Abstract
Background.
Little is known about the effect of intentional weight loss and subsequent weight regain on cardiometabolic risk factors in older adults. The objective of this study was to determine how cardiometabolic risk factors change in the year following significant intentional weight loss in postmenopausal women, and if observed changes were affected by weight and fat regain.
Methods.
Eighty, overweight and obese, older women (age = 58.8±5.1 years) were followed through a 5-month weight loss intervention and a subsequent 12-month nonintervention period. Body weight/composition and cardiometabolic risk factors (blood pressure; total, high-density lipoprotein, and low-density lipoprotein cholesterol; triglycerides; fasting glucose and insulin; and Homeostatic Model Assessment of Insulin Resistance) were analyzed at baseline, immediately postintervention, and 6- and 12-months postintervention.
Results.
Average weight loss during the 5-month intervention was 11.4±4.1kg and 31.4% of lost weight was regained during the 12-month follow-up. On average, all risk factor variables were significantly improved with weight loss but regressed toward baseline values during the year subsequent to weight loss. Increases in total cholesterol, triglycerides, glucose, insulin, and Homeostatic Model Assessment of Insulin Resistance during the postintervention follow-up were significantly (p < .05) associated with weight and fat mass regain. Among women who regained weight, model-adjusted total cholesterol (205.8±4.0 vs 199.7±2.9mg/dL), low-density lipoprotein cholesterol (128.4±3.4 vs 122.7±2.4mg/dL), insulin (12.6±0.7 vs 11.4±0.7mg/dL), and Homeostatic Model Assessment of Insulin Resistance (55.8±3.5 vs 50.9±3.7mg/dL) were higher at follow-up compared with baseline.
Conclusions.
For postmenopausal women, even partial weight regain following intentional weight loss is associated with increased cardiometabolic risk. Conversely, maintenance of or continued weight loss is associated with sustained improvement in the cardiometabolic profile.
Key Words: Weight loss, Weight regain, Aging.
OVERWEIGHT and obesity are significant public health problems, with recent estimates suggesting that 72.3% and 64.1% of adult men and women, respectively, have a body mass index (BMI) greater than or equal to 25kg/m2 (1). In women, postmenopausal status is associated with higher prevalence of overweight and obesity (2). Excessive body weight often predisposes to chronic disease, and accordingly, overweight and obese postmenopausal women are at increased risk of developing several cardiometabolic conditions (3–7).
Treatments for overweight and obesity, which result in significant weight loss, frequently yield immediate improvement in the cardiometabolic risk profile (8). For most, however, long-term weight loss maintenance remains elusive (9). Data show that about one third of individuals who lose weight will regain all of their lost weight within 1 year, and almost all individuals regress to their baseline weight within 5 years (10,11). Although nearly 60% of overweight women report trying to lose weight ([12] and previous data suggest that most will be unable to maintain their weight loss), the effect of a cycle of intentional weight loss followed by weight regain on cardiometabolic risk in overweight and obese postmenopausal women is not known. Such data are necessary to assess the long-term benefits and risks of intentional weight loss in this population.
Observational studies in younger adults show that weight cycling is not associated with increased cardiometabolic risk (13–15). Further, weight regain in this population is not associated with adverse effects on body composition or fat distribution (16,17). However, recent data from our group suggest that, for postmenopausal women, body composition is negatively impacted by weight regain following intentional weight loss (18). Specifically, after a 13% weight reduction, women who regained at least 2kg of lost weight within a year experienced a greater accretion of fat mass, relative to lean mass. Due to the known association between elevated body fat and cardiovascular disease risk, preferential regain of fat suggests that cardiometabolic risk factors may be worse in older adults who regain weight after intentional weight loss, although further investigation is warranted.
Therefore, the purpose of this study was to determine how cardiometabolic risk factors change in the year following significant intentional weight loss in postmenopausal women and if observed, changes are affected by weight and fat regain. We also assessed whether these risk factors were worse 1 year after intentional weight loss compared with baseline values in women who experienced weight regain.
Methods
Study Design Overview
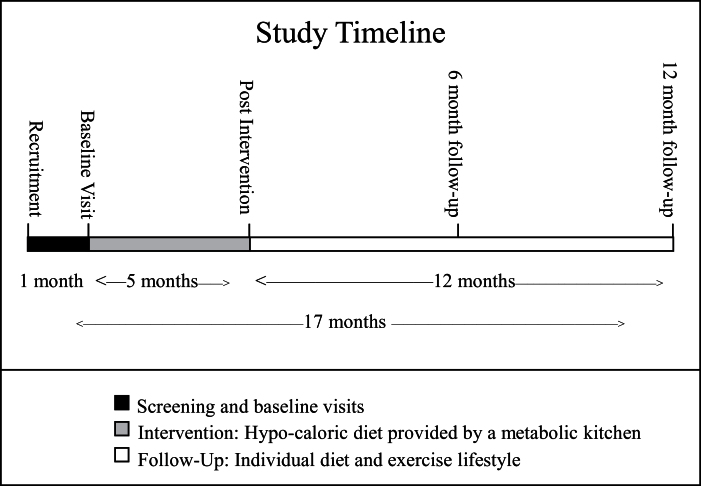
This article presents follow-up data from a randomized, clinical trial (NCT00664729), originally designed to determine whether the intensity of aerobic exercise affects the loss of abdominal adipose tissue in abdominally obese, postmenopausal women, under controlled conditions of isocaloric deficit. Study design and recruitment details were previously published (19). Briefly, this study was a 5-month trial comparing caloric restriction alone to caloric restriction plus moderate-intensity aerobic exercise and caloric restriction plus vigorous-intensity exercise. Women were recruited and enrolled based on the following criteria: (a) abdominal obesity (BMI: 25–40 and waist circumference >88cm), (b) age (50–70 years), (c) postmenopausal status (no menses for >1 year), (d) nonsmoking (for >1 year), (e) not on hormone replacement therapy, (f) sedentary (<15-min exercise 2 times/wk in the past 6 months), and (g) weight stable (<5% weight change) for more than or equal to 6 months before enrollment. After the caloric restriction interventions, women were asked to return for 6- and 12-month follow-up visits to assess changes in body weight, body composition, and cardiometabolic risk. A visual of the study time line is provided in Figure 1. The study was approved by the Wake Forest School of Medicine Institutional Review Board, and all participants signed an approved informed consent document.
Figure 1.
Study timeline.
Weight Loss Interventions and Follow-up
The weight loss intervention was previously described in detail (19). Briefly, 112 women were enrolled in a 5-month program of controlled underfeeding, of which 95 completed. Individual energy needs were calculated from each participant’s measured resting metabolic rate and an activity factor based on self-reported daily activity. The degree of caloric restriction was adjusted so that total caloric deficit (~400 kcal/d; 2800 kcal/wk) was similar for all groups.
At the end of the 5-month weight loss intervention, women were asked to return for anthropometric and cardiometabolic measurements at 6- and 12-months postintervention (11- and 17-months, respectively). Women were provided one counseling session with a registered dietitian concerning strategies for maintenance of weight loss at the end of the 5-month weight loss intervention. With the exception of scheduling phone calls and the 6- and 12-month follow-up visits, no other contact by study-related personnel was provided during the 12-month follow-up period. A total of 80 women completed their assigned 5-month weight loss intervention and had at least one follow-up time point weight and risk factor measurement.
Anthropometrics, Body Composition, and Cardiometabolic Risk Factor Measurements
Body weight and cardiometabolic risk factors were measured at baseline, immediately after the 5-month weight loss intervention and 6- and 12-months postintervention. Measurement details are previously reported (19). Study staff responsible for data collection were blinded to group assignment. Height and weight were measured with shoes and outer garments removed. BMI was calculated as weight in kilograms (kg) divided by the square of height in meters (m) and waist (ie, minimal) circumference (cm) was measured in triplicate. Whole-body fat and lean mass was measured by dual-energy x-ray absorptiometry (Hologic Delphi A 11.0 QDR, Bedford, MA).
Blood pressure was measured in the right arm, using a conventional mercury sphygmomanometer, with the participant in a seated position after having rested quietly for 10–15 minutes. Participants sat with feet flat on the floor and legs uncrossed and were asked not to talk during the rest period or during the measurement. An appropriate cuff size was used and systolic and diastolic blood pressures were defined as the average of two repeated measures.
Blood samples were collected in ethylenediaminetetraacetic acid-treated evacuated tubes by venipuncture in the early morning after a 10-hour fast, and plasma triglycerides, total cholesterol, high-density lipoprotein (HDL) cholesterol, and low-density lipoprotein (LDL) cholesterol were measured by standardized hospital laboratory methods. LDL:HDL ratio was calculated by dividing LDL cholesterol concentration by the HDL cholesterol concentration at each time point. Plasma glucose was measured with the glucose hexokinase method (Bayer Diagnostics, Tarrytown, NY). Plasma insulin was determined by a chemiluminescent immunoassay with the use of an IMMULITE analyzer (Diagnostics Products Corporation, Los Angeles, CA). Insulin sensitivity (Homeostatic Model Assessment of Insulin Resistance; HOMA) was calculated as the product of the fasting values of glucose (expressed as mg/dL) and insulin (expressed as µU/mL) divided by the constant 22.5 (20).
Statistical Analysis
Weight regain status was defined based on whether a participant gained greater than or equal to 2kg during the postintervention period (ie, either 6- or 12-month postintervention data point used). Women who gained greater than or equal to 2kg were labeled as “regainers,” whereas those who did not regain 2kg or continued losing weight were considered “maintainers.” Outcome measures included change in systolic blood pressure (mm Hg), diastolic blood pressure (mm Hg), total cholesterol (mg/dL), LDL cholesterol (mg/dL), HDL cholesterol (mg/dL), triglycerides (mg/dL), LDL:HDL ratio, glucose (mg/dL), fasting insulin (µIU/mL), and HOMA (mg/dL × µU/mL).
Cardiometabolic risk factor values were summarized by simple means and standard deviations (SD) at each of the 4 time points: baseline, 5-months (postintervention), 11 months (6-months postintervention), and 17 months (12-months postintervention). Means and SDs were further stratified by weight regain status. Longitudinal postintervention associations between change in weight and change in cardiometabolic risk factor values at 6- and 12 months were estimated using mixed linear models, adjusting for age, race, baseline BMI, randomization arm, baseline outcome measure, and 5-month outcome measure. The same model was used to assess the longitudinal association between change in fat mass and waist circumference with change in cardiometabolic risk factor value. An analysis of covariance model assessed the 12-month postintervention difference in risk factor values between weight maintainers and regainers, adjusting for age, race, randomization arm, baseline BMI, baseline risk factor value, and 5-month risk factor value. Finally, the 12-month postintervention mean cardiometabolic risk factor values for weight maintainers and regainers were compared with baseline values using a mixed-model profile analysis to test for an interaction of risk factor trend by weight regain group, adjusting age, race, baseline BMI, randomization arm, and postintervention risk factor values. p Values are presented, testing for a group (weight maintainers vs weight regainers) by time interaction or whether groups’ mean cardiometabolic risk factor values at 12-months postintervention differ from baseline means. All analyses were performed using SAS v9.2 using a Type I error rate of 0.05.
Results
Of the 112 original DEMO participants, 80 returned for at least one 6- or 12-month follow-up measurement. Mean age of the study sample at baseline was 58.8 ± SD = 5.1, and 56 (70%) were Caucasian. No significant differences between the 80 returning participants and 32 nonreturners were observed with regard to age (p = .15), race (p = .09), baseline, or postintervention body weight (p = .68 and .40, respectively).
Participant Weight Loss and Regain
As previously reported, no significant differences between randomization arms were observed with regard to magnitude or composition of weight lost or subsequent weight regained (18,19); therefore, results are presented across treatment groups. Average weight loss during the 5-month intervention was 11.4±4.1kg (range = −20.9 to −2.5kg), with 70.1±0.2% coming from fat mass and 29.9±0.2% coming from lean mass. On average, 4.1±6.8kg (31.4% of lost weight) was regained during the 12-month follow-up period, and of the 80 women, 58 (72.5%) were classified as regainers (ie, ≥2kg regain), whereas 22 (27.5%) were maintainers. Regainers had a mean baseline age of 58.6±4.8 years and 38 (65.5%) were Caucasian, whereas maintainers had a mean baseline age of 59.4±5.9 years and 18 (81.8%) were Caucasian. Previously published modeling results of regainers revealed fat mass was regained to a greater degree than lean mass (18). Of the maintainers, 12 continued losing weight during the postintervention period, and of the regainers, 13 women regained all lost weight by the 12-month follow-up visit.
Change in Cardiometabolic Risk Over Time and by Weight Regain Status
Unadjusted cardiometabolic risk factors by time point are presented in Table 1. All variables (except HDL cholesterol and the LDL:HDL ratio) were significantly improved immediately postintervention compared with baseline (all p < .05). On average, the weight loss interventions reduced systolic blood pressure by 8.8±15.3mm Hg, diastolic blood pressure by 5.3±10.1mm Hg, total cholesterol by 13.4±22.1mg/dL, LDL cholesterol by 6.5±17.8mg/dL, HDL cholesterol by 2.0±6.4mg/dL, triglycerides by 23.4±41.9mg/dL, glucose by 5.3±9.7mg/dL, insulin by 3.6±4.7mg/dL, and HOMA by 18.6±25.9mg/dL × µU/mL. These results did not differ by weight regain status.
Table 1.
Summary of Mean Cardiometabolic Risk Factors by Time Point
| Cardiometabolic Risk Factor | Baseline | Postintervention | 6-Mo Follow-Up | 12-Mo Follow-Up | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | M | SD | n | M | SD | n | M | SD | n | M | SD | |
| Systolic blood pressure (mm Hg) | 76 | 128.0 | 18.0 | 72 | 118.0 | 16.3 | 75 | 125.9 | 16.6 | 69 | 125.1 | 16.2 |
| Diastolic blood pressure (mm Hg) | 76 | 75.3 | 8.6 | 72 | 69.8 | 9.9 | 75 | 72.7 | 10.1 | 69 | 74.6 | 9.5 |
| Total cholesterol (mg/dL) | 80 | 204.4 | 32.1 | 80 | 191.0 | 31.7 | 73 | 207.6 | 29.6 | 68 | 202.8 | 38.0 |
| LDL cholesterol (mg/dL) | 80 | 125.5 | 28.5 | 80 | 119.0 | 29.5 | 73 | 128.3 | 28.9 | 68 | 124.5 | 34.6 |
| HDL cholesterol (mg/dL) | 80 | 53.2 | 11.4 | 80 | 51.2 | 9.2 | 73 | 60.0 | 12.3 | 68 | 57.0 | 14.0 |
| Triglycerides (mg/dL) | 80 | 128.9 | 52.5 | 80 | 105.4 | 37.7 | 73 | 99.2 | 42.5 | 68 | 106.5 | 45.8 |
| LDL–HDL ratio | 80 | 2.5 | 0.7 | 80 | 2.4 | 0.7 | 73 | 2.2 | 0.7 | 68 | 2.3 | 1.0 |
| Glucose (mg/dL) | 76 | 98.2 | 13 | 75 | 92.0 | 8.5 | 70 | 90 | 12.1 | 66 | 96.5 | 16.2 |
| Insulin (µIU/mL) | 76 | 11.9 | 7.0 | 75 | 7.9 | 4.7 | 70 | 10.6 | 6.3 | 66 | 10.9 | 6.0 |
| HOMA (mg/dL × µU/ml) | 76 | 54.0 | 39.6 | 75 | 33.1 | 21.1 | 70 | 44.1 | 29.6 | 66 | 48.3 | 29.9 |
Note: HDL = high-density lipoprotein; HOMA = homeostatic model assessment of insulin resistance; LDL = low-density lipoprotein; M = mean; SD = standard deviation.
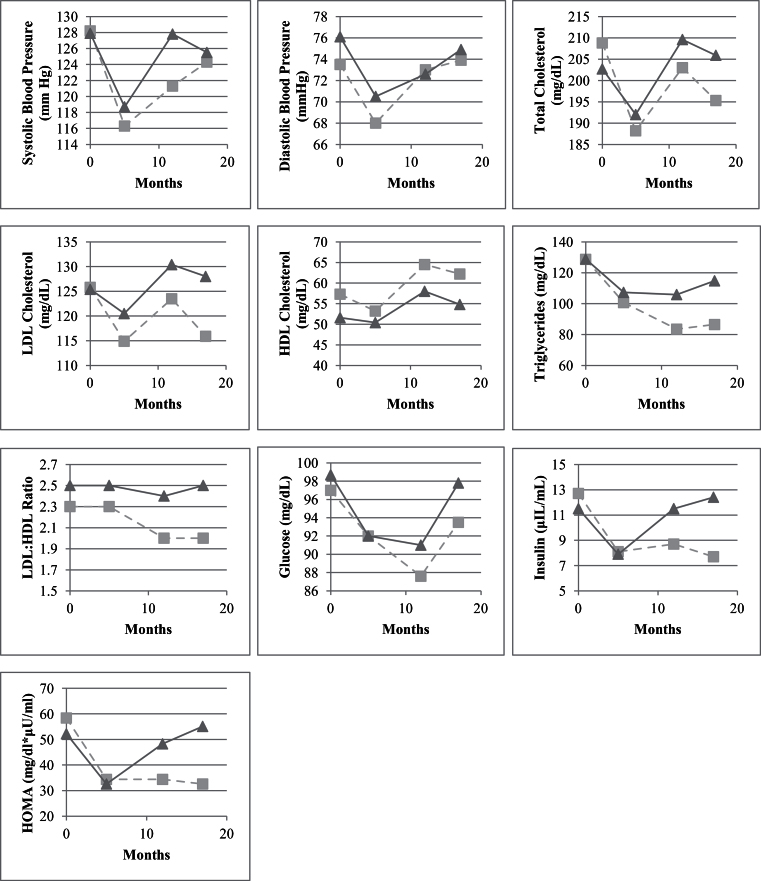
Examination of changes in cardiometabolic risk factors during postintervention follow-up revealed that, on average, all risk factors regressed toward baseline values during the follow-up period. However, this trend was notably different for regainers versus maintainers. Of particular interest, 12-month postintervention values for triglycerides, insulin, LDL:HDL ratio, and HOMA were all comparable with immediate postintervention values in women who were classified as maintainers, whereas 12-month postintervention values for total, LDL, and HDL cholesterol; insulin; and HOMA were slightly higher than baseline values in women classified as regainers (see Figure 2).
Figures 2.
Graphical summary of change in cardiometabolic risk factors by time point and weight regain status, where time point 1 = baseline; time point 2 = immediately postintervention; time point 3 = 6-months postintervention; and time point 4 = 12-months postintervention.
Relationships Between Changes in Cardiometabolic Risk Factors and Changes in Body Weight
Relationships between changes in cardiometabolic risk factors and changes in body weight and composition during the postintervention follow-up (including 6- and 12-month postintervention time points) are presented in Table 2. Parameter estimates indicate the model-adjusted change in risk factor value per unit change in weight (kg), fat mass (kg), and waist circumference (cm) during the postintervention period, after adjustment for baseline and postintervention risk factor values. Changes in total cholesterol, triglycerides, glucose, insulin, and HOMA values are directly associated with weight changes (all p < .05), whereas changes in systolic blood pressure and LDL:HDL show a marginal association with changes in weight (0.44±0.23; p = .06 and 0.02±0.01; p = .07, respectively). The observed risk factors showed similar relationships with changes in fat mass, with the addition of systolic blood pressure achieving statistical significance (p = .03). However, waist circumference appears to be a less sensitive predictor of cardiometabolic risk, only achieving a significant association with triglycerides (p = .04). Overall, this analysis indicates that increases in weight and total body fat during the postintervention period tend to be associated with increased (or worsened) cardiometabolic risk factors.
Table 2.
Change in Cardiometabolic Risk Factors by Change in Body Weight and Composition
| Weight (kg) | Fat Mass (kg) | Waist Circumference (cm) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Risk Factor Change | SE | p Value | Risk Factor Change | SE | p Value | Risk Factor Change | SE | p Value |
| Systolic blood pressure (mm Hg) | 0.44 | 0.23 | .06 | 0.68 | 0.31 | .03 | 0.36 | 0.24 | .14 |
| Diastolic blood pressure (mm Hg) | 0.08 | 0.15 | .60 | 0.19 | 0.20 | .34 | 0.23 | 0.16 | .16 |
| Total cholesterol (mg/dL) | 0.92 | 0.41 | .03 | 1.87 | 0.51 | <.001 | 0.69 | 0.42 | .10 |
| LDL cholesterol (mg/dL) | 0.58 | 0.35 | .11 | 1.14 | 0.44 | .01 | 0.51 | 0.37 | .17 |
| HDL cholesterol (mg/dL) | –0.08 | 0.14 | .59 | –0.05 | 0.18 | .80 | –0.04 | 0.12 | .75 |
| Triglycerides (mg/dL) | 1.75 | 0.50 | <.01 | 2.70 | 0.67 | <.001 | 1.13 | 0.53 | .04 |
| LDL–HDL ratio | 0.02 | 0.01 | .07 | 0.34 | 0.20 | .09 | 0.16 | 0.20 | .43 |
| Glucose (mg/dL) | 0.41 | 0.18 | .02 | 0.03 | 0.01 | .01 | 0.01 | 0.01 | .30 |
| Insulin (µIU/mL) | 0.45 | 0.08 | <.01 | 0.53 | 0.10 | <.001 | 0.33 | 0.08 | <.001 |
| HOMA (mg/dL × µU/mL) | 2.10 | 0.35 | <.01 | 2.27 | 0.48 | <.001 | 1.58 | 0.40 | <.001 |
Notes: HDL = high-density lipoprotein; HOMA = homeostatic model assessment of insulin resistance; LDL = low-density lipoprotein; M = mean; SE = standard error.
Model-adjusted estimates control for age, race, baseline fat mass, randomization arm, and baseline and postintervention risk factor value. Values for risk factor change are given per 1kg increase in weight.
Model-adjusted mean 12-month follow-up cardiometabolic risk factor differences stratified by weight regain status indicate generally improved risk factors for participants who maintain their weight loss. Compared with weight maintainers, women classified as regainers had a significantly higher change in total cholesterol (17.1±7.0mg/dL; p = .02), LDL cholesterol (14.5±5.8mg/dL; p = .01), triglycerides (21.2±9.1mg/dL; p = .02), LDL/HDL ratio (0.35±0.16; p = 0.03), insulin (4.5±1.3 µIU/mL; p < .01), and HOMA (20.5±6.1mg/dL × µU/mL; p < .01).
Cardiometabolic Risk Factor Comparisons From Baseline to 12-Months Postintervention
Table 3 shows cardiometabolic risk factor comparisons from baseline to 12-months postintervention by weight regain status. The model-adjusted estimates control for age, race, baseline BMI, randomization arm, and postintervention risk factor values. Significant group × time interactions, indicating differential risk factor trends after the 12-month follow-up (from baseline) by weight regain status, were observed for total cholesterol, LDL cholesterol, triglycerides, insulin, and HOMA (p for interaction < .05). For these variables, the 12-month follow-up time point indicates increased (ie, worsened) risk factor values compared with baseline for weight regainers, with the exception of triglycerides, which were reduced from baseline in both groups, but significantly higher in regainers compared with maintainers at the 12-month postintervention time point (109.2±5.2 vs 88.7±8.4, respectively). However, these risk factor values were still improved relative to baseline in weight maintainers. Systolic blood pressure was marginally reduced (3.6±2.1mm Hg; p = .08) and HDL cholesterol was elevated (3.4±1.0mg/dL; p < .01) from baseline in both groups.
Table 3.
Model-Adjusted Cardiometabolic Risk Factor Comparisons From Baseline to 12-Months Postintervention
| Baseline | 12-Months Postintervention | p Value | |||||
|---|---|---|---|---|---|---|---|
| Cardiometabolic Risk Factor | Status | M | SE | M | SE | Difference From Baseline | Group × Time Interaction |
| Systolic blood pressure (mm Hg) | Maintainers | 127.6 | 3.3 | 121.9 | 3.8 | .08 | .51 |
| Regainers | 126.9 | 2.0 | 124.2 | 2.5 | |||
| Diastolic blood pressure (mm Hg) | Maintainers | 74.1 | 2.0 | 73.3 | 2.2 | .47 | .88 |
| Regainers | 75.4 | 1.2 | 74.2 | 1.4 | |||
| Total cholesterol (mg/dL) | Maintainers | 209.6 | 4.9 | 194.8 | 6.4 | .20 | <.01 |
| Regainers | 199.7 | 2.9 | 205.8 | 4.0 | |||
| LDL cholesterol (mg/dL) | Maintainers | 128.3 | 4.0 | 117.9 | 5.4 | .40 | <.01 |
| Regainers | 122.7 | 2.4 | 128.4 | 3.4 | |||
| HDL cholesterol (mg/dL) | Maintainers | 55.4 | 1.5 | 59.0 | 2.2 | <.01 | .89 |
| Regainers | 52.5 | 0.9 | 55.9 | 1.4 | |||
| Triglycerides (mg/dL) | Maintainers | 128.3 | 9.3 | 88.7 | 8.4 | <.01 | .03 |
| Regainers | 123.9 | 5.6 | 109.2 | 5.2 | |||
| LDL–HDL ratio | Maintainers | 2.4 | 0.1 | 2.2 | 0.2 | .09 | .06 |
| Regainers | 2.5 | 0.1 | 2.5 | 0.1 | |||
| Glucose (mg/dL) | Maintainers | 96.9 | 2.4 | 92.8 | 3.3 | .07 | .37 |
| Regainers | 98.1 | 1.5 | 96.7 | 2.1 | |||
| Insulin (µIU/mL) | Maintainers | 12.9 | 1.1 | 7.8 | 1.1 | .03 | <.01 |
| Regainers | 11.4 | 0.7 | 12.6 | 0.7 | |||
| HOMA (mg/dL × µU/mL) | Maintainers | 59.1 | 5.9 | 32.9 | 5.2 | .02 | <.01 |
| Regainers | 50.9 | 3.7 | 55.8 | 3.5 | |||
Notes: HDL = high-density lipoprotein; HOMA = homeostatic model assessment of insulin resistance; LDL = low-density lipoprotein; M = mean; SE = standard error.
Model-adjusted estimates control for age, race, baseline body mass index (BMI), randomization arm, and postintervention risk factor values. Weight regain status was defined based on whether a participant gained greater than or equal to 2kg during the postintervention period (ie, either 6- or 12-month postintervention data point used). Women who gained greater than or equal to 2kg were labeled as “regainers,” whereas those who did not regain 2kg, or continued losing weight, were considered “maintainers.”
Discussion
The purpose of this study was to determine how cardiometabolic risk factors change in the year following significant intentional weight loss in postmenopausal women and whether observed changes were associated with weight regain status. Results demonstrate that more than two third of women regained at least 2kg of lost weight within 12 months of completing an intensive weight loss program. This cycle of weight regain following intentional weight loss was associated with deleterious long-term changes in several cardiometabolic risk factors, with fasting insulin and the HOMA estimate of insulin resistance most adversely affected by weight regain. Findings are particularly striking given that, on average, women classified as regainers still weighed less at 12-months follow-up than they did at baseline. Conversely, maintenance of (or continued) weight loss was associated with sustained improvement in the cardiometabolic risk profile; although the greatest improvement was seen immediately after weight loss.
Weight loss in overweight and obese individuals is known to yield immediate improvements in cardiometabolic risk, even in older adults (21–24). However, data examining the change in cardiometabolic risk factors during a period of weight regain following structured and intentional weight loss are scarce. Contrary to our results, an earlier study of young (25–45 years) obese women followed prospectively through a 30-month weight loss and maintenance program, found no adverse changes in lipid levels, blood pressure, waist-to-hip ratio, or percent body fat in weight regainers compared with weight stable women (13). However, this finding may not apply to older adults. Age is a significant predictor of cardiometabolic disease, and several lines of evidence suggest that weight cycling in late life may have unfavorable health effects. For example, longitudinal data suggest that, in older adults, self-reported intentional weight cycling contributes to loss of lean mass (25), increases risk of developing cardiovascular disease (26), and predicts future physical limitation and mortality (27). Further, we recently reported that fat mass is regained to a greater degree than lean mass in postmenopausal women who experienced a cycle of weight regain following intentional weight loss (18). Findings from this study extend this work by showing that weight (and similarly, fat) regain is further associated with a worsened cardiometabolic risk profile in this population.
Importantly, although weight loss maintenance was associated with prolonged improvements in most cardiometabolic risk factors, it is worth noting that even among women who maintained their weight loss, systolic and diastolic blood pressure, and to a lesser extent, fasting glucose, returned to baseline values within a year after weight loss. This observation indicates that other factors other than weight change may be driving this association. Further, even though several risk factor values continued on downward (ie, improved) trajectories in women who maintained their weight loss, the magnitude of improvement in each risk factor was most pronounced during the weight loss phase. This finding is in agreement with other studies showing that the degree of caloric restriction, rather than weight loss, may be more strongly associated with cardiometabolic benefit (28,29).
Strengths of this study design include measurements of multiple cardiometabolic risk factors before and after intentional weight loss, as well as a relatively long follow-up period. However, results of our study are limited to postmenopausal women and should not be considered definitive because we did not have a weight-stable group to serve as a control for intentional weight loss.
In conclusion, results from this study demonstrate that a cycle of weight regain following intentional weight loss is associated with deleterious long-term effects on cardiometabolic risk factors in postmenopausal women; however, weight loss maintenance is associated with sustained improvements. Results highlight the need for future research to develop effective strategies to promote the maintenance of weight loss in this population.
Funding
This work was supported by the National Institute of Health (R01-AG/DK20583, R01-HL093713, and F32-AG039186), Wake Forest University Claude D Pepper Older Americans Independence Center (P30-AG21332), and Wake Forest University General Clinical Research Center (M01-RR07122).
References
- 1. Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012; 307: 491–497 [DOI] [PubMed] [Google Scholar]
- 2. Pérez JA, Garcia FC, Palacios S, Pérez M. Epidemiology of risk factors and symptoms associated with menopause in Spanish women. Maturitas. 2009; 62: 30–36 [DOI] [PubMed] [Google Scholar]
- 3. Schenck-Gustafsson K. Risk factors for cardiovascular disease in women. Maturitas. 2009; 63: 186–190 [DOI] [PubMed] [Google Scholar]
- 4. Page JH, Rexrode KM, Hu F, Albert CM, Chae CU, Manson JE. Waist-height ratio as a predictor of coronary heart disease among women. Epidemiology. 2009; 20: 361–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rexrode KM, Hennekens CH, Willett WC, et al. A prospective study of body mass index, weight change, and risk of stroke in women. JAMA. 1997; 277: 1539–1545 [DOI] [PubMed] [Google Scholar]
- 6. Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995; 122: 481–486 [DOI] [PubMed] [Google Scholar]
- 7. Cushman M, Kuller LH, Prentice R, et al. Estrogen plus progestin and risk of venous thrombosis. JAMA. 2004; 292: 1573–1580 [DOI] [PubMed] [Google Scholar]
- 8. Poirier P, Giles TD, Bray GA, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006; 113: 898–918 [DOI] [PubMed] [Google Scholar]
- 9. McGuire MT, Wing RR, Hill JO. The prevalence of weight loss maintenance among American adults. Int J Obes Relat Metab Disord. 1999; 23: 1314–1319 [DOI] [PubMed] [Google Scholar]
- 10. Weiss EC, Galuska DA, Kettel Khan L, Gillespie C, Serdula MK. Weight regain in U.S. adults who experienced substantial weight loss, 1999-2002. Am J Prev Med. 2007; 33: 34–40 [DOI] [PubMed] [Google Scholar]
- 11. Methods for voluntary weight loss and control. NIH Technology Assessment Conference Panel. Consensus Development Conference, 30 March to 1 April 1992 Ann Intern Med. 1993; 119: 764–770 [PubMed] [Google Scholar]
- 12. Bish CL, Blanck HM, Serdula MK, Marcus M, Kohl HW, 3rd, Khan LK. Diet and physical activity behaviors among Americans trying to lose weight: 2000 Behavioral Risk Factor Surveillance System. Obes Res. 2005; 13: 596–607 [DOI] [PubMed] [Google Scholar]
- 13. Wing RR, Jeffery RW, Hellerstedt WL. A prospective study of effects of weight cycling on cardiovascular risk factors. Arch Intern Med. 1995; 155: 1416–1422 [PubMed] [Google Scholar]
- 14. Jeffery RW, Wing RR, French SA. Weight cycling and cardiovascular risk factors in obese men and women. Am J Clin Nutr. 1992; 55: 641–644 [DOI] [PubMed] [Google Scholar]
- 15. Petersmarck KA, Teitelbaum HS, Bond JT, Bianchi L, Hoerr SM, Sowers MF. The effect of weight cycling on blood lipids and blood pressure in the Multiple Risk Factor Intervention Trial Special Intervention Group. Int J Obes Relat Metab Disord. 1999; 23: 1246–1255 [DOI] [PubMed] [Google Scholar]
- 16. Graci S, Izzo G, Savino S, et al. Weight cycling and cardiovascular risk factors in obesity. Int J Obes Relat Metab Disord. 2004; 28: 65–71 [DOI] [PubMed] [Google Scholar]
- 17. Jebb SA, Goldberg GR, Coward WA, Murgatroyd PR, Prentice AM. Effects of weight cycling caused by intermittent dieting on metabolic rate and body composition in obese women. Int J Obes. 1991; 15: 367–374 [PubMed] [Google Scholar]
- 18. Beavers KM, Lyles MF, Davis CC, Wang X, Beavers DP, Nicklas BJ. Is lost lean mass from intentional weight loss recovered during weight regain in postmenopausal women? Am J Clin Nutr. 2011; 94: 767–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nicklas BJ, Wang X, You T, et al. Effect of exercise intensity on abdominal fat loss during calorie restriction in overweight and obese postmenopausal women: a randomized, controlled trial. Am J Clin Nutr. 2009; 89: 1043–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28: 412–419 [DOI] [PubMed] [Google Scholar]
- 21. Yassine HN, Marchetti CM, Krishnan RK, Vrobel TR, Gonzalez F, Kirwan JP. Effects of exercise and caloric restriction on insulin resistance and cardiometabolic risk factors in older obese adults–a randomized clinical trial. J Gerontol A Biol Sci Med Sci. 2009; 64: 90–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shah K, Stufflebam A, Hilton TN, Sinacore DR, Klein S, Villareal DT. Diet and exercise interventions reduce intrahepatic fat content and improve insulin sensitivity in obese older adults. Obesity (Silver Spring). 2009; 17: 2162–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Katzel LI, Bleecker ER, Colman EG, Rogus EM, Sorkin JD, Goldberg AP. Effects of weight loss vs aerobic exercise training on risk factors for coronary disease in healthy, obese, middle-aged and older men. A randomized controlled trial. JAMA. 1995; 274: 1915–1921 [DOI] [PubMed] [Google Scholar]
- 24. Nicklas BJ, Dennis KE, Berman DM, Sorkin J, Ryan AS, Goldberg AP. Lifestyle intervention of hypocaloric dieting and walking reduces abdominal obesity and improves coronary heart disease risk factors in obese, postmenopausal, African-American and Caucasian women. J Gerontol A Biol Sci Med Sci. 2003; 58: 181–189 [DOI] [PubMed] [Google Scholar]
- 25. Lee JS, Visser M, Tylavsky FA, et al. Weight loss and regain and effects on body composition: the Health, Aging, and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2010; 65: 78–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. French SA, Folsom AR, Jeffery RW, Zheng W, Mink PJ, Baxter JE. Weight variability and incident disease in older women: the Iowa Women’s Health Study. Int J Obes Relat Metab Disord. 1997; 21: 217–223 [DOI] [PubMed] [Google Scholar]
- 27. Arnold AM, Newman AB, Cushman M, Ding J, Kritchevsky S. Body weight dynamics and their association with physical function and mortality in older adults: the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2010; 65: 63–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Williams KV, Mullen ML, Kelley DE, Wing RR. The effect of short periods of caloric restriction on weight loss and glycemic control in type 2 diabetes. Diabetes Care. 1998; 21: 2–8 [DOI] [PubMed] [Google Scholar]
- 29. Wing RR, Blair EH, Bononi P, Marcus MD, Watanabe R, Bergman RN. Caloric restriction per se is a significant factor in improvements in glycemic control and insulin sensitivity during weight loss in obese NIDDM patients. Diabetes Care. 1994; 17: 30–36 [DOI] [PubMed] [Google Scholar]