]
- 9.Faden A.I. Chan P.H. Longar S. Alterations in lipid metabolism, Na+,K+-ATPase activity, and tissue water content of spinal cord following experimental traumatic injury. J. Neurochem. 1987;48:1809–1816. doi: 10.1111/j.1471-4159.1987.tb05740.x. [DOI] [PubMed] [Google Scholar]
- 10.Anderson D.K. Hall E.D. Pathophysiology of spinal cord trauma. Ann. Emerg. Med. 1993;22:987–992. doi: 10.1016/s0196-0644(05)82739-8. [DOI] [PubMed] [Google Scholar]
- 11.Murphy E.J. Behrmann D. Bates C.M. Horrocks L.A. Lipid alterations following impact spinal cord injury in the rat. Mol. Chem. Neuropathol. 1994;23:13–26. doi: 10.1007/BF02858504. [DOI] [PubMed] [Google Scholar]
- 12.King V.R. Huang W.L. Dyall S.C. Curran O.E. Priestley J.V. Michael-Titus A.T. Omega-3 fatty acids improve recovery, whereas omega-6 fatty acids worsen outcome, after spinal cord injury in the adult rat. J. Neurosci. 2006;26:4672–4680. doi: 10.1523/JNEUROSCI.5539-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu N.K. Zhang Y.P. Titsworth W.L. Jiang X. Han S. Lu P.H. Shields C.B. Xu X.M. A novel role of phospholipase A2 in mediating spinal cord secondary injury. Ann Neurol. 2006;59:606–619. doi: 10.1002/ana.20798. [DOI] [PubMed] [Google Scholar]
- 14.Titsworth W.L. Cheng X. Ke Y. Deng L. Burckardt K.A. Pendleton C. Liu N.K. Shao H. Cao Q.L. Xu X.M. Differential expression of sPLA2 following spinal cord injury and a functional role for sPLA2-IIA in mediating oligodendrocyte death. Glia. 2009;57:1521–1537. doi: 10.1002/glia.20867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang W. Bhavsar A. Ward R.E. Hall J.C. Priestley J.V. Michael-Titus A.T. Arachidonyl trifluoromethyl ketone is neuroprotective after spinal cord injury. J. Neurotrauma. 2009;26:1429–1434. doi: 10.1089/neu.2008.0835. [DOI] [PubMed] [Google Scholar]
- 16.Liu N.K. Xu X.M. Phospholipase A2 and its molecular mechanism after spinal cord injury. Mol. Neurobiol. 2010;41:197–205. doi: 10.1007/s12035-010-8101-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lang-Lazdunski L. Blondeau N. Jarretou G. Lazdunski M. Heurteaux C. Linolenic acid prevents neuronal cell death and paraplegia after transient spinal cord ischemia in rats. J. Vasc. Surg. 2003;38:564–575. doi: 10.1016/s0741-5214(03)00473-7. [DOI] [PubMed] [Google Scholar]
- 18.Huang W.L. King V.R. Curran O.E. Dyall S.C. Ward R.E. Lal N. Priestley J.V. Michael-Titus A.T. A combination of intravenous and dietary docosahexaenoic acid significantly improves outcome after spinal cord injury. Brain. 2007;130:3004–3019. doi: 10.1093/brain/awm223. [DOI] [PubMed] [Google Scholar]
- 19.Ward R.E. Huang W. Curran O.E. Priestley J.V. Michael-Titus A.T. Docosahexaenoic acid prevents white matter damage after spinal cord injury. J. Neurotrauma. 2010;27:1769–1780. doi: 10.1089/neu.2010.1348. [DOI] [PubMed] [Google Scholar]
- 20.Lim S.N. Huang W. Hall J.C. Ward R.E. Priestley J.V. Michael-Titus A.T. The acute administration of eicosapentaenoic acid is neuroprotective after spinal cord compression injury in rats. Prostaglandins Leukot. Essent. Fatty Acids. 2010;83:193–201. doi: 10.1016/j.plefa.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Hall J.C. Priestley J.V. Perry V.H. Michael-Titus A.T. Docosahexaenoic acid, but not eicosapentaenoic acid, reduces the early inflammatory response following compression spinal cord injury in the rat. J. Neurochem. 2012;121:738–750. doi: 10.1111/j.1471-4159.2012.07726.x. [DOI] [PubMed] [Google Scholar]
- 22.Figueroa J.D. Cordero K. Baldeosingh K. Torrado A.I. Walker R.L. Miranda J.D. Leon M.D. Docosahexaenoic acid pretreatment confers protection and functional improvements after acute spinal cord injury in adult rats. J. Neurotrauma. 2012;29:551–566. doi: 10.1089/neu.2011.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.López-Vales R. Redensek A. Skinner T.A. Rathore K.I. Ghasemlou N. Wojewodka G. DeSanctis J. Radzioch D. David S. Fenretinide promotes functional recovery and tissue protection after spinal cord contusion injury in mice. J. Neurosci. 2010;30:3220–3226. doi: 10.1523/JNEUROSCI.5770-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holly L.T. Blaskiewicz D. Wu A. Feng C. Ying Z. Gomez-Pinilla F. Dietary therapy to promote neuroprotection in chronic spinal cord injury. J. Neurosurg. Spine. 2012;17:134–140. doi: 10.3171/2012.5.SPINE1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gruner J.A. A monitored contusion model of spinal cord injury in the rat. J. Neurotrauma. 1992;9:123–128. doi: 10.1089/neu.1992.9.123. [DOI] [PubMed] [Google Scholar]
- 26.Basso D.M. Beattie M.S. Bresnahan J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 27.Basso D.M. Beattie M.S. Bresnahan J.C. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 28.Ferguson A.R. Hook M.A. Garcia G. Bresnahan J.C. Beattie M.S. Grau J.W. A simple post hoc transformation that improves the metric properties of the BBB scale for rats with moderate to severe spinal cord injury. J. Neurotrauma. 2004;21:1601–1613. doi: 10.1089/neu.2004.21.1601. [DOI] [PubMed] [Google Scholar]
- 29.Ohta T. Masutomi N. Tsutsui N. Sakairi T. Mitchell M. Milburn M.V. Ryals J.A. Beebe K.D. Guo L. Untargeted metabolomic profiling as an evaluative tool of fenofibrate-induced toxicology in Fischer 344 male rats. Toxicol. Pathol. 2009;37:521–535. doi: 10.1177/0192623309336152. [DOI] [PubMed] [Google Scholar]
- 30.Evans A.M. DeHaven C.D. Barrett T. Mitchell M. Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal. Chem. 2009;81:6656–6667. doi: 10.1021/ac901536h. [DOI] [PubMed] [Google Scholar]
- 31.Dehaven C.D. Evans A.M. Dai H. Lawton K.A. Organization of GC/MS and LC/MS metabolomics data into chemical libraries. J Cheminform. 2010;2:9. doi: 10.1186/1758-2946-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosas O.R. Figueroa J.D. Torrado A.I. Rivera M. Santiago J.M. Konig-Toro F. Miranda J.D. Expression and activation of ephexin is altered after spinal cord injury. Devel. Neurobiol. 2011;71:595–607. doi: 10.1002/dneu.20848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lowry O.H. Rosebrough N.J. Farr A.L. Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 34.Marteinsdottir I. Horrobin D.F. Stenfors C. Theodorsson E. Mathé A.A. Changes in dietary fatty acids alter phospholipid fatty acid composition in selected regions of rat brain. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1998;22:1007–1021. doi: 10.1016/s0278-5846(98)00052-9. [DOI] [PubMed] [Google Scholar]
- 35.Rapoport S.I. Brain arachidonic and docosahexaenoic acid cascades are selectively altered by drugs, diet and disease. Prostaglandins Leukot. Essent. Fatty Acids. 2008;79:153–156. doi: 10.1016/j.plefa.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rapoport S.I. In vivo fatty acid incorporation into brain phosholipids in relation to plasma availability, signal transduction and membrane remodeling. J. Mol. Neurosci. 2001;16:243–261. doi: 10.1385/JMN:16:2-3:243. 279–284. [DOI] [PubMed] [Google Scholar]
- 37.Umemura K. Toshima Y. Asai F. Nakashima M. Effect of dietary docosahexaenoic acid in the rat middle cerebral artery thrombosis model. Thromb. Res. 1995;78:379–387. doi: 10.1016/0049-3848(95)00071-x. [DOI] [PubMed] [Google Scholar]
- 38.Little S.J. Lynch M.A. Manku M. Nicolaou A. Docosahexaenoic acid-induced changes in phospholipids in cortex of young and aged rats: a lipidomic analysis. Prostaglandins Leukot. Essent. Fatty Acids. 2007;77:155–162. doi: 10.1016/j.plefa.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 39.Lin Y.H. Shah S. Salem N., Jr. Altered essential fatty acid metabolism and composition in rat liver, plasma, heart and brain after microalgal DHA addition to the diet. J. Nutr. Biochem. 2011;22:758–765. doi: 10.1016/j.jnutbio.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Resnick D.K. Schmitt C. Miranpuri G.S. Dhodda V.K. Isaacson J. Vemuganti R. Molecular evidence of repair and plasticity following spinal cord injury. Neuroreport. 2004;15:837–839. doi: 10.1097/00001756-200404090-00020. [DOI] [PubMed] [Google Scholar]
- 41.Fouad K. Krajacic A. Tetzlaff W. Spinal cord injury and plasticity: opportunities and challenges. Brain Res. Bull. 2011;84:337–342. doi: 10.1016/j.brainresbull.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 42.Rossignol S. Frigon A. Recovery of locomotion after spinal cord injury: some facts and mechanisms. Annu. Rev. Neurosci. 2011;34:413–440. doi: 10.1146/annurev-neuro-061010-113746. [DOI] [PubMed] [Google Scholar]
- 43.Gwak Y.S. Hains B.C. Johnson K.M. Hulsebosch C.E. Locomotor recovery and mechanical hyperalgesia following spinal cord injury depend on age at time of injury in rat. Neurosci. Lett. 2004;362:232–235. doi: 10.1016/j.neulet.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 44.Abbott S.K. Else P.L. Atkins T.A. Hulbert A.J. Fatty acid composition of membrane bilayers: Importance of diet polyunsaturated fat balance. Biochim. Biophys. Acta. 2012;1818:1309–1317. doi: 10.1016/j.bbamem.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 45.Cruz C.D. Cruz F. Spinal cord injury and bladder dysfunction: new ideas about an old problem. ScientificWorldJournal. 2011;11:214–234. doi: 10.1100/tsw.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salem N., Jr. Hullin F. Yoffe A.M. Karanian J.W. Kim H.Y. Fatty acid and phospholipid species composition of rat tissues after a fish oil diet. Adv. Prostaglandin Thromboxane Leukot. Res. 1989;19:618–622. [PubMed] [Google Scholar]
- 47.Horrocks L.A. Farooqui A.A. Docosahexaenoic acid in the diet: its importance in maintenance and restoration of neural membrane function. Prostaglandins Leukot. Essent. Fatty Acids. 2004;70:361–372. doi: 10.1016/j.plefa.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 48.Birch D.G. Birch E.E. Hoffman D.R. Uauy R.D. Retinal development in very-low-birth-weight infants fed diets differing in omega-3 fatty acids. Invest. Ophthalmol. Vis. Sci. 1992;33:2365–2376. [PubMed] [Google Scholar]
- 49.Greiner R.S. Moriguchi T. Hutton A. Slotnick B.M. Salem N., Jr. Rats with low levels of brain docosahexaenoic acid show impaired performance in olfactory-based and spatial learning tasks. Lipids. 1999;34(Suppl.):S239–S243. doi: 10.1007/BF02562305. [DOI] [PubMed] [Google Scholar]
- 50.Moriguchi T. Greiner R.S. Salem N., Jr. Behavioral deficits associated with dietary induction of decreased brain docosahexaenoic acid concentration. J. Neurochem. 2000;75:2563–2573. doi: 10.1046/j.1471-4159.2000.0752563.x. [DOI] [PubMed] [Google Scholar]
- 51.Oster T. Pillot T. Docosahexaenoic acid and synaptic protection in Alzheimer's disease mice. Biochim. Biophys. Acta. 2010;1801:791–798. doi: 10.1016/j.bbalip.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 52.Agrawal R. Gomez-Pinilla F. “Metabolic syndrome” in the brain: deficiency in omega-3-fatty acid exacerbates dysfunctions in insulin receptor signaling and cognition. J. Physiol. 2012;590:2485–2499. doi: 10.1113/jphysiol.2012.230078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moriguchi T. Loewke J. Garrison M. Catalan J.N. Salem N., Jr Reversal of docosahexaenoic acid deficiency in the rat brain, retina, liver, serum. J. Lipid Res. 2001;42:419–427. [PubMed] [Google Scholar]
- 54.Youyou A. Durand G. Pascal G. Piciotti M. Dumont O. Bourre J.M. Recovery of altered fatty acid composition induced by a diet devoid of n-3 fatty acids in myelin, synaptosomes, mitochondria, and microsomes of developing rat brain. J. Neurochem. 1986;46:224–228. doi: 10.1111/j.1471-4159.1986.tb12950.x. [DOI] [PubMed] [Google Scholar]
- 55.Mure P.Y. Galdo M. Compagnone N. Bladder function after incomplete spinal cord injury in mice: quantifiable outcomes associated with bladder function and efficiency of dehydroepiandrosterone as a therapeutic adjunct. J. Neurosurg. 2004;100(Suppl.):56–61. doi: 10.3171/spi.2004.100.1.0056. [DOI] [PubMed] [Google Scholar]
- 56.Wang Q. Liu L. Pei L. Ju W. Ahmadian G. Lu J. Wang Y. Liu F. Wang Y.T. Control of synaptic strength, a novel function of Akt. Neuron. 2003;38:915–928. doi: 10.1016/s0896-6273(03)00356-8. [DOI] [PubMed] [Google Scholar]
- 57.Barco A. Marie H. Genetic approaches to investigate the role of CREB in neuronal plasticity and memory. Mol. Neurobiol. 2011;44:330–349. doi: 10.1007/s12035-011-8209-x. [DOI] [PubMed] [Google Scholar]
- 58.Yamashima T. ‘PUFA-GPR40-CREB signaling’ hypothesis for the adult primate neurogenesis. Prog. Lipid Res. 2012;51:221–231. doi: 10.1016/j.plipres.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 59.Gao Y. Deng K. Hou J. Bryson J.B. Barco A. Nikulina E. Spencer T. Mellado W. Kandel E.R. Filbin M.T. Activated CREB is sufficient to overcome inhibitors in myelin and promote spinal axon regeneration in vivo. Neuron. 2004;44:609–621. doi: 10.1016/j.neuron.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 60.Yip P.K. Wong L.F. Sears T.A. Yanez-Munoz R.J. McMahon S.B. Cortical overexpression of neuronal calcium sensor-1 induces functional plasticity in spinal cord following unilateral pyramidal tract injury in rat. PLoS Biol. 2010;8:e1000399. doi: 10.1371/journal.pbio.1000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yune T.Y. Park H.G. Lee J.Y. Oh T.H. Estrogen-induced Bcl-2 expression after spinal cord injury is mediated through phosphoinositide-3-kinase/Akt-dependent CREB activation. J. Neurotrauma. 2008;25:1121–1131. doi: 10.1089/neu.2008.0544. [DOI] [PubMed] [Google Scholar]
- 62.Akbar M. Calderon F. Wen Z. Kim H.Y. Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival. Proc Natl Acad Sci USA. 2005;102:12997. doi: 10.1073/pnas.0502903102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McDonald J.W. Sadowsky C. Spinal-cord injury. Lancet. 2002;359:417–425. doi: 10.1016/S0140-6736(02)07603-1. [DOI] [PubMed] [Google Scholar]
- 64.Boden B.P. Jarvis C.G. Spinal injuries in sports. Phys. Med. Rehabil. Clin. N. Am. 2009;20:55–68. doi: 10.1016/j.pmr.2008.10.014. vii. [DOI] [PubMed] [Google Scholar]
- 65.Weaver F.M. Burns S.P. Evans C.T. Rapacki L.M. Goldstein B. Hammond M.C. Provider perspectives on soldiers with new spinal cord injuries returning from Iraq and Afghanistan. Arch. Phys. Med. Rehabil. 2009;90:517–521. doi: 10.1016/j.apmr.2008.09.560. [DOI] [PubMed] [Google Scholar]
- 66.Anderson K.D. Targeting recovery: priorities of the spinal cord-injured population. J. Neurotrauma. 2004;21:1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- 67.Pikov V. Wrathall J.R. Coordination of the bladder detrusor and the external urethral sphincter in a rat model of spinal cord injury: effect of injury severity. J. Neurosci. 2001;21:559–569. doi: 10.1523/JNEUROSCI.21-02-00559.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Groat W.C. Araki I. Vizzard M.A. Yoshiyama M. Yoshimura N. Sugaya K. Tai C. Roppolo J.R. Developmental and injury induced plasticity in the micturition reflex pathway. Behav. Brain Res. 1998;92:127–140. doi: 10.1016/s0166-4328(97)00185-x. [DOI] [PubMed] [Google Scholar]
- 69.Grillner S. Dubuc R. Control of locomotion in vertebrates: spinal and supraspinal mechanisms. Adv. Neurol. 1988;47:425–453. [PubMed] [Google Scholar]
- 70.Mori S. Matsui T. Kuze B. Asanome M. Nakajima K. Matsuyama K. Stimulation of a restricted region in the midline cerebellar white matter evokes coordinated quadrupedal locomotion in the decerebrate cat. J. Neurophysiol. 1999;82:290–300. doi: 10.1152/jn.1999.82.1.290. [DOI] [PubMed] [Google Scholar]
- 71.Hulsebosch C.E. Hains B.C. Crown E.D. Carlton S.M. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res. Rev. 2009;60:202–213. doi: 10.1016/j.brainresrev.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Krassioukov A. Wolfe D.L. Hsieh J.T. Hayes K.C. Durham C.E. Quantitative sensory testing in patients with incomplete spinal cord injury. Arch. Phys. Med. Rehabil. 1999;80:1258–1263. doi: 10.1016/s0003-9993(99)90026-6. [DOI] [PubMed] [Google Scholar]
- 73.Baumgärtner U. Magerl W. Klein T. Hopf H.C. Treede R.D. Neurogenic hyperalgesia versus painful hypoalgesia: two distinct mechanisms of neuropathic pain. Pain. 2002;96:141–151. doi: 10.1016/s0304-3959(01)00438-9. [DOI] [PubMed] [Google Scholar]
- 74.Kloos A.D. Fisher L.C. Detloff M.R. Hassenzahl D.L. Basso D.M. Stepwise motor and all-or-none sensory recovery is associated with nonlinear sparing after incremental spinal cord injury in rats. Exp. Neurol. 2005;191:251–265. doi: 10.1016/j.expneurol.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 75.Bourre J.M. Dumont O.S. Piciotti M.J. Pascal G.A. Durand G.A. Dietary alpha-linolenic acid deficiency in adult rats for 7 months does not alter brain docosahexaenoic acid content, in contrast to liver, heart and testes. Biochim. Biophys. Acta. 1992;1124:119–122. doi: 10.1016/0005-2760(92)90087-c. [DOI] [PubMed] [Google Scholar]
- 76.Rapoport S.I. Rao J.S. Igarashi M. Brain metabolism of nutritionally essential polyunsaturated fatty acids depends on both the diet and the liver. Prostaglandins Leukot. Essent. Fatty Acids. 2007;77:251–261. doi: 10.1016/j.plefa.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fujieda Y. Ueno S. Ogino R. Kuroda M. Jonsson T.J. Guo L. Bamba T. Fukusaki E. Metabolite profiles correlate closely with neurobehavioral function in experimental spinal cord injury in rats. PLoS ONE. 2012;7:e43152. doi: 10.1371/journal.pone.0043152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brossard N. Croset M. Pachiaudi C. Riou J.P. Tayot J.L. Lagarde M. Retroconversion and metabolism of [13C]22:6n-3 in humans and rats after intake of a single dose of [13C]22:6n-3-triacylglycerols. Am. J. Clin. Nutr. 1996;64:577–586. doi: 10.1093/ajcn/64.4.577. [DOI] [PubMed] [Google Scholar]
- 79.Vidgren H.M. Agren J.J. Schwab U. Rissanen T. Hanninen O. Uusitupa M.I. Incorporation of n-3 fatty acids into plasma lipid fractions, and erythrocyte membranes and platelets during dietary supplementation with fish, fish oil, and docosahexaenoic acid-rich oil among healthy young men. Lipids. 1997;32:697–705. doi: 10.1007/s11745-997-0089-x. [DOI] [PubMed] [Google Scholar]
- 80.Benatti P. Peluso G. Nicolai R. Calvani M. Polyunsaturated fatty acids: biochemical, nutritional and epigenetic properties. J. Am. Coll. Nutr. 2004;23:281–302. doi: 10.1080/07315724.2004.10719371. [DOI] [PubMed] [Google Scholar]
- 81.Arterburn L.M. Hall E.B. Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am. J. Clin. Nutr. 2006;83(Suppl.):1467S–1476S. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- 82.López-Vales R. Ghasemlou N. Redensek A. Kerr B.J. Barbayianni E. Antonopoulou G. Baskakis C. Rathore K.I. Constantinou-Kokotou V. Stephens D. Shimizu T. Dennis E.A. Kokotos G. David S. Phospholipase A2 superfamily members play divergent roles after spinal cord injury. FASEB J. 2011;25:4240–4252. doi: 10.1096/fj.11-183186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hulsebosch C.E. DeWitt D.S. Jenkins L.W. Prough D.S. Traumatic brain injury in rats results in increased expression of Gap-43 that correlates with behavioral recovery. Neurosci. Lett. 1998;255:83–86. doi: 10.1016/s0304-3940(98)00712-5. [DOI] [PubMed] [Google Scholar]
- 84.Kretschmer T. England J.D. Happel L.T. Liu Z.P. Thouron C.L. Nguyen D.H. Beuerman R.W. Kline D.G. Ankyrin G and voltage gated sodium channels colocalize in human neuroma—key proteins of membrane remodeling after axonal injury. Neurosci. Lett. 2002;323:151–155. doi: 10.1016/s0304-3940(02)00021-6. [DOI] [PubMed] [Google Scholar]
- 85.Wu A. Ying Z. Gomez-Pinilla F. Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience. 2008;155:751–759. doi: 10.1016/j.neuroscience.2008.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Simopoulos A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. (Maywood) 2008;233:674–688. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- 87.Bays H. Clinical overview of Omacor: a concentrated formulation of omega-3 polyunsaturated fatty acids. Am. J. Cardiol. 2006;98:71i–76i. doi: 10.1016/j.amjcard.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 88.Mayer K. Schaefer M.B. Seeger W. Fish oil in the critically ill: from experimental to clinical data. Curr. Opin. Clin. Nutr. Metab. Care. 2006;9:140–148. doi: 10.1097/01.mco.0000214573.75062.0a. [DOI] [PubMed] [Google Scholar]