Abstract
Exposure to secondhand smoke (SHS) exposure is increased the risk of heart disease included atherosclerosis and coronary disease. Aging is a physiology process involving progressive impairment of normal heart functions, due to an increasing vulnerability, which reduces the ability of survive. However, it is not clear pathological condition in aging exposure to SHS. The aim of this study was to examine SHS exposure in aging-related disease. The rats were placed in SHS exposure chamber and exposed to 10 cigarettes for 30 min, twice a day, 5 days/ one week for 1 month. After 4 weeks secondhand smoke exposure, rats left ventricular (LV) underwent morphological and function study with echocardiography. Histopathology of left ventricular sections were stained with Hematoxylin-Eosin staining and related left ventricular hypertrophy protein expression levels by Western blot analysis. After 4 weeks SHS exposure, LV weight showed significant increased. On the other hand, from echocardiography result, we found EF (%) and FS (%) were apparently decreased in aging SHS exposure. IVS, LVID and LVPW at diastolic diameters were increased in aging SHS exposure. However, in aging systolic diameters always preserved. Here we did not show that. Moreover, we observed enlargement morphology of the LV and LV well thickness of increase. In addition, we found LV hypertrophy marker protein, calcineurin/NFATc4, was only increased in aging and aging SHS exposure. Our study suggests that SHS exposure and aging will altercate left ventricular hypertrophy.
Keywords: apoptosis, survival signaling pathway, secondhand smoke, left ventricular hypertrophy
Secondhand smoke (SHS) exposure increase the risk of heart disease, including progression atherosclerosis, decreased heart rate variability, increased arterial stiffness, and increased risk of coronary disease events. Left ventricular hypertrophy has been observed in rabbits exposed to SHS. Left ventricular hypertrophy leads to ventricular remodeling and increases the risk of a cardiovascular event and mortality (1,2,3). Most of SHS is harmful and cause human diseases, especially in aging (4,5). However, the role of SHS exposure in human cardiac aging remains unclear. Age-related cardiac diseases is associated with numerous molecular and biochemical changes in the heart. These changes affect protein function and cardiac morphology result in alterations of cell death signals and cell survival signals. The biochemical changes also affect mitochondrial membrane anti-apoptosis and apoptosis proteins expression levels [6]. Human cardiac aging generates a complex phenotype. Experimental evidence in animal models indicates attenuation in cardioprotective pathways with aging, yet limit myocardial dysfunction information in the aging. It is available regarding aging-related changes also in the human heart. However, left ventricular hypertrophy (LVH) is an initial adaptive response. There are a lot of compensatory mechanisms to increased cardiac work-load and stimulation left ventricular sustains (7). Secondhand smoke (SHS) exposure increase the risk of heart disease, including progression atherosclerosis (8), decreased heart rate variability, increased arterial stiffness (9), and increased risk of coronary disease events. Left ventricular hypertrophy has been observed in rabbits exposed to SHS (10). Left ventricular hypertrophy leads to ventricular remodeling and increases the risk of a cardiovascular event and mortality. During LVH development, there are a imalance of progressive remodeling at cellular levels, such as calcineurin/NFATc4 (11). Therefore, we detected the molecular mechanisms behind SHS exposure in the aging to identify the pathological of cardiac disease disorder and elusive.
MATERIALS AND METHODS
Animals
We purchased male Hamster rats (Young: 6 weeks of age; body weight, 132.5 ± 4.61 g; Old: 18 months of age, body weight, 130.2 ± 6.04 g) from National Science Council Animal Center. The animals were housed six in individual cages in an environmentally controlled animal room, and water was provided tap water. All animals were handled according to the guidelines of the Taiwan Society for Laboratory Animals Sciences for the care and use of laboratory animals in temperature and humidity controlled chambers.
Experimental design and secondhand smoke (SHS) exposure treatment
Rats were divided into two age groups, young adult and old male which were divided into two subgroups and treated for 4 weeks secondhand smoke (SHS) exposure as follows: (1) Control (C), comprising 6 animals not exposed to cigarette secondhand smoke. (2) Secondhand smokers (S), comprising 6 animals exposed to cigarette secondhand smoke (SHS). The rats were placed in whole-body exposure chambers and exposed to 10 cigarettes. Filtered air was introduced into the chamber at a low rate. Rats were exposed to cigarette smoke for 30 min, twice a day, 5 days/week for 1 month. Room temperature was maintained at 22–25°C, and relative humidity was approximately 40%.
Echocardiography
Transthoracic echocardiography was performed at baseline and at 2 and 4 weeks after second hand smoke exposure (SHS) using a Hewlett-Packard Sonos 5500 ultrasound machine with a 15-MHz linear-array transducer, as described previously 12 Animals were anesthetized with a half dose of the ketamine-xylazine-atropine mixture used. M-mode images were recorded and analyzed offline. The following parameters were measured and calculated: left ventricular interior diastolic dimention (LVIDd), Interventricular septum at diastolic (IVSd), LV posterior wall thickness at diastole (LVPWd), fractional shortening (FS%) and ejection fractional (EF%).
Histological analysis by Hematoxylin-Eosin stain and Masson’s Trichrome Staining
Cross section of left ventricle were cut 10 um thick and placed on slides. Slides were prepared by deparaffinization and dehydration. They were passed through a series of graded alcohols from 100% to 95% to 75%, 15 min each.
For assessment of left ventricle cross-sectional area and extracellular space, cross sections were stained with hematoxylin or masson’s trichrome incubated for 5 min at room temperature. After rinsing with water, each slide was then soaked with 85% alcohol, 100% alcohol for 15 min. Stained sections were then rinsed with PBS and air dried before mounting. Left ventricles stained with hematoxylin-eosin or masson’s trichrome from the following groups: male young controls (MYC), male young smoke (MYS), male old control (MOC) and male old smoke (MOS) (X10 resolution with 200um calibration bar).
Western blot
We prepared the tissue extract samples as described above. 12.5% SDS-PAGE was carried out with polyacrylamide gels. The samples were electrophoresed at 100 V for 1 hr. Electrophoresed proteins were transferred to nitrocellulose paper using a Hoefer Scientific Instruments Transphor unit at 100 mA for 2 hr. We incubated nitrocellulose papers in blocking buffer for 2 hr at room temperature. Monoclonal antibodies (calcineurin and NFATc4) were diluted 1:200 in antibody binding buffer (TBS). Incubations were performed at room temperature for 3.5 hr. We washed the immunoblots three times in 50 mL blotting buffer for 10 min and then immersed in the second antibody solution containing alkaline phosphatase goat anti-rabbit IgG for 1 hr and diluted 1,000-fold in binding buffer. The filters were then washed in blotting buffer for 10 min three times. Color development was presented in ECL chemiluminescence.
Statistical analysis
All data examined are expressed as mean±SEM. For Western blot and analysis, quantitation was carried out by scanning and analyzing the intensity of the hybridization signals using FUJIFILM Imagine program. Statistical analysis of the data was performed using SigmaStat software. When assessing multiple groups, comparison between groups was made using a one-way analysis of variance (ANOVA) with Tukey’s post hoc test. Unpaired, two-sided Student’s t test was used when indicated. A p value of less than 0.05 and 0.01 were considered to be statistically significant.
RESULTS
Development of left ventricular and left ventricular function on Echocardiography in SHS exposure and in aging SHS exposure rats
Echocardiography of rat model of SHS exposure was presented in young adult (6-weeks-old) through old aged (18-mo-old) rats, we found left ventricular internal dimension at end diastole (LVIDd), left ventricular posterior wall thickness at end diastole (LVPWd), Interventricular septal at end diastole (IVSd) were increased in SHS exposure and aging SHS exposure group (Figure 1). Ejection Fraction (EF%) and Fraction Shortening (FS%) were progressive impairment in SHS and aging, especially in aging SHS exposure. *p<0.05, **p<0.01 significantly difference compared with male young control (MYC); #p<0.05, ##p<0.01 significantly difference compared with male old age (MOC).
Figure 1.
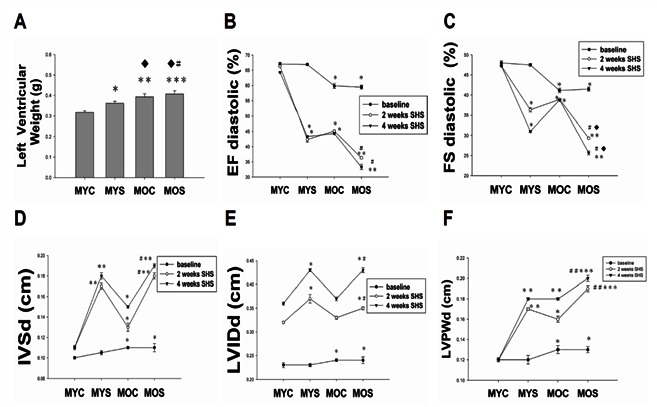
Echocardiography is a primary imaging method in the assessment of cardiac function. A. Left ventricular weight (g). B. Fractional shorting (FS%). C. Ejection fraction (EF%). D. Interventricular septum at diastolic (IVSd). E. left ventricular interior diastolic dimention (LVIDd). F. LV posterior wall thickness at diastole (LVPWd). *p<0.05, **p<0.01, ***p<0.001 vs. male young control (MYC); ♦p<0.05, ♦♦p<0.01, ♦♦♦p<0.001 vs. male young smoke (MYS); #p<0.05, ##p<0.01, ###p<0.001 vs. male old smoke (MOC).
On the other hand, we also observed LV weight increase from Figure 1A.
Histological and morphology analysis of left ventricular by Hematoxylin-Eosin and Masson’s Trichrome Staining
According to Figure 2 left ventricular (LV) cross sections were stained with hematoxylin-eosin and Masson’s Trichrome Staining to visualization of morphology and Histological. We observed that LV wall thickness and chamber were increased in SHS exposure and aging SHS exposure groups. A phase of left ventricular hypertrophy (LVH) during adaptive stress and overload is individual left ventricular myocytes grown in length and/or width as compensated hypertrophy. In aging exposure to SHS of the LV cross-sections by hematoxylin/eosin staining, we found that left ventricular muscle fibers interstitial was broad, decreased in conduction density and disordered more than normal. The muscle fiber was arranged relaxtion and the interstitial spaces were broad. Morphometric samples are at x100 magnification. On the other hand, left ventricular (LV) cross sections were stained with Masson’s trichrome for visualization of morphology and identification of location. We focused on the LV as aging -related cardiac remodeling are more prevalent in LV than other parts of the heart. We observed dramatic remodeling of the LV from the aging groups including secondhand smoke (SHS) exposure when compared with left ventricular sections from the young animals. Substantial enlargement of connective tissue existed as a result of aging and exposure to SHS rats. Remarkably, left ventricular cross sections from the aging exposure to SHS group exhibited more aging-related remodeling.
Figure 2.
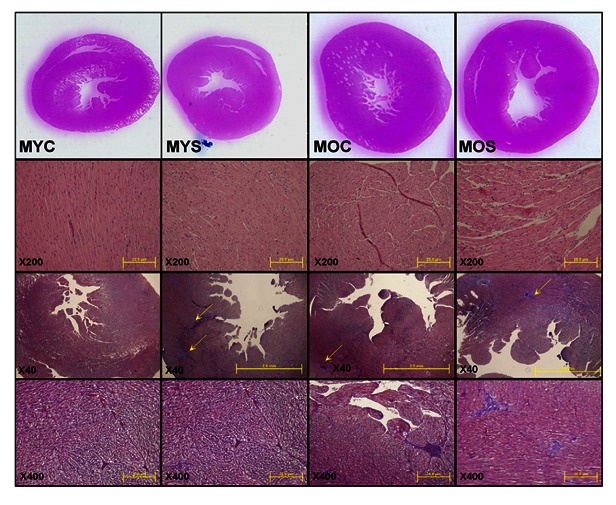
Histopathologic of left ventricular in aging and aging SHS exposure by Hematoxylin-Eosin and Masson’s Trichrome Staining. All data described are from young control (MYC), male young secondhand smoke (MYS), male old control (MOC), and male old secondhand smoke (MOS) groups of Hamster rats as outlined in experimental design. The images of myocardial architecture were magnified 40x, 200x and 400x. Arrow head represent collagen accumulation.
Calcineurin/NFATc4 signaling pathway induced left ventricular hypertrophy only in aging
In recent years, aging have constructed as physiological adaption response. The aging also induced cardiac hypertrophy similar as disease-induced. Calcineurin/NFAT are the Ca2+_ dependent serine/threonine protein-phosphatase calcineurin which was identified as a central pro-hypertrophic signaling molecule in the myocardium in pathological cardiac hypertrophy. Our data showing continued activation of calcineurin/NFAT throughtout the aging induced LV hypertrophy. Calcineurin/NFAT function was to maintain the LV hypertrophic profile of disease-dependent, but calcineurin/NFAT activation was increased only in aging and aging exposure to SHS (*p<0.05, **p<0.01 vs MYC; #p<0.05, ##p<0.01 vs MOS). Interestingly, there is no significantly difference in the young age exposure to SHS compared with young control.
DISCUSSION
Secondhand smoke (SHS) exposure increases the risk of coronary heart disease, especially in elderly age and is associated with an increased risk of atherosclerotic heart disease (12). The old age is a strong independent predictor for death, and morbidity in patient with structural heart disease (13). Therefore, the old age is also a major risk factor associated with poor cardiovascular outcome and reduces endogenous cardioprotection. There were 25.7% of students exposed to SHS at home, 34.2% outside of the home and 18.3% were exposed to SHS at home and outside of the home. Human cardiac aging generates a complex phenotype. Experimental evidence in animal models indicates attenuation in cardioprotective pathways with aging, yet limit myocardial dysfunction information in the aging. It is available regarding age-related changes also in the human heart (14). Age heart of normal old aging changes can produce mimic cardiac diseases, including coronary arteries, myocardial infarction, cardiac valves, aortic regurgitation. Age affects cardiovascular the same as secondhand smoke exposure. Age-related changes in left ventricular morphology and function, for example, decrease in myocyte number, increase in myocyte size (15). Secondhand smoke exposure (SHS) is linked to a number of harmful health outcomes. It causes of morbidity and mortality. There are a lot of evidences indicated that SHS exposure is a formidable health hazard (16). Because of a toxic air contaminant causes annual lung cancer and cardiovascular disease. Here, we want to know SHS exposure associated with the aging. First, we know the old annual human always accompany with atherosclerotic vascular disease (17). Second, we know secondhand smoke (SHS) exposure is associated with an increased risk of atherosclerotic heart diseased and cardiac events (18). Here, we found the aging-relatived changes will lead to cardiovascular pathological outcomes the same cardiac adaptations as SHS exposure. Thus, we found that a related changes cardiac morphology and LV function declined in aging exposure to SHS by echocardiography (Figure 1 and Figure 2). However, It is now unclear that several signaling molecules play unique roles in the regulation of pathological (SHS) and physiological cardiac hypertrophy (aging) (19). On the other hand, pathologic cardiac hypertrophy molecular markers, calcineurin and NFAT, were increased only in aging. There is no significantly difference in SHS exposure in young age. There are some papers elucidated SHS exposure maybe is safe (20).
Figure 3.
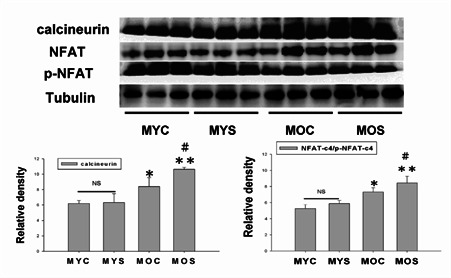
Stimulation of calcineurin and NFAT activation in aging cardiomyocyts. Calcineurin, NFAT and p-NFAT protein expression were increased in aging and aging exposure to SHS by western blot analysis. Quantification of densitometry analysis of protein levels. *p<0.05, **p<0.01, significantly difference compared with the male young control (MYC). #p<0.05, ##p<0.01, significantly difference compared with the male old control (MOC).
Acknowledgments
This work was supported by a grant from the National Science Council, Republic of China.
References
- [1].Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation. 2005;111:2684–98. doi: 10.1161/CIRCULATIONAHA.104.492215. [DOI] [PubMed] [Google Scholar]
- [2].Hamer M, Stamatakis E, Kivimaki M, Lowe GD, Batty GD. Objectively measured secondhand smoke exposure and risk of cardiovascular disease: what is the mediating role of inflammatory and hemostatic factors? J Am Coll Cardiol. 2010;56:18–23. doi: 10.1016/j.jacc.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lu SQ, Fielding R, Hedley AJ, Wong LC, Lai HK, Wong CM, Repace JL, McGhee SM. Secondhand smoke (SHS) exposures: workplace exposures, related perceptions of SHS risk, and reactions to smoking in catering workers in smoking and nonsmoking premises. Nicotine Tob. 2011;13:344–52. doi: 10.1093/ntr/ntr001. [DOI] [PubMed] [Google Scholar]
- [4].Rudatsikira E, Muula AS, Siziya S. Exposure to environmental tobacco smoke among adolescents in Kampala-Uganda. East Afr J Public Health. 2002;6:197–9. doi: 10.4314/eajph.v6i2.51770. [DOI] [PubMed] [Google Scholar]
- [5].Olivetti G, Melissari M, Capasso JM, Anversa P. Cardiomyopathy of the aging human heart. Myocyteloss and reactive cellular hypertrophy. Circ Res. 1991;68:1560–8. doi: 10.1161/01.res.68.6.1560. [DOI] [PubMed] [Google Scholar]
- [6].Lakatta EG. Cardiovascular regulatory mechanisms in advanced age. Physiol Rev. 1993;73:413–67. doi: 10.1152/physrev.1993.73.2.413. [DOI] [PubMed] [Google Scholar]
- [7].Nadal-Ginard B, Kajstura J, Leri A, Anversa P. Myocytedeath, growth, and regeneration in cardiac hypertrophy and failure. Circ Res. 2003;92:139–50. doi: 10.1161/01.res.0000053618.86362.df. [DOI] [PubMed] [Google Scholar]
- [8].Xu F, Ji J, Li L, Chen R, Hu W. Activation of adventitial fibroblasts contributes to the early development of atherosclerosis: a novel hypothesis that complements the “Response-to-Injury Hypothesis” and the “Inflammation Hypothesis”. Med Hypotheses. 2007;69:908–12. doi: 10.1016/j.mehy.2007.01.062. [DOI] [PubMed] [Google Scholar]
- [9].Wilcox JN, Scott NA. Potential role of the adventitia in arteritis and atherosclerosis. Int J Cardiol. 1996;54(Suppl):S21–35. doi: 10.1016/s0167-5273(96)02811-2. [DOI] [PubMed] [Google Scholar]
- [10].Weeks SG, Glantz SA, De Marco T, Rosen AB, Fleischmann KE. Secondhand smoke exposure and quality of life in patients with heart failure. Arch Intern Med. 2011;171:1887–93. doi: 10.1001/archinternmed.2011.518. [DOI] [PubMed] [Google Scholar]
- [11].Boyle P, Maisonneuve P. Lung cancer and tobacco smoking. Lung Cancer. 1995;12:167–81. doi: 10.1016/0169-5002(95)00443-5. [DOI] [PubMed] [Google Scholar]
- [12].Castardeli E, Duarte DR, Minicucci MF, Azevedo PS, Matsubara BB, Matsubara LS, Campana AO, Paiva SA, Zornoff LA. Exposure time and ventricular remodeling induced by tobacco smoke exposure in rats. Med Sci Monit. 2008;14:BR62–66. [PubMed] [Google Scholar]
- [13].Waller BF. The old-age heart: normal aging changes which can produce or mimic cardiac disease. Clin Cardiol. 1988;11:513–7. doi: 10.1002/clc.4960110802. [DOI] [PubMed] [Google Scholar]
- [14].Waller BF, Bloch T, Barker BG, Roe SJ, Hawley DA, Pless JC, Eble JN. The old-age heart: aging changes of the normal elderly heart and cardiovascular disease in 12 necropsy patients aged 90 to 101 years. Cardiol Clin. 1984;2:753–79. [PubMed] [Google Scholar]
- [15].Quarta G, Husain SI, Flett AS, Sado DM, Chao CY, Tomé Esteban MA, McKenna WJ, Pantazis A, Moon JC. Arrhythmogenic right ventricular cardiomyopathy mimics: role of cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2013;15:16. doi: 10.1186/1532-429X-15-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Venn A, Britton J. Exposure to secondhand smoke and biomarkers of cardiovascular disease risk in never-smoking adults. Circulation. 2007;115:990–5. doi: 10.1161/CIRCULATIONAHA.106.648469. [DOI] [PubMed] [Google Scholar]
- [17].Lakatta EG. Cardiovascular aging research: the next horizons. J Am Geriatr Soc. 1999;47:613–25. doi: 10.1111/j.1532-5415.1999.tb02579.x. [DOI] [PubMed] [Google Scholar]
- [18].Raya TE, Gaballa M, Anderson P, Goldman S. Left ventricular function and remodeling after myocardial infarction in aging rats. Am J Physiol. 1997;273:H2652–8. doi: 10.1152/ajpheart.1997.273.6.H2652. [DOI] [PubMed] [Google Scholar]
- [20].Anversa P, Palackal T, Sonnenblick EH, Olivetti G, Meggs LG, Capasso JM. Myocyte cell loss and myocyte cellular hyperplasia in the hypertrophied aging rat heart. Circ Res. 1990;67:871–85. doi: 10.1161/01.res.67.4.871. [DOI] [PubMed] [Google Scholar]
- [21].Li XM, Ma YT, Yang YN, Liu F, Chen BD, Han W, Zhang JF, Gao XM. Downregulation of survival signalling pathways and increased apoptosis in the transition of pressure overload-induced cardiac hypertrophy to heart failure. Clin Exp Pharmacol Physiol. 2009;36:1054–61. doi: 10.1111/j.1440-1681.2009.05243.x. [DOI] [PubMed] [Google Scholar]


