Abstract
Background.
Age trends in estradiol and estrone levels in men and how lifestyle factors, comorbid conditions, testosterone, and sex hormone–binding globulin affect these age trends remain poorly understood, and were examined in men of the Framingham Heart Study.
Methods.
Estrone and estradiol concentrations were measured in morning fasting samples using liquid chromatography tandem mass spectrometry in men of Framingham Offspring Generation. Free estradiol was calculated using a law of mass action equation.
Results.
There were 1,461 eligible men (mean age [±SD] 61.1±9.5 years and body mass index [BMI] 28.8±4.5kg/m2). Total estradiol and estrone were positively associated with age, but free estradiol was negatively associated with age. Age-related increase in total estrone was greater than that in total estradiol. Estrone was positively associated with smoking, BMI, and testosterone, and total and free estradiol with diabetes, BMI, testosterone, and comorbid conditions; additionally, free estradiol was associated negatively with smoking. Collectively, age, BMI, testosterone, and other health and behavioral factors explained only 18% of variance in estradiol, and 9% of variance in estrone levels. Men in the highest quintile of estrone levels had significantly higher age and BMI, and a higher prevalence of smoking, diabetes, and cardiovascular disease than others, whereas those in the highest quintile of estradiol had higher BMI than others.
Conclusions.
Total estrone and estradiol levels in men, measured using liquid chromatography tandem mass spectrometry, revealed significant age-related increases that were only partially accounted for by cross-sectional differences in BMI, diabetes status, and other comorbidities and health behaviors. Longitudinal studies are needed to confirm these findings.
Key Words: Age trends, Estrogen levels in men, LC-MS/MS, Age-related changes in estrone and estradiol, Determinants of estrogen levels in men.
THE estrogens have been linked to the pathophysiology of gynecomastia, osteoporosis, inflammation, and cardiovascular disease (CVD), but the precise biologic role of the circulating estrone and estradiol in men remains poorly understood (1,2). The function of estrone, which is at least as abundant in the circulation as estradiol but is less potent than estradiol in some bioassays (3), has been even less well studied as compared with estradiol.
In men, circulating estradiol is derived partly from direct testicular secretion and partly from peripheral aromatization of testosterone, whereas circulating estrone is derived predominantly from peripheral conversion of delta 4-androstenedione (3–10). A decline in testosterone and androstenedione levels with aging would, therefore, be expected to result in lower estradiol and estrone levels, respectively, in older men as compared with younger men. However, the data on age-related changes in estradiol levels are conflicting. Although some studies have reported lower estradiol levels in older men than in young men (11–15), others have noted stable (16,17) or even rising estradiol levels with age (18). Very few studies have investigated the age trends in estrone levels (14,15). Additionally, few studies have interpreted age trends in estrone and estradiol levels in the context of age-related changes in lifestyle and health-related factors. The estradiol levels were measured in most studies using direct immunoassays, whose accuracy in the low range prevalent in men has been questioned (19–21).
Using cross-sectional data from the Framingham Heart Study (FHS), we examined the age distribution of estradiol and estrone concentrations and the relationship of these hormones to body mass index (BMI), total testosterone, sex hormone–binding globulin (SHBG), diabetes mellitus, C-reactive protein, and lifestyle factors such as smoking and alcohol consumption in a sample of community-dwelling men. We also determined how comorbid conditions, lifestyle factors, inflammation, testosterone, and SHBG levels influence the age trends in estrone and estradiol levels. We adjusted the analyses for SHBG, the major binding protein for circulating estradiol that has been associated with metabolic disorders, which may indirectly affect estrogen levels. We measured estrone and estradiol levels using liquid chromatography tandem mass spectrometry (LC-MS/MS), widely accepted as the method with the highest accuracy and sensitivity (19–21).
Methods
Study Sample
The FHS design and methods have been described (22). Briefly, the original cohort was recruited from Framingham, Massachusetts in 1948 to identify risk factors for CVD. In 1971, the study enrolled a second-generation cohort (the Offspring cohort)—5,124 of the original participants’ adult children and their spouses. The men of the Offspring cohort who attended examination 7 (1998–2001) were eligible for the present study (n = 1,625). Men with missing estrone and estradiol measurements (n = 159) and those with prostate cancer undergoing androgen deprivation therapy (n = 5) were excluded, yielding a sample size of 1,461 for current analyses.
Ascertainment of Covariate Data
Diabetes mellitus was defined as a fasting blood glucose higher than 126mg/dL and/or the use of diabetes medication. Obesity was defined as BMI higher than 30kg/m2. Cardiovascular events included coronary artery disease (angina pectoris, myocardial infarction, sudden or nonsudden death attributable to coronary artery disease), congestive heart failure, cerebrovascular disease (stroke or transient ischemic attack), and intermittent claudication. Cancer was ascertained by self-report of physician diagnosis supported by medical records when available. A simple comorbidity indicator captured the presence of CVD and/or cancer (one or more vs neither) by the definitions above. A standard single item self-rated health measure was used, that is, “In general, how is your health now?” Response options included “poor,” “fair,” “good,” or “excellent.” The responses were reduced to a binary variable for analyses; good self-rated health (included responses of good and excellent) versus poor self-rated health (included responses of poor or fair health). The men who reported smoking at least one cigarette per day during the past year were categorized as smokers. Alcohol consumption was measured in ounces per month and was categorized into those who consumed no alcohol, those who consumed between 1–14 ounces per month, and those who consumed more than 14 ounces per month.
Hormone Assays
The FHS samples were obtained between 7:30 and 9:30 am after an overnight fast, aliquoted and frozen immediately, and stored at −80°C until the time of assay. The stability of FHS samples in storage has been evaluated previously by measuring the concentrations of cholesterol and triglycerides prior to freezing and storage at –80°C at examination cycle 5 in 1991–1995 with repeated measurement in 2007 (23). The concentrations of these analytes were unchanged over a 15-year period of storage at −80°C using processes that are similar to those used for samples included in these analyses (23).
Estradiol and estrone levels were measured using LC-MS/MS after derivatization with dansyl chloride (24). Twenty μL estrone-d4 and estradiol-d3 were added to 200 μL serum, extracted with 1.2mL methyl t-butyl ether (24), derivatized using dansyl chloride (3.7 mmol/L) in sodium carbonate (10 mmol/L, pH 10.5) at 60ºC for 10 minutes, diluted in 50 μL acetonitrile and water (1:1 volume ratio of acetonitrile and water), and injected in a C1 cartridge. Two-dimensional chromatographic separation was used. First-dimension separation was performed using a mobile phase consisting of methanol with 10 mmol/L formic acid and 10 mmol aqueous formic acid. The effluent was directed to second-dimension separation on a Germini Phenyl C6 column 100×2.0mm with 3 μm particles; the mobile phase consisted of acetonitrile with 10 mmol/L formic acid and 10 mmol/L aqueous formic acid (24). Effluent of the first column was directed to waste during more than 90% of the analysis time; therefore, unreacted dansyl chloride and most derivatization byproducts are not transferred to the analytical column. Effluent from the analytical column was directed onto an API 4000 triple quadrupole mass spectrometer (Applied Biosystems/MDS Sciex) with TurboIonSpray ion source.
The quadrupoles Q1 and Q3 were tuned to unit resolution and instrument was operated in positive ion mode with V-Spray ion source (650ºC), voltage 5000V, and entrance potential 10V. Two MS/MS transitions were monitored for each compound: for estrone, 504>171 and 504>156; for estrone-d4, 508>171 and 508>156; for estradiol, 506>171 and 506>156; for estradiol-d5, 509>171 and 509>156. The peak area ratio of the two transitions was used to assess possible interferences in each sample (25). Each assay included controls containing low and high concentrations of estrone and estradiol, and negative controls consisting of 0.05% bovine serum albumin; if concentrations in the controls were outside 2 SDs of the historically observed values, the results were not accepted. The limit of quantitation for both hormones was 2 pg/mL. Interassay coefficient of variations for estrone were 4.5%, 7.7%, and 6.9% at concentrations of 8, 77, and 209 pg/mL, respectively, and for estradiol 6.9%, 7.0%, and 4.8% at concentrations of 8, 77, and 206 pg/mL, respectively. The validation of the method, including comparisons with other LC-MS/MS methods, has been published (24). In a proficiency testing program, the estradiol and estradiol concentrations obtained using this method agreed well with target values.
We measured total testosterone using a validated LC-MS/MS assay (26). The limit of quantitation was 2ng/dL. Interassay coefficient of variations were 15.8% at 12.0ng/dL, 7.7% at 241ng/dL, and 4.4% at 532ng/dL, respectively. Free testosterone and estradiol were calculated using a published law of mass action solution that utilizes association constants estimated from a systematic review of published studies and an iterative numerical method to derive free androgen and estrogen fractions simultaneously (26).
SHBG levels were measured using an immunofluorometric assay (DELFIA-Wallac, Inc., Turku, Finland) (25,26). Interassay coefficient of variations were 8.3%, 7.9%, and 10.9%, and intraassay coefficient of variations 7.3%, 7.1%, and 8.7%, respectively, in the low, medium, and high pools. The analytical sensitivity of the assays was 0.5 nmol/L.
Statistical Analyses
Descriptive statistics estimating population distributional characteristics were obtained. Data analyses were conducted in two stages. First, exploratory analyses were performed to evaluate the distribution of estrone and estradiol and identify outliers using Tukey’s fences procedure. Because estrone, estradiol, total testosterone, and SHBG values were not distributed normally, log-transformed values were used. Multiple regression models were built sequentially by first estimating models including only age as a covariate, then adding BMI, then incorporating measurements of total testosterone, and finally including all other predictors as a group to determine the contribution of these factors to predictive value of the model. In view of the potential for threshold associations, the men in the highest quintile for each of the two estrogens were compared with the men in the remaining combined quintiles on the descriptive characteristics. All analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
Results
Cohort Characteristics
The baseline characteristics of the analytical sample are reported in Table 1; the mean age was 61.1 (range 37–89) years and mean BMI 28.8kg/m2. This subcohort is predominantly Caucasian. Eighteen percent had CVD, 16% had diabetes mellitus, and 10% had cancer. Thirty-two percent of the men had BMI at least 30kg/m2.
Table 1.
Description of the Analytical Sample, Mean (SD), or Count (percent), by Age
| Age, y | ||||||
|---|---|---|---|---|---|---|
| 30–39 (N = 10) | 40–49 (N = 147) | 50–59 (N = 515) | 60–69 (N = 475) | 70–79 (N = 288) | 80+ (N = 26) | |
| Current smoking | 1 (10%) | 35 (24%) | 91 (18%) | 48 (10%) | 14 (5%) | — |
| Alcohol consumption, ounces/mo | ||||||
| 0 | 4 (40%) | 34 (23%) | 112 (22%) | 123 (26%) | 112 (39%) | 11 (42%) |
| 1–14 | 2 (20%) | 61 (41%) | 214 (42%) | 178 (38%) | 81 (28%) | 9 (35%) |
| >14 | 4 (40%) | 52 (35%) | 187 (36%) | 173 (36%) | 93 (33%) | 6 (23%) |
| Body mass index, kg/m2 | 27.9 (4.5) | 28.9 (4.9) | 29.0 (4.7) | 29.2 (4.6) | 27.93 (3.8) | 26.8 (4.1) |
| Body mass index, kg/m2 | ||||||
| <25 | 3 (30%) | 29 (20%) | 82 (16%) | 86 (18%) | 65 (23%) | 11 (42%) |
| 25–30 | 4 (40%) | 69 (47%) | 263 (51%) | 216 (45%) | 151 (53%) | 8 (31%) |
| 30–35 | 3 (30%) | 32 (22%) | 121 (24%) | 118 (25%) | 57 (20%) | 5 (19%) |
| >35 | — | 16 (11%) | 49 (10%) | 55 (12%) | 13 (5%) | 2 (8%) |
| Cardiovascular disease | — | 2 (1%) | 51 (10%) | 99 (21%) | 91 (32%) | 15 (58%) |
| Diabetes | — | 8 (5%) | 64 (12%) | 80 (17%) | 61 (21%) | 13 (50%) |
| Cancer | 1 (10%) | 2 (1%) | 16 (3%) | 56 (12%) | 64 (22%) | 3 (12%) |
| Estrone, pg/mL | 44.2 (9.1) | 48.0 (18.1) | 50.3 (18.2) | 52.9 (18.6) | 52.4 (18.3) | 58.2 (18.1) |
| Estradiol, pg/mL | 25.1 (7.4) | 25.7 (12.5) | 25.7 (8.7) | 27.1 (9.0) | 27.5 (9.6) | 29.7 (11.4) |
| Free estradiol, pg/mL | 0.54 (0.11) | 0.56 (0.27) | 0.53 (0.19) | 0.53 (0.18) | 0.49 (0.18) | 0.46 (0.17) |
Note: To convert estrone and estradiol to pg/mL, multiply by 3.7 and 3.67, respectively.
Distribution of Estrone and Total and Free Estradiol Levels by Age
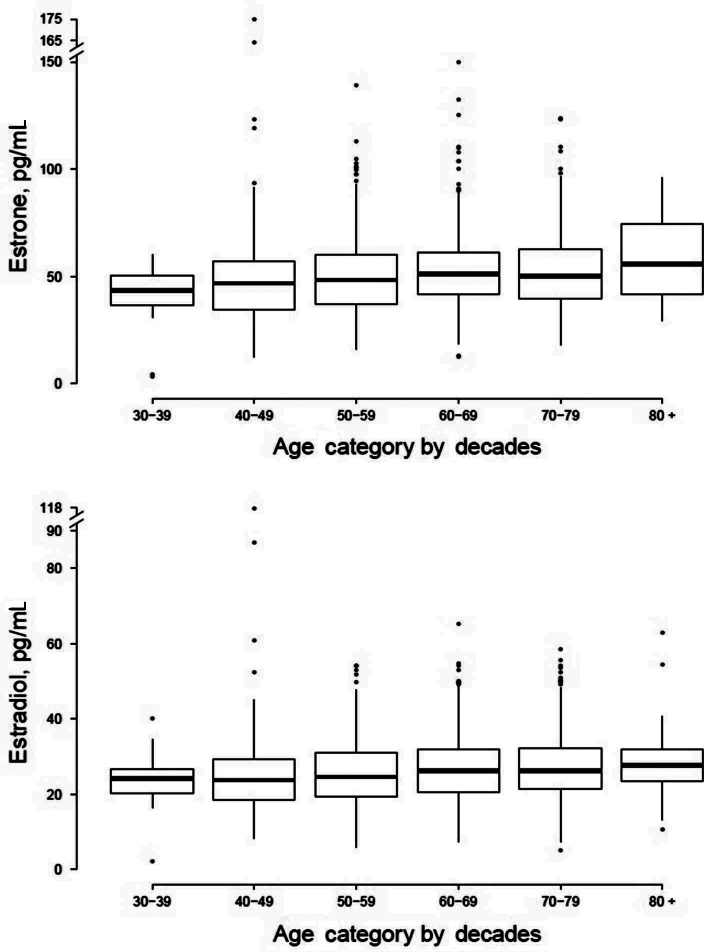
Means and SDs of estrone and estradiol by age decade are given in Table 1; full distributional characteristics are provided in Figure 1. Serum estrone and estradiol concentrations were positively associated with age; unadjusted estimates indicated a 3.6% increase in estrone (p = .0003) and a corresponding 3.4% increase in mean estradiol concentrations (p = .0007) per cross-sectional 10 years increase in age. However, free estradiol levels were negatively associated with age (unadjusted estimate: 4.3% cross-sectional decrease per decade age), suggesting that the higher observed total estradiol levels in older men as compared with younger men are likely due to higher SHBG levels in older men.
Figure 1.
Distribution of estrone and estradiol (pg/mL) by decades of age. Box-and-whisker plot display the median value (heavy horizontal bar), 25th and 75th percentiles (lighter horizontal bars), and range of outlying values. To convert estrone and estradiol to pmol/L, multiply by 3.70 and 3.67, respectively.
Multivariate Correlates of Estrone and Estradiol
Multivariable analyses showed that both estrone and estradiol levels were positively associated with chronological age, BMI, total testosterone, and C-reactive protein levels (Table 2). Current smoking was displayed significant adjusted association with estrone levels, and with free but not total estradiol. BMI was associated with both estrone and estradiol, whereas diabetes displayed a significant association with total estradiol but not estrone levels. Total testosterone was more strongly associated with total estradiol than with free estradiol or estrone, though each association was highly statistically significant. Though unadjusted age trends in estrone and estradiol were similar, proportionate age-related cross-sectional increases in estradiol were of somewhat lesser magnitude than that for estrone after adjustment for covariates (Table 2).
Table 2.
Multivariable Regression Results
| Estrone | Estradiol | Free Estradiol | ||||
|---|---|---|---|---|---|---|
| Estimate (95% CI) | p | Estimate (95% CI) | p | Estimate (95% CI) | p | |
| Age, 10 y | 4.6 (2.4, 6.8) | <.0001 | 3.5 (1.4, 5.5) | .0007 | −5.2 (−7.3, −3.1) | <.0001 |
| Smoking, % | 11.7 (5.6, 18.1) | .0001 | −1.5 (−6.5, 3.8) | .58 | −8.1 (−13.3, −2.6) | .0042 |
| Alcohol, ounces/mo | ||||||
| 0 | ||||||
| 1–14 | −1.3 (−5.7, 3.4) | .59 | −0.4 (−4.6, 4.0) | .86 | 1.5 (−3.2, 6.5) | .53 |
| >14 | 2.9 (−1.8, 7.7) | .23 | −2.6 (−6.7, 1.8) | .24 | −0.41 (−5.1, 4.5) | .87 |
| Body mass index, kg/m2 | 1.7 (1.2, 2.1) | <.0001 | 1.8 (1.3, 2.2) | <.0001 | 2.0 (1.5, 2.4) | <.0001 |
| Diabetes | 2.9 (−2.3, 8.5) | .28 | 5.9 (0.9, 11.3) | .02 | 5.2 (−0.39, 11.1) | .07 |
| Poor self-rated health | 3.4 (−3.8, 11.1) | .36 | 1.5 (−5.2, 8.6) | .67 | −0.86 (−8.01, 6.8) | .82 |
| Comorbidity (CVD/cancer) | 2.8 (−1.7, 7.6) | .22 | 4.7 (0.3, 9.2) | .03 | 6.5 (1.6, 11.6) | .008 |
| CRP* | 0.8 (0.02, 1.5) | .04 | 0.7 (−0.02, 1.4) | .06 | 0.7 (−0.06, 1.5) | .07 |
| Total testosterone* | 6.7 (4.7, 8.7) | <.0001 | 16.9 (14.7, 19.0) | <.0001 | 7.6 (5.4, 9.8) | <.0001 |
Notes: CRP = C-reactive protein; CVD = cardiovascular disease. Slope estimates quantify cross-sectional percentage trends in hormones per unit change in variables at left. For instance, adjusted estrone values exhibit a mean cross-sectional increase of approximately 4.6% per 10 y difference in age.
*Estimated cross-sectional difference between two participants whose CRP or total testosterone concentrations differ by 50%.
Models assessing the effect of the sequential addition of covariates on estimated age trends are provided in Table 3. The magnitude of age trends was generally robust to control for covariates. The overall power of the model to account for variation in hormone levels was limited; as quantified by R 2 statistics, age and BMI together accounted for only 3.8% of the variance in estrone and 1.9% of the variance in estradiol levels. In the case of estradiol, the addition of total testosterone after age and BMI increased the variance explained to 17.3%. The addition of other health and behavioral factors to both models provided little improvement in the explanatory ability of the model. Collectively, age, BMI, testosterone, and other health and behavioral factors explained only 18% of variance in estradiol, and 9% of variance in estrone levels.
Table 3.
Estimated Trends in Hormones Per Decade Age After Adjustment for Major Covariates
| Covariates Included in Models | ||||||||
|---|---|---|---|---|---|---|---|---|
| Age only | Age, BMI | Age, BMI, total testosterone | All covariates | |||||
| Estimate (95% CI) | p | Estimate (95% CI) | p | Estimate (95% CI) | p | Estimate (95% CI) | p | |
| Estrone | 3.6 (1.6, 5.6) | .0003 | 4.2 (2.2, 6.2) | <.0001 | 4.8 (2.9, 6.8) | <.0001 | 4.6 (2.4, 6.8) | <.0001 |
| Total estradiol | 3.4 (1.4, 5.4) | .0007 | 3.7 (1.7, 5.7) | .0002 | 5.2 (3.4, 7.1) | <.0001 | 3.5 (1.4, 5.5) | .0007 |
| Free estradiol | −4.3 (−6.2, −2.3) | <.0001 | −3.7 (−5.6, −1.7) | .0002 | −3.0 (−4.9, −1.1) | .0023 | −5.2 (−7.3, −3.1) | <.0001 |
Notes: BMI = body mass index. Slope estimates quantify cross-sectional percentage trends in hormones per decade of age. Moving left to right, models are adjusted for covariates listed at the top of each column.Covariates included in the final model are age, BMI, total testosterone, smoking, alcohol consumption, self-rated health, comorbidity, and C-reactive protein. Full information for the final (rightmost) model is presented in Table 2.
Characteristics of Men with the Highest Estrone and Estradiol Levels
To identify the factors associated with high estrone and estradiol levels, we compared men in the highest quintile of estrone and estradiol levels with those in the lower quintiles (Supplementary Table 1). The men in the highest quintile of estrone and estradiol levels had significantly higher BMI than those in other quintiles. The prevalence of diabetes and CVD was higher in men in the highest quintile of estrone levels than those in other quintiles.
Discussion
In this FHS cohort, total estrone and estradiol levels were significantly positively associated with age and were higher in older men than in young men. However, free estradiol levels revealed a negative association with age, suggesting that the higher total estradiol levels in older men in comparison with young men may be related to the increased SHBG levels in older men. Although some studies have reported higher total estradiol levels in older men than young men, other studies have reported either no age-related change or even lower estradiol levels in older men than in young men (11–15,27–34). Some of the differences in the age trends in estradiol levels among epidemiological studies may be related to the differences in the age range of the study populations, the prevalence of comorbid conditions, and the assays used for measurements of estrone and estradiol levels. The poor precision and inaccuracy in the low range of the immunoassays used in some previous studies may have masked the age trends in estradiol levels in those studies.
The age-related increase in total estradiol and estrone levels is particularly remarkable because the circulating concentrations of their precursors, testosterone, and delta 4-androstenedione, respectively, decline significantly with age. We have reported previously (30) that older men administered graded doses of testosterone enanthate had higher estradiol levels than younger men, after adjusting for testosterone dose, suggesting relatively greater conversion of testosterone to estradiol in older men than in younger men, partly related to the higher percentage fat mass in older men. Age-related increase in SHBG levels may also contribute to the higher observed total estradiol levels.
Although the role of estradiol in regulating bone health and in pathogenesis of gynecomastia in men has been appreciated, the important role of estradiol in regulating cardiometabolic health, physical and sexual function, and adipose tissue remains incompletely understood. The biologic role of E1 in men is even less well understood; estrone has usually been lumped together with estradiol under the broad term “estrogens.” Our study provides novel information about age trends in estrone levels and the health and behavioral factors that are associated with estrone levels in men. Our data show that the age-related increase in estrone levels was somewhat greater than that in estradiol levels, and the factors associated with estrone levels differed from those associated with estradiol levels. In multivariate models, smoking had a significant positive effect on estrone levels but was negatively associated with free estradiol levels. Testosterone levels were more strongly associated with estradiol than with estrone levels. Although BMI appeared to have an independent relation to both estradiol and estrone, diabetes was associated only with the latter after adjustment for other factors. The relationship between estrogens and diabetes may be bidirectional; hyperinsulinemia has been reported to upregulate CYP19 aromatase; conversely, estrogens downregulate insulin receptor expression and signaling through estrogen receptor-α (35,36). These data suggest that the factors that regulate the aromatization of androstenedione to estrone in men may differ from those that regulate the aromatization of testosterone to estradiol; these differences might partly be explained by the fact that androstenedione, rather than testosterone, is the direct precursor for estrone: smoking is known to increase circulating levels of adrenal androgens (37,38).
Age and BMI, which have been viewed traditionally as major determinants of estrogen levels in men (11,17,18), explained only a small amount of variation in estrone and estradiol levels. In addition, in regression models for either hormone, after the inclusion of age, BMI, and testosterone, all the other factors that were studied explained only a small part of the overall variance, suggesting that important additional regulators of circulating estrone and estradiol levels remain unrecognized.
This investigation of the age trends in estradiol and estrone has some notable strengths. We measured hormone levels using LC-MS/MS, the method with the highest specificity and sensitivity (19–21). The FHS cohort had a wide age range in contrast to some other cohorts that included only older men. The FHS samples were collected in morning in fasting state, and stored frozen at −80°C. An additional unique aspect of this investigation is that we examined the age-related changes in estrone and estradiol levels in the context of lifestyle factors, testosterone, SHBG, and other health-related factors that might affect age trends in estrone and estradiol levels.
Our study also has some limitations. Only associations, but not causality, can be determined from epidemiological studies and reverse causality cannot be excluded. The current study was cross-sectional in nature; longitudinal studies are needed to confirm these findings. The FHS population is predominantly Caucasian and geographically limited, therefore, these findings may not be generalizable to other race/ethnicities or residents of other geographic regions. However, the age trends of total and free testosterone and SHBG and their associations with outcomes in FHS are similar to those reported in other large cohorts such as the European Male Aging Study and MrOS (26). Estrone and estradiol levels were measured in single morning samples in a manner similar to what clinicians might do in clinical practice, but single measurements may not reflect hormone levels over time. We did not measure serum androstenedione, which is an immediate precursor to estrone and which also declines with advancing age (39,40). The percentage of body fat for any given BMI increases with age; it is possible that the age effect is partly the result of residual confounding due to the inability to fully adjust for body composition. Even though C-reactive protein did not have a significant interaction with age for either estrogen, the role of other inflammatory markers, such as interleukin-6 and interleukin-11, and cytokines in the regulation of aromatization, as has been decribed previously, cannot be excluded (39).
In summary, the age trends in estrone and estradiol levels, measured using LC-MS/MS in community-dwelling men, revealed some notable differences, and were affected differentially by BMI and diabetes. A large part of the variation in circulating estrogen levels in men cannot be explained on the basis of the expected health and behavioral factors, including age and BMI. Longitudinal studies are needed to confirm these age trends, and to elucidate the regulators of estrogen levels in men and the mechanistic basis of age trends.
Funding
This project was supported by National Institute on Aging grant 1RO1AG31206 to S.B. and R.S.V. Additional support was provided by the Boston Claude D. Pepper Older Americans Independence Center (5P30AG031679) and by a grant from the CDC Foundation. The Framingham Heart Study is supported by the National Heart, Lung and Blood Institute’s contract N01-HC-25195.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Acknowledgment
Disclosure statement: The authors have nothing to disclose.
References
- 1. Sudhir K, Komesaroff PA. Cardiovascular actions of estrogens in men. J Clin Endocrinol Metab. 1999; 84: 3411–3415 [DOI] [PubMed] [Google Scholar]
- 2. Cushman M, Legault C, Barrett-Connor E, et al. Effect of postmenopausal hormones on inflammation-sensitive proteins: the Postmenopausal Estrogen/Progestin Interventions (PEPI) Study. Circulation. 1999; 100: 717–722 [DOI] [PubMed] [Google Scholar]
- 3. Gordon EE, Villee CA. An in vitro assay for estradiol-17beta and estrone. Endocrinology. 1956; 58: 150–157 [DOI] [PubMed] [Google Scholar]
- 4. Gruber CJ, Tschugguel W, Schneeberger C, Huber JC. Production and actions of estrogens. N Engl J Med. 2002; 346: 340–352 [DOI] [PubMed] [Google Scholar]
- 5. Longcope C, Kato T, Horton R. Conversion of blood androgens to estrogens in normal adult men and women. J Clin Invest. 1969; 48: 2191–2201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Longcope C, Layne DS, Tait JF. Metabolic clearance rates and interconversions of estrone and 17beta-estradiol in normal males and females. J Clin Invest. 1968; 47: 93–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hembree WC, Bardin CW, Lipsett MB. A study of estrogen metabolic clearance rates and transfer factors. J Clin Invest. 1969; 48: 1809–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vermeulen A, Kaufman JM, Goemaere S, van Pottelberg I. Estradiol in elderly men. Aging Male. 2002; 5: 98–102 [PubMed] [Google Scholar]
- 9. Schneider G, Kirschner MA, Berkowitz R, Ertel NH. Increased estrogen production in obese men. J Clin Endocrinol Metab. 1979; 48: 633–638 [DOI] [PubMed] [Google Scholar]
- 10. Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005; 26: 833–876 [DOI] [PubMed] [Google Scholar]
- 11. Orwoll E, Lambert LC, Marshall LM, et al. Testosterone and estradiol among older men. J Clin Endocrinol Metab. 2006; 91: 1336–1344 [DOI] [PubMed] [Google Scholar]
- 12. Simon D, Preziosi P, Barrett-Connor E, et al. The influence of aging on plasma sex hormones in men: the Telecom Study. Am J Epidemiol. 1992; 135: 783–791 [DOI] [PubMed] [Google Scholar]
- 13. Ferrini R, Barrett-Connor E. Sex hormones and age: a cross-sectional study of testosterone and estradiol and their bioavailable fractions in community-dwelling men. Am J Epidemiol. 1998; 147: 750–754 [DOI] [PubMed] [Google Scholar]
- 14. Leifke E, Gorenoi V, Wichers C, Von Zur Mühlen A, Von Büren E, Brabant G. Age-related changes of serum sex hormones, insulin-like growth factor-1 and sex-hormone binding globulin levels in men: cross-sectional data from a healthy male cohort. Clin Endocrinol (Oxf). 2000; 53: 689–695 [DOI] [PubMed] [Google Scholar]
- 15. Gray A, Feldman HA, McKinlay JB, Longcope C. Age, disease and changing sex hormone levels in middle-aged men: results of the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 1991; 73: 1016–1025 [DOI] [PubMed] [Google Scholar]
- 16. Denti L, Pasolini G, Sanfelici L, et al. Aging-related decline of gonadal function in healthy men: correlation with body composition and lipoproteins. J Am Geriatr Soc. 2000; 48: 51–58 [DOI] [PubMed] [Google Scholar]
- 17. Muller M, den Tonkelaar I, Thijssen JH, Grobbee DE, van der Schouw YT. Endogenous sex hormones in men aged 40-80 years. Eur J Endocrinol. 2003; 149: 583–589 [DOI] [PubMed] [Google Scholar]
- 18. Bjørnerem A, Straume B, Midtby M, et al. Endogenous sex hormones in relation to age, sex, lifestyle factors, and chronic diseases in a general population: the Tromsø Study. J Clin Endocrinol Metab. 2004; 89: 6039–6047 [DOI] [PubMed] [Google Scholar]
- 19. Stanczyk FZ, Cho MM, Endres DB, Morrison JL, Patel S, Paulson RJ. Limitations of direct estradiol and testosterone immunoassay kits. Steroids. 2003; 68: 1173–1178 [DOI] [PubMed] [Google Scholar]
- 20. Lee JS, Ettinger B, Stanczyk FZ, et al. Comparison of methods to measure low serum estradiol levels in postmenopausal women. J Clin Endocrinol Metab. 2006; 91: 3791–3797 [DOI] [PubMed] [Google Scholar]
- 21. Bhasin S, Zhang A, Coviello A, et al. The impact of assay quality and reference ranges on clinical decision making in the diagnosis of androgen disorders. Steroids. 2008; 73: 1311–1317 [DOI] [PubMed] [Google Scholar]
- 22. Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951; 41: 279–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ingelsson E, Massaro JM, Sutherland P, et al. Contemporary trends in dyslipidemia in the Framingham Heart Study. Arch Intern Med. 2009; 169: 279–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kushnir MM, Rockwood AL, Bergquist J, et al. High-sensitivity tandem mass spectrometry assay for serum estrone and estradiol. Am J Clin Pathol. 2008; 129: 530–539 [DOI] [PubMed] [Google Scholar]
- 25. Rockwood AL, Nelson GJ, Yue B, Urry FM. Assessing analytical specificity in quantitative analysis using tandem mass spectrometry. Clin Biochem. 2005; 38: 319–327 [DOI] [PubMed] [Google Scholar]
- 26. Bhasin S, Pencina M, Jasuja GK, et al. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohorts. J Clin Endocrinol Metab. 2011; 96: 2430–2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Orwoll ES, Nielson CM, Labrie F, et al. Osteoporotic Fractures in Men (MrOS) Research Group Evidence for geographical and racial variation in serum sex steroid levels in older men. J Clin Endocrinol Metab. 2010; 95: E151–E160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mellström D, Vandenput L, Mallmin H, et al. Older men with low serum estradiol and high serum SHBG have an increased risk of fractures. J Bone Miner Res. 2008; 23: 1552–1560 [DOI] [PubMed] [Google Scholar]
- 29. Travison TG, Nguyen AH, Naganathan V, et al. Changes in reproductive hormone concentrations predict the prevalence and progression of the frailty syndrome in older men: the concord health and ageing in men project. J Clin Endocrinol Metab. 2011; 96: 2464–2474 [DOI] [PubMed] [Google Scholar]
- 30. Lakshman KM, Kaplan B, Travison TG, et al. The effects of injected testosterone dose and age on the conversion of testosterone to estradiol and dihydrotestosterone in young and older men. J Clin Endocrinol Metab. 2010; 95: 3955–3964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schneider G, Kirschner MA, Berkowitz R, Ertel NH. Increased estrogen production in obese men. J Clin Endocrinol Metab. 1979; 48: 633–638 [DOI] [PubMed] [Google Scholar]
- 32. Maggio M, Ceda GP, Lauretani F, et al. Estradiol and inflammatory markers in older men. J Clin Endocrinol Metab. 2009; 94: 518–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nakhai Pour HR, Grobbee DE, Muller M, van der Schouw YT. Association of endogenous sex hormone with C-reactive protein levels in middle-aged and elderly men. Clin Endocrinol (Oxf). 2007; 66: 394–398 [DOI] [PubMed] [Google Scholar]
- 34. de Ronde W, van der Schouw YT, Muller M, et al. Associations of sex-hormone-binding globulin (SHBG) with non-SHBG-bound levels of testosterone and estradiol in independently living men. J Clin Endocrinol Metab. 2005; 90: 157–162 [DOI] [PubMed] [Google Scholar]
- 35. Williams G. Aromatase up-regulation, insulin and raised intracellular oestrogens in men, induce adiposity, metabolic syndrome and prostate disease, via aberrant ER-α and GPER signalling. Mol Cell Endocrinol. January 5 2012; 351: 269–278 [DOI] [PubMed] [Google Scholar]
- 36. Gonzalez C, Alonso A, Grueso NA, et al. Role of 17beta-estradiol administration on insulin sensitivity in the rat: implications for the insulin receptor. Steroids. 2002; 67: 993–1005 [DOI] [PubMed] [Google Scholar]
- 37. Hautanen A, Mänttäri M, Kupari M, et al. Cigarette smoking is associated with elevated adrenal androgen response to adrenocorticotropin. J Steroid Biochem Mol Biol. 1993; 46: 245–251 [DOI] [PubMed] [Google Scholar]
- 38. Field AE, Colditz GA, Willett WC, Longcope C, McKinlay JB. The relation of smoking, age, relative weight, and dietary intake to serum adrenal steroids, sex hormones, and sex hormone-binding globulin in middle-aged men. J Clin Endocrinol Metab. 1994; 79: 1310–1316 [DOI] [PubMed] [Google Scholar]
- 39. Zietz B, Hrach S, Schölmerich J, Straub RH. Differential age-related changes of hypothalamus - pituitary - adrenal axis hormones in healthy women and men - role of interleukin 6. Exp Clin Endocrinol Diabetes. 2001; 109: 93–101 [DOI] [PubMed] [Google Scholar]
- 40. Couillard C, Gagnon J, Bergeron J, et al. Contribution of body fatness and adipose tissue distribution to the age variation in plasma steroid hormone concentrations in men: the HERITAGE Family Study. J Clin Endocrinol Metab. 2000; 85: 1026–1031 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.